Abstract
Background: The transforming growth factor-β1 (TGF-β1) gene -509C/T polymorphism has been suggested to be associated with increased coronary artery disease (CAD) risk. However, the individual studies results are still inconsistent.
Objective and methods: To investigate the relationship between TGF-β1 gene -509C/T polymorphism and CAD, a meta-analysis involving 11,701 participants from 8 individual studies was conducted. The pooled odds ratios (ORs) and their corresponding 95% confidence intervals were evaluated by using random or fixed effect models.
Results: A significant association between TGF-β1 gene -509C/T polymorphism and CAD was detected in the total population under allelic (OR: 1.130, 95% CI: 1.060–1.200, P = 0.0001), recessive (OR: 1.390, 95% CI: 1.100–1.750, P = 0.006), dominant (OR: 0.857, 95% CI: 0.785–0.935, P = 2.507 × 10−4), homozygous (OR: 1.258, 95% CI: 1.098–1.442, P = 0.001), heterozygous (OR: 1.147, 95% CI: 1.046–1.257, P = 0.003), and additive genetic models (OR: 1.131, 95% CI: 1.063–1.204, P = 5.442 × 10−5). In the subgroup analysis, there was a significant association between them in Chinese population under all of the genetic models (P < 0.05), except under the heterozygous genetic model (P > 0.05). In the Caucasian subgroup, a significant association between them was also detected under all of the genetic models (P < 0.05), except under the recessive genetic model (P > 0.05).
Conclusions: TGF-β1 gene -509C/T polymorphism was significantly associated with increased CAD risk. The people with T allele of TGF-β1 gene -509C/T polymorphism might be predisposed to CAD.
Keywords: TGF-β1, -509C/T, polymorphism, coronary artery disease
Introduction
The coronary artery disease (CAD) morbidity rose markedly which presented a rejumvanation trend (Zou et al., 2013). According to the mortality and disability rate, CAD is the most important disease in the United States of America (USA) and other industrialized countries. In the nearly one million patients who died of the cardiovascular disease in USA every year, CAD accounted for 50%. About 1.5 million USA patients were attacked by acute myocardial infarction which was almost caused by coronary atherosclerosis (Yang et al., 2006). CAD is the myocardial ischemia or necrosis disease caused by the artery lumen stenosis due to the coronary atherosclerosis. The CAD pathogenesis might be the combined actions result by heredity and environmental factors. The single nucleotide polymorphism is the important genetic factor which influences the CAD susceptibility.
The human transforming growth factor-β1 (TGF-β1) gene, located in 19q13 chromosome, spans 2.5 kb which consists of 7 exons and 6 introns. The researches have confirmed that TGF-β1 could increase the extracellular matrix, inhibit the vascular smooth muscle cells proliferation, promote the vascular endothelial cells, and regulate the local inflammation. Thus TGF-β1 could influence the CAD progress (Aihara et al., 2010). The -509C/T (rs1800469) mutation, located in the upstream of the TGF-β1 gene transcriptional initiation site, is the negative regulation region. C/T mutation alters the affinity of the promoter and transcription factor which cause the reduced TGF-β1 transcription level and participate in the CAD progression (Yokota et al., 2000).
Although many studies on the relationship between TGF-β1 gene -509C/T polymorphism and CAD have been performed so far, the individual studies results were still conflicting. In 2014, Pei et al. found the TT genotype of the TGF-β1 gene -509C/T polymorphism increased the CAD risk in a Chinese population (Pei et al., 2014). In addition, in another Chinese population Zou et al. also got the similar conclusion in 2013 (Zou et al., 2013). On the contrary, in 2006, Koch et al. did not find any significant genotype distributions difference of TGF-β1 gene -509C/T gene polymorphism between control and CAD patients and they concluded that the TGF-β1 gene -509C/T polymorphism was not associated with CAD in a Caucasian population (Koch et al., 2006). In 2009, Drenos got the similar negative conclusion in another Caucasian population (Drenos et al., 2009).
In the current study, a meta-analysis involving 6,377 CAD patients and 5,324 controls from eight separate studies was performed (Supplement S1). The current meta-analysis will help us further estimate the relationship of TGF-β1 gene -509C/T polymorphism and CAD and formulate a novel individualized therapy strategy of CAD.
Materials and methods
Publication search and inclusion criteria
The terms as “TGF-β1,” “-509C/T,” “polymorphism” and “coronary artery disease” were used to retrieve the electronic databases including China Biological Medicine Database, China National Knowledge Infrastructure, PubMed, Embase, and Web of Science. The last research was updated on December 21, 2016 with the publication years ranging from 1996 to 2014.
The recruited studies should conform to such major criteria as the follows: (a) Estimation of the association of TGF-β1 gene -509C/T polymorphism and CAD. (b) CAD was diagnosed by coronary angiography and the criteria was defined as the luminal narrowing in at least one coronary artery was no less than 50%. The patients with any previous history of documented CAD, with or without coronary revascularization were also classified to be CAD group (Li et al., 2016). The healthy population in the similar period without coronary artery stenosis was included in the control group. (c) The case-control or cohort studies published officially should be included. (d) The control group genotype member should follow the Hardy-Weinberg equilibrium (HWE).
Data extraction
According to a standard protocol, the data was drawn out. The meta-analysis was performed by three investigators; two of whom were responsible for searching out the individual studies in duplicate, and the third one was to resolve the disputes between the two investigators. Those studies that did not meet the major selection criteria, that were repeated publications, or that supplied inadequate data were got rid of the present meta-analysis. If similar data was found in different papers by the same author group, the data was only adopted for one time. The following items as publication year, the first author's name, ethnicity, country, genotyping method, matching criteria, genotype number, and total number of cases and controls should be listed in the data table.
Statistical analyses
Six genetic models as the allelic (T allele distribution frequency), recessive (TT vs. CC+CT), dominant (CC vs. TT+CT), homozygous (TT vs. CC), heterozygous (CT vs. CC), and additive (T vs. C) genetic models were adopted in the present meta-analysis. The odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were used to compare the association of TGF-β1 gene -509C/T polymorphism and CAD. The heterogeneity among the studies was calculated by Chi-square-based Q-tests and the significance was set at P < 0.05 level (Cochran, 1968). If heterogeneity existed among the individual studies, the random-effect model (DerSimonian and Laird method) would be used (Dersimonian and Laird, 2015). Or else, the fixed-effect model was adopted (the Mantel–Haenszel method) (Mantel and Haenszel, 1959). The pooled OR was assessed by Z test with significance set at P < 0.05 level.
The HWE was evaluated by Fisher's exact test with significance set at P < 0.05 level. The potential publication bias was assessed by funnel plot. The funnel plot symmetry was evaluated by using the Egger's linear regression test on the natural logarithm scale of the OR with significance set at P < 0.05 level (Egger et al., 1997). The statistical analyses were performed by using Revman 5.0 and Stata 12.0 software (StataCorp, College Station, TX, USA).
Results
Studies and populations
All of information was abstracted from 6,377 CAD cases and 5,324 controls (Table 1) (Cambien et al., 1996; Syrris et al., 1998; Koch et al., 2006; Crobu et al., 2008; Drenos et al., 2009; Sudomoina et al., 2010; Zou et al., 2013; Pei et al., 2014). Sixteen manuscripts were found out by the retrieval process, among which eight papers conformed to the inclusion criteria. Among the eight excluded studies, three papers were of reviews character, and two manuscripts violated the HWE (Sie et al., 2006; Najar et al., 2011). Three papers were not involved with TGF-β1 gene -509C/T polymorphism or CAD (Supplement S2). Six countries were included in the present meta-analysis as China, Italia, German, United Kingdom, France, and Russia. They belong to Chinese and Caucasian subgroups respectively. Two individual studies were included in the Chinese subgroup and six individual studies were included in the Caucasian subgroup.
Table 1.
Characteristics of the investigated studies of the association between the TGF-β1 gene -509C/T polymorphism and CAD.
| Author | Year | Country | Ethnicity | Genotype | CAD | Control | Matching criteria | sample size (CAD/control) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | CC | CT | TT | |||||||
| Zou SM | 2013 | China | Han | PCR-LDR | 49 | 153 | 138 | 49 | 128 | 94 | Age, ethnicity,HDL-C | 340/271 |
| Pei F | 2014 | China | Han | PCR-RFLP | 97 | 217 | 143 | 111 | 201 | 101 | Age, sex, ethnicity, smoker, HP | 457/413 |
| Crobu F | 2008 | Italia | Caucasian | PCR-RFLP | 67 | 87 | 47 | 80 | 92 | 29 | Ethnicity | 201/201 |
| Koch W | 2006 | German | Caucasian | PCR-RFLP | 1581 | 1659 | 417 | 564 | 508 | 139 | Ethnicity | 3657/1211 |
| Syrris P | 1998 | UK | Caucasian | PCR-SSCP | 301 | 284 | 70 | 124 | 97 | 23 | Age, ethnicity | 655/244 |
| Cambien F | 1996 | FR,NIE | Caucasian | PCR-SSCP | 240 | 257 | 66 | 263 | 297 | 69 | Ethnicity | 563/629 |
| Sudomoina MA | 2010 | Russia | Caucasian | PCR-RFLP | 77 | 150 | 37 | 90 | 103 | 19 | Ethnicity | 264/212 |
| Drenos F | 2009 | England | Caucasian | PCR-RFLP | 120 | 100 | 20 | 1090 | 885 | 168 | Ethnicity | 240/2143 |
CAD, coronary artery disease; HDL-C, high density lipoprotein-cholesterol; HP, hypertension; UK, United Kingdom; FR, France; NIE, Northern Ireland. PCR-LDR, Polymerase chain reaction-ligase detection reaction; PCR-RFLP, Polymerase chain reaction-restriction fragment length polymorphism; PCR-SSCP, Polymerase Chain Reaction-Single Strand Conformation Polymerase; Case-control study design were adopted in the above studies.
Pooled analyses
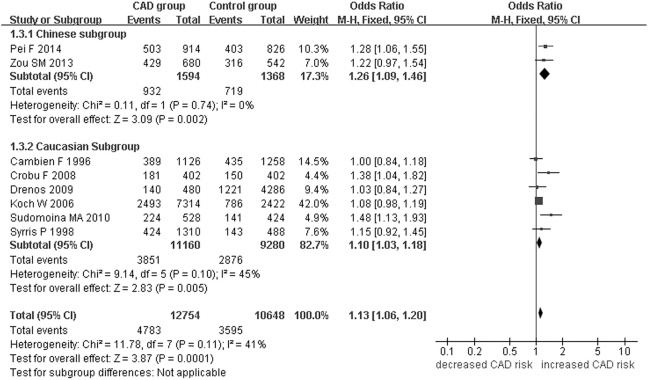
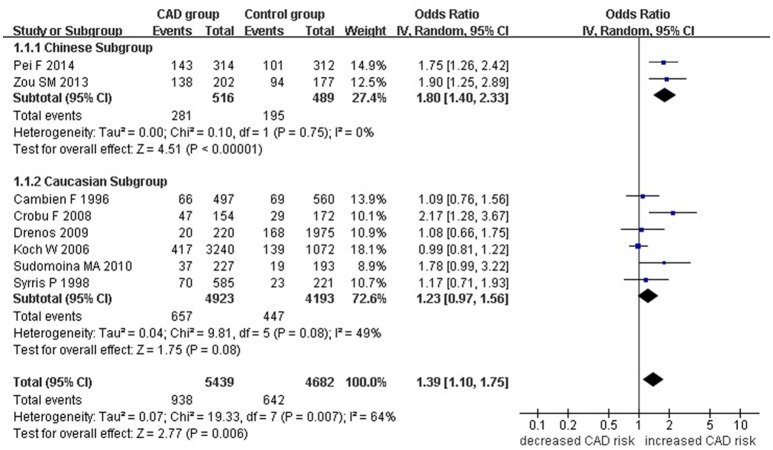
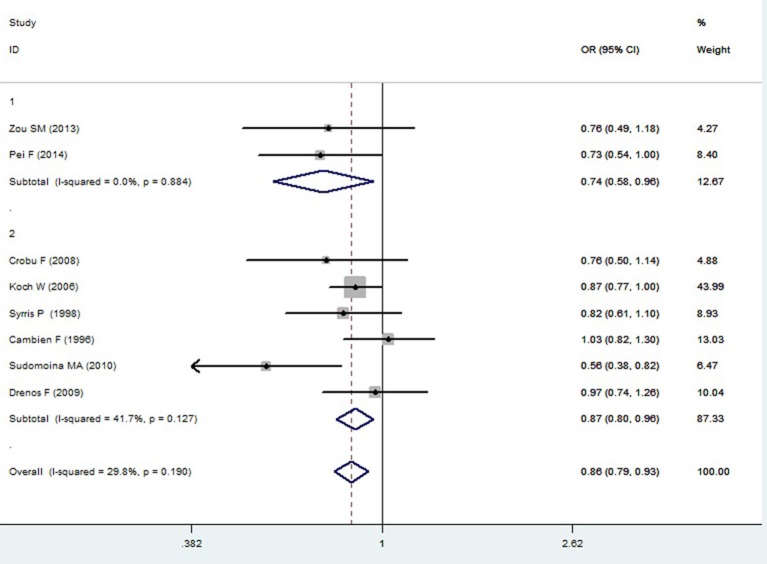
A significant association between TGF-β1 gene -509C/T polymorphism and CAD was detected in the total population under allelic (OR: 1.130, 95% CI: 1.060–1.200, P = 0.0001), recessive (OR: 1.390, 95% CI: 1.100–1.750, P = 0.006), dominant (OR: 0.857, 95% CI: 0.785–0.935, P = 2.507 × 10−4), homozygous (OR: 1.258, 95% CI: 1.098–1.442, P = 0.001), heterozygous (OR: 1.147, 95% CI: 1.046–1.257, P = 0.003), and additive genetic models (OR: 1.131, 95% CI: 1.063–1.204, P = 5.442 × 10−5).
In the subgroup analysis, there was a significant association between them in Chinese population under all of the genetic models (P < 0.05), except under the heterozygous genetic model (P >0.05).
In the Caucasian subgroup, a significant association between them was also detected under all of the genetic models (P < 0.05), except under the recessive genetic model (P > 0.05).
In the subgroup analysis, there was a significant association between them in Chinese population under allelic (OR: 1.260, 95% CI: 1.090–1.460, P = 0.005), recessive (OR: 1.800, 95% CI: 1.400–2.330, P = 3.241 × 10−6), dominant (OR: 0.743, 95% CI: 0.577-0.957, P = 0.022), homozygous (OR: 1.561, 95% CI: 1.164–2.092, P = 0.003), and additive genetic models (OR: 1.259, 95% CI: 1.088–1.458, P = 0.002). However, no significant association between TGF-β1 gene -509C/T polymorphism and CAD was found under heterozygous genetic model (OR: 1.222, 95% CI: 0.933–1.600, P = 0.146).
In the Caucasian subgroup, a significant association between them was also detected under allelic (OR: 1.100, 95% CI: 1.030–1.180, P = 0.002), dominant (OR: 0.873, 95% CI: 0.796-0.958, P = 0.004), homozygous (OR: 1.187, 95% CI: 1.017–1.384, P = 0.029), heterozygous (OR: 1.137, 95% CI: 1.031–1.254, P = 0.010), and additive genetic models (OR: 1.105, 95% CI: 1.031–1.184, P = 0.005). However, no significant association was detected under recessive genetic model (OR: 1.230, 95% CI: 0.970–1.560, P = 0.08) (Table 2, Figures 1–6).
Table 2.
Summary of meta-analysis of association between the TGF-β1 gene -509C/T polymorphism and CAD.
| Genetic model | Pooled OR (95% CI) | Z-value | P-value | Study number | CAD size | Control size | Pheterogeneity(I2%) |
|---|---|---|---|---|---|---|---|
| Allelic genetic model | 1.130 (1.060–1.200) | 3.87 | 0.0001* | 8 | 6377 | 5324 | 0.11 (41.0) |
| Chinese subgroup | 1.260 (1.090–1.460) | 2.83 | 0.005* | 2 | 797 | 684 | 0.74 (0) |
| Caucasian subgroup | 1.100 (1.030–1.180) | 3.09 | 0.002* | 6 | 5580 | 4640 | 0.10 (45.0) |
| Recessive genetic model | 1.390 (1.100–1.750) | 2.77 | 0.006* | 8 | 6377 | 5324 | 0.007* (64.0) |
| Chinese subgroup | 1.800 (1.400–2.330) | 4.51 | 3.241 × 10−6* | 2 | 797 | 684 | 0.75 (0) |
| Caucasian subgroup | 1.230 (0.970–1.560) | 1.75 | 0.08 | 6 | 5580 | 4640 | 0.08 (49.0) |
| Dominant genetic model | 0.857 (0.785–0.935) | 3.48 | 2.507 × 10−4* | 8 | 6377 | 5324 | 0.190 (29.8) |
| Chinese subgroup | 0.743 (0.577–0.957) | 2.30 | 0.022* | 2 | 797 | 684 | 0.884 (0) |
| Caucasian subgroup | 0.873 (0.796–0.958) | 2.87 | 0.004* | 6 | 5580 | 4640 | 0.127 (41.7) |
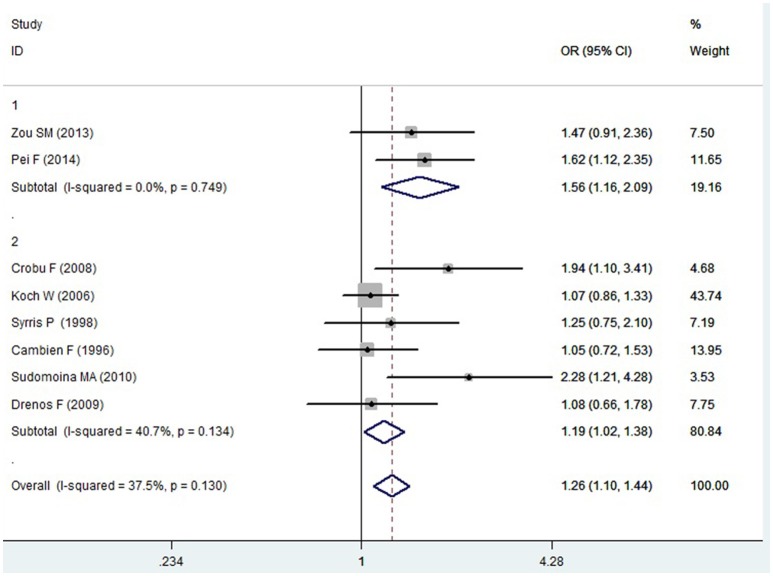
| Homozygous genetic model | 1.258 (1.098–1.442) | 3.30 | 0.001* | 8 | 6377 | 5324 | 0.130 (37.5) |
| Chinese subgroup | 1.561 (1.164–2.092) | 2.98 | 0.003* | 2 | 797 | 684 | 0.749 (0) |
| Caucasian subgroup | 1.187 (1.017–1.384) | 2.18 | 0.029* | 6 | 5580 | 4640 | 0.134 (40.7) |
| Heterozygous genetic model | 1.147 (1.046–1.257) | 2.92 | 0.003* | 8 | 6377 | 5324 | 0.407 (2.9) |
| Chinese subgroup | 1.222 (0.933–1.600) | 1.45 | 0.146 | 2 | 797 | 684 | 0.909 (0) |
| Caucasian subgroup | 1.137 (1.031–1.254) | 2.58 | 0.010* | 6 | 5580 | 4640 | 0.224 (28.2) |
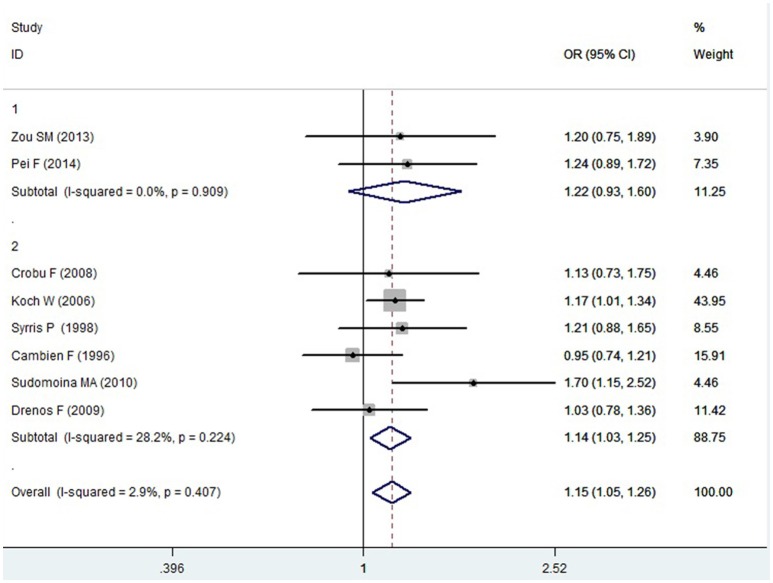
| Additive genetic model | 1.131 (1.063–1.204) | 3.87 | 5.442 × 10−5* | 8 | 6377 | 5324 | 0.108 (40.6) |
| Chinese subgroup | 1.259 (1.088–1.458) | 3.09 | 0.002* | 2 | 797 | 684 | 0.744 (0) |
| Caucasian subgroup | 1.105 (1.031–1.184) | 2.83 | 0.005* | 6 | 5580 | 4640 | 0.104 (45.3) |
P ≤ 0.05. CAD, coronary artery disease; CI, confidence interval; OR, odds ratio; CAD size, the total number of CAD cases; control size, the total number of control group; Allelic genetic model, T allele distribution frequency; recessive genetic model: TT vs. CC+CT, Dominant genetic model: CC vs. TT+CT; Homozygous genetic mode, TT vs. CC; Heterozygous genetic model, CT vs. CC; Additive genetic model, total T allele vs. total C allele.
Figure 1.
Forest plot of CAD associated with TGF-β1 gene -509C/T polymorphism under an allelic genetic model (distribution of T allelic frequency of TGF-β1 gene -509C/T polymorphism).
Figure 6.
Forest plot of CAD associated with TGF-β1 gene -509C/T polymorphism under an additive genetic model (T vs. C).
Figure 2.
Forest plot of CAD associated with TGF-β1 gene -509C/T polymorphism under a recessive genetic model (TT vs. CC+CT).
Figure 3.
Forest plot of CAD associated with TGF-β1 gene -509C/T polymorphism under a dominant genetic model (CC vs. TT+CT).
Figure 4.
Forest plot of CAD associated with TGF-β1 gene -509C/T polymorphism under a homozygous genetic model (TT vs. CC).
Figure 5.
Forest plot of CAD associated with TGF-β1 gene -509C/T polymorphism under a heterozygous genetic model (CT vs. CC).
There was no significant heterogeneity in the whole population under all of the genetic models (Pheterogeneity > 0.05) except under the recessive genetic model (Pheterogeneity < 0.05). In the subgroup analysis stratified by ethnicity, no heterogeneity was detected either in the Chinese subgroup or in the Caucasian subgroup under the recessive genetic model (Pheterogeneity > 0.05).
Bias diagnostics
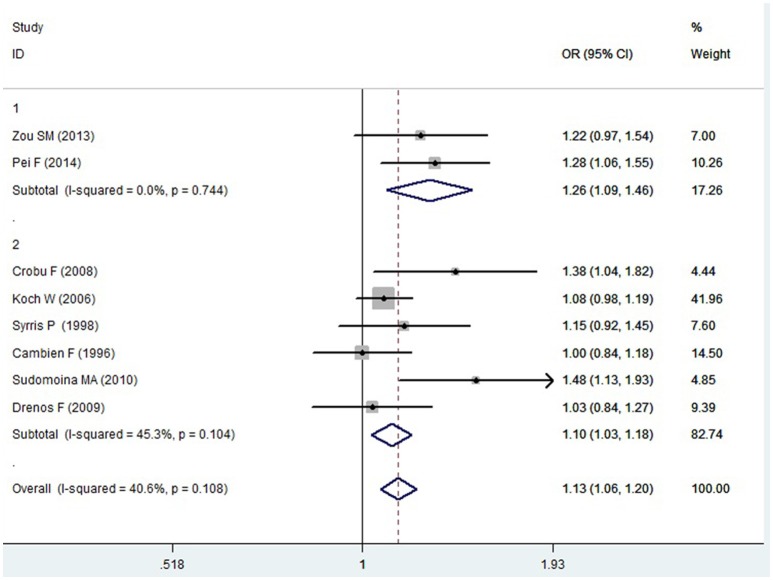
The publication bias among the individual studies was evaluated by using the funnel plot and Egger's test. No publication bias was visualized in the funnel plot under the allelic genetic model (Figure 7). Additionally, no significant difference was detected in the Egger's test yet, which indicated that there was no publication bias in the current meta-analysis by using allelic genetic model (T = 2.04, P = 0.088).
Figure 7.
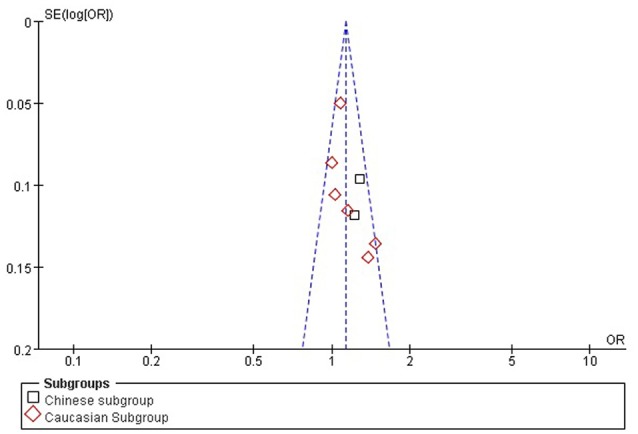
The funnel plot for studies of the association of CAD associated with TGF-β1 gene -509C/T polymorphism under an allelic genetic model (distribution of T allelic frequency of TGF-β1 gene -509C/T polymorphism). The horizontal and vertical axis correspond to the OR and confidence limits. OR, odds ratio, SE; standard error.
Discussion
In the current meta-analysis, a significant association was detected in the whole population between TGF-β1 gene -509C/T polymorphism and CAD under allelic (OR: 1.130), recessive (OR: 1.390), dominant (OR: 0.857), homozygous (OR: 1.258), heterozygous (OR: 1.147), and additive genetic models (OR: 1.131). In the subgroup analysis by ethnicity, a significant association in Chinese subgroup was also found under allelic (OR: 1.260), recessive (OR: 1.800), dominant (OR: 0.743), homozygous (OR: 1.561), and additive genetic models (OR: 1.259). A significant association was also found in the Caucasian subgroup under allelic (OR: 1.100), recessive (OR: 1.230), dominant (OR: 0.873), homozygous (OR: 1.187), heterozygous (OR: 1.137), and additive genetic models (OR: 1.105). In conclusion, it was implied that the carriers with T allele of TGF-β1 gene -509C/T polymorphism might increase CAD risk, both in the Chinese and Caucasian population.
No significant heterogeneity was detected in the whole population under all of the genetic models except the recessive genetic model. Under the recessive genetic model, a significant heterogeneity was detected in the whole population (Pheterogeneity = 0.007). In the subsequent subgroup analysis stratified by ethnicity, the heterogeneity did not exist any longer in the Chinese or Caucasian subgroup (P > 0.05).
The pathogenesis of CAD include coronary atherosclerosis, embolism, dissecting aneurysm, coronaritis, malformation of coronary artery, metabolic disease, infection, trauma and et al, of which coronary atherosclerosis accounts for more than 90%. Atherosclerosis is proliferative inflammatory lesions. The pathological changes include the vascular endothelial cells dysfunction, the vascular smooth muscle cells (VSMCs) proliferation and migration, the extracelluar matrix proliferation, the inflammatory cells infiltration, the fibrous cap formation, and rupture and angiogenesis (Yang et al., 2006).
Recently the researches about the association of some cytokines and CAD have become the research hot, particularly the TGF-β1. TGF-β1 is the disulfide bonded dimer which consists of two 12.5 kDa polypeptide chains. The newly synthesized TGF-β1 is the inactive precursor protein which is constituted with 391 amino acid residues. The 25 kDa active TGF-β1 is generated by the enzymolysis in vivo or disposed by acid, urea and protease in vitro. TGF-β1 plays the multifunctional regulation role as inhibiting the inflammatory cytokines, regulating the cells proliferation and differentiation, embryonic development, damage repair and angiogenesis through the endocrine, and paracrine mechanisms (Feinberg et al., 2000).
TGF-β1 plays the important roles in the coronary atherosclerosis. TGF-β1 could down regulate many cytokines level and function. It can inhibit TNF-α synthesis and reduce its effect. TGF-β1 can decrease the VCAM-1 expression induced by the inflammatory cytokines and inhibit the leukocytes adhering to the endothelial cells (Agassandian et al., 2015). TGF-β1 can decrease the IFN-γ effect. TGF-β1 can reduce the macrophage activity by decreasing the IL-1 activity (Ruscetti et al., 1992; Gamble et al., 1993; Park et al., 2000). TGF-β1 mainly has 4 kinds of functions. (1) TGF-β1 inhibits the VSMCs migration and proliferation; (2) TGF-β1 induces the apoptosis of leukocytes which participates in the vascular injury; (3) TGF-β1 reduces the adherence of the inflammatory cells to the endothelial cells; (4) TGF-β1 helps the vascular protection by inhibiting the human metalloenzyme and unstability reaction in the instable plaque (Wahl et al., 1989; Mccaffrey et al., 1999; Blobe et al., 2000). TGF-β1 gene -509C/T mutation might decrease the affinity of the promoter and transcription factor, reduce TGF-β1 transcription level and promote the coronary atherosclerosis.
In 2012, Morris et al. performed a meta-analysis on the association of CAD with TGF-β1 gene -509C/T polymorphism. In this meta-analysis, they concluded that T alleles of rs1800469 in TGF-β1 were associated with complications of CAD (Morris et al., 2012). However, only four individual studies were included. In 2012, Lu et al. performed another meta-analysis on the association of CAD with TGF-β1 gene -509C/T polymorphism. They found that T allele carriers of rs1800469 in TGF-β1 have a 15% increased risk of CAD. In their meta-analysis, only seven individual studies were included. Among these included individual studies, one study by Sie deviating from the HWE was not excluded (Lu et al., 2012). Hence, the results from the two above meta-analysises published before were not as accurate as that deduced from the current meta-analysis.
However, there were still some limitations in the present meta-analysis. The current meta-analysis is still short of prospective or large-scale studies on the association of TGF-β1 gene -509C/T polymorphism and CAD. The serum TGF-β1 level was influenced not only by the TGF-β1 gene -509C/T polymorphism, but also by other polymorphism as -800 G/A, 868 T/C, 913 G/C, and 11929 C/T gene polymorphism. Many other environmental factors as age, smoke, gender, obesity, dyslipidemia, diabetes mellitus, and hypertension might affect the CAD development. Since these factors were not matched in most of individual studies, it was difficult to analyze their effect on the CAD progress (Lu et al., 2012). Additionally, according to our knowledge it is >1000-fold confirmed association between given polymorphism and the risk of CAD. It might not influence our every day clinical practice concerning diagnosis or therapy yet. However, it can help us to screen out the susceptible population for CAD and prevent the CAD progression in advance. Therefore, the association of TGF-β1 gene -509C/T polymorphism and CAD is important to the prevention of CAD in the high risk population.
As CAD is a polygenic disease, various micro-effect genes might have an effect on the CAD risk. Other genes polymorphisms as CD14 gene −159C/T polymorphism, Interleukin-6 C-572G polymorphism, methylenetetrahydrofolate reductase gene C677T polymorphism, intercellular adhesion molecule-1 gene E469K polymorphism, ATP-binding cassette transporter A1 gene R219K polymorphism, apo A5 gene −1131T/C, FgB −455G/A, −148C/T, and CETP gene TaqIB polymorphisms, plasminogen activator inhibitor-1 gene 4G/5G polymorphism might increase the CAD risk (Li, 2012a,b; Li et al., 2012, 2013, 2015a,b; Yanyan, 2012).
In short, TGF-β1 gene -509C/T polymorphism was significantly associated with increased CAD risk both in the Chinese and Caucasian population. The persons with the T allele of TGF-β1 gene -509C/T polymorphism might be predisposed to CAD risk. This conclusion might guide us to establish a novel CAD individualized treatment. In consideration of the above limitations, more researches on the association of TGF-β1 gene -509C/T polymorphism and CAD needed to be conducted to further confirm the conclusions in the future.
Author contributions
Conceived and designed the experiments: YL and YZ. Performed the experiments: YL and GG. Analyzed the data: YL and HG. Contributed reagents/material/analysis tools: YL and XY. Wrote the manuscript, reference collection, data management, statistical analyses, paper writing, and study design: YL.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (NSFC 81100073 to YL), Excellent Young and Middle-Aged Teachers Assistance Program of Nanjing Medical University for YL (2013–2015, JX2161015034), Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Thank all our colleagues working in the Department of geriatrics, the First Affiliated Hospital of Nanjing Medical University.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphys.2017.00108/full#supplementary-material
References
- Agassandian M., Tedrow J. R., Sembrat J., Kass D. J., Zhang Y., Goncharova E. A., et al. (2015). VCAM-1 is a TGF-β1 inducible gene pregulated in idiopathic pulmonary fibrosis. Cell. Signal. 27, 2467–2473. 10.1016/j.cellsig.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara K., Ikeda Y., Yagi S., Akaike M., Matsumoto T. (2010). Transforming growth factor-β1 as a common target molecule for development of cardiovascular diseases, renal insufficiency and metabolic syndrome. Cardiol. Res. Pract. 2011:175381. 10.4061/2011/175381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobe G. C., Schiemann W. P., Lodish H. F. (2000). Role of transforming growth factor beta in human disease. N. Engl. J. Med. 342, 1350–1358. 10.1056/NEJM200005043421807 [DOI] [PubMed] [Google Scholar]
- Cambien F., Ricard S., Troesch A., Mallet C., Generenaz L., Evans A., et al. (1996). Polymorphisms of the transforming growth factor-beta 1 gene in relation to myocardial infarction and blood pressure. The Etude Cas-Temoin de l'Infarctus du Myocarde (ECTIM) Study. Hypertension 28, 881–887. 10.1161/01.HYP.28.5.881 [DOI] [PubMed] [Google Scholar]
- Cochran W. G. (1968). The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics 24, 295–313. 10.2307/2528036 [DOI] [PubMed] [Google Scholar]
- Crobu F., Palumbo L., Franco E., Bergerone S., Carturan S., Guarrera S., et al. (2008). Role of TGF-β1 haplotypes in the occurrence of myocardial infarction in young Italian patients. BMC Med. Genet. 9:13. 10.1186/1471-2350-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersimonian R., Laird N. (2015). Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 45, 139–145. 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenos F., Talmud P. J., Casas J. P., Smeeth L., Palmen J., Humphries S. E., et al. (2009). Integrated associations of genotypes with multiple blood biomarkers linked to coronary heart disease risk. Hum. Mol. Genet. 18, 2305–2316. 10.1093/hmg/ddp159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M., Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M. W., Jain M. K., Werner F., Sibinga N. E., Wiesel P., Wang H., et al. (2000). Transforming growth factor-beta 1 inhibits cytokine-mediated induction of human metalloelastase in macrophages. J. Biol. Chem. 275, 25766–25773. 10.1074/jbc.M002664200 [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Khew-Goodall Y., Vadas M. A. (1993). Transforming growth factor-beta inhibits E-selectin expression on human endothelial cells. J. Immunol. 150, 4494–4503. [PubMed] [Google Scholar]
- Koch W., Hoppmann P., Mueller J. C., Schomig A., Kastrati A. (2006). Association of transforming growth factor-beta1 gene polymorphisms with myocardial infarction in patients with angiographically proven coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 26, 1114–1119. 10.1161/01.ATV.0000217747.66517.11 [DOI] [PubMed] [Google Scholar]
- Li X., Liu M., Sun R., Zeng Y., Chen S., Zhang P. (2016). Atherosclerotic coronary artery disease: the accuracy of measures to diagnose preclinical atherosclerosis. Exp. Ther. Med. 12, 2899–2902. 10.3892/etm.2016.3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Y. (2012a). Methylenetetrahydrofolate reductase C677T gene polymorphism and coronary artery disease in a Chinese Han population: a meta-analysis. Metab. Clin. Exp. 61, 846–852. 10.1016/j.metabol.2011.10.013 [DOI] [PubMed] [Google Scholar]
- Li Y. Y. (2012b). Plasminogen activator inhibitor-1 4G/5G gene polymorphism and coronary artery disease in the Chinese Han population: a meta-analysis. PLoS ONE 7:e33511. 10.1371/journal.pone.0033511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Y., Wang X. M., Zhou C. W., Xu J., Qian Y. (2015a). CD14 gene-159C/T polymorphism and coronary artery disease: a meta-analysis involving 4467 subjects. Int. J. Clin. Exp. Med. 8, 12149–12160. [PMC free article] [PubMed] [Google Scholar]
- Li Y. Y., Wu X. Y., Xu J., Qian Y., Zhou C. W., Wang B. (2013). Apo A5 -1131T/C, FgB -455G/A, -148C/T, and CETP TaqIB gene polymorphisms and coronary artery disease in the Chinese population: a meta-analysis of 15,055 subjects. Mol. Biol. Rep. 40, 1997–2014. 10.1007/s11033-012-2257-9 [DOI] [PubMed] [Google Scholar]
- Li Y. Y., Zhang H., Qin X. Y., Lu X. Z., Yang B., Chen M. L. (2012). ATP-binding cassette transporter A1 R219K polymorphism and coronary artery disease in Chinese population: a meta-analysis of 5,388 participants. Mol. Biol. Rep. 39, 11031–11039. 10.1007/s11033-012-2006-0 [DOI] [PubMed] [Google Scholar]
- Li Y. Y., Zhou C. W., Xu J., Qian Y., Wang X. M. (2015b). Interleukin-6 C-572G gene polymorphism and coronary artery disease in Asian: a meta-analysis of 2511 subjects. Int. J. Clin. Exp. Med. 8, 8995–9003. [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Boer J. M., Barsova R. M., Favorova O., Goel A., Muller M., et al. (2012). TGFB1 genetic polymorphisms and coronary heart disease risk: a meta-analysis. BMC Med. Genet. 13:39. 10.1186/1471-2350-13-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N., Haenszel W. (1959). Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 22, 719–748. [PubMed] [Google Scholar]
- Mccaffrey T. A., Du B., Fu C., Bray P. J., Sanborn T. A., Deutsch E., et al. (1999). The expression of TGF-β receptors in human atherosclerosis: evidence for acquired resistance to apoptosis due to receptor imbalance. J. Mol. Cell. Cardiol. 31, 1627–1642. 10.1006/jmcc.1999.0999 [DOI] [PubMed] [Google Scholar]
- Morris D. R., Moxon J. V., Biros E., Krishna S. M., Golledge J. (2012). Meta-analysis of the association between transforming growth factor-beta polymorphisms and complications of coronary heart disease. PLoS ONE 7:e37878. 10.1371/journal.pone.0037878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najar R. A., Ghaderian S. M., Panah A. S. (2011). Association of transforming growth factor-β1 gene polymorphisms with genetic susceptibility to acute myocardial infarction. Am. J. Med. Sci. 342, 365–370. 10.1097/MAJ.0b013e318215908a [DOI] [PubMed] [Google Scholar]
- Park S. K., Yang W. S., Lee S. K., Ahn H., Park J. S., Hwang O., et al. (2000). TGF-β(1) down-regulates inflammatory cytokine-induced VCAM-1 expression in cultured human glomerular endothelial cells. Nephrol. Dial. Transplant 15, 596–604. 10.1093/ndt/15.5.596 [DOI] [PubMed] [Google Scholar]
- Pei F., Huang J., Huang J., Tang S. Q., Li B. C., Liu Z., et al. (2014). Association of -509C/T polymorphism in the TGF-β1 gene promoter and coronary heart disease and its conventional risk factors in chongqing han population. Chin. J. Arterioscler. 22, 939–944. 10.1016/j.mgene.2016.06.006 [DOI] [Google Scholar]
- Ruscetti F. W., Dubois C. M., Jacobsen S. E., Keller J. R. (1992). Transforming growth factor beta and interleukin-1: a paradigm for opposing regulation of haemopoiesis. Baillieres. Clin. Haematol. 5, 703–721. 10.1016/S0950-3536(11)80013-2 [DOI] [PubMed] [Google Scholar]
- Sie M. P., Uitterlinden A. G., Bos M. J., Arp P. P., Breteler M. M., Koudstaal P. J., et al. (2006). TGF-β 1 polymorphisms and risk of myocardial infarction and stroke: the Rotterdam Study. Stroke 37, 2667–2671. 10.1161/01.STR.0000244779.30070.1a [DOI] [PubMed] [Google Scholar]
- Sudomoina M. A., Sukhinina T. S., Barsova R. M., Favorov A. V., Shakhnovich R. M., Titov B. V., et al. (2010). [Complex analsis of association of inflammation genes with myocardial infarction]. Mol. Biol. 44, 463–471. 10.1134/S0026893310030088 [DOI] [PubMed] [Google Scholar]
- Syrris P., Carter N. D., Metcalfe J. C., Kemp P. R., Grainger D. J., Kaski J. C., et al. (1998). Transforming growth factor-beta1 gene polymorphisms and coronary artery disease. Clin. Sci. 95, 659–667. 10.1042/cs0950659 [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Mccartney-Francis N., Mergenhagen S. E. (1989). Inflammatory and immunomodulatory roles of TGF-β. Immunol. Today 10, 258–261. 10.1016/0167-5699(89)90136-9 [DOI] [PubMed] [Google Scholar]
- Yang C. K., Lan Y. H., Yang S. J. (2006). Clinical significance of serum transforming growth factor -β1 measurement in patients with coronary heart disease. Chinese Primary Health Care 20, 88–90. 10.3969/j.issn.1001-568X.2006.04.067 [DOI] [Google Scholar]
- Yanyan L. (2012). Intercellular adhesion molecule-1 E469K gene polymorphism and coronary artery disease in the Chinese population: a meta-analysis involving 3065 subjects. Clin. Cardiol. 35, 55–60. 10.1002/clc.20972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota M., Ichihara S., Lin T. L., Nakashima N., Yamada Y. (2000). Association of a T29–>C polymorphism of the transforming growth factor-beta1 gene with genetic susceptibility to myocardial infarction in Japanese. Circulation 101, 2783–2787. 10.1161/01.CIR.101.24.2783 [DOI] [PubMed] [Google Scholar]
- Zou S. M., Chen J. M., Yang X. L., Jing X., Liu X. G., Xiong X. D. (2013). Correlation between TGF-β1 -509C/T polymorphism and genetic susceptibility to coronary artery disease. Chin. J. Geriatr. Heart Brain Vessel Dis. 15, 361–364. 10.3969/j.issn.1009-0126.2013.04.008 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.