Abstract
Background
Acinetobacter baumannii has emerged as an increasingly important and successful opportunistic human pathogen due to its ability to withstand harsh environmental conditions, its characteristic virulence factors and quick adaptability to stress.
Methods
We developed a clinically relevant murine model of A. baumannii traumatic wound infection to determine the effect of local wound environment on A. baumannii virulence. Mice underwent rectus muscle crush injury combined with ischemia created by epigastric vessel ligation, followed by A. baumannii inoculation. Reiterative experiments were performed using 1) a mutant deficient in the production of the siderophore acinetobactin, or 2) iron supplementation of the wound milieu. Mice were euthanized 7 days later and rectus muscle analyzed for signs of clinical infection, HIF1α accumulation, bacterial abundance and colony morphotype. To determine the effect of wound milieu on bacterial virulence, the Galleria mellonella infection model was utilized.
Results
The combination of rectus muscle injury with ischemia and A. baumannii inoculation resulted in 100% incidence of clinical wound infection that was significantly higher compared to other groups (n=15/group, p<0.0001). The highest level of wound infection was accompanied with the highest level of A. baumannii colonization (p<0.0001) and the highest degree of HIF1α accumulation (p<0.05). A. baumannii strains isolated from injured/ischemic muscle with clinical infection displayed a rough morphotype and a higher degree of virulence as judged by G. mellonella killing assay as compared to smooth morphotype colonies isolated from injured muscle without clinical infection (100% vs. 60%, n=30 Log-Rank test, p=0.0422). Iron supplementation prevented wound infection (n=30, p<0.0001) and decreased HIF1α (p=0.039643). Similar results of decrease in wound infection and HIF1α were obtained when A. baumannii wild type was replaced with its derivative mutant ΔBasD deficient in acinetobactin production.
Conclusions
The ability of A. baumannii to cause infections in traumatized wound relies on its ability to scavenge iron and can be prevented by iron supplementation to the wound milieu.
Keywords: Acinetobacter baumannii, ΔBasD, wound injury, ischemia, infection, iron, HIF1α
Background
Over the past thirty years, Acinetobacter baumannii has gained recognition as an increasingly important opportunistic human pathogen. Worldwide, an increasing number and severity of A. baumannii outbreaks have occurred in various settings, such as hospitals, long-term care facilities, military bases, and within the civilian community (1–4). A. baumannii’s ability to persist in harsh environmental conditions coupled with its alarmingly rapid rate of antibiotic resistance acquisition places this pathogen as one of the six most problematic multi-drug resistant (MDR) healthcare-associated pathogens worldwide (5). Although A. baumannii is known for its ability to cause a broad range of infectious complications, from skin and soft tissue infections to ventilator-associated pneumonia and catheter-related blood stream infections (6), here we focus on A. baumannii wound infections as they relate to tissue trauma and host stress.
A. baumannii traumatic wound infections have become a topic of recent interest with reports of increasing incidences of outbreaks among victims of combat injuries and natural disasters (7). Recent data from Iraq and Afghanistan identify highly resistant strains of A. baumannii to be some of the most common organisms causing severe and often lethal wound infections (4, 8, 9). Trauma related A. baumannii burn wound infections and osteomyelitis are also emerging as commonly encountered problems in the injured military personnel (10–12). In the nonmilitary setting, A. baumannii has been reported to be a cause of surgical site infections in some institutions (13, 14) and an infrequent cause of skin and soft tissue infections in the intensive care unit setting (1). Especially troubling are the antimicrobial resistance profiles of A. baumannii wound infections, with reports of epidemics caused by multidrug-resistant strains as well as strains resistant to all available antimicrobials (15).
The increasing success of A. baumannii as a human pathogen lies in its virulence factors as well as its quick adaptability to changing environmental conditions (16). The virulence factors responsible for the long-term survival and wide-spread transmissibility in the health care environment include the A. baumannii’s ability to attach to and persist on solid and otherwise abiotic surfaces in the form of a biofilm as well as its ability to resist desiccation and disinfection (17, 18). A. baumannii is significantly less permeable to biocidal substances, harbors efflux pumps that facilitate its persistence on nosocomial surfaces, and thrives in the presence of ethanol (19). A. baumannii has been shown to easily spread from infected patients to the environment through the aerosol route and can survive in the nosocomial environment for up to 13 days (20). Importantly, A. baumannii is rarely found outside of the healthcare setting and exhibits a low human carrier rate (20). To illustrate this point, A. baumannii has been recovered from all military treatment facilities sampled on the aeromedical evacuation route from Iraq and Afghanistan; however, A. baumannii was found on the skin of only 1 of 160 screened patients and in 1 of 49 soil samples (genetically separate from the clinical isolates), suggesting that its reservoir of infection persists within treatment facilities but rarely beyond (3). These characteristics coupled with the ability of A. baumannii to use horizontal gene transfer to acquire multi-drug resistance, make this a particularly problematic and virulent pathogen.
A. baumannii has developed additional virulence factors that allow it to evade host defenses. One important innate host immune response is the sequestration of essential metal nutrients such as iron away from pathogens, termed nutritional immunity (21, 22). A. baumannii has developed a variety of high-affinity iron acquisition systems to overcome nutritional immunity and scavenge iron away from the host (22) Production of the acinetobactin siderophore is one such mechanism (23). Iron-limited conditions upregulate A. baumannii genes involved in iron acquisition as well as genes involved in growth, motility and respiration (24). Deletion and insertion inactivation of genes coding for acinetobactin synthesis and transport impairs A. baumannii virulence and reduces its lethality when tested with the Galleria mellonella and the mouse sepsis models (23). We therefore hypothesize that iron depletion plays a significant role in A. baumannii traumatic wound infection pathogenesis and posit that iron abundance in the wound milieu prevents in-vivo virulence activation and protects the host from wound infection.
In order to inform molecular mechanisms responsible for host morbidity and mortality due to A. baumannii wound infections, there is a growing need to develop clinically relevant animal models (25). The aim of this study was to establish a reproducible murine model of A. baumannii wound infection. We demonstrate that A. baumannii wound infection success is dependent on the degree of tissue trauma, with the requirement of tissue injury for abscess development and increased virulence with addition of tissue ischemia. We also show that iron abundance in the wound milieu prevents in-vivo virulence activation and protects the host from wound infection, suggesting a key role of iron in A. baumannii infection pathogenesis. Our findings inform potential targets for treatment and prevention strategies against this highly problematic pathogen.
Methods
Bacteria
A. baumannii ATCC 19606T and its derivative mutant ΔbasD (26), deficient in the production of siderophore acinetobactin, were used in the experiments. A. baumannii was plated on tryptic soy agar (TSA) from a frozen stock and grown overnight at 37°C. A few colonies were then suspended in liquid TSB medium, sub-grown for 1 hour on a heated shaker and then adjusted to an OD600 of 0.5. Bacterial suspension was centrifuged at 3,300 × g for 10 min, the excess TSB removed and the remaining pellet was re-suspended in the same volume of 0.9% normal saline. The resulting microbial suspension was used for wound inoculation.
Mouse model of Acinetobacter baumannii wound infection
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Chicago (IACUC protocol 72090). Seven- to nine-week old male C57B/L6 mice were used for all experiments. Mice were routinely fed tap water and Harland Teklad feed (Madison, WI) under 12-h light/dark cycles. All animals were allowed to acclimate for at least 48 hours before surgery. Knowing that tissue trauma related to combat wounds, such as blast injuries and gunshot wounds, is often associated with vascular injury leading to tissue ischemia and necrosis, we designed a more clinically relevant model incorporating both, injury and ischemia. A vertical skin incision was made under sterile conditions and the rectus abdominis muscle was exposed by undermining the skin. Muscle injury was performed using forceps to lightly crush the rectus muscle. Ischemia was induced by double suture ligation of the rectus muscle superiorly and inferiorly with care taken to include the epigastric vascular bundle in the suture ligature. The wound was then inoculated with 100 μL of A. baumannii suspension (106 CFU/mL) in 0.9% normal saline for 2 minutes, followed by washout with 0.9% normal saline and skin closure. Mice were randomly assigned into four groups (n=15/group): 1. A. baumannii (AB) inoculation without muscle injury or ischemia (sham + AB), 2. Muscle injury alone followed by A. baumannii inoculation (Injury + AB), 3. Muscle ischemia only followed by A. baumannii inoculation (Isch + AB), 4. Muscle injury and ischemia followed by A. baumannii inoculation (Injury/Isch + AB). Mice were euthanized on post-operative day 7 (POD7) and wounds were examined for the presence of gross abscesses. The rectus muscle was excised, homogenized in 10% glycerol and cultured on MacConkey agar. Rectus muscle tissues were assayed for HIF1-α expression as described below.
Role of iron in A. baumannii wound infection
To assess the role of iron in A. baumannii wound infection, reiterative experiments were performed using the above model. Following A. baumannii inoculation, 200 μL of 100 mM FeCl3 (Sigma Aldrich) in 0.9% normal saline was used as a washout solution prior to skin closure in place of 0.9% normal saline alone (n=30/group). Mice were euthanized on POD7 and wounds were examined for the presence of gross abscesses. The rectus muscle was excised, homogenized in 10% glycerol and cultured on MacConkey agar. Rectus muscle tissues were also used in HIF1-α assays as described below.
HIF1α assay
HIF1α tissue levels were assayed by western blot analysis. Resected rectus muscle tissues were weighed, homogenized in 10% glycerol using a sterile glass tissue grinder (Corning), snap frozen in liquid nitrogen and kept in −80°C. Ice-cold lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% Triton X-100, and phenylmethylsulfonyl fluoride, 0.1 mM (Sigma)] was added to the homogenized tissues at the ratio of 10:1 and incubated for 15 min on ice. Following centrifugation at 9,300 × g for 15 minutes, the supernatants were boiled for 5 min with Laemmli sample buffer (Novex), electrophoresed through 10% SDS–polyacrylamide gels, and then transferred onto polyvinylidene difluoride membranes (Immobilon-P, Millipore). The membranes were blocked for 1 hour in TBS Tween-20 buffer (ThermoScientific) with 4% nonfat dry milk (LabScientific). Membranes were then incubated with primary anti-HIF-1α antibody (Novus Biologicals), followed by the corresponding horseradish peroxidase-coupled secondary antibody (at dilutions recommended by the vendors). Beta-actin was used as an internal housekeeping protein control. The membranes were developed using ECL Western Blotting Detection Reagents (GE Healthcare). Densitometry analyses were performed using ImageJ software.
A. baumannii virulence assay using Galleria mellonella
Last-instar (VII) larvae of the wax moth G. mellonella in the weight range of 250–350 mg were stored in the dark and used within 1 week of shipment (Best Bet, Inc., Blackduck, MN). Intrahaemocoelic injection of 10 μl of A. baumannii suspension diluted to OD600 of 0.2 was performed using the KDS-100 single syringe pump (KD Scientific). Larvae were placed between the thumb and forefinger and needle was carefully placed in the cuticle. A. baumannii isolates were recovered from sham + AB muscle samples from mice that did not display gross abscess formation but were nonetheless culture positive, as well as and from Injury/Isch + AB muscle samples with gross abscess formation. Isolates were grown overnight on MacConkey agar and subgrown for 1 hour in liquid TSB media. The suspension was then diluted to OD600 of 0.2 in 0.9% normal saline and 10 μl injected into the larvae to determine differences in larvae mortality and hence virulence among the tested strains. After each injection, the larva was carefully removed away from the needle and placed in a 5-cm petri dish. Fifteen larvae were used per group in two replicates. The larvae were then incubated at 37°C in the dark and followed for mortality over the next 72 hours. Dead larvae were recognized by lack of movement and color change to black.
Statistics
Statistical analysis was performed using GraphPad Prism 7.00 (GraphPad Software, La Jolla, California). Categorical variables were compared using chi-square test, and continuous variables using Student’s t-test. Non-parametric statistical analysis with Bonferroni correction for multiple comparisons was used for the infection analysis in mice. Galleria mellonella survival curves were created using Kaplan-Meier method with log-rank test. Statistical significance was established at P ≤ 0.05.
Results
Muscle injury and ischemia are synergistic and sufficient for A. baumannii wound infection in mice
Gross infection determined by presence of an abscess was noted in 100% of mice on POD7 following rectus muscles injury and ischemia with AB inoculation (Injury/Isch + AB) (Fig. 1A). None of the control groups without A. baumannii inoculation (i.e. ischemia alone, injury alone, or ischemia and injury) developed clinically detectable infections. Concurrently, we confirmed 0% rate of abscess formation in the sham + AB group, 0% abscess formation in the Isch + AB group and 20 % rate of abscess formation in the Injury + AB group, this was however not a statistically significant difference (n=15 per group, p=0.68; Bonferroni corrected α′=0.0024) (Fig. 1B). These findings significantly differed from the 100% rate of abscess formation in the Injury/Isch + AB group (n=15 per group, *p<0.0001; Bonferroni corrected α′=0.0024) (Fig. 1B).
Figure 1. Muscle injury and ischemia are required for Acinetobacter baumannii wound infection in mice.
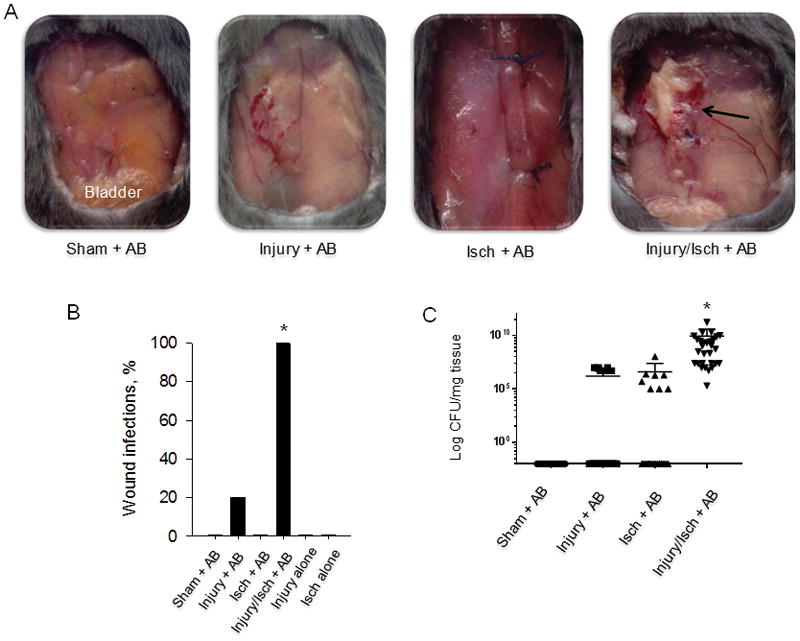
(A), Images of wounds on post-operative day 7 (POD7) in four treatment groups of mice. Black arrow denotes an abscess. (B), Percent of animals with gross wound infection in each group (n=15/group). The rate of abscess formation in Injury + AB group reached 20% but was not significantly higher compared to Sham + AB group (P=0.68; Bonferroni corrected α′=0.0024). The rate of abscess formation in the Injury/Isch + AB group was significantly greater than in all other groups (p < 0.0001; Bonferroni corrected α′=0.0024) (C), Bacterial density of A. baumannii in mouse tissues. All injured and ischemic tissue samples (Inj/Isch + AB) displayed significantly greater A. baumanni colonization compared to other groups (n=30; 15 mice/group × 2 tissue samples each, *p<0.0001, Mann-Whitney test). A negligible level of contamination (seen as black dots on the × axis) was found in majority of samples in other groups. Each dot on graph represents CFU value of each tissue sample. AB (A. baumannii), Injury (muscle injury), Isch (muscle ischemia).
A. baumannii densely colonizes injured ischemic muscle tissues
Upon culture of homogenized rectus muscle wound tissues at the time of mice sacrifice at POD7, wounds subjected to muscle injury, ischemia and A. baumannii inoculation (Injury/Isch + AB) displayed 1010 average colony forming unit (CFU) count per milligram of tissue that was significantly higher compared to the other groups (n=30 tissue samples/group, *p<0.0001) (Fig. 1C). Only few tissue samples in Injury + AB and Isch + AB groups were colonized by A. baumannii at the average 106 CFU/mg tissue, these two groups did not significantly differ from one another (n=30, p=0.776).
HIF1α expression is greatest in injured and ischemic tissues infected by A. baumannii
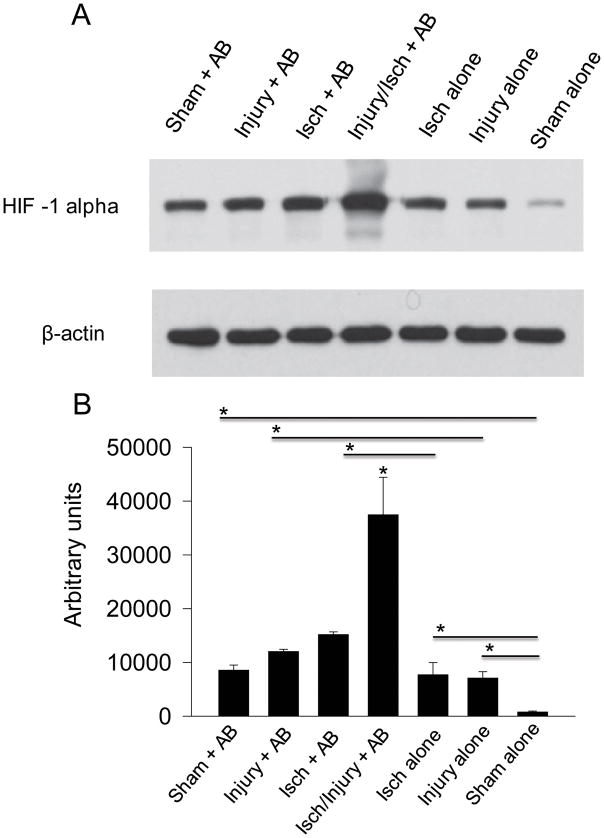
The groups of mice with at least one insult (Injury alone or Ischemia alone) had elevated levels of HIF1α compared to rectus muscle of non-treated mice (sham alone) (p=0.018348 and 0.049987, respectively) (Fig. 2). A. baumannii (AB) inoculation led to an increase in HIF1α in all groups (Sham + AB vs Sham alone, p=0.0076; Injury + AB vs Injury alone, p=0.031782; and Isch + AB vs Ischemia alone, p=0.045198), highlighting the role of A. baumannii (AB) in HIF1α expression. The triple insult consisting of injury, ischemia and A. baumannii (Injury/Isch + AB) led to ~3 fold increase in HIF1α production compared to Isch + AB (p=0.045954) and Injury + AB (p=0.035776) (Fig. 2).
Figure 2. HIF1α is most abundant in injured ischemic tissues exposed to A. baumannii.
(A), Western blot analysis of muscle tissues isolated on POD7. (B), Protein band densities evaluated by ImageJ (n=3, *p<0.05). AB (A. baumannii), Injury (muscle injury), Isch (muscle ischemia). Error bars indicate standard deviation.
Iron plays a critical role in the pathogenesis of A. baumannii wound infection
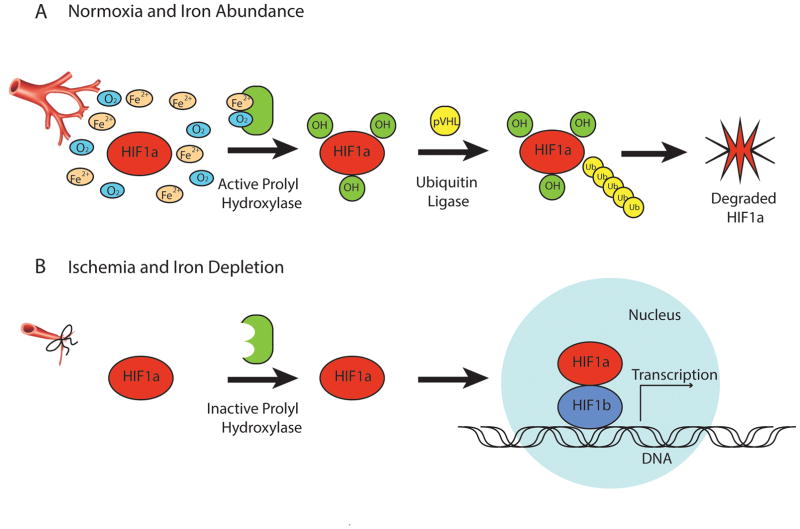
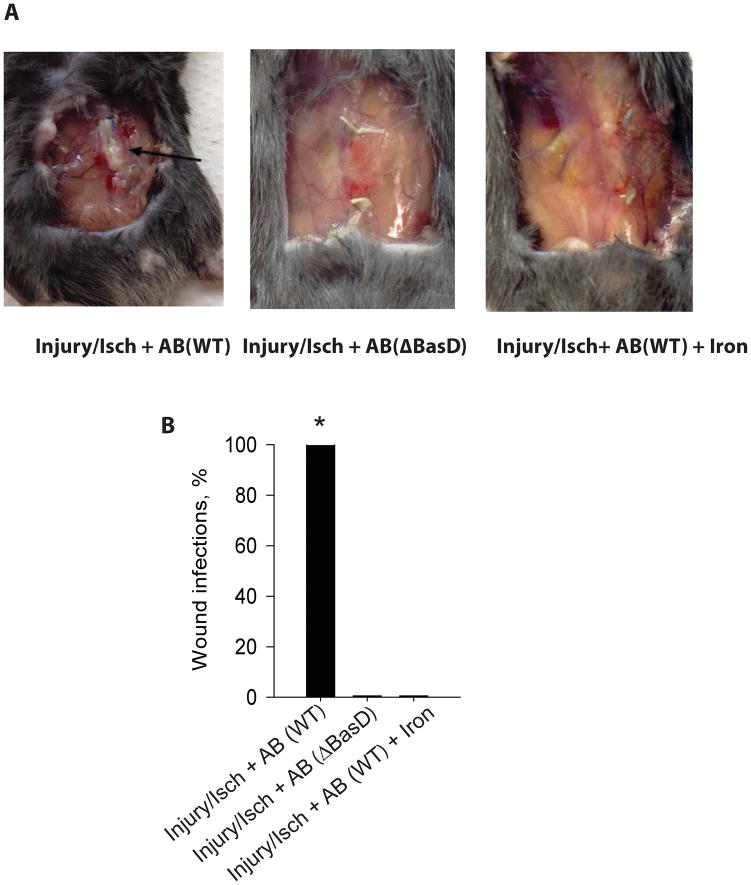
Since iron is a cofactor of HIF1α prolyl hydroxylase required for HIF1α ubiquitination (Fig. 3), we next hypothesized that the high level of HIF1α accumulation in the tissue of the Injury/Isch + AB animal group may be related, in part, to A. baumannii’s ability to locally scavenge iron from the wound milieu away from host tissues. We therefore introduced iron into the wound milieu of our murine model of A. baumannii wound infection. The wounds washed out with 200 μL of 100 mM FeCl3 solution following muscle injury, ischemia and A. baumannii inoculation, did not develop wound infections (n=30 per group, *p<0.0001) (Fig. 4). Reiterative experiments were performed using an A. baumannii ATCC 19606T ΔBasD mutant deficient in the synthesis of the iron scavenging siderophore acinetobactin and hence without ability to sequester iron from the local environment. Results indicated that following muscle injury, ischemia and ΔBasD A. baumannii inoculation, none of 30 animals developed wound infections (Fig. 4).
Figure 3. The role of oxygen and iron in HIF1α degradation.
(A), In the presence of normoxia and iron abundance, HIF1α undergoes hydroxylation by active form of prolyl hydroxylase, which requires oxygen and iron cofactors. This is followed by proteasomal degradation of HIF1α mediated by ubiquitin ligase. (B), Limitation of oxygen and iron, characteristic of injured and ischemic tissues, are essential for post-translational HIF1α stabilization and accumulation, affecting gene transcription.
Figure 4. Iron plays a critical role in A. baumannii wound infection.
(A), Images of wounds on POD7 in three treatment groups of mice. Black arrow denotes an abscess. (B), Percent of animals with gross wound infection in each group (n=30/group, *p<0.0001). AB (A. baumannii), Injury (muscle injury), Isch (muscle ischemia), WT (wild type), ΔBasD (A. baumannii mutant deficient in scavenging iron).
Injured and ischemic tissues exposed to A. baumannii produce lower levels of HIF1α in the presence of iron
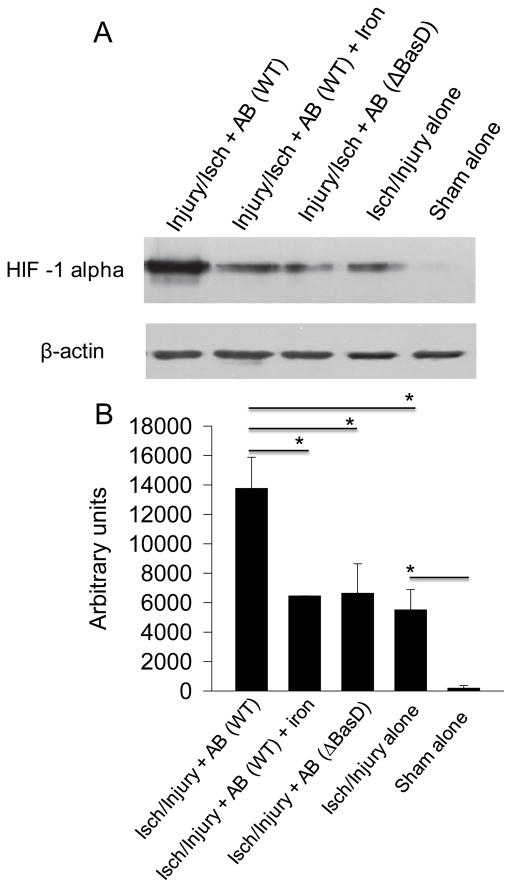
We next evaluated the role of iron in the accumulation of HIF1α in wound tissues using Injury/isch + AB groups of mice. The presence of A. baumannii significantly increased HIF1α expression (p=0.043862). However, wounds supplemented with iron contained decreased levels of HIF1α (p=0.039643) (Fig. 5). Similarly, HIF1α level was decreased when WT strain of A. baumannii was replaced by ΔBasD deficient in iron scavenging (injury/Isch/WT vs injury/Isch/ΔBasD, p=0.044488).
Figure 5. Abundance of iron reduces HIF1α in injured ischemic tissues exposed to A. baumannii.
A), Western blot analysis of muscle tissues isolated on POD7. (B), Protein band densities evaluated by ImageJ (n=3, *p<0.05). AB (A. baumannii), Injury (muscle injury), Isch (muscle ischemia), WT (wild type), ΔBasD (A. baumannii mutant). Error bars indicate standard deviation.
A. baumannii isolates from injured ischemic tissues display a more virulent phenotype
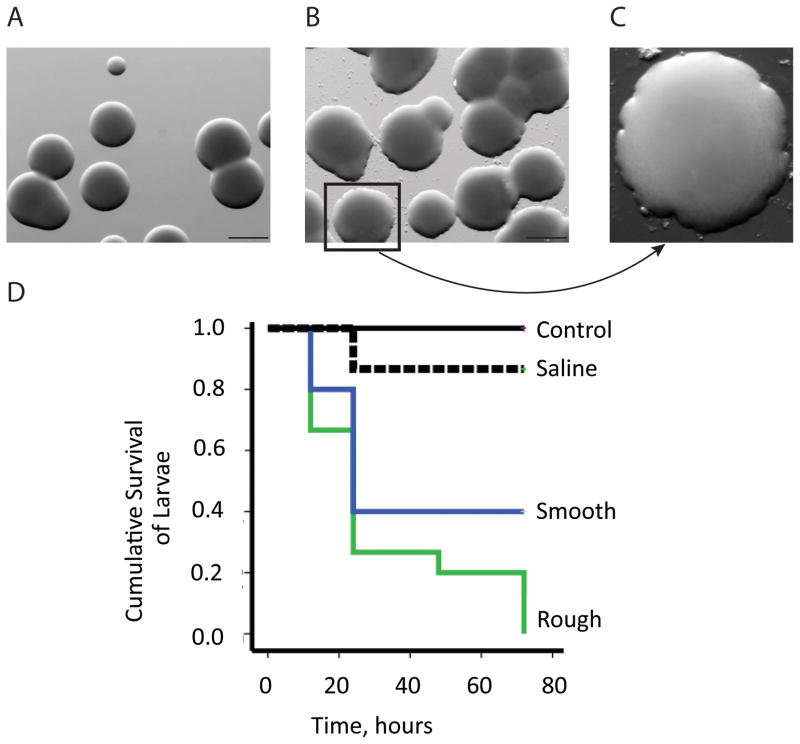
We have noted that A. baumannii shifted its colony morphotype to a rough-edge appearance and larger colonies when cultured directly from abscesses of injured and ischemic muscle (Fig. 6A). To determine whether this change in morphotype correlates with enhanced virulence, we used a G. mellonella infection model, an established invertebrate animal model already used to study the virulence of different pathogens including A. baumannii (23, 27). Seventy-two hours after intrahaemocoelic injection, rough colony morphotypes caused significantly higher mortality rates compared to the smooth colony morphotype (100% vs. 60% respectively, n=30, Log-Rank test, p=0.0422) (Fig. 6B). The morphotype change was not stable in culture reverting back to the smooth morphotype following several subcultures in nutrient-rich TSA medium.
Figure 6. A. baumannii isolates from injured ischemic tissues have higher virulence phenotype.
(A), Smooth A. baumannii morphotype colonies isolated from abscess-negative wounds (1mm size bar). (B), Rough A. baumannii morphotype isolated from abscess-positive wounds (1mm size bar). (C) Magnification of a rough colony. (D), Kaplan-Meier survival curves of G. mellonella injected with smooth and rough morphotype colonies. Control larvae were not injected with any solution. Saline group larvae served as negative control in which the injection of 10 μl of sterile saline solution was performed (n=30, P <0.05 comparing mortality induced by smooth vs. rough morphotypes).
Discussion
While the epidemiology of A. baumannii wound infections has been extensively studied, their pathogenicity remains poorly understood. In order to elucidate the molecular mechanisms responsible for A. baumannii wound infections, we established a reproducible and clinically relevant murine model of traumatic A. baumannii wound infection. We provide evidence that the pathogenesis of A. baumannii wound infections is dependent on the degree of tissue trauma and the composition of the wound milieu. We have previously shown that the wound environment plays a significant contributory role in infectious pathogenesis using a Pseudomonas aeruginosa wound infection model in which rectus muscle injury providing contact with fascia was sufficient for P. aeruginosa to cause wound infection through activation of its iron scavenging systems (28). However, as our current work demonstrated, a more severe insult combining muscle injury with ischemia was required for A. baumannii wound infection in mice. This difference may reflect a higher capacity of P. aeruginosa to trigger its virulence in response to multiple host derived signals (28–34), and hence explain, in part, its reputation as a highly virulent pathogen. Without the local microenvironmental cues present in a wound complicated by injury and ischemia, A. baumannii may not be sufficiently activated to cause a clinical wound infection.
A. baumannii’s most potent virulence activation system relies on its ability to respond to iron limitation through iron chelation (35, 36). We therefore hypothesized that the ability of A. baumannii to cause traumatic wound infection in Injury/Ischemia mouse model relies on its ability to scavenge iron from the wound milieu. We chose the combination of injury and ischemia to model A. baumannii wound infections as it reflects the clinical soft-tissue infections associated with war trauma in which A. baumannii is the lead pathogen (4, 37). It is well known that ischemic tissues of traumatized wounds are characterized by overexpression and stabilization of a master transcriptional regulator HIF1α (38). HIF1α expression is linked to iron metabolism where it senses and regulates iron content in tissues (39). It is a compensatory mechanism aimed at increasing the oxygen content of tissues through the activation of various genes encoding proteins such as transferrin and ferritin (39). Ferritin-mediated iron sequestration can stabilize HIF1α by inhibiting its hydroxylation (Fig. 3) (40). It can also induce bacteria to activate their iron sequestration systems (i.e., siderophores) that compete for iron with ferritin (21, 41, 42). These interactions can lower tissue-associated iron levels and induce further accumulation of HIF1α in an iterative loop of signal exchange. Our data clearly show evidence for a synergistic effect of injury, ischemia and A. baumannii exposure on the development of traumatic A. baumannii wound infection related to increased HIF1α production. These intersecting systems between tissue trauma compensatory mechanisms and bacterial virulence expression are dampened by the presence of iron in the wound milieu as competition for iron is eliminated. While it may seem counterintuitive that supplementation of iron, a known growth factor for bacteria, actually prevented wound infection in this model, the interrelationship between iron scavenging by A. baumannii and the role of iron in post-translational HIF1α stabilization and accumulation can play a yet-unrecognized role. Results from the present study suggest that iron excess could promote the ubiquitination of HIF1α and shut down the expression of bacterial virulence factors.
While HIF1α is an essential mediator of the cellular response network to hypoxia, its overexpression has been shown to associate with poor clinical outcomes (43–45). Additionally, pathogenic bacteria have been shown to recognize HIF1α and respond to its accumulation with enhanced virulence (29) and to further enhance HIF1α production in host tissues (46, 47). As such, the accumulation of HIF1α in our model may serve as a quorum sensing signal for A. baumannii, enhancing its virulence and leading to wound infection. However, whether excess accumulation of HIF1α in injured and ischemic tissues infected by A. baumannii is just a biomarker of oxygen and iron deficiency or whether it is directly responsible for A. baumannii success at causing an infection, requires further clarification.
There is clearly a complex molecular dialogue that occurs between host tissues and invading pathogens that, dependent on local environmental cues and resources, shifts the clinical trajectory from either pathogen clearance to gross infection. This can be seen in the group of animals with non-traumatized wounds that failed to develop clinical infection despite contamination with significant inoculum of A. baumannii, as well as in the ability of the wound environment to select for the two reported morphotypes, rough and smooth, that significantly vary in virulence. The context-dependency of this response clearly involves a complex interrelationship between iron and iron-scavenging mechanisms of both the invading pathogen and the host tissues. The use of high resolution multi-omic analyses will facilitate the identification and understanding of the bacterial factors involved in this process.
In summary, here we present a novel model of A. baumannii wound infection that demonstrates the critical role of the wound environment and bacterial functions in the pathogenesis of these critical infections and the emergence of morphotypes with enhanced virulence. Manipulation of iron within traumatic wounds at risk for A. baumannii infection could represent a novel anti-virulence approach to prevent this lethal infection.
Acknowledgments
This work was supported by NIH grant 5R01 GM062344 and DDRCC grant P30DK42086.
Footnotes
The data was partially presented at the 34th Surgical Infection Society Meeting Baltimore, Maryland, May 1–3, 2014. It has not been published elsewhere.
Conflict of interest statement: No conflicts are declared.
Author contributions: IF performed study design and experiments; MK analyzed and interpreted the data, performed a literature search, and wrote the draft of the manuscript; NB performed experiments, analyzed and interpreted the data, participated in the experimental design; AZ, JD, LC performed experiments and analyzed the data; LA analyzed the data and critically revised the manuscript; OZ and JA designed and analyzed the experiments and wrote the manuscript.
References
- 1.Gaynes R, Edwards JR National Nosocomial Infections Surveillance S. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–54. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 2.Sengstock DM, Thyagarajan R, Apalara J, Mira A, Chopra T, Kaye KS. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis. 2010;50(12):1611–6. doi: 10.1086/652759. [DOI] [PubMed] [Google Scholar]
- 3.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44(12):1577–84. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 4.Sebeny PJ, Riddle MS, Petersen K. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin Infect Dis. 2008;47(4):444–9. doi: 10.1086/590568. [DOI] [PubMed] [Google Scholar]
- 5.Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG Antimicrobial Availability Task Force of the Infectious Diseases Society of A. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42(5):657–68. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 6.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5(12):939–51. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 7.Garzoni C, Emonet S, Legout L, Benedict R, Hoffmeyer P, Bernard L, Garbino J. Atypical infections in tsunami survivors. Emerg Infect Dis. 2005;11(10):1591–3. doi: 10.3201/eid1110.050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Be NA, Allen JE, Brown TS, Gardner SN, McLoughlin KS, Forsberg JA, Kirkup BC, Chromy BA, Luciw PA, Elster EA, et al. Microbial profiling of combat wound infection through detection microarray and next-generation sequencing. J Clin Microbiol. 2014;52(7):2583–94. doi: 10.1128/JCM.00556-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK. Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis. 2007;45(4):409–15. doi: 10.1086/520029. [DOI] [PubMed] [Google Scholar]
- 10.Davis KA, Moran KA, McAllister CK, Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis. 2005;11(8):1218–24. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schafer JJ, Mangino JE. Multidrug-resistant Acinetobacter baumannii osteomyelitis from Iraq. Emerg Infect Dis. 2008;14(3):512–4. doi: 10.3201/eid1403.070128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keen EF, 3rd, Robinson BJ, Hospenthal DR, Aldous WK, Wolf SE, Chung KK, Murray CK. Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns. 2010;36(6):819–25. doi: 10.1016/j.burns.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Bano J, Cisneros JM, Fernandez-Cuenca F, Ribera A, Vila J, Pascual A, Martinez-Martinez L, Bou G, Pachon J Grupo de Estudio de Infeccion H. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol. 2004;25(10):819–24. doi: 10.1086/502302. [DOI] [PubMed] [Google Scholar]
- 14.Cisneros JM, Reyes MJ, Pachon J, Becerril B, Caballero FJ, Garcia-Garmendia JL, Ortiz C, Cobacho AR. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin Infect Dis. 1996;22(6):1026–32. doi: 10.1093/clinids/22.6.1026. [DOI] [PubMed] [Google Scholar]
- 15.Valencia R, Arroyo LA, Conde M, Aldana JM, Torres MJ, Fernandez-Cuenca F, Garnacho-Montero J, Cisneros JM, Ortiz C, Pachon J, et al. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect Control Hosp Epidemiol. 2009;30(3):257–63. doi: 10.1086/595977. [DOI] [PubMed] [Google Scholar]
- 16.Antunes LC, Visca P, Towner KJ. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 2014;71(3):292–301. doi: 10.1111/2049-632X.12125. [DOI] [PubMed] [Google Scholar]
- 17.Rajamohan G, Srinivasan VB, Gebreyes WA. Biocide-tolerant multidrug-resistant Acinetobacter baumannii clinical strains are associated with higher biofilm formation. J Hosp Infect. 2009;73(3):287–9. doi: 10.1016/j.jhin.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Mortensen BL, Skaar EP. Host-microbe interactions that shape the pathogenesis of Acinetobacter baumannii infection. Cell Microbiol. 2012;14(9):1336–44. doi: 10.1111/j.1462-5822.2012.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nwugo CC, Arivett BA, Zimbler DL, Gaddy JA, Richards AM, Actis LA. Effect of ethanol on differential protein production and expression of potential virulence functions in the opportunistic pathogen Acinetobacter baumannii. PLoS One. 2012;7(12):e51936. doi: 10.1371/journal.pone.0051936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Getchell-White SI, Donowitz LG, Groschel DH. The inanimate environment of an intensive care unit as a potential source of nosocomial bacteria: evidence for long survival of Acinetobacter calcoaceticus. Infect Control Hosp Epidemiol. 1989;10(9):402–7. doi: 10.1086/646061. [DOI] [PubMed] [Google Scholar]
- 21.Skaar EP. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 2010;6(8):e1000949. doi: 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberg ED. Iron availability and infection. Biochim Biophys Acta. 2009;1790(7):600–5. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Gaddy JA, Arivett BA, McConnell MJ, Lopez-Rojas R, Pachon J, Actis LA. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect Immun. 2012;80(3):1015–24. doi: 10.1128/IAI.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eijkelkamp BA, Hassan KA, Paulsen IT, Brown MH. Investigation of the human pathogen Acinetobacter baumannii under iron limiting conditions. BMC Genomics. 2011;12:126. doi: 10.1186/1471-2164-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McConnell MJ, Actis L, Pachon J. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev. 2013;37(2):130–55. doi: 10.1111/j.1574-6976.2012.00344.x. [DOI] [PubMed] [Google Scholar]
- 26.Dorsey CW, Tomaras AP, Connerly PL, Tolmasky ME, Crosa JH, Actis LA. The siderophore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology. 2004;150(Pt 11):3657–67. doi: 10.1099/mic.0.27371-0. [DOI] [PubMed] [Google Scholar]
- 27.Ramarao N, Nielsen-Leroux C, Lereclus D. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J Vis Exp. 2012;(70):e4392. doi: 10.3791/4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M, Christley S, Khodarev NN, Fleming I, Huang Y, Chang E, Zaborina O, Alverdy JC. Pseudomonas aeruginosa wound infection involves activation of its iron acquisition system in response to fascial contact. J Trauma Acute Care Surg. 2015;78(4):823–9. doi: 10.1097/TA.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel NJ, Zaborina O, Wu L, Wang Y, Wolfgeher DJ, Valuckaite V, Ciancio MJ, Kohler JE, Shevchenko O, Colgan SP, et al. Recognition of intestinal epithelial HIF-1alpha activation by Pseudomonas aeruginosa. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G134–42. doi: 10.1152/ajpgi.00276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaborina O, Lepine F, Xiao G, Valuckaite V, Chen Y, Li T, Ciancio M, Zaborin A, Petrof EO, Turner JR, et al. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog. 2007;3(3):e35. doi: 10.1371/journal.ppat.0030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu L, Estrada O, Zaborina O, Bains M, Shen L, Kohler JE, Patel N, Musch MW, Chang EB, Fu YX, et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309(5735):774–7. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]
- 32.Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, Poroyko V, Liu DC, Zaborina O, Alverdy JC. Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Ann Surg. 2012;255(2):386–93. doi: 10.1097/SLA.0b013e3182331870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaborina O, Zaborin A, Romanowski K, Babrowski T, Alverdy J. Host stress and virulence expression in intestinal pathogens: development of therapeutic strategies using mice and C. elegans. Curr Pharm Des. 2011;17(13):1254–60. doi: 10.2174/138161211795703771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holm A, Vikstrom E. Quorum sensing communication between bacteria and human cells: signals, targets, and functions. Front Plant Sci. 2014;5:309. doi: 10.3389/fpls.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorsey CW, Beglin MS, Actis LA. Detection and analysis of iron uptake components expressed by Acinetobacter baumannii clinical isolates. J Clin Microbiol. 2003;41(9):4188–93. doi: 10.1128/JCM.41.9.4188-4193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modarresi F, Azizi O, Shakibaie MR, Motamedifar M, Mosadegh E, Mansouri S. Iron limitation enhances acyl homoserine lactone (AHL) production and biofilm formation in clinical isolates of Acinetobacter baumannii. Virulence. 2015;6(2):152–61. doi: 10.1080/21505594.2014.1003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerrero DM, Perez F, Conger NG, Solomkin JS, Adams MD, Rather PN, Bonomo RA. Acinetobacter baumannii-associated skin and soft tissue infections: recognizing a broadening spectrum of disease. Surg Infect (Larchmt) 2010;11(1):49–57. doi: 10.1089/sur.2009.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conde E, Alegre L, Blanco-Sanchez I, Saenz-Morales D, Aguado-Fraile E, Ponte B, Ramos E, Saiz A, Jimenez C, Ordonez A, et al. Hypoxia inducible factor 1-alpha (HIF-1 alpha) is induced during reperfusion after renal ischemia and is critical for proximal tubule cell survival. PLoS One. 2012;7(3):e33258. doi: 10.1371/journal.pone.0033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peyssonnaux C, Nizet V, Johnson RS. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle. 2008;7(1):28–32. doi: 10.4161/cc.7.1.5145. [DOI] [PubMed] [Google Scholar]
- 40.Siegert I, Schodel J, Nairz M, Schatz V, Dettmer K, Dick C, Kalucka J, Franke K, Ehrenschwender M, Schley G, et al. Ferritin-Mediated Iron Sequestration Stabilizes Hypoxia-Inducible Factor-1alpha upon LPS Activation in the Presence of Ample Oxygen. Cell Rep. 2015;13(10):2048–55. doi: 10.1016/j.celrep.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Fischbach MA, Lin H, Liu DR, Walsh CT. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol. 2006;2(3):132–8. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- 42.Chu BC, Garcia-Herrero A, Johanson TH, Krewulak KD, Lau CK, Peacock RS, Slavinskaya Z, Vogel HJ. Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals. 2010;23(4):601–11. doi: 10.1007/s10534-010-9361-x. [DOI] [PubMed] [Google Scholar]
- 43.Baba Y, Nosho K, Shima K, Irahara N, Chan AT, Meyerhardt JA, Chung DC, Giovannucci EL, Fuchs CS, Ogino S. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol. 2010;176(5):2292–301. doi: 10.2353/ajpath.2010.090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vainrib M, Golan M, Amir S, Dang DT, Dang LH, Bar-Shira A, Orr-Urtreger A, Matzkin H, Mabjeesh NJ. HIF1A C1772T polymorphism leads to HIF-1alpha mRNA overexpression in prostate cancer patients. Cancer Biol Ther. 2012;13(9):720–6. doi: 10.4161/cbt.20554. [DOI] [PubMed] [Google Scholar]
- 45.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9(9):609–17. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178(12):7516–9. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 47.Holden VI, Breen P, Houle S, Dozois CM, Bachman MA. Klebsiella pneumoniae Siderophores Induce Inflammation, Bacterial Dissemination, and HIF-1alpha Stabilization during Pneumonia. MBio. 2016;7(5) doi: 10.1128/mBio.01397-16. [DOI] [PMC free article] [PubMed] [Google Scholar]