Abstract
The amygdala (AMG) has been repeatedly implicated in the processing of threatening and negatively valenced stimuli and multiple fMRI paradigms have reported personality, genetic, and psychopathological associations with individual differences in AMG activation in these paradigms. Yet the interchangeability of activations in these probes has not been established, thus it remains unclear if we can interpret AMG responses on specific tasks as general markers of its reactivity. In this study we aimed to assess if different tasks that have been widely used within the Affective Neuroscience literature consistently recruit the AMG.
Method
Thirty-two young healthy subjects completed four fMRI tasks that have all been previously shown to probe the AMG during processing of threatening stimuli: the Threat Face Matching (TFM), the Cued Aversive Picture (CAP), the Aversive and Erotica Pictures (AEP) and the Screaming Lady paradigm (SLp) tasks. Contrasts testing response to aversive stimuli relative to baseline or neutral stimuli were generated and correlations between activations in the AMG were calculated across tasks were performed for ROIs of the AMG.
Results
The TFM, CAP and AEP, but not the SLp, successfully recruit the AMG, among other brain regions, especially when contrasts were against baseline or nonsocial stimuli. Conjunction analysis across contrasts showed that visual cortices (VisCtx) were also consistently recruited. Correlation analysis between the extracted data for right and left AMG did not yield significant associations across tasks. By contrast, the extracted signal in VisCtx showed significant associations across tasks (range r=0.511–r=0.630).
Conclusions
Three of the four paradigms revealed significant AMG reactivity, but individual differences in the magnitudes of AMG reactivity were not correlated across paradigms. By contrast, VisCtx activation appears to be a better candidate than the AMG as a measure of individual differences with convergent validity across negative emotion processing paradigms.
Keywords: Threat Processing, Affective Neuroscience, Amygdala, Visual Cortex, Convergent Validity
1. INTRODUCTION
Research within affective neuroscience has repeatedly implicated the amygdala (AMG) in the processing of threatening and negative emotional stimuli in both non-human animal studies and human lesion and neuroimaging studies (LeDoux, 1994; Zald, 2003). Multiple fMRI paradigms have been used to demonstrate amygdalar activation in response to threat and negative emotion stimuli. Many of these studies use tasks that either induce Pavlovian fear learning (usually by subtracting the brain response to a neutral stimulus (CS−) from the response to a conditioned stimulus (CS+)) (Buchel et al., 1998); or examine automatic responses to visual threat or negative emotional stimuli such as angry or fearful faces or aversive pictures. The latter have predominantly subtracted responses to non-threatening visual stimuli from responses towards either facial stimuli expressing aversive emotions or sets of more generally aversive scenes (Adolphs, 2008). A number of these tasks have been applied as probe tasks of amygdalar functioning in different patient groups (Broome et al., 2015), across individuals with different personality traits (Clauss et al., 2015; Kennis et al., 2013) and in studies of development and genetics (Fisher et al., 2015; Wu et al., 2016).
Although research in this area has made substantial advances, a number of questions remain about the interpretation of findings from these tasks (Church et al., 2010; Friston et al., 1996; Price and Friston, 1997). Principal among these is whether any given task can be used as a general marker of the region’s functioning. For instance, does a measure of the amygdala’s response to one task (say responses to a threat face matching task) provide enough generalizability that it predicts responses to another probe task (such as fear conditioning). If the tasks are to be interpreted as a general measure of amygdalar reactivity (and as a marker of a relatively general psychological construct) one would want to see evidence of convergent validity; that is, responses across tasks should be correlated. If they are not significantly correlated, interpretations of the activations should be much more limited, for instance being described with specificity to a particular task, rather than treated as a general marker of AMG reactivity or as a biomarker for a broad process of emotional processing writ large (Wise and Tracey, 2006). From a psychometric standpoint it is thus striking that, to date, studies have not directly examined the convergent validity of individual differences in AMG activation across tasks described to test similar constructs.
Within this research area there is an additional interpretational issue that relates to the psychological constructs typically inferred to be reflected by the amygdalar activation. Specifically, the studies are often interpreted as indexing threat reactivity, or a highly similar construct related to threat processing (Adolphs, 2008). However, in some cases the primary contrasts utilized do not provide a completely clean comparison between threat and nonthreat conditions. For instance, in the frequently used Threat Face Matching (TFM) paradigm developed by Hariri and colleagues (Hariri et al., 2002a; Hariri et al., 2002b), the negatively valenced emotional faces are often contrasted with geometric shapes leaving unclear whether differential activations across subjects are related to exposure to the emotional faces or are actually related to heightened responses to faces in general.
In this study we aimed to assess if different tasks that have been widely used within the Affective Neuroscience literature consistently recruit the AMG. We selected four tasks that have all been previously shown to probe the AMG during processing of threatening stimuli. One task was based on Pavlovian fear conditioning (Lau et al., 2008) and three tasks were based on visual processing of aversive stimuli. Of the latter, one used facial expressions (Hariri et al., 2002a; Hariri et al., 2002b), one used aversive scenes (Heinzel et al., 2005) and another added a cue for the presentation of aversive scenes (Nitschke et al., 2006). All of these tasks use validated sets of stimuli that have been shown to consistently trigger threat processing. We examined whether AMG activity during the target conditions (response to threatening stimuli) is correlated across the four tasks to test for convergent validity, and thereby determine whether AMG responses can be readily interpreted as reflecting the same underlying construct across the fMRI paradigms, regardless of design.
2. METHODS
2.1. Participants
We recruited a convenience sample of thirty-two young, self-reported healthy subjects (23.13+− 3.62 y.o. and 17 males). All subjects gave written informed consent and the study was approved by Vanderbilt’s Internal Review Board.
2.2. Imaging Stimuli and Tasks
Participants completed four standard AMG probe tasks distributed across six functional runs. Figure 1 schematizes each task. The task order was the same for all participants.
Fig. 1.
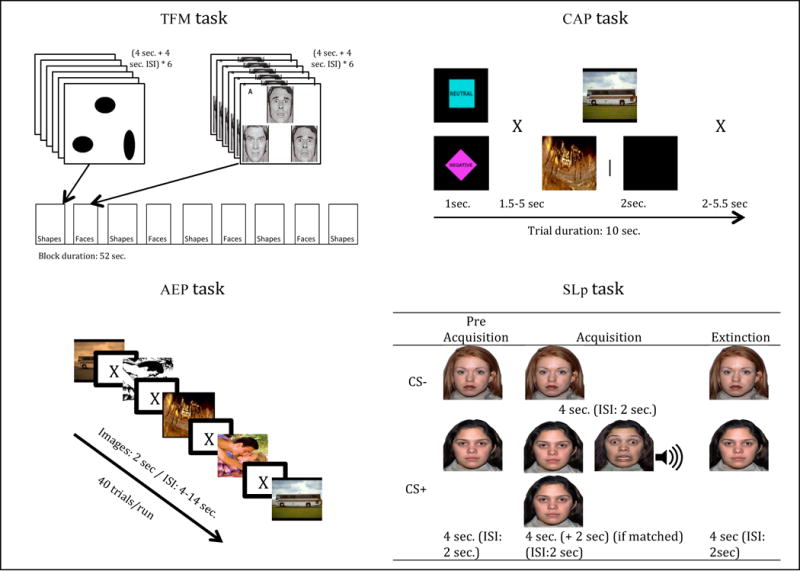
Schematized figure of the tasks performed in the MR Scan.
In the first functional run, participants performed the TFM task (Hariri et al., 2002a; Hariri et al., 2002b), which required subjects to match faces based on their emotional expressions. Brain response to faces was compared to a sensorimotor control task, in which subjects had to match one of two geometric shapes with a simultaneously presented target shape. The TFM task consisted of a total of 4 blocks depicting facial emotional expressions (emotional blocks) interleaved with 5 blocks with geometrical shapes (sensorimotor blocks). Participants were presented with 2 different faces or shapes on the bottom of the screen and one on the top of the screen and were asked to select which of the two faces or shapes on the bottom matched the identical image on top. Facial expressions included angry, fearful, surprise and neutral and were balanced in terms of gender. Each block consisted of 6 slides, which were each presented for 4 seconds, with a 4 second interstimulus interval (ISI).
During the next four functional runs, participants performed two tasks that included stimuli from the International Affective Picture System (IAPS) (Lang, 2008); the main difference between the two tasks was the presence or absence of a cue allowing anticipation of the valence of upcoming stimuli and the specific stimuli utilized.
The first of these tasks, the Cued Aversive Picture Task (CAP) included 2 functional runs and followed the design of a task implemented by Nitschke (Nitschke et al., 2009), in which trials included a consistent cue indicating the nature of the picture (either negative or neutral) that participants were about to view. The advantage of this cued approach is it allows modeling of both the response to the stimulus and processes related to the anticipation of an emotional stimulus. Each run consisted of 15 neutral trials and 25 negative trials. Negative and Neutral images were chosen based on their scores on arousal and valence, were matched by general content (scenes, people, things), were balanced in luminosity, and were preceded by a consistent cue. The intertrial and interstimuli interval (ISI) were reduced from that of the original design due to time constraints. In our version of the task, each trial lasted 10 sec. On 20% of the cued negative trials, subjects saw a blank screen to facilitate the identification of activity related to the cue versus the negative pictures.
The second picture viewing task, the Aversive and Erotica Picture Task (AEP) also involved 2 functional runs, and followed a design implemented by Heinzel (Heinzel et al., 2005) in which participants were exposed to emotional pictures without cueing, Participants were asked to press a button as quickly as possible whenever they saw a picture. Stimuli for this task consisted of a random selection of 40 images from a set of 20 neutral, 20 erotica and 20 negative. Images were shown for 2 seconds each with a jittered ISI (4–14 sec.).
The last task (1 functional run) was a threat appraisal paradigm using both visual and auditory aversive stimuli (“the screaming lady” paradigm or SLp) (Lau et al., 2008). This paradigm has shown equivalent threat conditioning to paradigms using other aversive stimuli (Britton et al., 2011). This experiment had three phases. During the pre-acquisition phase individuals were shown faces of two different females (12 trials: 6 CS− and 6 CS+). During the acquisition phase individuals were shown the same faces but one of them (CS+) was followed by two aversive stimuli: a picture of the same female with an expression of fear at high intensity paired with a shrieking female scream (52 trials: 20 CS−; 12 CS+ unmatched with scream and 20 CS+ matched with scream). During last phase, the extinction phase, individuals were shown the same two female faces not followed by any aversive stimuli (20 trials: 10 CS− and 10 CS+). CS− and CS+ unmatched images were shown for 4 seconds, CS+ matched stimuli was shown for 6 seconds; an ISI of 2 seconds was used.
Participants completed a few practice trials before the scanning session to familiarize them with the tasks. None of the IAPS slides from the practice version of the tasks were used during the scanning session.
2.3. Image data acquisition
Prior to the imaging session, participants were trained on all of the tasks. Participants were placed supine in the scanner, wearing headphones to muffle noise and deliver auditory stimuli. Head fixation was limited with foam padding. Participants viewed target stimuli through a mirror mounted on the head coil. The stimuli were projected onto a screen using a computer-activated LCD projection system. Task administration was triggered by the scanner and synced to the image acquisition using ePrime software (Psychology Software Tools, Pittsburgh, PA) on a PC computer. Participants’ responses were collected using a MRI compatible response keypad.
Anatomical and functional images were acquired on one of two identical 3-T Phillips Achieva scanners with a 32-channel head coil. Blood Oxygenation Level Dependent (BOLD) sensitive functional images were acquired using a gradient echo-planar imaging (EPI) sequence (TR=2000ms, TE=25ms, 38 slices, ascending acquisition, voxel size = 3×3×3, with 0.3 mm interslice gap, FA= 90°, FOV = 240 mm). A total of 206 volumes were acquired for the TFM run; 203 volumes for each CAP run; 173 for each AEP run and 274 volumes for the SLp run. A high-resolution MP-RAGE T1-weighted anatomical scan was acquired for each participant (duration of 4′32.8″, 170 sagittal slices, voxel size 1×1×1mm, FOV=256mm) to provide anatomical reference for normalization and displaying of functional data.
2.4. Image processing and analysis
2.4.1. Pre-processing
Functional imaging data were preprocessed and analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; see www.fil.ion.ucl.ac.uk/spm) running in MatLab R2014b (Mathworks, Natick, Massachussets). The functional images were reoriented to the anterior/posterior commissures (AC–PC) plane, realigned to the first image of the scanning session and coregistered to each subject’s anatomical image. Segmentation of anatomical images was completed using the VBM 8 toolbox. The spatial normalization parameters of the grey matter segmentation map were applied to the realigned fMRI time series from each subject to transform the images into MNI space. Finally, normalized images were smoothed with an isotropic Gaussian kernel of 8 mm full-width at half-maximum (FWHM).
2.4.2. First-level analysis
For each participant and task, a general linear model (GLM) was estimated with a canonical hemodynamic response function including time and dispersion derivatives. Motion parameters obtained from realignment were included in the GLM as covariates. The TFM task’s GLM modeled each emotional block and the sensorimotor blocks. The CAP task’s GLM modeled cues (neutral and negative) and images (neutral, negative and black screen). The AEP task’s GLM modeled neutral, negative and erotica images. The SLp task’s GLM modeled CS+ and CS− in the preacquisition phase. CS−, CS+ unmatched and CS+matched in the acquisition phase and CS+ and CS− in the extinction phase.
The data and model were high-pass filtered to a cutoff of 128 sec. After parameter estimation, T-contrasts were computed for each target condition, relative to their respective control condition/s and for each condition relative to baseline.
2.4.3. Second-level analysis
The significance threshold for all the resulting statistical maps was set at p<0.001unc, with a cluster-wise corrected threshold (FWEc) of p<0.05. All tests were performed at both whole brain level (for descriptive purposes) and masked with a bilateral AMG template obtained from the WFU-Pickatlas toolbox (Maldjian et al., 2003). One sample t-tests for each task and contrast of interest were performed to assess recruitment of the AMG. Age, gender and MR scanner on which images were acquired were used as covariates.
Using MarsBaR (Brett, 2002), the percent signal change in right and left AMG was obtained for the contrasts assessing response to threat (against baseline or control) in each task. Correlation analyses were performed to test whether individual differences in the level of AMG activation was in each of four contrasts involving negative stimuli was consistent across tasks. Similar post-hoc analysis were performed for right and left visual cortices (VisCtx), as this area showed robust activations in multiple tasks, and has previously been found to be heavily modulated by emotional salience in fMRI studies (Lang et al., 1998). We performed two sets of correlation analyses. First we examined the correlations among the contrasts that produced the largest AMG activation in each task. We performed a second analysis examining the correlations for aversive vs. neutral contrasts for each task. In each case, we applied a Bonferroni correction for the number of correlations assessed in each analysis. In order to ensure that single subjects were not driving effects, we performed a jackknife analysis in which the correlation was repeated 31 times excluding one subject each time. We considered the result to be stable if they remained significant at least at the p <0.05 uncorrected level in each of the 31 runs.
3. RESULTS
Fig. 2 and Supplemental Table 1 show the statistically significant second-level main effects for the contrasts of interest for each task at a whole brain corrected level. AMG activations can be seen for the TFM, CAP and AEP. However, the SLp did not successfully recruit any brain region at a whole brain significance level, and was therefore excluded from subsequent analyses. For the three remaining tasks the AMG activations emerged across multiple contrasts, inferior occipital regions were also consistently recruited across different contrasts. The contrasts that recruited the most total voxels within the AMG in each task were: negative vs. baseline for the CAP, negative vs. shapes for the TFM, and negative vs. baseline for AEP. Figure 2D shows a conjunction map for the three contrasts, indicating their overlap in the AMG and VisCtx bilaterally.
Fig. 2.
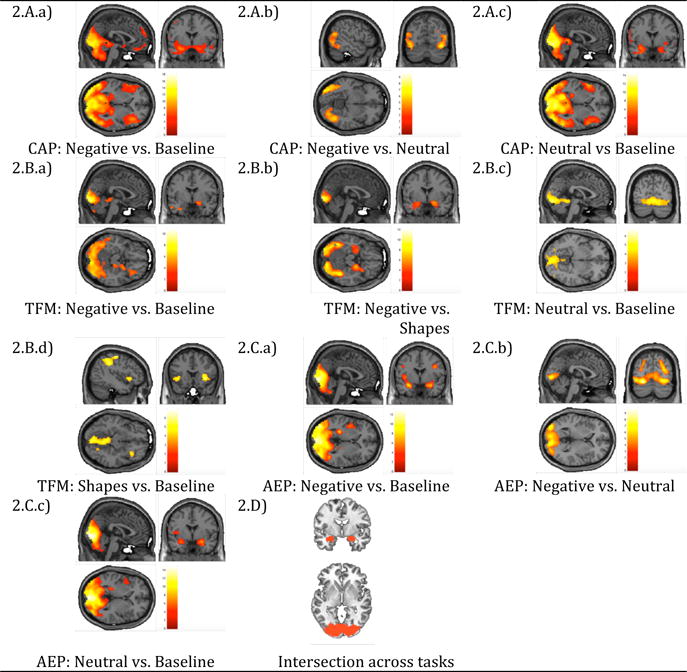
Whole brain level significantly (PFWE-cluster<0.05) recruited regions for our contrasts of interest and intersection map across the CAP (2.A.a), TFM (2.B.b) and AEP (2.C.a) maps. (Supplemental Table 1 provides detailed information on activations).
Table 1 details AMG recruitment across tasks and contrasts. Although the AMG was activated by the TFM, CAP and AEP, activations to negative stimuli only reached statistical significance in contrasts with baseline or geometrical shapes, rather than in contrasts to neutral pictures or faces. This suggests a lack of specificity to threat or negative valence. In addition, neutral stimuli activated the amygdala in contrasts with baseline in both the CAP and AEP, which further confirms this lack of specificity to threat.
Table 1.
Recruitment of AMG for contrasts of interest and tasks
| Cluster p(FWE-corr) | ke | Peak pFWE-corr | T | Right (R)/Left (L) | |
|---|---|---|---|---|---|
|
|
|||||
| CAP: Negative images vs. baseline | 0.001 | 6 3 |
<0.001 | 9.7 5 |
L |
| 0.001 | 6 5 |
<0.001 | 7.8 5 |
R | |
| CAP: Neutral images vs. baseline | 0.001 | 5 4 |
<0.001 | 6.9 2 |
L |
| 0.001 | 5 0 |
<0.001 | 4.9 5 |
R | |
| TFM: Negative emotions vs. baseline | 0.003 | 2 3 |
0.002 | 4.7 8 |
R |
| 0.026 | 1 | 0.023 | 3.2 1 |
L | |
| TFM: Negative emotions vs. Shapes | 0.002 | 5 3 |
<0.001 | 6.7 1 |
R |
| 0.002 | 5 4 |
0.002 | 4.6 0 |
L | |
| AEP: Negative images vs. baseline | 0.001 | 5 7 |
<0.001 | 6.9 9 |
R |
| 0.002 | 4 7 |
<0.001 | 8.6 0 |
L | |
| AEP: Neutral images vs. baseline | 0.001 | 6 6 |
<0.001 | 6.6 8 |
R |
| 0.002 | 4 2 |
<0.001 | 5.7 2 |
L | |
In order to test the convergent validity across tasks, we performed correlational analyses between the extracted beta values for the AMG for conditions involving threat stimuli with the specific contrast based on the contrast producing the largest AMG activation: Negative vs. baseline in the CAP and AEP, and TFM threat vs. shapes. Table 2a shows the results for the right (top-right part of the table) and left AMG (bottom-right part of table). Because there were 6 correlations (3 per side × 2), we applied a Bonferroni correction resulting in a p<0.008 significance threshold. None of the correlations in either AMG reached statistical significance. We note that use of a more liberal statistical significance threshold would not change these conclusions, as none of the 6 correlation values exceeded r > .164, and some values were negative (Supplemental Table 2 specifies p-values and provides non-parametric correlation statistics for these associations).
Table 2.
Pearson Correlation Coefficients between beta values for threat processing constrasts recruiting most AMG studied in each task within ipsilateral right (black shade) and left (grey shade) AMG masks (2.a) and VisCtx masks (2.b).
| 2.a) | 2.b) | |||||
|---|---|---|---|---|---|---|
| 1 | 0.075 | −0.350 | A - CAP: Negative Images vs. baseline | 1 | 0.323 | 0.542* |
| 0.035 | 1 | −0.050 | B - TFM: Negative Faces vs Shapes | 0.342 | 1 | 0.511* |
| −0.128 | 0.164 | 1 | C - AEP: Negative Images vs. baseline | 0.615* | 0.630* | 1 |
| A | B | C | A | B | C | |
p<0.008
Scatter plots in Supplemental Figure 1 and extended statistics in Supplemental Table 2.a
By contrast, when we performed correlations for the same contrasts in the right and left VisCtx, significant correlations arose particular for correlations with the AEP, which explained 29% of the variance in the right visual cortex of the CAP (r=0.542, p=0.002), and 26% of the variance for the TFM (r=0.511, p=0.005); and 38% of the variance in the left visual cortex of the CAP (r=0.615,p=0.0002), and 40% of the variance for the TFM (r=0.630, p=0.0002) (see Table 2b). All these results survived jackknife analysis.
As a complementary analysis, we also examined correlations using contrasts in which aversive/threat stimuli were contrasted with neutral stimuli of the same general characteristics (i.e., neutral IAPS images, or neutral faces). This should reflect a greater specificity of interpretation because the neutral stimuli provide a better sensory control than baseline or shapes. Table 3a shows results for correlational analysis of the extracted signal of the right and left AMG across 3 contrasts in which negative stimuli in the CAP, AEP and TFM were contrasted with neutral stimuli (as opposed to baseline or shapes). The results do not reach rigorous Bonferorni levels for statistical significance. However, there was a positive correlation between the TFM (negative vs. neutral) and AEP (negative vs. neutral) for the left amygdala (r=.445) that met uncorrected significance (Supplemental Table 3 specifies p-values and provides non-parametric correlation statistics for these associations). None of the other 5 correlations approached significance at this liberal uncorrected threshold.
Table 3.
Pearson Correlation Coefficients between beta values for threat processing constrasts against neutral conditions studied in each task within ipsilateral right (black shade) and left (grey shade) AMG masks (3.a) and VisCtx masks (3.b).
| 3.a) | 3.b) | |||||
|---|---|---|---|---|---|---|
| 1 | −0.151 | −0.346 | A - CAP: Negative vs. Neutral Images | 1 | −0.027 | −0.018 |
| 0.079 | 1 | 0.278 | B - TFM: Negative vs Neutral Emotion Faces | 0.006 | 1 | 0.510a |
| −0.230 | 0.445 | 1 | C - AEP: Negative vs Neutral Images | −0.107 | 0.539a | 1 |
| A | B | C | A | B | C | |
Survived a threshold of p<0.008 but not jacknife.
Scatter plots in Supplemental Figure 2 and extended statistics in Supplemental Table 3.a
VisCtx activation showed Bonferroni-corrected significant correlations between the TFM (negative vs. neutral) and AEP (negative vs. neutral) bilaterally (see Table 3b). However, these correlations did not survive jackknife analyses for these contrasts (indicating that the result were sensitive to removal of a single subject). Scatter plots for the associations in Table 2 and Table 3 are shown in Supplemental Figures 1 and 2, respectively. Supplemental Figure 3 shows the mean extracted values of the contrasts used in Table 2 and Table 3 for both AMG and VisCtx.
Complementary analyses to test habituation of both AMG and VisCtx are detailed in the supplemental materials.
4. DISCUSSION
A primary aim of this study was to test if individual differences in AMG recruitment are consistent across common fMRI probe paradigms. Such probe tasks have been widely used in studies of genetics, psychopathology, and personality. Demonstration of similar responsivity across tasks would indicate that individual differences in AMG response reflects similar underlying functional or neural constructs across tasks and support generalizability in the interpretation of results in the multiple studies using these paradigms in different research domains.
All of the paradigms used in this study assess some aspect of threat or aversive processing. Two of the tasks assessed for correlations used IAPS images (CAP and AEP); the other task used facial expressions (TFM) as threatening or aversive stimuli. While the IAPS pictures have been described as biologically relevant stimuli (important for our survival); emotional faces are biologically significant because of their social relevance (Sakaki et al., 2012). As such, it seemed reasonable to expect that activations related to this threat processing would show correlations across tasks. However, we found little evidence that the AMG response is generalizable, since only one of 12 possible correlations across tasks in the right or left AMG reached even liberal levels of statistical significance (and this one case is equivocal in that it did not survive correction for multiple comparisons). The contrasts of threat/aversive stimuli against baseline or shapes produced no correlations in the AMG suggesting little generalizability of activations in these contrasts.
The only AMG correlation to reach liberal levels of significance arose in the left AMG for contrasts between of aversive vs. neutral viewing (AEP), and threat vs. neutral faces (TFM). In this one case, activation in the left AMG in one task predicted just under 20% of variance in the other condition. This may suggest that if one is to find convergent validity of AMG probe tasks, that it may be easier to see them in contrasts against neutral stimuli, although this speculation is limited by the lack of more conservative levels of statistical significance in any contrasts involving the AMG.
The lack of association across tasks is especially striking when comparing associations of right and left AMG activity between the CAP and the AEP, as both tasks use the same stimulus class, the IAPS. The main differences between the tasks are that presentation of stimuli is cued in the CAP and is not in the AEP and the inclusion of erotica in the AEP. (Bradley et al., 2003; Edmiston et al., 2013). Cueing may lead to both anticipatory responses and preparatory emotion regulation that directly impacts the response to the actual images. Previous studies have shown differential behavioral responses depending on whether the aversive stimulus is cued or unpredictable (Baas et al., 2009) and, while we are not aware of any studies that have compared the AMG response between cued and non-cued image viewing paradigms, it has been reported that the inclusion of anticipatory signals modifies the brain response to aversive stimuli (Denny et al., 2014). Erotic images have been observed to cause activations that can be both broader and of higher intensity than threatening pictures (Bradley et al., 2003; Edmiston et al., 2013). This may lead to a differential scaling or anchoring of aversive and neutral images, with a corresponding change in activations. It may thus be the case that the inclusion of an anticipatory signal to visual stimuli, or the inclusion of erotica images, modifies AMG functionality in response to those stimuli robustly enough to eliminate a correlation between CAP and AEP tasks. Such a possibility could be consistent with the idea that emotion regulation processes critically impact AMG responses to emotionally salient stimuli (Ochsner et al., 2004).
Overall, the lack of robust associations between AMG responses across tasks gives little support for the use of AMG response to a given task as a comparable or interchangeable measure of threat response across tasks. Our results instead suggest that the subtle differences in threat and aversive visual processing paradigms can alter the interpretation of AMG activation despite the use of similar stimuli or contrasts. Depending upon the paradigm, AMG responses may represent several different constructs or processes rather than a generic response to threat or aversive stimulation. While this interpretational specificity has been repeatedly suggested by researchers (Costafreda et al., 2008; Pessoa and Adolphs, 2010; Zald, 2003), the current study is one of the few empirical studies to provide data comparing tasks aimed at testing the same constructs directly. From a perspective of individual differences, these data suggest that individuals do not show a generic AMG reactivity that is observable across paradigms. Rather, to the extent that the activations reflect trait differences, they are highly task- or stimulus-specific trait differences. In psychometric terms, our results appear indicative of poor convergent validity across tasks, at least if we wish to consider the activations to be indicative of general AMG reactivity, or general responsivity to negative stimuli. In the absence of convergent validity, we would suggest caution in describing these paradigms as explicit “amygdala probes” without characterizing the specific features of the probes.
4.1. Theoretical specificity of AMG probes
Given the above discussion, it becomes important to characterize the functional and behavioral features of tasks or contrasts engaging the AMG. Passive viewing of IAPS images (both cued and without cue) as well as the TFM task, recruited the AMG successfully. In this respect, our results are consistent with the literature on AMG recruitment in threat and negative emotional paradigms (Hariri et al., 2002b; Sabatinelli et al., 2005; Zald, 2003). Nevertheless, we cannot conclude that such recruitment is unique to either threat or negative emotional processing. In the tasks where subjects were passively viewing IAPS images, the AMG was recruited equally when participants were viewing negative and neutral images, with no preference for negative over neutral stimuli. For the TFM task, the AMG was recruited when participants were matching negative emotional expressions relative to shapes, but not when comparing negative emotional expressions with neutral emotional expressions. Contrasts with neutral stimuli are important as they provide a level of interpretational specificity that is not present in contrasts with baseline conditions in which there is weak or no control for basic sensory perceptual features and might be argued to have an inherent lack of interpretational specificity. On the surface these findings appear to contradict the large body of literature reporting preferential activation of the AMG by emotional stimuli (see (Zald, 2003) for review). There are several possible explanations for this apparent discrepancy. First, it is possible that some aspect of our methodology, such as having a smaller number of runs, fewer trials, or a subtle undetected technical issue limited our ability to detect threat-specific AMG signal. If this is the case, then these results do not challenge common interpretations of amygdalar functions. However, it must be noted that we were able to see substantial amygdalar activation in these tasks as long as we contrasted the negative emotional conditions with baseline or geometrical shapes.
Though the AMG appears highly sensitive to threatening and aversive stimuli, substantial evidence indicates that it does not respond exclusively to these stimuli, but rather it appears attuned to the relevance of the stimuli rather than their valence (Costafreda et al., 2008; Sakaki et al., 2012; Stillman et al., 2015). Our results (except for the SLp, see below) support this idea. We asked participants to press a button as quickly as they could after seeing any image in both the CAP and AEP, so all images were behaviorally relevant for participants. This might explain why the AMG was recruited across different types of stimuli, but did not show a bias towards threatening stimuli. The TFM task included stimuli that differed in their nature (emotional faces vs. shapes) and their valence (aversive vs. neutral). For this task, the AMG showed a preference for the facial expressions (vs. shapes) but did not show a preference for negative facial expressions over neutral expressions. Overall, our results suggest that, despite the use of aversive or threatening stimuli across these probe tasks, caution needs to be taken when interpreting results as specific measures of threat response or emotional processing. In order to be able to interpret AMG activity as a direct measure of a threat response, researchers arguably need to utilize paradigms and contrasts that show selective threat effects. We have not demonstrated such selectivity in this study. The lack of robust activations in contrasts against neutral stimuli poses a paradox in that the only place where we see any evidence of a positive correlation between AMG activations were in contrasts of threat/aversive stimuli against neutral stimuli despite the failure to see group activations in these specific contrasts.
4.2. Factors that may alter the consistency of AMG recruitment
We did not test for correlations with the SLp because it did not successfully recruit the AMG, contrary to previous results in the literature (Haddad et al., 2015; Lau et al., 2008). Our main contrast of interest for this task was the comparison between CS+ (unmatched) vs CS−, following previous studies that have reported AMG recruitment, mainly in adolescents, but in adult populations as well (Lau et al., 2011). This contrast has also been reported to engage other brain regions (Haddad et al., 2015). We cannot determine whether this lack of overall recruitment was due to habituation across our imaging session or if it was a task effect. It is worth noting that the order of the tasks was not randomized across participants. We ordered the tasks based on the intensity of threatening stimuli, from least to most intense to try to minimize habituation of the AMG over the course of the imaging session. AMG responses are known to be highly sensitive to habituation (Breiter et al., 1996)(often assessed by the inclusion of linear terms such as trial number (Fischer et al., 2003; Plichta et al., 2014; Zald, 2003)). As such, it is possible that habituation contributed to a reduced sensitivity to threat stimuli in the later tasks, with SLp being particularly impacted as the last task. If habituation occurs at similar rates across subjects, associations between convergent tasks should be high even if the net intensity of responses is decreasing over the course of study. That said, the rate at which AMG habituates may be influenced by clinical variables that lead to differential responses across individuals (Avery and Blackford, 2016). Such individual differences in habituation rate might contribute to the lack of consistency of AMG recruitment across tasks. It is notable in this regard that we do indeed see evidence of within task AMG habituation in the AEP task, but not the CAP task, and no correlation in the rates of habituation during the AEP and CAP (see supplemental materials). Such results leave open the possibility that differential rates of habitution contribute to indivdiual differences in activations across task, but suggest that such effects, if relevant, are likely quite complex to model, as they may differ across indivdiuals depending upon the tasks in question. If there are task-specific, and possibly nonlinear individual differences in rates of habituation, this may decrease correlations across tasks even if applied in a counter-balanced or multi-session design. Thus, while concerns about habituation effects clearly warrant consideration, such concerns may also limit the extent to which we can draw inferences about general AMG reactivity from a single task given that such responses may be differentially impacted by habituation across subjects. It is worth noting, though, that habituation also occurs in visual regions (Avery and Blackford, 2016; Britton et al., 2008), and these areas were significantly correlated across tasks. Importantly, indivdiual differences in the rate at which the VisCtx habituates also showed evidence of consistency across tasks (see supplemental material).
4.3. Visual Regions: relevance to threat processing and convergent validity
When combining the whole brain maps across tasks (except for the SLp) the VisCtx emerged as a common area of activation by threat/aversive stimuli across the CAP, AEP and TFM tasks. This activation occurred both in contrasts with baseline and importantly in contrasts with neutral stimuli. Enhancement of visual regions for affective stimuli (regardless of valence) is well-established in the imaging literature (Goldberg et al., 2014; Lang et al., 1998), but has rarely been considered as a potential marker of emotional processing in its own right. Because of the existence of projections from the AMG to early visual regions (Adolphs and Spezio, 2006), it has often been assumed that the AMG causes a feedback modulation of visual regions based on its evaluation of stimuli as emotionally relevant. However, recent data suggests that the modulation of VisCtx is not exclusively dependent upon the AMG (Edmiston et al., 2013; Pessoa and Adolphs, 2010).
In contrast to the AMG, individual differences in the degree of recruitment of the VisCtx appeared substantially more consistent across tasks. In terms of the two tasks using IAPS images, both the right and left VisCtx showed consistency in responding to aversive stimuli relative to baseline, regardless of the difference in the presence of cueing. Particularly strong associations were also found between the AEP and the TFM, which employ different stimuli (scenes vs. faces). Overall, these associations may be indicative of a shared functionality of the VisCtx in the processing of threatening and aversive stimuli presented in different contexts (cued and non cued) or for different types (scenes or faces). It is worth noting that this consistency is not universal as the CAP and TFM (which differed both in cuing and content) were not significantly correlated. Nevertheless, the VisCtx seems to be a better candidate than the AMG as a measure that demonstrates convergent validity across widely used threat and aversive processing fMRI paradigms. Surprisingly little research has addressed the genetic, environmental, or behavioral phenotypic correlates of visual cortex responses to emotional stimuli. Given the present results, consideration of visual cortex as a marker of individual differences in affective processing appears warranted. Interestingly, given that individual differences in habitatuion across tasks was correlated in this region, this signal may provide a useful area for assessing trait differences in habituation processes.
4.4 Future Directions
While the above analyses raise questions about the ability to assume that AMG reactivity to brief tasks represents a general measure of AMG reactivity, it clearly remains an a priori area of interest for studies in affective and clinical neuroscience. To be able to make more general, task-nonspecific interpretations of AMG reactivity it may be necessary to use more latent trait types of measures in which an investigator uses a group of measures (or in this case a group of contrasts across multiple tasks) to estimate a general reactivity of the region. At least for the left AMG, the moderate correlations for the TFM and AEP threat/aversive vs. neutral contrasts, suggest that those tasks could be used together to tap a general AMG reactivity construct. The core difficulty with such a latent trait approach is that it requires substantial scanning time, requiring multiple tasks, each with hopefully enough trials to have reasonable internal consistency and test-retest reliability. This could be hard to achieve in many studies in which multiple tasks assessing different neural systems or functions are included. If habituation is a limiting factor, it could also require multi-session protocols. That said, such approaches may be necessary if we want to draw general conclusions from existing AMG probe tasks.
Supplementary Material
Acknowledgments
Funding:
This work was supported by the National Institutes of Mental Health [grant number: R01-MH098098].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18:166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Prog Brain Res. 2006;156:363–378. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Avery SN, Blackford JU. Slow to warm up: the role of habituation in social fear. Soc Cogn Affect Neurosci. 2016 doi: 10.1093/scan/nsw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas JM, Mol N, Kenemans JL, Prinssen EP, Niklson I, Xia-Chen C, Broeyer F, van Gerven J. Validating a human model for anxiety using startle potentiated by cue and context: the effects of alprazolam, pregabalin, and diphenhydramine. Psychopharmacology (Berl) 2009;205:73–84. doi: 10.1007/s00213-009-1516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behav Neurosci. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brett MA,JL, Valabregue R, Poline JB. Neuroimage. Sendai, Japan: 2002. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain. [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: the role of threat appraisal and fear learning. Depress Anxiety. 2011;28:5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Shin LM, Barrett LF, Rauch SL, Wright CI. Amygdala and fusiform gyrus temporal dynamics: responses to negative facial expressions. BMC Neurosci. 2008;9:44. doi: 10.1186/1471-2202-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome MR, He Z, Iftikhar M, Eyden J, Marwaha S. Neurobiological and behavioural studies of affective instability in clinical populations: a systematic review. Neurosci Biobehav Rev. 2015;51:243–254. doi: 10.1016/j.neubiorev.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Church JA, Petersen SE, Schlaggar BL. The “Task B problem” and other considerations in developmental functional neuroimaging. Hum Brain Mapp. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, Blackford JU. The nature of individual differences in inhibited temperament and risk for psychiatric disease: A review and meta-analysis. Prog Neurobiol. 2015:127–128. doi: 10.1016/j.pneurobio.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Denny BT, Ochsner KN, Weber J, Wager TD. Anticipatory brain activity predicts the success or failure of subsequent emotion regulation. Soc Cogn Affect Neurosci. 2014;9:403–411. doi: 10.1093/scan/nss148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston EK, McHugo M, Dukic MS, Smith SD, Abou-Khalil B, Eggers E, Zald DH. Enhanced visual cortical activation for emotional stimuli is preserved in patients with unilateral amygdala resection. J Neurosci. 2013;33:11023–11031. doi: 10.1523/JNEUROSCI.0401-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Res Bull. 2003;59:387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Grady CL, Madsen MK, Strother SC, Knudsen GM. 5-HTTLPR differentially predicts brain network responses to emotional faces. Hum Brain Mapp. 2015;36:2842–2851. doi: 10.1002/hbm.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RS, Dolan RJ. The trouble with cognitive subtraction. Neuroimage. 1996;4:97–104. doi: 10.1006/nimg.1996.0033. [DOI] [PubMed] [Google Scholar]
- Goldberg H, Preminger S, Malach R. The emotion-action link? Naturalistic emotional stimuli preferentially activate the human dorsal visual stream. Neuroimage. 2014;84:254–264. doi: 10.1016/j.neuroimage.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Haddad AD, Bilderbeck A, James AC, Lau JY. Fear responses to safety cues in anxious adolescents: Preliminary evidence for atypical age-associated trajectories of functional neural circuits. J Psychiatr Res. 2015;68:301–308. doi: 10.1016/j.jpsychires.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002a;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002b;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Heinzel A, Bermpohl F, Niese R, Pfennig A, Pascual-Leone A, Schlaug G, Northoff G. How do we modulate our emotions? Parametric fMRI reveals cortical midline structures as regions specifically involved in the processing of emotional valences. Brain Res Cogn Brain Res. 2005;25:348–358. doi: 10.1016/j.cogbrainres.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Kennis M, Rademaker AR, Geuze E. Neural correlates of personality: an integrative review. Neurosci Biobehav Rev. 2013;37:73–95. doi: 10.1016/j.neubiorev.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. University of Florida; Gainesville, FL: 2008. [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Grillon C, Leibenluft E, Lissek S, Norcross M, Shiffrin N, Pine DS. Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci U S A. 2011;108:4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, Jenness J, Ernst M, Grillon C, Pine DS. Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. J Am Acad Child Adolesc Psychiatry. 2008;47:94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion, memory and the brain. Sci Am. 1994;270:50–57. doi: 10.1038/scientificamerican0694-50. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin NH. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Grimm O, Morgen K, Mier D, Sauer C, Haddad L, Tost H, Esslinger C, Kirsch P, Schwarz AJ, Meyer-Lindenberg A. Amygdala habituation: a reliable fMRI phenotype. Neuroimage. 2014;103:383–390. doi: 10.1016/j.neuroimage.2014.09.059. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. Neuroimage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sakaki M, Niki K, Mather M. Beyond arousal and valence: the importance of the biological versus social relevance of emotional stimuli. Cogn Affect Behav Neurosci. 2012;12:115–139. doi: 10.3758/s13415-011-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman PE, Van Bavel JJ, Cunningham WA. Valence asymmetries in the human amygdala: task relevance modulates amygdala responses to positive more than negative affective cues. J Cogn Neurosci. 2015;27:842–851. doi: 10.1162/jocn_a_00756. [DOI] [PubMed] [Google Scholar]
- Wise RG, Tracey I. The role of fMRI in drug discovery. J Magn Reson Imaging. 2006;23:862–876. doi: 10.1002/jmri.20584. [DOI] [PubMed] [Google Scholar]
- Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, Monk CS, Phan KL. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


