Abstract
BACKGROUND
Freezing of gait (FOG) affects approximately 50% of people with Parkinson Disease (PD), impacting quality of life and placing financial and emotional strain on the individual and caregivers. People with PD and FOG have similar deficits in motor adaptation and cognition as individuals with cerebellar lesions, indicating the cerebellum may play a role in FOG.
OBJECTIVE
To examine potential differences in cerebellar volumes and their relationships with cognition between PD with (FOG+) and without FOG (FOG−).
METHODS
Sixty-three participants were divided into two groups, FOG+ (n=25) and FOG− (n=38), based on the New Freezing of Gait Questionnaire. Cognitive assessment included Trail Making, Stroop, Verbal Fluency, and Go-NoGo executive function tasks. All participants completed structural T1- and T2-weighted MRI scans. Imaging data were processed with FreeSurfer and the Spatially Unbiased Infratentorial toolbox to segment the cerebellum into individual lobules.
RESULTS
FOG+ performed significantly worse on phonemic verbal fluency (F(1, 22)=7.06, p=0.01) as well as the Go-NoGo task (F(1, 22)=9.00, p=0.004). We found no differences in cerebellar volumes between groups (F(4, 55)=1.42, p=0.24), but there were significant relationships between verbal fluency measures and lobule volumes in FOG−.
CONCLUSIONS
These findings underscore the need for longitudinal studies to better characterize potential changes in cerebellar volume, cognitive function, and functional connectivity between people with PD with and without FOG.
Keywords: Parkinson disease, Gait, Cerebellum, Cognition, Neuroimaging
Introduction
Freezing of gait (FOG) affects approximately 50% of people with Parkinson disease (PD), with increasing prevalence as the disease progresses [1]. FOG is characterized by a temporary inability to make a step during walking or turning but can also affect upper limbs, speech, and eye movements. FOG increases the risk of falling, which can lead to serious health complications such as broken hips or wrists, placing financial and emotional strain on the individual and caregivers [2].
Although the underlying mechanisms of FOG remain unclear, the cerebellum has been implicated as a potential contributor to FOG. Recent research on cerebellar functional connectivity in PD with FOG (FOG+) showed altered connectivity between the cerebellum and other subcortical and cortical structures [3, 4]. Notably, diffusion tensor imaging (DTI) research showed altered connectivity through the pedunculopontine nucleus (PPN) in FOG+ compared to PD without FOG (FOG−) and healthy controls [3]. FOG+ also show decreased after effects compared to FOG− following locomotor adaptation [5], indicating impaired retention of the learned adaptation. Individuals with cerebellar damage show similar deficits, displaying poor adaptation to, and retention after, removal of kinetic or visual perturbations [6]. While impaired retention of adaptation in FOG+ may relate to basal ganglia disruption, learning in these adaptation paradigms is relatively rapid and likely depends on the cerebellum. Therefore, deficits in rapid adaptation performance suggest FOG+ may have cerebellar impairment.
In addition to motor adaptation, growing evidence suggests that the cerebellum also has a role in cognition. For example, individuals with posterior cerebellar lesions display deficits in verbal fluency, spatial cognition, and working memory [7]. Anatomical studies [8] as well as functional MRI studies [9] show that the posterior lobe of the cerebellum, particularly the crus I and crus II lobules, connects with the frontal cortex. Given this, the cerebellum may not only affect FOG in PD but could also affect cognition through its functional and anatomical connections with the frontal cortex.
People with PD develop cognitive impairment as the disease progresses , affecting set shifting, planning, and attention [10]. Deficits in executive function correlate with disruption of the fronto-striatal network [11]; however, dysfunction of the basal ganglia may also disrupt connections with the cerebellum, thereby affecting the fronto-cerebellar circuit. Further, FOG+ and FOG− differ in cognitive performance, including conflict resolution, response control , and verbal fluency [12]. Previous research suggests that dysfunction of the cognitive control network [13] may elicit freezing episodes. Specifically, FOG+ may fail to utilize frontal regions to respond to perturbations in gait, causing a freezing episode [14]. Therefore, research is needed to determine whether relationships exist between cognitive impairment and cerebellar structure and/or function in FOG+.
While several imaging studies examined relationships between cognition and cortical volume in PD [15], few studies included the cerebellum. Though one study showed decreased cognitive performance correlated with decreased overall cerebellar volume in PD [16], no studies examined potential differences in the individual cerebellar lobules between FOG+ and FOG− or their relationship to cognition. Each cerebellar lobule connects with distinct cortical regions and could therefore be affected by PD to different extents. Specifically, crus I and crus II anatomically connect to the prefrontal cortex [8], an area associated with executive function. Because FOG and cognition may relate to the cerebellum, and FOG+ have decreased cognitive performance, we hypothesized that volumes of the crus I and II would be significantly decreased in FOG+ compared to FOG−. Additionally, we aimed to examine potential relationships between cerebellar lobule volumes and cognition. We hypothesized that crus I and II volumes in the FOG+ group would correlate with cognitive performance based on their anatomical connections with the frontal cortex.
Materials and Methods
Participants
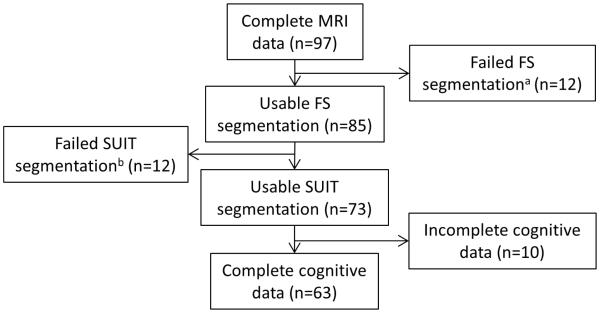
All participants were part of a larger exercise intervention trial [17]. Study inclusion criteria were 1) clinical diagnosis of idiopathic PD, 2) clear benefit from levodopa, 3) Hoehn & Yahr stages I-IV, 4) ability to walk independently for at least three meters, and 5) score ≥ 24 on the Mini Mental Status Exam (MMSE). For the present analyses, participants needed 1) T1- and T2-weighted structural MRI scans, 2) successful cortical and cerebellar segmentation with FreeSurfer software (v5.3.0, http://surfer.nmr.mgh.harvard.edu/) and the Spatially Unbiased Infratentorial (SUIT) toolbox [18] and 3) a complete cognitive dataset. Of the 120 participants, 63 participants met all inclusion criteria (Figure 1). Participants were divided into FOG+ and FOG−based on the New Freezing of Gait Questionnaire (N-FOGQ) [19]. A higher N-FOGQ score indicates a greater quantity and severity of freezing episodes in daily life and serves as a measure of freezing severity. Individuals who reported freezing episodes in the past month were categorized as FOG+ (n=25). All behavioral assessments and MRI scans were performed OFF medications, defined as ≥12-hours without anti-PD medications. This study was approved by the Human Research Protection Office of Washington University in St Louis, and all participants provided informed written consent.
Figure 1.
Participant flow diagram. Data for the present analyses came from a pool of participants who participated in a larger exercise intervention trial. Participants were included if 1) they had both a T1- and T2-weighted structural MRI scan; 2) FreeSurfer and the SUIT toolbox could properly segment the brain; and 3) they had a complete cognitive dataset.
aFailed FreeSurfer segmentation due to poor MRI quality or motion artifact.
bFailed SUIT segmentation defined as significant volume bleed into CSF or cortex, improper lobule segmentation, or exclusion of cerebellar cortex.
MRI acquisition and image processing
Participants completed MRI scans while OFF anti-PD medications on a Siemens TRIO 3T scanner (Erlangen, Germany) at Washington University School of Medicine. Participants completed whole brain structural T1-weighted (TR=2400ms, TI=1000ms, TE=3.16ms, FA=8°, 0.9mm3 voxels, 8:09min) and T2-weighted (TR=3200ms, TE=455ms, 1.0mm3 voxels, 4:43min) scans.
Structural scans were automatically segmented using FreeSurfer. After FreeSurfer processing, scans were visually inspected for proper segmentation and manually edited and reprocessed as necessary. Manual edits failed to successfully segment scans from 12 participants (out of 97), removing them from further analysis (Figure 1).
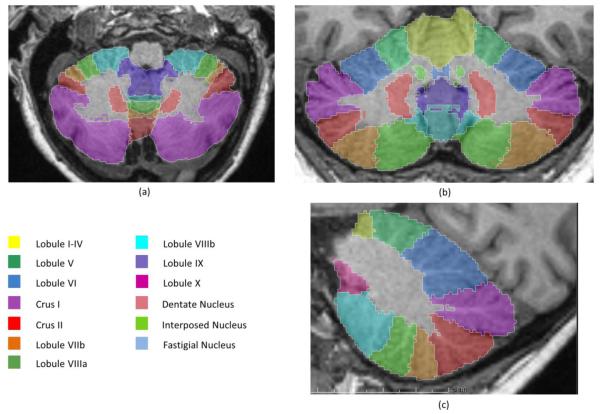
The cerebellum’s location puts it at risk for spatial warping during atlas normalization, making volume estimates of the cerebellum unreliable. To avoid this potential bias, we used the SUIT toolbox to segment the cerebellum into individual lobules, providing better estimates of the volume of each lobule (Figure 2). The SUIT toolbox provided volume measures for individual lobules (bilateral lobules I-IV, V, VI, crus I, crus II, VIIb, VIIIa, VIIIb, IX, and X) as well as total cerebellar volume. To optimize SUIT cerebellar segmentation for older adults, we applied FreeSurfer’s cerebellar white matter mask from each participant’s segmentation to remove white matter voxels, providing grey matter volumes for each lobule. In addition, we used the FreeSurfer-generated cortical ribbon and the T2-weighted CSF boundary to reduce inclusion of surrounding cortex and CSF during SUIT segmentation. Accurate SUIT segmentation was determined through visual inspection for each participant. Of the 85 participants’ scans processed, SUIT failed to properly segment 12 scans, removing them from further analysis.
Figure 2.
SUIT segmentation of the cerebellum. Lobules are bilateral with vermal portions in the middle. Lobules shown in (a) transverse, (b) coronal, and (c) sagittal views.
Cognitive assessment
Participants completed a short cognitive battery to assess executive function. Specific tests included Trail Making to measure attention and set-shifting [20], Delis-Kaplan Executive Function System (D-KEFS) Color-Word Interference test (i.e., Stroop) to measure attention and inhibition [21], and Verbal Fluency to measure response generation and flexibility [21]. As the primary dependent measures of cognitive performance, we computed the difference between Trails A and Trails B (Trails B time – Trails A time; Trails), the difference between the inhibition and color conditions of Stroop (Inhibition time – color time), and the total number of correct responses for each section of Verbal Fluency (Phonemic VF, Semantic VF, and Switch Accuracy VF).
In addition to these standard neuropsychological measures, participants completed a computerized Go-NoGo (GNG) test of response inhibition. The GNG task requires participants to respond to certain stimuli (Go trials) while withholding responses to other stimuli (NoGo trials). We calculated discriminability (Pr) and bias (Br) scores as previously described [22] using an individual’s hit and false alarm rates. Hit rate equals the number of correct responses divided by the total number of Go trials. False alarm rate equals the number of incorrect responses divided by the total number of NoGo trials. Discriminability measures the ability to differentiate between two trial types. A score of 0 indicates no discrimination and a score of 1 indicates perfect discrimination. Bias measures the likelihood to make one response over another response (i.e., does the participant make one response for all trials, regardless of trial type). A score of 0.5 indicates no bias, whereas a score >0.5 indicates a tendency towards responding to No-Go trials [22]. To avoid dividing by zero for the calculation of Pr and Br, hit rates of 1 were replaced with (ng − 0.5)/ng and false alarm rates of 0 were replaced with 0.5/nng, where ng is the number of Go trials, and nng is the number of No-Go trials [23]. Of the 73 participants with successful FreeSurfer and SUIT segmentations, 10 had incomplete cognitive data and were removed from further analysis.
Statistical analyses
Statistical analyses were performed with SPSS (IBM Analytics, version 24) with a statistical significance threshold of α<0.05. Analyses included the omnibus, multivariate analysis of covariance (MANCOVA) as well as follow-up analysis of covariance (ANCOVA). Partial correlations were used to control for demographics (age, education, disease duration) and estimated total intracranial volume (eTIV) when appropriate. Bonferroni correction was used when appropriate to correct for multiple comparisons. Participants with scores on any executive function task greater than 2.5 standard deviations above or below the grand mean were removed as outliers from analyses involving these data. This criterion excluded four participants from each group. To identify outliers in the lobule volumes, each participant’s lobule volumes were normalized to the participant’s total cerebellar volume. We set having ≥25% of normalized lobule volumes (7 lobules or more) being ±2.5 standard deviations from the normalized grand mean as our threshold for exclusion as an outlier. No participants met the exclusion criterion as no participant had more than 3 out of 28 lobules ±2.5 standard deviations from the normalized grand mean.
Results
The two groups were well-matched for demographics (Table 1), except for duration of PD diagnosis (t(61)=−2.97, p=0.004), indicating that FOG+ had a longer average disease duration. To control for this potential confound, analyses included a disease duration covariate when appropriate. As expected, the FOG+ group reported higher scores on the N-FOGQ compared to FOG− (t(61)=−11.47, p<0.001).
Table 1.
Group demographic characteristics.
| Demographics | FOG− (n=38) | FOG+ (n = 25) | p-value |
|---|---|---|---|
| Age (years) | 63.9 (9.6) | 66.7 (10.1) | 0.27 |
| Sex (# female) | 18 | 11 | 1 |
| MMSE (median, range) | 29, 27 - 30 | 29, 24 - 30 | 0.16 |
| Hoehn & Yahr (stage, n) | -- | -- | 0.58 |
| I, 3 | I, 1 | ||
| II, 30 | II, 19 | ||
| III, 5 | III, 4 | ||
| IV, 0 | IV, 1 | ||
| Duration of Diagnosis (years) | 4.3 (3.2) | 7.5 (5.2) | 0.004* |
| Education (years) | 16.3 (2.4) | 15.4 (2.4) | 0.14 |
| N-FOGQ | 0 | 9.8 (5.3) | <0.001* |
Values denote mean (standard deviation), except where noted.
statistically significant (p<0.05)
Cognitive Performance
After removing outliers for executive function, the omnibus MANCOVA with the raw scores for each cognitive test as dependent variables, age, education, and duration of PD diagnosis as covariates, and group (FOG− or FOG+) as a fixed factor, was significant (F(7, 44)=3.35, p=0.006). Follow-up univariate ANCOVAs showed significant differences in phonemic VF (F(1, 22)=7.06, p=0.01) and Pr (F(1, 22)=9.00, p=0.004) (Table 2).
Table 2.
Cognitive performance measures.
| ANCOVA Measure | FOG−, n=34 | FOG+, n = 21 | p-value |
|---|---|---|---|
| Trails (seconds) | 60.5 (6.3) | 71.4 (7.6) | 0.21 |
| Stroop (seconds) | 32.1 (4.7) | 33.8 (4.7) | 0.58 |
| Phonemic VF | 42.6 (2.2) | 33.8 (2.6) | 0.01* |
| Semantic VF | 39.7 (1.6) | 37.1 (1.7) | 0.68 |
| Switch Accuracy VF | 12.9 (0.5) | 11 (0.6) | 0.17 |
| Pr | 0.76 (0.03) | 0.63 (0.04) | 0.004* |
| Br | 0.45 (0.007) | 0.46 (0.007) | 0.41 |
Values denote mean (standard deviation) after removing outliers based on cognitive performance.
statistically significant (p<0.05)
Cerebellar Lobules
To test for grey matter volume differences in crus I and II between groups, we ran a MANCOVA with the SUIT grey matter volumes for crus I and II, bilaterally. The overall MANCOVA (covariates: age, disease duration, eTIV) showed no significant differences between groups (F(4, 55)=1.42, p=0.24). As an exploratory analysis, we ran a MANCOVA for differences in all lobules between groups (covariates: age, disease duration, eTIV), which showed a trend towards significance (F(21, 38)=1.65, p=0.09). Additional exploratory univariate ANCOVAs for each lobule, using an adjusted significance threshold of α <0.01, did not yield significant differences for any lobules (all p>0.02).
Relationships between cognition, freezing severity, and cerebellar volume
All participants
We first explored possible relationships between volume and cognitive performance within PD, regardless of freezing status. A third-order, partial correlation controlling for age, education, and eTIV showed a significant relationship between Trails and right crus II volume (partial r=−0.30, p=0.03). We also ran exploratory third-order, partial correlations for the other lobules controlling for age, education, and eTIV, with Bonferroni multiple comparisons correction (α<0.003). This analysis showed significant correlations between Switch Accuracy VF and lobule I-IV (left: partial r=−0.48, p<0.001; right: partial r=−0.44, p=0.001), lobule V (left: partial r=−0.54, p<0.001; right: partial r=−0.47, p=0.001), and left lobule VI (partial r=−0.53, p<0.001). To ensure duration of PD diagnosis did not act as a confounder, we ran bivariate correlations between disease duration and age, education, measures of executive function, lobular and total cerebellar volumes, and NFOG-Q total (FOG+ only). No correlations reached significance (all p≥0.09).
FOG+
Next, we ran the same third-order, partial correlations, using only FOG+ data. The third-order, partial correlation for cognitive measures and crus I and II volumes revealed a trend towards a relationship between Trails and right crus I (partial r=0.42, p=0.08) and between Stroop and left crus I (partial r=−0.43, p=0.07). The exploratory, third-order, partial correlation for cognitive performance and all lobule volumes showed no significant relationships (all p>0.005).
We also ran second-order, partial correlations on the FOG+ data, examining relationships between freezing severity, cognitive performance, and cerebellar lobule volumes. A second-order, partial correlation, controlling for age and education, showed a significant relationship between semantic VF and N-FOGQ score (partial r=−0.46, p=0.048).
A second-order, partial correlation, controlling for age and eTIV, found no significant relationships between crus I and II volumes and N-FOGQ score (all p>0.22). An exploratory second-order partial correlation between all lobules and N-FOGQ, controlling for age and eTIV and significant threshold set at α<0.003 (Bonferroni correction for multiple comparisons) showed no significant relationships (all p>0.04).
FOG−
We ran the same third-order, partial correlations using only FOG− data. The partial correlation between cognitive performance and crus I and II volumes showed a significant relationship between Pr and crus II (right: partial r=−0.44, p=0.01; left: partial r=−0.33, p=0.07). In addition, Switch Accuracy VF significantly correlated with left lobule I-IV (partial r=−0.53, p=0.002), lobule V (left: partial r=−0.62, p<0.001; right: partial r=−0.54, p=0.002), and left lobule IV (partial r=−0.62, p<0.001).
Discussion
Based on multiple lines of research implicating the cerebellum in FOG, we investigated potential cerebellar volumetric differences between people with PD with and without FOG. Improved cerebellar segmentation methods revealed similar total cerebellar volume and individual lobular volumes between our groups of FOG+ and FOG−. PD with FOG did not have smaller cerebellar volumes, despite obvious motor deficits and impaired cognition. Further, no relationships between cerebellar volume and cognitive function or motor severity emerged for PD with FOG. These results suggest that in our sample cerebellar volume did not account for either the motor or cognitive deficits associated with FOG in PD.
Though FOG+ and FOG− did not differ in cerebellar volumes, the SUIT toolbox provided accurate volumes by using nonlinear normalization methods with a cerebellum-specific template rather than whole-brain alignment methods. Our methods prevented spatial warping that could bias volume calculations. Because cerebellar volume decreases with age [24], we defined the CSF boundary around the cerebellum using T2-weighted scans to optimize SUIT cerebellar segmentation for older adult brains. This optimized accuracy of segmentation, preventing inclusion of surrounding CSF and cortex into lobule volume calculations. Previous research examining volumetric differences in the cerebellum between FOG+ and FOG− reports conflicting results. Kostic et al. [25] did not find significant cerebellar volume differences between FOG+ and FOG−; however Herman et al. [26] found significantly lower cerebellar volume in FOG+ compared to FOG−. Our results may help clarify discrepancies in the current cerebellar volumetric literature in FOG+ and FOG−.
Although the present results suggest cerebellar volume is not associated with FOG in PD, it remains possible that other aspects of cerebellar pathology and function contribute to FOG in PD and its associated cognitive deficits. For example, cerebellar acetylcholinesterase activity was significantly reduced in PD compared to controls, which suggests disproportionate degeneration of cholinergic pathways compared to overall tissue degradation [27]. Cholinergic projections in the PPN are reduced in PD compared to controls [28], and DTI in FOG+ showed PPN connectivity with the cerebellum to be non-existent and corticopontine projections to be increased, suggesting an important function of the corticopontine-cerebellar pathways in the manifestation of FOG [3].
Further, reduced acetylcholine receptor density in the cerebellum may contribute to cognitive comorbidities in PD [29]. Importantly, dopaminergic replacement therapies have heterogeneous effects on cognition and the effects diminish over time [30], suggesting that the cholinergic system may also play a significant role in cognition and PD. Altogether, while volume of the cerebellum may not predict cognitive performance in FOG+, its involvement in freezing of gait requires further research, particularly in measures of cholinergic transmission and connectivity with the cerebral cortex
These findings raise the question of whether the tissue measured with structural MRI has the same functional integrity in FOG+ and FOG−. Indeed, FOG+ demonstrate reduced functional connectivity between the cerebellum and the supplementary motor area [31].Therefore, while grey matter volume measures may not capture cerebellar differences between FOG+ and FOG−, the pathophysiology of FOG may still involve the cerebellum.
Our results regarding executive dysfunction in FOG+ agree with previous research demonstrating cognitive differences between FOG+ and FOG−. Similar to our findings, Amboni et al. [32] also reported reduced verbal fluency for FOG+ compared to FOG−. PD with FOG also performed worse on the GNG task, consistent with previous reports of impaired performance compared to FOG− [33]. However, neither cognitive function nor severity of freezing symptoms correlated with cerebellar volume in our sample of PD with FOG. Further, duration of PD diagnosis did not correlate with executive function, indicating that disease duration was not a confounding factor in our analyses.
Interestingly, our results show FOG− have negative relationships between cerebellar lobule volumes and executive function, indicating decreased volume corresponded with better executive function. O’Callaghan et al. [34] found that smaller cerebellar volume correlated with increased functional connectivity between the cerebellum and the fronto-parietal network in PD compared to controls. Importantly, they used the SUIT toolbox to assess cerebellar volume. Combining across studies, these results suggest that better executive function may relate to greater functional connectivity between the cerebellum and the fronto-parietal network; conversely, worse executive function, as associated with FOG+, may relate to reduced functional connectivity between the cerebellum and the fronto-parietal network. Reduced connectivity could affect the ability to make corrective movements, requiring a higher degree of cognitive effort in order to successfully make necessary adjustments. This fits with the theory of “executive function overload” that proposes an inability of FOG+ to properly recruit frontal networks, particularly during dual-task conditions, leading to a freezing episode [14]. While speculative, this warrants further research investigating cerebellar functional connectivity and its relationship with the motor and cognitive features associated with FOG in PD.
Our study may have limited generalizability due to inclusion of only mildly to moderately affected PD participants. While duration of PD diagnosis did not significantly correlate with cerebellar volume in our sample, cerebellar atrophy may occur later in the disease course or with greater disease severity. Future research should examine more severe PD participants. Further, the use of a cross-sectional design limits the ability to examine changes over time. Longitudinal studies that track PD participants over several years may clarify the cerebellum’s role in disease progression and whether it influences the development of FOG. Additionally, studies may benefit from using a more comprehensive cognitive battery. Other aspects of cognition such as memory, attention, and visuospatial processing may also be affected in FOG and involve the cerebellum [12].
As freezing of gait can lead to falls and debilitating injuries that decrease a person’s quality of life, greater understanding of the mechanisms behind FOG is needed. We used a novel approach for measuring cerebellar volumes in PD with and without FOG, examining whether individual cerebellar lobule volumes differed between the two groups and whether executive function relates to cerebellar volume in PD with FOG. We found no evidence of differences in cerebellar volumes between groups, despite having a well-differentiated sample. Instead, we found strong negative relationships between executive function and cerebellar volume in FOG− that may reflect functional connectivity differences between FOG+ and FOG−. By conducting longitudinal studies on volume, cognition, and functional connectivity in PD with FOG, the role of the cerebellum in the multifactorial mechanisms behind FOG can be ascertained.
Acknowledgements
We thank all members of the Campbell Laboratory and The Movement Science Research Center for their contributions to data collection and analyses. We are especially grateful to our participants and their caregivers. This work was supported by the National Institutes of Health [NICHD T32HD007434, NINDS R01NS077959, NINDS R01NS075321]; the Greater St. Louis Chapter of the American Parkinson Disease Association (APDA); Parkinson’s Disease Foundation/Parkinson Study Group; and the APDA Advanced Research Center at Washington University in St. Louis.
Footnotes
Conflict of interest
The authors have no conflict of interst to report.
References
- [1].Macht M, Kaussner Y, Moller JC, Stiasny-Kolster K, Eggert KM, Kruger HP, Ellgring H. Predictors of freezing in Parkinson's disease: A survey of 6,620 patients. Movement Disorders. 2007;22:953–956. doi: 10.1002/mds.21458. [DOI] [PubMed] [Google Scholar]
- [2].Vercruysse S, Gilat M, Shine JM, Herernans E, Lewis S, Nieuwboer A. Freezing beyond gait in Parkinson's disease: A review of current neurobehavioral evidence. Neuroscience and Biobehavioral Reviews. 2014;43:213–227. doi: 10.1016/j.neubiorev.2014.04.010. [DOI] [PubMed] [Google Scholar]
- [3].Schweder PM, Hansen PC, Green AL, Quaghebeur G, Stein J, Aziz TZ. Connectivity of the pedunculopontine nucleus in parkinsonian freezing of gait. Neuroreport. 2010;21:914–916. doi: 10.1097/WNR.0b013e32833ce5f1. [DOI] [PubMed] [Google Scholar]
- [4].Wang M, Jiang SM, Yuan YS, Zhang L, Ding J, Wang JW, Zhang JJ, Zhang KZ, Wang J. Alterations of functional and structural connectivity of freezing of gait in Parkinson's disease. Journal of Neurology. 2016;263:1583–1592. doi: 10.1007/s00415-016-8174-4. [DOI] [PubMed] [Google Scholar]
- [5].Nemanich ST, Earhart G. Prism adaptation in Parkinson disease: comparing reaching to walking and freezers to non-freezers. Experimental Brain Research. 2015;233:2301–2310. doi: 10.1007/s00221-015-4299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Current Opinion in Neurobiology. 2006;16:645–649. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- [7].Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- [8].Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nature Reviews Neuroscience. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- [9].O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and Overlapping Functional Zones in the Cerebellum Defined by Resting State Functional Connectivity. Cerebral Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Biundo R, Calabrese M, Weis L, Facchini S, Ricchieri G, Gallo P, Antonini A. Anatomical Correlates of Cognitive Functions in Early Parkinson's Disease Patients. Plos One. 2013;8 doi: 10.1371/journal.pone.0064222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Lange KW, Robbins TW. Fronto-Striatal Cognitive Deficits at Different Stages of Parkinsons-Disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- [12].Peterson DS, King LA, Cohen RG, Horak FB. Cognitive Contributions to Freezing of Gait in Parkinson Disease: Implications for Physical Rehabilitation. Physical Therapy. 2016;96:659–670. doi: 10.2522/ptj.20140603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- [14].Shine JM, Naismith SL, Lewis SJG. The pathophysiological mechanisms underlying freezing of gait in Parkinson's Disease. Journal of Clinical Neuroscience. 2011;18:1154–1157. doi: 10.1016/j.jocn.2011.02.007. [DOI] [PubMed] [Google Scholar]
- [15].Ibarretxe-Bilbao N, Tolosa E, Junque C, Marti MJ. MRI and Cognitive Impairment in Parkinson's Disease. Movement Disorders. 2009;24:S748–S753. doi: 10.1002/mds.22670. [DOI] [PubMed] [Google Scholar]
- [16].Camicioli R, Gee M, Bouchard TP, Fisher NJ, Hanstock CC, Emery DJ, Martin WRW. Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism & Related Disorders. 2009;15:187–195. doi: 10.1016/j.parkreldis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- [17].Earhart GM, Duncan RP, Huang JL, Perlmutter JS, Pickett KA. Comparing interventions and exploring neural mechanisms of exercise in Parkinson disease: a study protocol for a randomized controlled trial. Bmc Neurology. 2015;15 doi: 10.1186/s12883-015-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- [19].Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N. Reliability of the new freezing of gait questionnaire: Agreement between patients with Parkinson's disease and their carers. Gait & Posture. 2009;30:459–463. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- [20].Spreen O, Strauss E. A compendium of neuropsychological tests : administration, norms, and commentary. Oxford University Press; New York: 1991. [Google Scholar]
- [21].Delis DC, Kaplan E, Kramer JH. The Psychological Corporation. San Antonio: 2001. [Google Scholar]
- [22].Hershey T, Campbell MC, Videen TO, Lugar HM, Weaver PM, Hartlein J, Karimi M, Tabbal SD, Perlmutter JS. Mapping Go-No-Go performance within the subthalamic nucleus region. Brain. 2010;133:3625–3634. doi: 10.1093/brain/awq256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods Instruments & Computers. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- [24].Luft AR, Skalej M, Schulz JB, Welte D, Kolb R, Burk K, Klockgether T, Voigt K. Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cerebral Cortex. 1999;9:712–721. doi: 10.1093/cercor/9.7.712. [DOI] [PubMed] [Google Scholar]
- [25].Kostic VS, Agosta F, Pievani M, Stefanova E, Jecmenica-Lukic M, Scarale A, Spica V, Filippi M. Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology. 2012;78:409–416. doi: 10.1212/WNL.0b013e318245d23c. [DOI] [PubMed] [Google Scholar]
- [26].Herman T, Rosenberg-Katz K, Jacob Y, Giladi N, Hausdorff JM. Gray Matter Atrophy and Freezing of Gait in Parkinson's Disease: Is the Evidence Black-on-White? Movement Disorders. 2014;29:134–139. doi: 10.1002/mds.25697. [DOI] [PubMed] [Google Scholar]
- [27].Gilman S, Koeppe RA, Nan B, Wang CN, Wang X, Junck L, Chervin RD, Consens F, Bhaumik A. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology. 2010;74:1416–1423. doi: 10.1212/WNL.0b013e3181dc1a55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Muller MLTM, Bohnen NI. Cholinergic Dysfunction in Parkinson's Disease. Current Neurology and Neuroscience Reports. 2013;13 doi: 10.1007/s11910-013-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Meyer PM, Strecker K, Kendziorra K, Becker G, Hesse S, Woelpl D, Hensel A, Patt M, Sorger D, Wegner F, Lobsien D, Barthel H, Brust P, Gertz HJ, Sabri O, Schwarz J. Reduced alpha 4 beta 2*-Nicotinic Acetylcholine Receptor Binding and Its Relationship to Mild Cognitive and Depressive Symptoms in Parkinson Disease. Archives of General Psychiatry. 2009;66:866–877. doi: 10.1001/archgenpsychiatry.2009.106. [DOI] [PubMed] [Google Scholar]
- [30].Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neuroscience and Biobehavioral Reviews. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- [31].Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, Horak FB. Functional Reorganization of the Locomotor Network in Parkinson Patients with Freezing of Gait. Plos One. 2014;9 doi: 10.1371/journal.pone.0100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Amboni M, Cozzolino A, Longo K, Picillo M, Barone P. Freezing of gait and executive functions in patients with Parkinson's disease. Movement Disorders. 2008;23:395–400. doi: 10.1002/mds.21850. [DOI] [PubMed] [Google Scholar]
- [33].Cohen RG, Klein KA, Nomura M, Fleming M, Mancini M, Giladi N, Nutt JG, Horak FB. Inhibition, Executive Function, and Freezing of Gait. Journal of Parkinsons Disease. 2014;4:111–122. doi: 10.3233/JPD-130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].O'Callaghan C, Hornberger M, Balsters JH, Halliday GM, Lewis SJG, Shine JM. Cerebellar atrophy in Parkinson's disease and its implication for network connectivity. Brain. 2016;139:845–855. doi: 10.1093/brain/awv399. [DOI] [PubMed] [Google Scholar]