Abstract
Purpose
Cardiac rehabilitation (CR) is a program of structured exercise and interventions for coronary risk factor reduction that reduces morbidity and mortality following a major cardiac event. Although a dose response relationship between number of CR sessions completed and health outcomes has been demonstrated, adherence with CR is not high. In this study we examined associations between number ofsessions completed within CR and patient demographics, clinical characteristics, smoking status, and socioeconomic status (SES).
Methods
Multiple LogisticRegression and Classification and Regression Tree (CART) modeling were used to examine associations between participant characteristics measured at CR intake and number of sessions completed in a prospectively collected CR clinical database (N=1658).
Results
Current smoking, lower-SES, non-surgical diagnosis, exercise-limiting comorbidities, and lower age independently predicted fewer sessions completed. The CART analysis illustrates how combinations of these characteristics (i.e., risk profiles) predict number of sessions completed. Those with the highest-risk profile for non-adherence (less than 65 years old, current smoker, lower-SES) completed on average 9 sessions while those with the lowest-risk profile (greater than 72 years old, not current smoker, higher-SES, surgical diagnosis) completed on average 27 sessions.
Conclusions
Younger individuals, as well as those who report smoking, economic challenges, or have a non-surgical diagnosis, may require additional support to maintain CR session attendance.
Introduction
Cardiac rehabilitation (CR) is a program consisting of individualized, structured, progressive exercise sessions and behavioral and pharmacological interventions for coronary risk factor reduction that is highly effective at reducing morbidity and mortality rates following a myocardial infarction (MI) or coronary revascularization.1-5 Cardiac rehabilitation participation is associated with a 20-30% reduction in cardiac re-hospitalizations and 26% decrease in cardiac mortality.6
Considering that CR is a highly effective treatment for reducing recurrent cardiac events, it is important to examine if patients are benefiting as much as possible from this effective service. Most insurance covers up to 36 sessions and the benefits of CR have been demonstrated to increase with the number of sessions attended, with each additional 6 cardiac rehabilitation sessions attended reducing risk of subsequent MI by 5-11%.7, 8 Given the increasing health benefits of attending additional sessions it is imperative to determine which patients are at risk of being less adherent by completing fewer sessions.
Several patient characteristics have been associated with early drop out of CR. Those who did not have a surgical diagnosis complete fewer sessions7-9 as do those with depressive symptoms.10-12 Comorbidities, such as diabetes, peripheral vascular disease, obesity, and chronic obstructive pulmonary disease, appear to also predict fewer sessions completed.13-16,8 Effects of smoking have been reviewed, with current smoking being a powerful predictor of fewer sessions completed.17 Lower-socioecomonic status (SES), measured by income, education, or having subsidized insurance (e.g. Medicaid), may also predict fewer sessions completed.8,10,16 However, data on effects on SES has largely been restricted to patients who are 65 or older (enrolled in Medicare). Evidence on effect of sex differences is mixed, with reviews of studies being unable to demonstrate a consistent direction of effect.9,10 Age appears to have a modal relationship with number of sessions attended, with the very young and the very old being at risk to complete fewer sessions.8,10 As is detailed above, a number of variables predicting early CR termination have been identified, however, it remains unclear how these variables may interact or whether certain risk-factor combinations are particularly strong predictors of dropout.
This study seeks to expand on the existing literature on predictors of number of CR sessions completed by examining the relative strength of the contributions of various factors, clinical and demographic, within a large, prospectively collected, clinical database. This study includes many characteristics that have previously been shown to be predictive of number of sessions completed but also expands upon the existing literature by including a measure of aerobic fitness (Peak Metabolic Equivalents) as well as focusing on socioeconomic status (SES). Often strongly associated with health-related behaviors, information regarding SES is not commonly collected in CR programs. Additionally, to facilitate examination of risk-factor combinations, or risk profiles, in predicting number of sessions completed we conducted a Classification and Regression Tree (CART) analysis. CART is a nonparametric procedure for dividing a population of interest into mutually exclusive subgroups based on a dependent variable of interest while identifying independent variables with the most explanatory power in accounting for that dependent variable. To our knowledge, no one has applied CART analyses to the issue of cardiac rehabilitation attendance allowing for a unique opportunity to rank patient characteristics based on relative importance and examine cumulative effects of different characteristics on number of sessions attended.
Materials and Methods
Participants
Data were extracted from a prospectively collected clinical database. The cohort was 1658 patients who had at least an entry stress test at the CR program at the University of Vermont Medical Center on January 1, 2010or later and who completed or dropped out of the program by December 31, 2014.
The primary outcome of interest was number of sessions completed out of a possible 36. The following variables were included in the analyses: age, sex, CR qualifying diagnosis (surgical or non-surgical), self-reported smoking status (current smoker vs. non/former smoker), socioeconomic status (lower-SES vs. higher-SES as defined below), body mass index (BMI), depression score (Geriatric Depression Scale, 0-4 vs. 5+), aerobic fitness level at intake (Peak Metabolic Equivalents), and exercise–limiting comorbidities (none or minimal vs. moderate or severe).
Billing information extracted from electronic health records was used to determine SES status. Lower-SES was defined as having Medicaid insurance during CR participation. Current smoking was defined as reporting current smoking (within past 30 days) at intake to the CR program. Attendance data for former (≥100 cigarettes lifetime but not past 30 days) and never smokers (< 100 cigarettes lifetime) were not significantly different (data not shown) and were thus combined into one group (former/never smokers). To assess aerobic fitness, a symptom-limited exercise tolerance test was performed to determine peak metabolic equivalents (METspeak). METspeak were estimated based on the highest speed and grades achieved during the exercise test18 and were available for 1459 patients (88%). The exercise- limiting comorbidities score was calculated as a combination of the presence and severity of conditions that could limit activities within the CR program and consisted of COPD, PVD, orthopedic problems, and CVA/stroke. Patients were grouped as having either no comorbidities or comorbidities that would minimally impact exercise capacity versus patients who had a comorbidity or a combination of comorbidities that moderately or severely interfered with CR physical activities. Depression status was determined based on scores from the Geriatric Depression Scale – short form (GDS).19 A score of 0-4 is considered in the normal range while 5 or higher is suggestive of depression. Depression scores were available for 968 (58%) of the patients included in the database.
Statistical methodology
Frequencies and descriptive statistics were generated for independent variables with complete or relatively complete data: sex, age, BMI, diagnosis, smoking status, SES, number of comorbidities, and METspeak. Univariate tests of association with the primary dependent variable (number of sessions completed) were conducted and variables significantly related to the outcome were then used in multiple regression analysis. An initial model predicting number of sessions was fit using independent variables univariately associated with the outcome. Any variable that did not contribute significantly to predicting the outcome was deleted from the model. Once a tentative final model was determined, variables that had been eliminated earlier in the process were reintroduced, one at a time, and retained only if they contributed to the model in the presence of the previously significant predictors. Once the next iteration of a final model was determined, all possible interactions were tested, one at a time. Since no interaction contributed to the model, the final model consisted entirely of main effects. Final beta estimates and 95% confidence intervals were generated. A normal probability plot of jackknife residuals, a plot of jackknife residuals versus predicted values of number of sessions, plots of jackknife residuals versus each predictor, plots of leverage and Cook’s D, and correlation matrices between the independent variables and the dependent variable, as well as across independent variables, were generated to examine outliers, homogeneity of variance across the outcome variable, normality, linearity, and collinearity. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). All tests used a 5% Type I error level.
A Classification and Regression Tree (CART) analysis20 was used to quantify which of the variables identified in logistic regression analyses were most important in predicting number of sessions completed and how combinations of those variables (risk profiles) affected number of sessions completed. CART is a nonparametric procedure for dividing a population of interest into mutually exclusive subgroups based on a dependent variable of interest, such as number of sessions in the current study21 and, in the process, identifying independent variables with the most explanatory power in accounting for that dependent variable. The process begins by identifying the single most important independent variable for dividing the total sample (parent node) into two groups (child nodes), using a predetermined branching criterion. Nodes are split based on their purity using the Gini impurityfunction.20 A “pure” node has no variability in the dependent variable. A completely “impure” node has a conditional probability of p(k|t) = 0.5, where k refers to the dependent variable and t refers to the node.22 A splitting or branching criterion “selects the split that has the largest difference between the impurity of the parent node and a weighted average of the impurity of the two child nodes.”21 We used the Gini impurity function to split nodes, repeating the process recursively with every subsample, until the subsample reached a minimum size or no further splits could be made. The tree was built using R’s rpart package.23,24 A fully saturated tree was produced initially, and then pruned by selecting the complexity parameter that minimized cross-validation error and setting a minimum sample size in terminal nodes (leaves) of n = 33. A hierarchy of variable importance was also generated.
Results
Univariate analyses
Patient characteristics can be seen in Table 1. Number of sessions completed differed significantly by all of the measured characteristics except sex and depression score. Younger age, lower- SES, having a non-surgical diagnosis, METspeak, having exercise-limiting comorbidities, BMI, and being a current smoker were significantly associated with completing fewer sessions and were included in the subsequent multivariate model.
Table 1.
Baseline Characteristics and p-Values of Univariate Tests Predicting Number of Sessions
| All (n = 1,658) | p | |
|---|---|---|
| Sex | ||
| Female | 451 (27.20%) | 0.862 |
| Male | 1207 (72.80%) | |
| Age (years) (M ± SD) | 63.86 ± 11.58 | <0.001 |
| BMI (M ± SD) | 29.59 ± 5.94 | <0.001 |
| Diagnosis | ||
| Nonsurgical | 1041 (62.79%) | <0.001 |
| Surgical | 581 (35.04%) | |
| Smoking status | ||
| Never or former | 1459 (88.00%) | <0.001 |
| Current | 190 (11.46%) | |
| SES | ||
| Higher | 1302 (78.53%) | <0.001 |
| Lower | 356 (21.47%) | |
| No. comorbidities | ||
| 0-2 | 1389 (83.78%) | <0.001 |
| 3+ | 269 (16.22%) | |
| estMETs (M ± SD) | 7.17 ± 2.93 | <0.001 |
| Depression (M ± SD)* | 2.99 ± 2.86 | 0.156 |
| No. sessions (M ± SD) | 20.28 ± 13.65 |
Data were missing for 690 participants (42%).
Multivariatelogistic regression analyses
Smoking status, SES, age, severity of comorbidities, and qualifying diagnosis contributed independently to predicting number of sessions completed in multivariate modeling (Table 2). METspeak and BMI were not significant independent predictors. Age and higher number of comorbidities were associated positively with number of sessions, while a non-surgical diagnosis, being a smoker, and lower SES were negatively associated with number of sessions.
Table 2.
Predictors of Number of Sessions
| Model 1
|
Model 2
|
||
|---|---|---|---|
| ß | ß | 95% CI | |
| Intercept | 18.88*** | 15.68*** | [11.00, 20.36] |
| Age | 0.16*** | 0.15*** | [0.09, 0.21] |
| BMI | -0.07 | ||
| Diagnosis | -1.92** | -2.25*** | [-3.58, -0.93] |
| Smoker | -6.11*** | -5.83*** | [-7.92, -3.74] |
| SES | -3.50** | -3.84*** | [-5.47, -2.20] |
| Comorbidities | 2.88** | 2.41** | [0.68, 4.14] |
| estMETs | -0.23 | ||
|
| |||
| R2 | 0.106 | 0.094 | |
| F | 24.36*** | 33.37*** | |
Note. CI = Confidence interval. Surgical is the reference for diagnosis. Never/Former smoker is the reference for smoker. Higher SES is the reference for SES. Low (0-2) is the reference for comorbidities.
p < .05.
p < .01.
p < .001.
Classification and regression tree (CART) analysis
The CART analysis created a hierarchy of the patient characteristic variables based on their level of importance in predicting number of sessions completed. Age was identified as the strongest predictor, followed by smoking status, SES, and then diagnosis (surgical or non-surgical). Figure 1 shows how combinations of different patient characteristics predict number of sessions attended. The node at the top of the figure represents the entire sample (n = 1,658) and the overall average number of sessions completed (20). The first branching of the sample was based on age, the strongest predictor. This split the sample into those less than 65 (52% of the sample and an average of 17 sessions completed) and those 65 or older (48% of the sample and an average of 24 sessions completed). The next most important characteristic for both the younger and older groups is being a current smoker, which predicts an average of eight fewer sessions completed than in former/never-smokers. This branching continues until further splitting of the sample would not result in significant improvement in the model. Nodes where further iterations are not informative are labeled terminal nodes and are located in the bottom two rows of Figure 1. These terminal nodes can be thought of as risk profiles that represent how many sessions a patient with that particular set of characteristics completed. Overall, the model identified 12 terminal nodes or risk profiles. The number of sessions completed within these different nodes ranged from a low of 9 for patients below 65 years of age who were current smokers and lower-SES to a high of 27 sessions completed for patients above the age of 72 who were nonsmokers, higher-SES, and had a surgical diagnosis.
Figure 1.
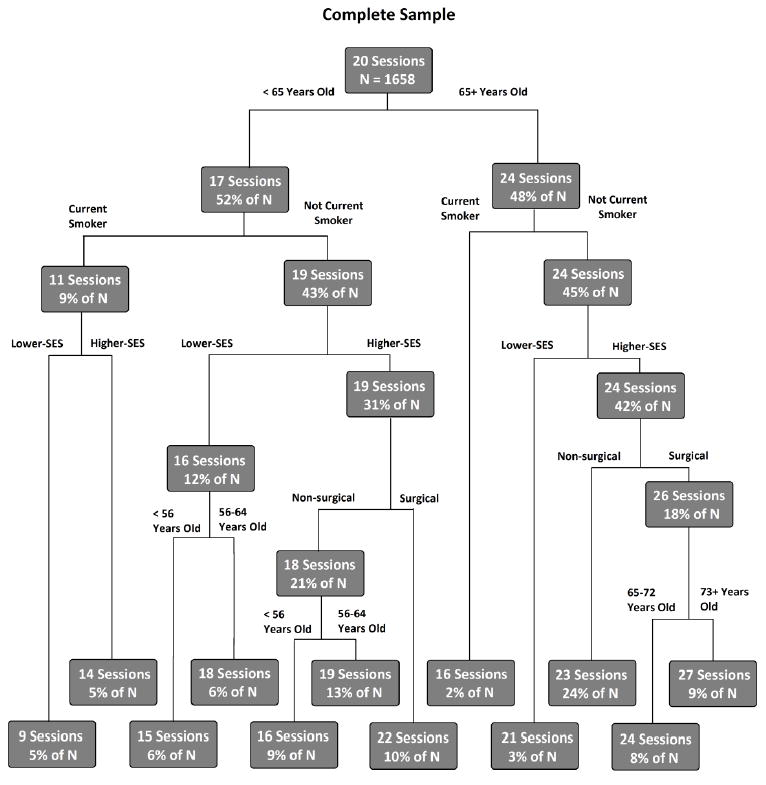
Classification and regression tree (CART) analysis showing cumulative effects of patient characteristics on number of sessions attended.
Discussion
The results of our analyses demonstrate that current smoking, lower-SES, younger age, and a non-surgical qualifying diagnosisare independent, robust predictors of completing fewer sessions of CR. The most powerful predictor of number of sessions completed was whether a patient was 65 or older. Smoking status and SES were also strong independent predictors of number of sessions completed. Number of sessions completed ranged from a low of only 9 to a high of 27 sessions depending on the combination of patient characteristics. Some additional interesting patterns are discernible from CART analyses. For example, being a current smoker highly restricts the number of sessions completed. Any variable combination that includes current smoking predicts no more than 16 sessions completed. Alternatively, being in the younger (<65 years) group allows for the greatest effect of other variables. Within the younger group, number of predicted sessions completed varies from 9 to 22 based on which other variables are present. Being in the older group (65+) seems the most protective, with all combinations within that first division predicted to complete at least 16 sessions. Current smoking and lower-SES are also less predictive in the older populations, likely due to the reduced number of smokers and lower-SES patients in the older age bracket. Not surprising, as smoking and lower SES are associated with reduced longevity.
Two of the strongest predictors of completing fewer sessions were being a current smoker and having lower-SES. This is unfortunate as these are also two populations that are at relatively increased medical risk following a cardiac event and would likely benefit most from additional sessions. Smokers, for example, are at especially high risk for subsequent morbidity and mortality following acute coronary events.25 Lower-socioeconomic (SES) populations are also generally higher risk patients having greater rates of smoking, higher fat diets, lower levels of fitness, more hypertension, higher prevalence of type 2 diabetes mellitus, and are more likely to be obese than more affluent populations.26-30 As would be expected, given high risk profiles, smokers and lower-SES patients are also more likely to be rehospitalized and have higher rates of mortality following an MI than their non-smoking, more affluent counterparts.25-27,31,32 Accordingly, these higher-risk subgroups should be specifically targeted and encouraged to not only enter CR but also complete the recommended duration in order to receive the greatest benefit possible.
Given the importance of smoking status and SES, with their clinical risk implications and their implications for number of CR sessions completed, they are both inadequately measured in many clinical settings. Despite being one of the strongest predictors of future cardiac events33,34, smoking is almost never objectively measured in clinical settings, such as with breath carbon monoxide or urine cotinine, most clinicians rely on self-reported smoking behavior. SES is also rarely recorded clinically. Clinics may avoid recording SES due to concerns about patient privacy or due to it not being viewed as clinically significant, despite SES being a predictor of many health-related behaviors.35-37
As lower-SES is a predictor of many negative health behaviors, perhaps it is not surprising that it is also a predictor of completing fewer CR sessions. What might be unexpected is that both smoking status and SES independently predict number of sessions completed with current smoking being the stronger predictor of the two variables. On a theoretical level this appears reasonable as one might expect that the best predictor of a negative health behavior is participation in another negative health behavior. This does have support in the literature. For example, in studies of treatment for drug dependence patients who smoked were less stable than those who did not, with smoking being a strong predictor of drug use and treatment success.38,39 Analyses of national survey data in Canada also support this idea, with the greatest correspondence between unhealthy behaviors being between smoking and physical inactivity and the second most common between smoking and drinking.40 This pattern also bears out in the area of medication adherence, where current smoking is a strong predictor of statin non-adherence.41 Similar effects can be seen in other studies of medication adherence in different populations.42,43
As research in predictors of CR attendance continues it is becoming increasingly clear which groups are at high risk for completing relatively few sessions. However, what needs further clarification is the cause of dropout. One common barrier for the younger patients and for working lower-income patients is the hardship of attending a program multiple times weekly, a program that is often open only during usual working hours. Many patients may work in a job that does not allow the flexibility to attend a program with limited hours. The barriers for attendance among those who smoke could be diverse. Those who smoke may believe that attending CR does not have enough benefit to be worth the effort, may have lower tolerance to exercise, may have other more pressing health concerns, or may not feel they are getting any benefit from attending CR.44,45 If the appropriate barriers within these various groups are identified CR staff can determine what interventions may be most useful, whether it is more education for the patient on the benefits of CR, a more intensive smoking cessation program, being open outside of normal working hours one day a week, or offering incentives for program completion.17,46 Smoking cessation interventions should be a particularly high priority given the strong associations between current smoking and both high morbidity and mortality combined with strong associations with poor CR attendance
This study does have some limitations that should be mentioned. Smoking status was based on self-report, which likely underestimates prevalence. Medical comorbidity scores were determined based on clinical definitions at intake, we were not able to determine whether comorbidities actually caused symptoms during exercise sessions. Additionally, depression scores were not available for a large portion of the population and the population was restricted to a single clinical site. Despite these limitations the findings of this study appear robust and could serve to inform clinicians and policy makers. Individuals who report smoking or financial challenges at CR intake, especially younger patients, will likely require additional support to complete the CR program. Patients in these higher-risk groups should be queried about potential barriers to participation and provided with information about all available forms of assistance. Additionally, as smoking is such a strong predictor of early drop out, increased support for cessation could potentially help maintain participation in CR. Given the high-risk profiles of both low-SES populations and those who smoke, intense interventions should be provided to help encourage these patients to enter and complete CR.
Acknowledgments
Funding Sources: This research was supported in part by National Institutes of Health Center of Biomedical Research Excellence award P20GM103644 from the National Institute of General Medical Sciences and Tobacco Centers of Regulatory Science award P50DA036114 from the National Institute on Drug Abuse and U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
DEG, PAA, and STH crafted the research strategy. PDS, JLR, AYC, and RJE collected the data and ran preliminary analyses. JSP and DEG ran the final analyses and created the figures and tables. DEG wrote the initial manuscript. All authors have read and approved of the manuscript.
Footnotes
Disclosures:
All authors report no conflicts of interest.
References
- 1.Ades PA. Cardiac rehabilitation and secondary prevention of coronary heart disease. N Engl J Med. 2001;345(12):892–902. doi: 10.1056/NEJMra001529. [DOI] [PubMed] [Google Scholar]
- 2.Balady GJ, Williams MA, Ades PA, et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: A scientific statement from the American heart association exercise, cardiac rehabilitation, and prevention committee, the council on clinical Cardiology; the councils on cardiovascular nursing, Epidemiology and prevention, and nutrition, physical activity, and metabolism; and the American association of cardiovascular and pulmonary rehabilitation. Circulation. 2007;115(20):2675–2682. doi: 10.1161/CIRCULATIONAHA.106.180945. [DOI] [PubMed] [Google Scholar]
- 3.Clark AM, Barbour RS, White M, MacIntyre PD. Promoting participation in cardiac rehabilitation: Patient choices and experiences. J AdvNurs. 2004;47(1):5–14. doi: 10.1111/j.1365-2648.2004.03060.x. [DOI] [PubMed] [Google Scholar]
- 4.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Wenger NK. Current status of cardiac rehabilitation. JACC. 2008;51(17):1619–1631. doi: 10.1016/j.jacc.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;7(7) doi: 10.1002/14651858.CD001800.pub2. CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly medicare beneficiaries. Circulation. 2009;121(1):63–70. doi: 10.1161/CIRCULATIONAHA.109.876383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suaya JA, Stason WB, Ades PA, Normand S-LT, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. JACC. 2009;54(1):25–33. doi: 10.1016/j.jacc.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 9.Jackson L, Leclerc J, Erskine Y, Linden W. Getting the most out of cardiac rehabilitation: a review of referral and adherence predictors. Heart. 2005;91(1):10–14. doi: 10.1136/hrt.2004.045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor GH, Wilson SL, Sharp J. Medical, psychological, and Sociodemographic factors associated with adherence to cardiac rehabilitation programs. JCN. 2011;26(3):202–209. doi: 10.1097/JCN.0b013e3181ef6b04. [DOI] [PubMed] [Google Scholar]
- 11.Glazer KM, Emery CF, Frid DJ, Banyasz RE. Psychological predictors of adherence and outcomes among patients in cardiac rehabilitation. JCR. 2002;22(1):40–46. doi: 10.1097/00008483-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 12.McGrady A, McGinnis R, Badenhop D, Bentle M, Rajput M. Effects of depression and anxiety on adherence to cardiac rehabilitation. JCRP. 2009;29(6):358–364. doi: 10.1097/HCR.0b013e3181be7a8f. [DOI] [PubMed] [Google Scholar]
- 13.Alter DA, Zagorski B, Marzolini S, Forhan M, Oh PI. On-site programmatic attendance to cardiac rehabilitation and the healthy-adherer effect. European Journal of Preventive Cardiology. 2015;22:1232–1246. doi: 10.1177/2047487314544084. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong MJ, Martin BJ, Arena R, et al. Patients with diabetes in cardiac rehabilitation. Med Sci Sports Exerc. 2014;46(5):845–850. doi: 10.1249/MSS.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 15.Forhan M, Zagorski BM, Marzonlini S, Oh P, Alter DA. Predicting exercise adherence for patients with obesity and diabetes referred to a cardiac rehabilitation and secondary prevention program. Canadian Journal of Diabetes. 2013;37(3):189–194. doi: 10.1016/j.jcjd.2013.03.370. [DOI] [PubMed] [Google Scholar]
- 16.Martin B, Hauer T, Arena R, et al. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation. 2012;126(6):677–687. doi: 10.1161/CIRCULATIONAHA.111.066738. [DOI] [PubMed] [Google Scholar]
- 17.Gaalema DE, Cutler AY, Higgins ST, Ades PA. Smoking and cardiac rehabilitation participation:Associations with referral, attendance and adherence. Prev Med. 2015;80:67–74. doi: 10.1016/j.ypmed.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Sports Medicine. 9. Baltimore, MD: Lippincott Williams and Wilkins; 2014. ACSM’s Guidelines for Exercise Testing and Prescription; pp. 123–127. [Google Scholar]
- 19.Burke WJ, Roccaforte WH, Wengel SP. The short form of the geriatric depression scale: A comparison with the 30-Item form. J Geriatr Psychiatry Neurol. 1991;4(3):173–178. doi: 10.1177/089198879100400310. [DOI] [PubMed] [Google Scholar]
- 20.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. Belmont, CA: Wadsworth; 1984. [Google Scholar]
- 21.Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: Methodological review and comparison with logistic regression. Ann Behav Med. 2003;26(3):172–181. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- 22.Lei Y, Nollen N, Ahluwahlia JS, Yu Q, Mayo MS. An application in identifying high-risk populations in alternative tobacco product use utilizing logistic regression and CART: A heuristic comparison. BMC Public Health. 2015;15:341. doi: 10.1186/s12889-015-1582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: [April 29, 2016]. http://www.R-project.org/. Published 2013. [Google Scholar]
- 24.Therneau T, Atkinson B, Ripley B. rpart: Recursive Partitioning. R package version 4.1-3. [April 29, 2016];R- project. 2015 Jun 29; http://CRAN.R-project.org/package=rpart.
- 25.Shen L, Peterson ED, Li S, et al. The association between smoking and long-term outcomes after non ST-segment elevation myocardial infarction in older patients. Am Heart J. 2013;166(6):1056–1062. doi: 10.1016/j.ahj.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Alter DA, Chong A, Austin PC, et al. Socioeconomic status and mortality after acute myocardial infarction. Ann Intern Med. 2006;144:82–93. doi: 10.7326/0003-4819-144-2-200601170-00005. [DOI] [PubMed] [Google Scholar]
- 27.Alter DA, Franklin B, Ko DT, et al. Socioeconomic status, functional recovery, and long-term mortality among patients surviving acute myocardial infarction. PLoS ONE. 2013;8(6):e65130. doi: 10.1371/journal.pone.0065130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan RHM, Gordon NF, Chong A, Alter DA. Influence of socioeconomic status on lifestyle behavior modifications among survivors of acute myocardial infarction. Am J Cardiol. 2008;102(12):1583–1588. doi: 10.1016/j.amjcard.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Govil SR, Weidner G, Merritt-Worden T, Ornish D. Socioeconomic status and improvements in lifestyle, coronary risk factors, and quality of life: The Multisite cardiac lifestyle intervention program. Am J Public Health. 2009;99(7):1263–1270. doi: 10.2105/AJPH.2007.132852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oberg EB, Fitzpatrick AL, Lafferty WE, LoGerfo JP. Secondary prevention of myocardial infarction with nonpharmacologic strategies in a Medicaid cohort. Preventing Chronic Disease. 2009;6(2):A52. [PMC free article] [PubMed] [Google Scholar]
- 31.Bernheim SM, Spertus JA, Reid KJ, et al. Socioeconomic disparities in outcomes after acute myocardial infarction. Am Heart J. 2007;153(2):313–319. doi: 10.1016/j.ahj.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 32.Lindenauer PK, Lagu T, Rothberg MB, et al. Income inequality and 30 day outcomes after acute myocardial infarction, heart failure, and pneumonia: Retrospective cohort study. BMJ. 2013;346:f521. doi: 10.1136/bmj.f521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow CK, Jolly S, Rao-Melacini P, Fox KAA, Anand SS, Yusuf S. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary Syndromes. Circulation. 2010;121(6):750–758. doi: 10.1161/CIRCULATIONAHA.109.891523. [DOI] [PubMed] [Google Scholar]
- 34.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease. JAMA. 2003;290(1):86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- 35.Gaalema DE, Higgins ST, Shepard DS, Suaya JA, Savage PD, Ades PA. State-by-state variations in cardiac rehabilitation participation are associated with educational attainment, income, and program availability. JCRP. 2014;34(4):248–254. doi: 10.1097/HCR.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins ST, Kurti AN, Redner R, et al. Co-occurring risk factors for current cigarette smoking in a U.S. nationally representative sample. Prev Med. 2016 doi: 10.1016/j.ypmed.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality. JAMA. 1998;279(21):1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 38.Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: A systematic review. Addiction. 2012;107(8):1404–1417. doi: 10.1111/j.1360-0443.2012.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug Alcohol Depend. 1996;40(3):195–201. doi: 10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- 40.de Ruiter WK, Cairney J, Leatherdale S, Faulkner G. The period prevalence of risk behavior co- occurrence among Canadians. Prev Med. 2016;85:11–16. doi: 10.1016/j.ypmed.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 41.Warren JR, Falster MO, Fox D, Jorm L. Factors influencing adherence in long-term use of statins. Pharmacoepidemiology and Drug Safety. 2013;22(12):1298–1307. doi: 10.1002/pds.3526. [DOI] [PubMed] [Google Scholar]
- 42.Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: A systematic review and meta-analysis. Ann Oncol. 2016;27(4):575–590. doi: 10.1093/annonc/mdv590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong MCS, Liu J, Zhou S, et al. The association between multimorbidity and poor adherence with cardiovascular medications. Int J Cardiol. 2014;177(2):477–482. doi: 10.1016/j.ijcard.2014.09.103. [DOI] [PubMed] [Google Scholar]
- 44.Asthana A, Piper ME, McBride PE, et al. Long-term effects of smoking and smoking cessation on exercise stress testing: Three-year outcomes from a randomized clinical trial. Am Heart J. 2012;163(1):81–87. doi: 10.1016/j.ahj.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vozoris NT, O’donnell DE. Smoking, activity level and exercise test outcomes in a young population sample without cardiopulmonary disease. J Sports Med Phys Fitness. 2014;55(7-8):787–796. [PubMed] [Google Scholar]
- 46.Pack QR, Johnson LL, Barr LM, et al. Improving cardiac rehabilitation attendance and completion through quality improvement activities and a Motivational program. JCRP. 2013;33(3):153–159. doi: 10.1097/HCR.0b013e31828db386. [DOI] [PMC free article] [PubMed] [Google Scholar]


