Abstract
Patients with Parkinson's disease (PD) often present with unilateral motor symptoms that eventually spread to the other side. This symptom lateralization is diagnostically important, as it serves to distinguish PD from other motor disorders with overlapping symptom profiles. Further, recent studies have shown that the side of symptom onset is important for prognosis, as there are differences in the rate of disease progression and the incidence of secondary symptoms between right- and left-dominant (RD, LD) patients. Physiologically, previous studies have shown asymmetrical decline in structure and metabolism throughout the basal ganglia, although connecting this directly to motor function has been difficult. To identify the neurophysiological basis of symptom laterality in PD, we recorded magnetoencephalography (MEG) during left- and right-hand movement paradigms in patients with PD who exhibited either RD or LD symptomatology. The beta oscillations serving these movements were then imaged using beamforming methods, and we extracted the time series of the peak voxel in the left and right primary motor cortices for each movement. In addition, each patient's symptom asymmetry was quantitated using the Unified Parkinson's Disease Rating Scale (UPDRS), which allowed the relationship between symptom asymmetry and neural asymmetry to be assessed. We found that LD patients had stronger beta suppression during movement, as well as greater post-movement beta rebound compared to patients with RD symptoms, independent of the hand that was moved. Interestingly, the asymmetry of beta activity during right-hand movement uniquely correlated with symptom asymmetry, such that the more LD the symptom profile, the more left-lateralized (i.e., contralateral to movement) the beta response; conversely, the more RD the symptom profile, the more right-lateralized (i.e., ipsilateral to movement) the beta response. This study is the first to directly probe the relationship between symptom asymmetry and the laterality of neural activity during movement in patients with PD, and suggests that LD patients have a fundamentally different and more “healthy” oscillatory pattern relative to RD patients.
Keywords: ERD, Oscillations, Magnetoencephalography, MEG, Movement disorders, Asymmetry
Highlights
-
•
Right-dominant expression of Parkinson's has been connected to faster progression.
-
•
Linkage between symptom asymmetry and cortical physiology remains unknown.
-
•
Cortical motor activity was measured in patients with left/right-dominant symptoms.
-
•
Patients with left-dominant symptoms had “healthier” pattern of motor responses.
-
•
Laterality of cortical activity during movement was related to symptom laterality.
1. Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by muscle rigidity, bradykinesia, resting tremor, impaired posture and balance, and speech and writing changes (Jankovic, 2008). PD initially emerges as a unilateral disorder, such that symptoms begin on one side of the body and spread to the other, although symptoms continue to be worse on the initially-affected side throughout the disease process (Djaldetti et al., 2006, Haaxma et al., 2010, Hoehn and Yahr, 2001, Lee et al., 1995, Riederer and Sian-Hulsmann, 2012, Uitti et al., 2005). This lateralization is diagnostically important, as it allows PD to be distinguished from other neurodegenerative disorders including essential tremor (Thenganatt and Louis, 2012), multiple system atrophy, and supranuclear palsy (Suchowersky et al., 2006). Interestingly, the side initially affected in PD has been recently associated with symptom trajectories. Indeed, a large-scale prospective study showed that patients with a right-dominant (RD) symptom profile had significantly more rapid progression of motor symptoms compared to those with a left-dominant (LD) symptom profile (Baumann et al., 2014). Patients with RD symptoms also showed significantly decreased muscle strength on both sides of the body compared to healthy controls, whereas LD patients showed no such differences (Frazzitta et al., 2015). Finally, LD symptomatology has been associated with longer disease duration, indicative of an extended period of survival after diagnosis, as well as delayed ambulatory inhibition compared to RD symptomatology (Munhoz et al., 2013). Taken together, these findings suggest that the side of symptom onset may hold important implications for predicting symptom trajectory in PD, and thus formulating prognoses. Nonetheless, the nature of symptom asymmetry in PD, especially the degree of asymmetry (i.e., how unilateral or bilateral symptoms present) and its neurophysiological origin, remains to be characterized.
Various studies have demonstrated asymmetrical subcortical structure and function in patients with PD (Abe et al., 2000, Choe et al., 1998, Eidelberg et al., 1990, Kempster et al., 1989, Morrish et al., 1995, Rinne et al., 1993). Overwhelmingly, researchers have found that the substantia nigra (SN) and putamen contralateral to the more affected side have greater degeneration and reduced dopamine uptake compared to homologous structures on the ipsilateral side. For example, Choe et al. (1998) used 1H-MRS to determine various metabolite levels in the SN and putamen of patients with unilateral PD. They found decreased N-acetylaspartate to creatine ratios (NAA/Cr; indicative of neuronal impairment) in both structures contralateral to the affected side, irrespective of whether patients were LD or RD (Choe et al., 1998). Similarly, many PET studies have demonstrated reduced endogenous dopamine, as well as reduced dopamine uptake, in the basal ganglia contralateral to the affected side, and that this asymmetry persists when Parkinson's symptoms become bilateral (Bohnen et al., 2006, Lin et al., 2014, Rinne et al., 1993). Most recently, diffusion tensor imaging in patients with PD has shown reduced fiber integrity throughout the nigrostriatal pathway, but especially contralateral to the more affected side (Wang et al., 2015, Zhang et al., 2015). In contrast to this subcortical work, very few studies have investigated potential neural asymmetries in the neocortex of patients with PD (Hall et al., 2014, Pollok et al., 2012). Overall, these studies show differential resting and movement-related neural activity in the hemisphere contralateral to the more affected side compared to the ipsilateral hemisphere, which suggests that these asymmetries transcend basal ganglia structures. However, these studies did not distinguish between RD and LD patients and thus, did not investigate whether the strength of such asymmetries might differ between these subtypes of patients with PD. Further, no study to date has connected the degree of symptom laterality to neural laterality in subcortical or cortical regions. Given the heterogeneity of symptom expression in PD and the substantial differences between RD and LD patient prognoses, understanding this relationship may provide critical new insight to disease progression.
A widely replicated finding in patients with PD undergoing deep brain stimulation (DBS) surgery is the presence of pathological beta activity (14–30 Hz) throughout the basal ganglia motor circuit (Brown, 2007, Cassidy et al., 2002, Hammond et al., 2007, Little and Brown, 2014, Litvak et al., 2011). Such beta activity is known to be critical for successful movement execution and its inherent time course has been well characterized. Briefly, about 1.0 s prior to movement onset there is a strong decrease in cortical beta activity, which has been termed the beta event-related desynchronization (ERD) response. This response appears to be generated by the bilateral primary motor cortices (stronger contralateral to movement), with weaker activity in the parietal, premotor, and supplementary motor areas (Gaetz et al., 2010, Heinrichs-Graham et al., 2016, Heinrichs-Graham and Wilson, 2016, Heinrichs-Graham and Wilson, 2015, Heinrichs-Graham et al., 2014b, Jurkiewicz et al., 2006, Wilson et al., 2014, Wilson et al., 2013, Wilson et al., 2010, Wilson et al., 2011). Approximately 0.5 s after movement offset, there is a strong resynchronization of beta activity that lasts approximately 2.0 s, termed the post-movement beta rebound (PMBR; (Gaetz et al., 2010, Heinrichs-Graham et al., 2014b, Jurkiewicz et al., 2006, Wilson et al., 2010, Wilson et al., 2011)). The beta ERD and PMBR have been reliably associated with movement planning/selection and active motor termination operations, respectively (Alegre et al., 2008, Alegre et al., 2004, Doyle et al., 2005, Grent-'t-Jong et al., 2014, Heinrichs-Graham and Wilson, 2015, Heinrichs-Graham and Wilson, 2016, Solis-Escalante et al., 2012, Tzagarakis et al., 2010), and are strongly modulated in the healthy aging brain (Heinrichs-Graham and Wilson, 2016, Rossiter et al., 2014). Importantly, recent work from our laboratory using noninvasive magnetoencephalography (MEG) has demonstrated pathologically-reduced beta activity in the motor cortices of patients with PD compared to healthy controls, both at rest and during transient movement (Heinrichs-Graham et al., 2014a, Heinrichs-Graham et al., 2014b). Specifically, we found reduced beta ERD (i.e., weaker suppression relative to baseline) and marginally reduced PMBR amplitude (i.e., less increase from baseline) in patients with PD compared to healthy controls. Taken together, these data indicate that beta oscillatory activity is critical to the dysfunction seen in motor circuits, and the overall pathophysiology of PD.
The primary goal of the current study was to determine whether symptom laterality in patients with PD (i.e., LD or RD) is associated with distinct aberrations in motor-related beta activity. To this end, we collected high-density MEG to examine oscillatory activity during two movement tasks in right-handed patients with PD who had either a LD or RD symptom profile. Movement-related beta oscillatory responses were then imaged using beamforming, and the level of symptom asymmetry was quantified using the Unified Parkinson's Disease Rating Scale (UPDRS). These data were then used to evaluate the relationship between neuronal activity and symptom asymmetry. Consistent with recent clinical studies showing differences in LD/RD patients, we hypothesized that patients who were LD would exhibit significantly stronger (i.e., more negative) beta ERD activity, as well as stronger (i.e., more positive) PMBR activity, compared to patients who were RD. The directionality of this hypothesis is in line with prior neurophysiological research showing that patients with PD have reduced motor-related responses compared to healthy controls (Heinrichs-Graham et al., 2014b, Pollok et al., 2012); thus, it is intuitive in this population that stronger motor-related responses are indicative of a “healthier” motor system. Secondly, we hypothesized that the pattern of neural asymmetry would reflect the pattern of symptom asymmetry across the two patient groups.
2. Methods
2.1. Subject selection and behavioral testing
We studied 27 right-handed adults (4 females) with well-documented PD. Four participants were excluded from analysis due to artifacts in their MEG data (2 participants) or no significant movement-related oscillatory response (1 participant). An additional participant was excluded due to the discovery of exclusionary criteria post-enrollment. The mean age of the remaining patients was 64.74 years (range: 52–78 years; see Table 1). All participants had been prescribed a regularly-monitored and unchanged dosage of antiparkinsonian medication for at least 2 months prior to study enrollment, and had showed a satisfactory clinical response to the particular antiparkinsonian medication(s). Exclusionary criteria included any medical illness affecting CNS function, neurological disorder(s) besides PD, history of head trauma, and current substance abuse. After complete description of the study to participants, written informed consent was obtained following the guidelines of the University of Nebraska Medical Center's Institutional Review Board, which approved the study protocol.
Table 1.
Clinical and demographic characteristics.
| Subject ID | Age (yrs) | Sex | Disease duration (yrs) | PD medications (type, dose) | (MDS)-UPDRS-III |
|---|---|---|---|---|---|
| 1 | 62 | M | 4 | Pram (4.5 mg), Ras (1 mg) | 22 |
| 2 | 70 | M | – | Pram (1.5 mg), CD/LD (25/100 mg) | 17 |
| 3 | 52 | M | 9 | CD/LD, Rop | 62 |
| 4 | 61 | M | – | Rop (1 mg) | 15 |
| 5 | 60 | M | 1 | Rop (1 mg) | 27 |
| 6 | 72 | F | 9 | Rop | 13 |
| 7 | 64 | F | 8 | Ras (1 mg) CD/LD (25/100 mg) | 34 |
| 8 | 67 | M | 3 | CD/LD (25/100 mg) | 23 |
| 9 | 69 | M | – | CD/LD (50/200 mg) | 52 |
| 10 | 69 | M | – | CD/LD | 37 |
| 11 | 52 | M | 6 | Rop (8 mg) | 14 |
| 12 | 64 | M | 7 | CD/LD (25/100 mg), Aman | 28 |
| 13 | 75 | M | 8 | CD/LD (25/100 mg) | 29 |
| 14 | 64 | M | 7 | CD/LD (10/100 mg), Rot (8 mg) | 53 |
| 15 | 61 | M | 8 | CD/LD (25/100 mg), Pram (1.5 mg), Sel (2 mg) | 35 |
| 16 | 55 | M | 6 | CD/LD (25/100 mg) | 21 |
| 17 | 78 | F | 16 | CD/LD (25/100 mg) | 36 |
| 18 | 73 | M | 8 | CD/LD (25/100 mg), Rot (4 mg) | 54 |
| 19 | 55 | M | 9 | Pram (1 mg), CD/LD (25/100 mg), Sel (5 mg) | 60 |
| 20 | 66 | M | 5 | Rop (3 mg), Ras (1 mg) | 40 |
| 21 | 61 | M | 3 | CD/LD (25/100 mg), Pram (1 mg) | 57 |
| 22 | 67 | M | 5 | CD/LD (25/100 mg) | 36 |
| 23 | 72 | M | 8 | CD/LD (25/100 mg) | 39 |
| 24a | 54 | F | 6 | Pram (4.5 mg), CD/LD (25/100 mg) | 27 |
| 25a | 76 | M | 4 | Ras (1 mg), CD/LD (50/200 mg) | 35 |
| 26a | 57 | M | 3 | Aman (100 mg), CD/LD (25/100 mg) | 31 |
| 27a | 75 | M | – | Pram (0.5 mg), CD/LD (25/100 mg) | 17 |
Notes: Pram = pramipexole; Ras = rasagiline; CD/LD = carbidopa/levodopa; Rop = ropinirole; Aman = amantadine; Sel = selegiline; Rot = rotigotine.
Excluded from analysis.
Parkinsonism was measured by a certified rater using either the UPDRS (12 patients) or the Movement Disorders Society-sponsored revision of the UPDRS (MDS-UPDRS (Goetz et al., 2007), 11 patients) in the practically-defined “off” state, which means following at least a 12-hour holiday from antiparkinsonian medications. In order to identify left- or right-dominance of symptoms, scores from each item on the (MDS-)UPDRS Part III that contained both a right and a left side component (e.g., right upper limb resting tremor, left upper limb resting tremor) were extracted, which provided left and right motor subscores for each individual. Symptom asymmetry was calculated using each patient's motor subscores by subtracting the total symptom score from the right side from the total symptom score from the left side. Negative values of symptom asymmetry (LIp) indicated LD of symptoms, while positive values indicated RD. Using this calculation, we divided our patient group by asymmetry of symptoms, such that 12 patients with PD exhibited symptoms that were LD (1 female), and 11 had symptoms that were RD (2 females). These groups were matched on sex, age, and whether their Parkinsonism had been rated using the UPDRS or the MDS-UPDRS (UPDRS: 6 of 12 LD patients; 5 of 11 RD patients). There was no significant difference in the sum of left and right UPDRS subscores between LD and RD patients with PD, t(21) = 1.749, p = 0.095, which ensures that disease severity was uniform between groups.
2.2. Experimental paradigm
All patients were scheduled for MEG early in the morning (i.e., 07:30–08:00) and a minimum of 12 h since their last dose of antiparkinsonian medication. During MEG recording, participants were seated with both arms resting on a table attached to their chair. Dual-plane accelerometer chips (Analog Devices Inc., model: ADXL103) were attached to each index fingertip to precisely quantify movement onset and the rate of acceleration (see Section 2.4), and to continuously monitor for intermittent tremor. Participants were instructed to fixate on a cross hair presented centrally and to perform a single tap of the right (or left) index finger each time a dot reached the 12 o'clock position (Fig. 1). This dot completed one full revolution, around a clock-like circle without numbers or tick marks every 6 s, which constituted one trial (Heinrichs-Graham et al., 2014b, Wilson et al., 2014, Wilson et al., 2013, Wilson et al., 2010). Left and right index finger movements were completed in separate blocks, with the order being randomized between participants. Each patient performed at least 105 trials for each task, and the total recording time was ~ 22 min. Of note, 19 patients (10 LD, 9 RD) completed both tasks, while the other four (2 LD, 2 RD) performed only the right finger-tapping task.
Fig. 1.
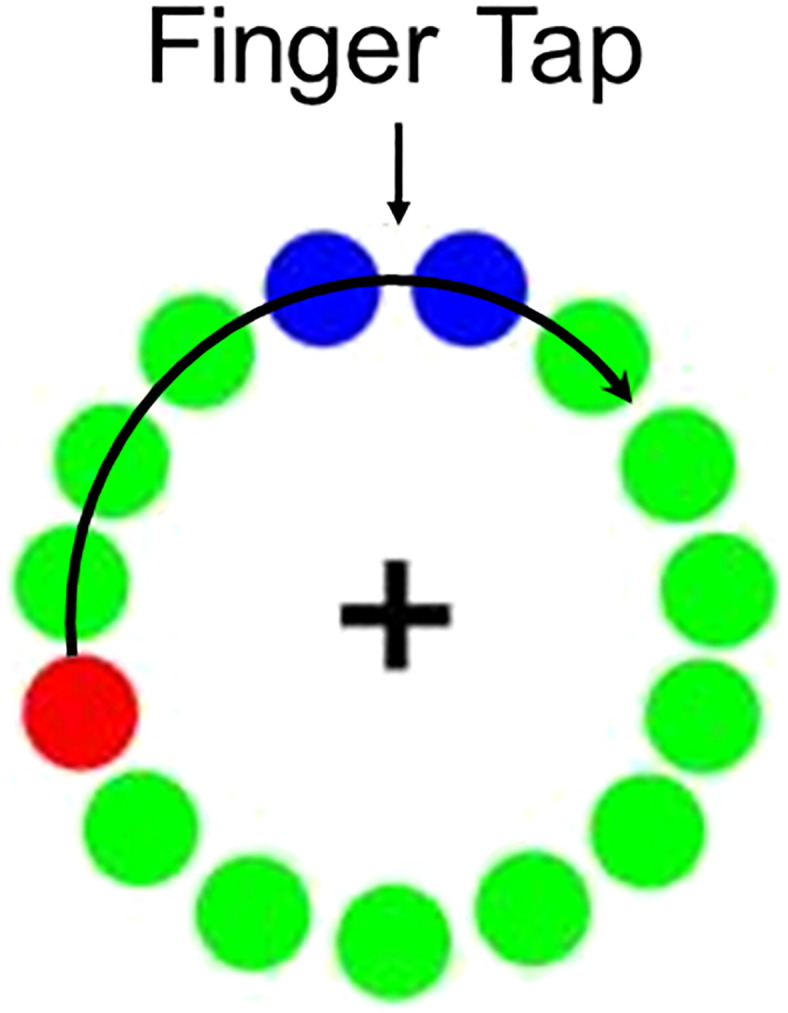
Motor task design. Participants fixated on the cross hair as the red dot moved in a clock-like rotation, displacing each green dot in turn. The red dot made one full rotation every 6 s. Participants were instructed to tap their left or right index finger each time the red dot reached the blue area. Left and right index finger movements were performed in separate blocks, and block order was randomized between participants.
2.3. MEG data acquisition & coregistration with structural MRI
All recordings were conducted in a one-layer magnetically-shielded room with active shielding engaged. Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz using an Elekta MEG system with 306 magnetic sensors (Elekta, Helsinki, Finland). Using MaxFilter (v2.2; Elekta), MEG data from each patient were individually corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola, 2006, Taulu et al., 2005). Each participant's MEG data were coregistered with structural T1-weighted MRI data prior to source space analyses using BESA MRI (Version 2.0). Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space. After beamformer analysis, each subject's functional images were also transformed into standardized space using the transform applied to the structural MRI volume and spatially resampled.
2.4. MEG time-frequency transformation and statistics
Cardiac artifacts were removed from the data using signal-space projection (SSP), which was accounted for during source reconstruction (Uusitalo and Ilmoniemi, 1997). The continuous magnetic time series was divided into epochs of 4.5 s duration, with 0.0 s defined as movement onset and the baseline defined as the − 2.0 to − 1.2 s time window (i.e., before movement onset). Movement onset was defined using the dual-plane accelerometers, which were attached to each index finger and digitized along with the MEG data at 1 kHz. The onset of movement was determined and quantified by a sharp increase in the amplitude of the accelerometer signal attached to the index finger being moved. Epochs containing artifacts were rejected based on a fixed threshold method, supplemented with visual inspection.
Artifact-free epochs were transformed into the time-frequency domain using complex demodulation (resolution: 2.0 Hz, 25 ms) and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized by dividing the power value of each time-frequency bin by the respective bin's baseline power, which was calculated as the mean power during the − 2.0 to − 1.2 s time period. The specific time-frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms. Each data point in the spectrograms was initially evaluated using a mass univariate approach based on the GLM. To reduce the risk of false positive results, a two-stage procedure involving nonparametric permutation testing was followed to control for Type 1 error. In the first stage, one-sample t-tests were conducted on each data point and the output spectrogram of t-values was thresholded at p < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the (p < 0.05) threshold, and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster-values and the significance level of the observed clusters (from stage one) was tested directly using this distribution (Ernst, 2004, Maris and Oostenveld, 2007). For each comparison, at least 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time-frequency windows that contained significant oscillatory events across all participants and corresponded to those of a priori interest (e.g., beta ERD, PMBR) were subjected to the beamforming analysis. Further details of this method and our processing pipeline can be found in recent papers (Heinrichs-Graham et al., 2014b, Wilson et al., 2014, Wilson et al., 2015).
2.5. MEG imaging
Cortical networks were imaged through an extension of the linearly constrained minimum variance vector beamformer (Gross et al., 2001), which employs spatial filters in the frequency domain to calculate source power for the entire brain volume. The single images were derived from the cross spectral densities of all combinations of MEG gradiometers averaged over the time-frequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images was normalized per participant using a separately averaged pre-stimulus noise period of equal duration and bandwidth (Hillebrand et al., 2005). MEG pre-processing and imaging used the Brain Electrical Source Analysis (BESA version 6.0) software. Normalized source power was computed for the selected time-frequency bands over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. Beamformer images were then averaged across participants, and coordinates corresponding to peak responses were identified for the left and right hand individually. We extracted virtual sensors corresponding to the peak voxel per region and task, and these were used to statistically evaluate neuronal activity in these brain regions between groups.
2.6. Statistical analysis
Oscillatory power for each response was extracted from the virtual sensors by averaging the total power over the same time-frequency bins that were determined previously by statistical analysis of the sensor-level data (see below). A linear mixed-model design was then employed in order to determine the relationship between side of movement (left or right index finger), hemisphere of response (contralateral or ipsilateral to movement), and affected side (LD or RD). Briefly, a linear mixed-model offers advantages over the general linear model, especially in cases of repeated-measures designs of unbalanced data (Jiang, 2007). Finally, correlations were computed to further resolve the relationship between variables on the patient level. All statistical analyses were performed using IBM SPSS Statistics (Release 23.0.0, Armonk, NY).
3. Results
All participants successfully completed the tasks. Across both groups, an average of 94.68 (SD: 10.44) artifact-free trials were used in the right hand analysis and 97.32 (SD: 9.32) were used in the left hand analysis. There were no significant differences between LD and RD groups on the number of trials used in either analysis, right hand: t(20) = 0.420, p = 0.679, left hand: t(17) = 1.030, p = 0.318. There were also no significant differences within group in the number of trials used per hand for those who performed both tasks, t(17) = 0.548, p = 0.591.
3.1. Sensor-level results
Sensor-level time-frequency spectrograms indicated the typical response pattern of peri-movement beta desynchronization followed by a PMBR in all patients. These spectrograms were statistically examined using one sample t-tests (across both groups) to derive the precise time-frequency bins for beamforming and subsequent virtual sensor analyses. Significant peri-movement beta ERD was found in a large number of sensors near sensorimotor regions in the 14–24 Hz range from about 1.0 s before movement onset until about 0.3 s after movement onset (p < 0.0001, corrected). There was also a significant beta synchronization (i.e., PMBR) that extended from about 0.5 s to 2.0 s after movement (p < 0.0001, corrected). To identify the neural origin of the beta ERD and PMBR, we imaged the time-frequency bin that showed the highest amplitude responses across all participants (Beta ERD: − 0.3 s to 0.2 s, 14–24 Hz; PMBR: 0.7 to 1.5 s, 14–24 Hz).
3.2. Neuroanatomical results
Analysis of the beamformer images of each group revealed strong desynchronization and subsequent rebound both centered on the motor hand knob region (Yousry et al., 1997) of the precentral gyrus contralateral to movement, as well as a smaller cluster in the same region of the ipsilateral hemisphere (Fig. 2). Peaks for the beta ERD and PMBR were distinct, in agreement with prior literature (Fry et al., 2016, Jurkiewicz et al., 2006). The beta ERD time courses for the peak voxel per hemisphere (ipsi- and contralateral) and hand (i.e., left and right) are shown in Fig. 3; the time series of the PMBR responses followed a very similar trajectory to those of the beta ERD activity. A linear mixed model was employed to determine the effects of and interactions between affected side (LD, RD), side of movement (left, right), and hemisphere (contralateral, ipsilateral) on beta ERD power and PMBR power, separately. The model of beta ERD power was significant, F(7,74) = 5.433, p < 0.001, and there was a significant main effect for each factor (affected side: F(1,74) = 11.119, p = 0.001); hand: F(1,74) = 16.023, p < 0.001; hemisphere: F(1,74) = 6.491, p = 0.013). No interactions between the variables were significant. Follow-up testing of model contrasts revealed that LD patients had significantly stronger (i.e., more negative) beta ERD responses, regardless of which finger was moved or hemisphere of response (t = 3.064, p = 0.003). Further, beta ERD responses were stronger on the side contralateral relative to the ipsilateral to the movement (t = 2.337, p = 0.022). Finally, both LD and RD patients showed stronger beta ERD in both the contralateral and ipsilateral precentral gyri when moving their left finger compared to their right finger (t = 3.861, p < 0.001). The linear mixed model of PMBR power was marginally significant, F(7,74) = 1.926, p = 0.077. The main effect of affected hand was significant, F(1,74) = 8.464, p = 0.005, but no other effects or interactions were significant (though a three-way interaction (affected hand, hand moved, and hemisphere) was marginal, F(1,74) = 3.815, p = 0.055). Follow-up contrast testing showed that LD patients exhibited stronger PMBR responses compared to RD patients, regardless of hemisphere or finger moved (t = 2.266, p = 0.027). Full results of each model can be found in Table 2.
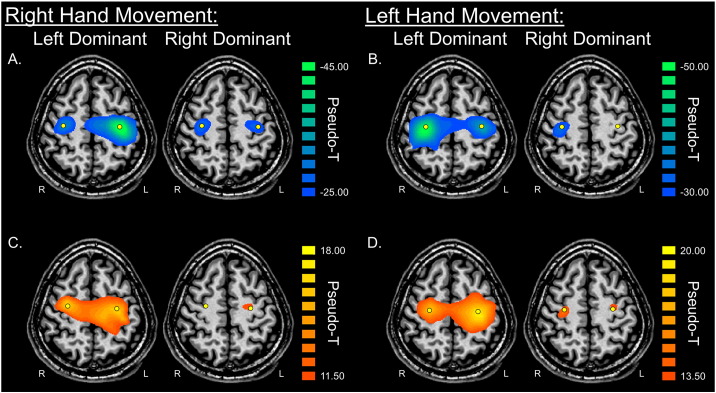
Fig. 2.
Peri-movement beta ERD and PMBR for patients with Parkinson's disease who exhibited left-dominant and right-dominant symptomatology. Group mean beamformer images (pseudo-t) of beta activity are shown for both the left-dominant (LD; left) and right-dominant (RD; right) patient groups. Peak voxels are denoted with a yellow dot. The top panel shows mean images of the a) right hand beta ERD and b) left hand beta ERD, while the bottom panel shows mean images of the c) right hand PMBR and d) left hand PMBR for each group. Patients in each group showed strong beta responses in the bilateral primary motor cortices, although activity was of noticeably lower power in the RD patient group compared to the LD patient group. As can be discerned, left hand movements generated higher-amplitude beta responses compared to right hand movements. Axial slices are shown in radiologic convention (right = left).
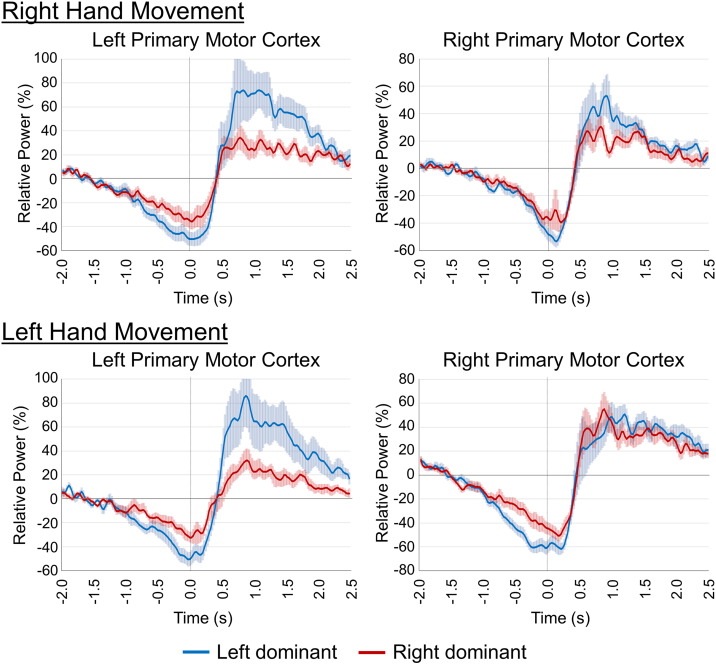
Fig. 3.
Temporal evolution of beta activity in the left and right primary motor cortices. To more precisely examine the dynamics of beta activity in patients with left-dominant (LD; blue line) and right-dominant (RD; red line) symptomatology, the beamformer images were averaged across both groups, and virtual sensors (i.e., voxel time series) were extracted from the peak voxels. Time series from the peak voxels of the beta ERD in the left and right primary motor cortices are shown. Those corresponding to the PMBR peaks showed similar trajectories (not shown), as the peak voxels were spatially adjacent to those of the beta ERD (see Fig. 1). In all panels, time (in s) is denoted on the x-axis (movement onset = 0.0 s) and relative power (expressed as percentage from baseline) is shown on the y-axis. Shaded areas around each line denote standard error of the mean (SEM). Patients with RD symptomatology showed reduced beta ERD responses (i.e., less desynchronization) prior to and during movement, as well as a reduced PMBR power (i.e., less synchronization) after movement, compared to patients with LD symptomatology.
Table 2.
Linear mixed model analysis.
| Beta ERD |
PMBR |
|||||
|---|---|---|---|---|---|---|
| F-value | df | p-Value | F-value | df | p-Value | |
| Effects | ||||||
| Corrected model | 5.433 | 7,74 | < 0.001 | 1.926 | 7,74 | 0.077 |
| Affected side (LD, RD) | 11.119 | 1,74 | 0.001 | 8.464 | 1,74 | 0.005 |
| Hand moved (left, right) | 16.023 | 1,74 | < 0.001 | 0.323 | 1,74 | 0.572 |
| Hemisphere (contra, ipsi) | 6.491 | 1,74 | 0.013 | 0.687 | 1,74 | 0.410 |
| Interactions | ||||||
| Affected side × hand | 1.071 | 1,74 | 0.304 | 0.070 | 1,74 | 0.792 |
| Affected side × hemisphere | 0.067 | 1,74 | 0.796 | 0.241 | 1,74 | 0.625 |
| Hand × hemisphere | 1.723 | 1,74 | 0.193 | 1.660 | 1,74 | 0.202 |
| Affected side × hand × hemisphere | 0.288 | 1,74 | 0.593 | 3.815 | 1,74 | 0.055 |
⁎Boldface denotes significance (p < 0.05).
⁎Contra = contralateral to movement, ipsi = ipsilateral to movement.
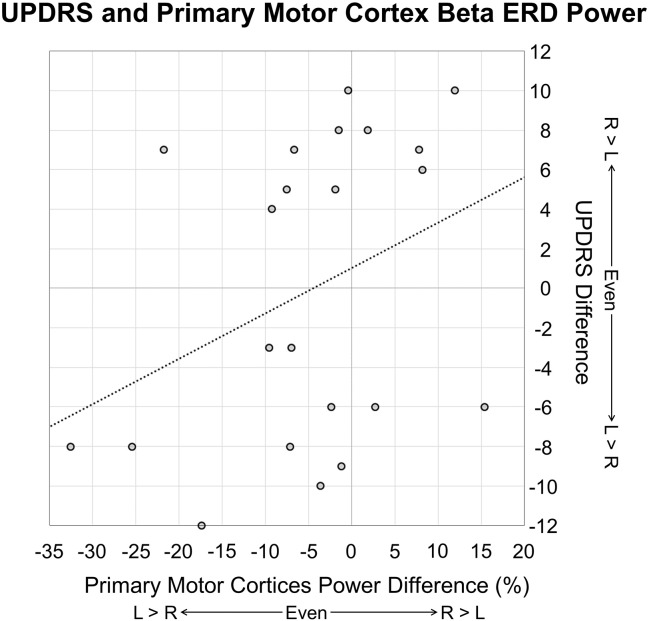
Finally, we calculated the partial correlation between neural asymmetry indices and symptom asymmetry, controlling for symptom severity (i.e., total UPDRS score from left and right subscores). We found a significant correlation between right hand beta ERD asymmetry and symptom asymmetry, r(15) = 0.530, p = 0.029, such that the more LD the symptom profile, the more left-lateralized the beta ERD, and in contrast, the more RD the symptom profile, the more right-lateralized the beta ERD response (see Fig. 4). We performed the same calculations using the PMBR amplitude from the left and right precentral gyri to determine the asymmetry of the PMBR response; values from the left hand correlated with symptom asymmetry, r(15) = 0.486, p = 0.048, such that the more right-lateralized the symptom profile, the more right-lateralized (i.e., contralateral to movement) the PMBR response, and vice versa. No other correlations were significant.
Fig. 4.
Relationship between symptom asymmetry and neural asymmetry. Asymmetry of UPDRS/MDS-UPDRS symptoms is shown on the y-axis, while asymmetry of right hand beta ERD power in the primary motor cortices is shown on the x-axis. Negative UPDRS asymmetry values reflect patients who had symptoms that were left-dominant, while positive values reflect patients whose symptoms were right-dominant. Negative neural asymmetry values reflect relatively greater beta ERD in the left (contralateral to movement) primary motor cortex, while positive neural asymmetry values reflect relatively greater beta ERD in the right (ipsilateral to movement) primary motor cortex. There was a significant relationship between symptom asymmetry and neural asymmetry, r(15) = 0.486, p = 0.048, such that the more left-lateralized the symptomatology, the more left-lateralized the beta ERD, and in contrast, the more right-lateralized the symptomatology, the more right-lateralized the beta ERD response, controlling for symptom severity.
4. Discussion
Our goal in this study was to identify the motor-related neural correlates of symptom asymmetry in patients with PD. We found that patients with LD symptomatology had significantly greater peri-movement beta ERD power (i.e., more negative relative to baseline) as well as greater PMBR activity (i.e., more positive relative to baseline) compared to patients with RD symptomatology, regardless of whether they were moving their right (less affected) or left (more affected) hand. Further, there was a significant correlation between symptom asymmetry and neural asymmetry for the right hand, such that the more left-lateralized the symptom profile, the more left-lateralized the beta ERD activity (i.e., greater beta ERD contralateral to movement), and the more right-lateralized the symptom profile, the more right-lateralized the beta ERD (i.e., greater beta ERD ipsilateral to movement). Below, we discuss the implications of these findings for understanding the cortical basis of symptom laterality in PD, and the recent discussions of distinct clinical outcomes among LD and RD patients.
Prior behavioral data suggests that patients with PD who have a LD symptom profile have a more favorable symptom trajectory than those with a RD profile, including a shallower symptom trajectory, longer disease duration, reduced muscle fatigue, and delayed ambulatory depletion (Baumann et al., 2014, Frazzitta et al., 2015, Munhoz et al., 2013). Despite the growing body of literature suggesting a behavioral dissimilarity between LD and RD patients with Parkinson's, the underlying neural mechanisms remain largely unknown. To at least some extent, this is because most studies to date have combined patients with LD and RD symptomatology, in order to focus on the more general effects of PD on brain structure and function. Our previous MEG investigation compared beta ERD and PMBR amplitude in patients with PD compared to healthy controls during the same finger-tapping movement of the right hand (Heinrichs-Graham et al., 2014b). We found that patients with PD had abnormally reduced beta ERD amplitude prior to and during movement, as well as marginally reduced PMBR amplitude following movement termination, compared to healthy age-matched adults (Heinrichs-Graham et al., 2014b). These results provided preliminary evidence that movement-related beta oscillatory activity may be a good candidate by which to investigate differences within the PD population. Indeed, in accordance with our hypothesis and in line with these findings, the current results show that patients who exhibited a LD symptom profile have significantly stronger beta ERD and PMBR responses (i.e., more healthy) during movement compared to those with RD symptomatology, regardless of whether they were moving their more-affected or less-affected side. This is in agreement with one recent single-photon emission computed tomography (SPECT) study by Scherfler and colleagues that examined levels of striatal dopamine transporter uptake between patients with PD and healthy controls, as well as between patients with LD and RD symptomatology (Scherfler et al., 2012). This study showed a reduction in dopamine transporter uptake between patients with PD and healthy controls, and that this reduction was more targeted to the left putamen than the right (Scherfler et al., 2012). Importantly, total dopamine uptake depletion in the left and right putamen was significantly greater in patients with RD compared to LD symptom profiles (Scherfler et al., 2012). Taken together, it is possible that the greater dopamine depletion in the putamen of patients with RD relative to LD symptomatology strongly alters the local physiology, and that these alterations disrupt brain activity in higher-order structures like the motor cortex, as evidenced by the reduction in beta ERD and PMBR power in the current study.
There is a wealth of positron emission tomography (PET) and magnetic resonance spectroscopy (MRS) data showing resting-state metabolic and dopamine receptor asymmetries in the basal ganglia of patients with PD, which correlate with the degree of symptom asymmetry (Abe et al., 2000, Choe et al., 1998, Eidelberg et al., 1990, Morrish et al., 1995, Rinne et al., 1993). However, few studies have examined aberrations in cortical neurophysiology while controlling for, but not directly investigating differences in, symptom laterality in patients with PD (Hall et al., 2014; (Pollok et al., 2012, Wu et al., 2015). Thus, the relationship between this subcortical asymmetry and cortical dynamics was largely unknown. In the current study, we found that there was a significant relationship between the asymmetry of beta ERD power during right hand movements in the contralateral and ipsilateral motor cortices and symptom asymmetry (LD, RD). We were initially surprised that this relationship was unique to the right hand, but we suggest that this might be due to the overall difference in beta ERD asymmetry between the left and right hands across patient groups. Basically, our results suggest that the healthy pattern of neural physiology (i.e., bilateral activation with greater activity in the hemisphere contralateral to movement) remains somewhat intact in patients with a LD symptom profile, regardless of whether the movement is with the left or right hand. On the contrary, patients who exhibit a RD profile show less lateralized (i.e., more aberrant) beta ERD responses during right hand movements compared to LD patients. This, coupled with the overall reduced beta ERD amplitude during both left and right movements and in both hemispheres in these patients, suggests a greater impact of RD symptomatology on overall physiology throughout the entire motor network, with particularly severe aberrations for the right hand, which is in line with prior behavioral and metabolic data.
In sum, our study was the first to directly compare the laterality of motor-related beta oscillatory responses with symptom asymmetry in patients with PD whose symptoms were either LD or RD. Patients who had LD symptomatology had significantly greater peri-movement beta ERD amplitude in the left primary motor cortex during right-hand movements compared to patients with RD symptomatology. Further, despite differences in overall beta power between LD and RD patients, we found a significant relationship between peri-movement beta ERD laterality and symptom asymmetry across groups in the right hand, such that the more LD the symptoms, the more LD the beta ERD response. Future work should aim to elucidate the underlying cause of behavioral and neurophysiological differences between patients with LD and RD symptom profiles. Further, recent studies have identified several other Parkinson's subtypes, based on motor subtypes, movement complications, etc. (Thenganatt and Jankovic, 2014), and the neurophysiology of these subtypes should also be the focus of future work. Thus, the complex interactions between Parkinson's subtype, side of disease onset, and physiology remain to be discovered and are likely critical. Nonetheless, this study highlights the importance of taking patient symptom heterogeneity into consideration in research and the clinic, and suggests that future clinical and translational research studies should strongly consider dividing patients with PD into LD and RD subgroups. This study also provides preliminary evidence of the neurophysiological basis of preferential behavioral outcomes of LD patients compared to RD patients. Future studies should evaluate potential treatment differences among LD and RD patients, and consider monitoring the course of disease progression in separate LD and RD subgroups.
Acknowledgements
This work was supported by NIH grant R01 MH103220 (TWW), NS036126 (HEG), and NS034239 (HEG), the Shoemaker Prize from the University of Nebraska Foundation (TWW), a Kinman-Oldfield Award for Neurodegenerative Research from the University of Nebraska Foundation (TWW), a Skate-a-Thon for Parkinson's Research Award from the University of Nebraska Medical Center (EH-G), and funding from the Nebraska Banker's Association (TWW). The Center for MEG at the University of Nebraska Medical Center was founded through an endowment from an anonymous donor. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Abe K., Terakawa H., Takanashi M., Watanabe Y., Tanaka H., Fujita N., Hirabuki N., Yanagihara T. Proton magnetic resonance spectroscopy of patients with parkinsonism. Brain Res. Bull. 2000;52:589–595. doi: 10.1016/s0361-9230(00)00321-x. [DOI] [PubMed] [Google Scholar]
- Alegre M., Gurtubay I.G., Labarga A., Iriarte J., Valencia M., Artieda J. Frontal and central oscillatory changes related to different aspects of the motor process: a study in go/no-go paradigms. Exp. Brain Res. 2004;159:14–22. doi: 10.1007/s00221-004-1928-8. [DOI] [PubMed] [Google Scholar]
- Alegre M., Alvarez-Gerriko I., Valencia M., Iriarte J., Artieda J. Oscillatory changes related to the forced termination of a movement. Clin. Neurophysiol. 2008;119:290–300. doi: 10.1016/j.clinph.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Baumann C.R., Held U., Valko P.O., Wienecke M., Waldvogel D. Body side and predominant motor features at the onset of Parkinson's disease are linked to motor and nonmotor progression. Mov. Disord. 2014;29:207–213. doi: 10.1002/mds.25650. [DOI] [PubMed] [Google Scholar]
- Bohnen N.I., Albin R.L., Koeppe R.A., Wernette K.A., Kilbourn M.R., Minoshima S., Frey K.A. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J. Cereb. Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr. Opin. Neurobiol. 2007;17:656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Cassidy M., Mazzone P., Oliviero A., Insola A., Tonali P., Di Lazzaro V., Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Choe B.Y., Park J.W., Lee K.S., Son B.C., Kim M.C., Kim B.S., Suh T.S., Lee H.K., Shinn K.S. Neuronal laterality in Parkinson's disease with unilateral symptom by in vivo 1H magnetic resonance spectroscopy. Investig. Radiol. 1998;33:450–455. doi: 10.1097/00004424-199808000-00005. [DOI] [PubMed] [Google Scholar]
- Djaldetti R., Ziv I., Melamed E. The mystery of motor asymmetry in Parkinson's disease. Lancet Neurol. 2006;5:796–802. doi: 10.1016/S1474-4422(06)70549-X. [DOI] [PubMed] [Google Scholar]
- Doyle L.M., Yarrow K., Brown P. Lateralization of event-related beta desynchronization in the EEG during pre-cued reaction time tasks. Clin. Neurophysiol. 2005;116:1879–1888. doi: 10.1016/j.clinph.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Eidelberg D., Moeller J.R., Dhawan V., Sidtis J.J., Ginos J.Z., Strother S.C., Cedarbaum J., Greene P., Fahn S., Rottenberg D.A. The metabolic anatomy of Parkinson's disease: complementary [18F]fluorodeoxyglucose and [18F]fluorodopa positron emission tomographic studies. Mov. Disord. 1990;5:203–213. doi: 10.1002/mds.870050304. [DOI] [PubMed] [Google Scholar]
- Ernst M.D. Permutation methods: a basis for exact inference. Stat. Sci. 2004;19:676–685. [Google Scholar]
- Frazzitta G., Ferrazzoli D., Maestri R., Rovescala R., Guaglio G., Bera R., Volpe D., Pezzoli G. Differences in muscle strength in parkinsonian patients affected on the right and left side. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A., Mullinger K.J., O'Neill G.C., Barratt E.L., Morris P.G., Bauer M., Folland J.P., Brookes M.J. Modulation of post-movement beta rebound by contraction force and rate of force development. Hum. Brain Mapp. 2016;37:2493–2511. doi: 10.1002/hbm.23189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W., Macdonald M., Cheyne D., Snead O.C. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. NeuroImage. 2010;51:792–807. doi: 10.1016/j.neuroimage.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Goetz C.G., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stebbins G.T., Stern M.B., Tilley B.C., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A.E., Lees A., Leurgans S., LeWitt P.A., Nyenhuis D., Olanow C.W., Rascol O., Schrag A., Teresi J.A., Van Hilten J.J., LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov. Disord. 2007;22:41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- Grent-'t-Jong T., Oostenveld R., Jensen O., Medendorp W.P., Praamstra P. Competitive interactions in sensorimotor cortex: oscillations express separation between alternative movement targets. J. Neurophysiol. 2014;112:224–232. doi: 10.1152/jn.00127.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Kujala J., Hamalainen M., Timmermann L., Schnitzler A., Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaxma C.A., Helmich R.C., Borm G.F., Kappelle A.C., Horstink M.W., Bloem B.R. Side of symptom onset affects motor dysfunction in Parkinson's disease. Neuroscience. 2010;170:1282–1285. doi: 10.1016/j.neuroscience.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Hall S.D., Prokic E.J., McAllister C.J., Ronnqvist K.C., Williams A.C., Yamawaki N., Witton C., Woodhall G.L., Stanford I.M. GABA-mediated changes in inter-hemispheric beta frequency activity in early-stage Parkinson's disease. Neuroscience. 2014;281:68–76. doi: 10.1016/j.neuroscience.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C., Bergman H., Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Wilson T.W. Coding complexity in the human motor circuit. Hum. Brain Mapp. 2015;36:5155–5167. doi: 10.1002/hbm.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Wilson T.W. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. NeuroImage. 2016;134:514–521. doi: 10.1016/j.neuroimage.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Kurz M.J., Becker K.M., Santamaria P.M., Gendelman H.E., Wilson T.W. Hypersynchrony despite pathologically reduced beta oscillations in patients with Parkinson's disease: a pharmaco-magnetoencephalography study. J. Neurophysiol. 2014;112:1739–1747. doi: 10.1152/jn.00383.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Wilson T.W., Santamaria P.M., Heithoff S.K., Torres-Russotto D., Hutter-Saunders J.A., Estes K.A., Meza J.L., Mosley R.L., Gendelman H.E. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson's disease. Cereb. Cortex. 2014;24:2669–2678. doi: 10.1093/cercor/bht121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Arpin D.J., Wilson T.W. Cue-related temporal factors modulate movement-related beta oscillatory activity in the human motor circuit. J. Cogn. Neurosci. 2016;28:1039–1051. doi: 10.1162/jocn_a_00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A., Singh K.D., Holliday I.E., Furlong P.L., Barnes G.R. A new approach to neuroimaging with magnetoencephalography. Hum. Brain Mapp. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 2001;57:S11–S26. [PubMed] [Google Scholar]
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jiang J. 1 ed. Springer-Verlag; New York: 2007. Linear and Generalized Linear Mixed Models and Their Applications. [Google Scholar]
- Jurkiewicz M.T., Gaetz W.C., Bostan A.C., Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. NeuroImage. 2006;32:1281–1289. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kempster P.A., Gibb W.R., Stern G.M., Lees A.J. Asymmetry of substantia nigra neuronal loss in Parkinson's disease and its relevance to the mechanism of levodopa related motor fluctuations. J. Neurol. Neurosurg. Psychiatry. 1989;52:72–76. doi: 10.1136/jnnp.52.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.S., Schulzer M., Mak E., Hammerstad J.P., Calne S., Calne D.B. Patterns of asymmetry do not change over the course of idiopathic Parkinsonism: implications for pathogenesis. Neurology. 1995;45:435–439. doi: 10.1212/wnl.45.3.435. [DOI] [PubMed] [Google Scholar]
- Lin S.C., Lin K.J., Hsiao I.T., Hsieh C.J., Lin W.Y., Lu C.S., Wey S.P., Yen T.C., Kung M.P., Weng Y.H. In vivo detection of monoaminergic degeneration in early Parkinson disease by (18)F-9-fluoropropyl-(+)-dihydrotetrabenzazine PET. J. Nucl. Med. 2014;55:73–79. doi: 10.2967/jnumed.113.121897. [DOI] [PubMed] [Google Scholar]
- Little S., Brown P. The functional role of beta oscillations in Parkinson's disease. Parkinsonism Relat. Disord. 2014;20(Suppl. 1):S44–S48. doi: 10.1016/S1353-8020(13)70013-0. [DOI] [PubMed] [Google Scholar]
- Litvak V., Jha A., Eusebio A., Oostenveld R., Foltynie T., Limousin P., Zrinzo L., Hariz M.I., Friston K., Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain. 2011;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Morrish P.K., Sawle G.V., Brooks D.J. Clinical and [18F] dopa PET findings in early Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 1995;59:597–600. doi: 10.1136/jnnp.59.6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz R.P., Espay A.J., Morgante F., Li J.Y., Teive H.A., Dunn E., Gallin E., Litvan I. Long-duration Parkinson's disease: role of lateralization of motor features. Parkinsonism Relat. Disord. 2013;19:77–80. doi: 10.1016/j.parkreldis.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollok B., Krause V., Martsch W., Wach C., Schnitzler A., Sudmeyer M. Motor-cortical oscillations in early stages of Parkinson's disease. J. Physiol. 2012;590:3203–3212. doi: 10.1113/jphysiol.2012.231316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P., Sian-Hulsmann J. The significance of neuronal lateralisation in Parkinson's disease. J. Neural Transm. 2012;119:953–962. doi: 10.1007/s00702-012-0775-1. [DOI] [PubMed] [Google Scholar]
- Rinne J.O., Laihinen A., Rinne U.K., Nagren K., Bergman J., Ruotsalainen U. PET study on striatal dopamine D2 receptor changes during the progression of early Parkinson's disease. Mov. Disord. 1993;8:134–138. doi: 10.1002/mds.870080203. [DOI] [PubMed] [Google Scholar]
- Rossiter H.E., Davis E.M., Clark E.V., Boudrias M.H., Ward N.S. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. NeuroImage. 2014;19:360–365. doi: 10.1016/j.neuroimage.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherfler C., Seppi K., Mair K.J., Donnemiller E., Virgolini I., Wenning G.K., Poewe W. Left hemispheric predominance of nigrostriatal dysfunction in Parkinson's disease. Brain. 2012;135:3348–3354. doi: 10.1093/brain/aws253. [DOI] [PubMed] [Google Scholar]
- Solis-Escalante T., Muller-Putz G.R., Pfurtscheller G., Neuper C. Cue-induced beta rebound during withholding of overt and covert foot movement. Clin. Neurophysiol. 2012;123:1182–1190. doi: 10.1016/j.clinph.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Suchowersky O., Reich S., Perlmutter J., Zesiewicz T., Gronseth G., Weiner W.J., Quality Standards Subcommittee of the American Academy of N Practice parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2006;66:968–975. doi: 10.1212/01.wnl.0000215437.80053.d0. [DOI] [PubMed] [Google Scholar]
- Taulu S., Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Taulu S., Simola J., Kajola M. Applications of the signal space separation method (SSS) IEEE Trans. Signal Process. 2005;53:3359–3372. [Google Scholar]
- Thenganatt M.A., Jankovic J. Parkinson disease subtypes. JAMA Neurol. 2014;71:499–504. doi: 10.1001/jamaneurol.2013.6233. [DOI] [PubMed] [Google Scholar]
- Thenganatt M.A., Louis E.D. Distinguishing essential tremor from Parkinson's disease: Bedside tests and laboratory evaluations. Expert Rev. Neurother. 2012;12(6):687–696. doi: 10.1586/ern.12.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C., Ince N.F., Leuthold A.C., Pellizzer G. Beta-band activity during motor planning reflects response uncertainty. J. Neurosci. 2010;30:11270–11277. doi: 10.1523/JNEUROSCI.6026-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitti R.J., Baba Y., Wszolek Z.K., Putzke D.J. Defining the Parkinson's disease phenotype: initial symptoms and baseline characteristics in a clinical cohort. Parkinsonism Relat. Disord. 2005;11:139–145. doi: 10.1016/j.parkreldis.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Uusitalo M.A., Ilmoniemi R.J. Signal-space projection method for separating MEG or EEG into components. Med. Biol. Eng. Comput. 1997;35:135–140. doi: 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang Q.X., Sun X., Vesek J., Mosher Z., Vasavada M., Chu J., Kanekar S., Shivkumar V., Venkiteswaran K., Subramanian T. MRI evaluation of asymmetry of nigrostriatal damage in the early stage of early-onset Parkinson's disease. Parkinsonism Relat. Disord. 2015;21:590–596. doi: 10.1016/j.parkreldis.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Wilson T.W., Slason E., Asherin R., Kronberg E., Reite M.L., Teale P.D., Rojas D.C. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 2010;73:75–84. doi: 10.1016/j.bandc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.W., Slason E., Asherin R., Kronberg E., Teale P.D., Reite M.L., Rojas D.C. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev. Neuropsychol. 2011;36:596–613. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.W., Heinrichs-Graham E., Robertson K.R., Sandkovsky U., O'Neill J., Knott N.L., Fox H.S., Swindells S. Functional brain abnormalities during finger-tapping in HIV-infected older adults: a magnetoencephalography study. J. NeuroImmune Pharmacol. 2013;8:965–974. doi: 10.1007/s11481-013-9477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.W., Heinrichs-Graham E., Becker K.M. Circadian modulation of motor-related beta oscillatory responses. NeuroImage. 2014;102(Pt 2):531–539. doi: 10.1016/j.neuroimage.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.W., Heinrichs-Graham E., Becker K.M., Aloi J., Robertson K.R., Sandkovsky U., White M.L., O'Neill J., Knott N.L., Fox H.S., Swindells S. Multimodal neuroimaging evidence of alterations in cortical structure and function in HIV-infected older adults. Hum. Brain Mapp. 2015;36:897–910. doi: 10.1002/hbm.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Hou Y., Hallett M., Zhang J., Chan P. Lateralization of brain activity pattern during unilateral movement in Parkinson's disease. Hum. Brain Mapp. 2015;36:1878–1891. doi: 10.1002/hbm.22743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry T.A., Schmid U.D., Alkadhi H., Schmidt D., Peraud A., Buettner A., Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(Pt 1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wu I.W., Buckley S., Coffey C.S., Foster E., Mendick S., Seibyl J., Schuff N. Diffusion tensor imaging of the nigrostriatal fibers in Parkinson's disease. Mov. Disord. 2015;30(9):1229–1236. doi: 10.1002/mds.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]