Abstract
Monocytes and macrophages (MΦs) play a central role in the pathogenesis of chronic Hepatitis C Virus (HCV) infection. The tissue microenvironment triggers monocyte differentiation into MΦs with polarization ranging within the spectrum of M1 (classical) to M2 (alternative) activation. Recently we demonstrated that HCV infection leads to monocyte differentiation into polarized MΦs which mediate stellate cell activation via TGFβ. In this study we aimed to identify the viral factor/s that mediate monocyte to MΦ differentiation. We performed co-culture experiments of healthy monocytes with exosome-packaged HCV, cell-free HCV, or single-stranded (ss) HCV RNA. Co-culture of monocytes with exosome-packaged HCV, cell-free HCV or ssHCV RNA induced differentiation into MΦs with high M2 surface marker expression and production of both pro- and anti-inflammatory cytokines. The HCV ssRNA-induced monocyte activation and differentiation into MΦ could be prevented by TLR7 or TLR8 knock-down. Furthermore, TLR7 or TLR8 stimulation, independent of HCV, caused monocyte differentiation and M2-MΦ polarization. In vivo, in chronic HCV-infected patients, we found increased expression of TLR7 and TLR8 in circulating monocytes that was associated with increased intracellular expression of pro-collagen. Furthermore, knockdown of TLR8 completely, and of TLR7 partially attenuated collagen expression in monocytes exposed to HCV suggesting the role of TLR8 and TLR7 in induction of fibrocytes in HCV-infection. We identified TLR7/8 as mediators of monocyte differentiation and M2 macrophage polarization during HCV-infection. Further, we demonstrated that HCV ssRNA and other TLR7/8 ligands promote macrophage polarization and generation of circulating fibrocytes.
Introduction
According to recent estimates by WHO, about 185 million people are chronically infected with Hepatitis C Virus (HCV), and more than 350,000 people die annually from HCV-related liver diseases (1, 2). HCV establishes chronicity in 50–80% of infected individuals leading to liver inflammation and fibrosis. Recent advances in HCV treatment has led to the development of new drugs that can achieve sustained virological response in more than 95% patients (3, 4). During its lifecycle, HCV generates both single-stranded (ss) and double-stranded (ds) RNA that are ligands for pattern recognition receptors (PRRs), Toll-like receptors (TLRs) and RNA helicase receptors, expressed by innate immune cells (5, 6). Monocytes and macrophages sense ssRNA by TLR7 and TLR8 whereas dsRNA is recognized by TLR3 and RIG-I localized in endosomes and cytosol (7–11).
Monocytes and macrophages are the primary mediators of the inflammatory response during HCV infection, where overproduction of tumor necrosis factor (TNF)α, abnormal interleukin (IL)-1, IL-10, and transforming growth factor (TGF)β influence the natural history of HCV infection (5, 12, 13). Macrophages possess functional plasticity mediated by microenvironment signals and can exist in any combinatorial spectrum of classically activated, M1 and alternatively activated, M2 populations (14–16). Classically activated (M1) macrophages have the role of effector cells in Th1 cellular immune responses. LPS and IFN-gamma (Th1 cytokine) polarize macrophages towards the M1 phenotype which induces the macrophage to produce large amounts of TNF, IL-12, and IL-23 which drives antigen-specific Th1 and Th17 cell inflammatory responses (17). M1 MΦs express CD86, MHC-II, CD40, and CD16 molecules as surface markers (18, 19). The alternatively activated (M2) macrophages are involved in tissue repair and immunosuppression. Macrophages exposed to the Th2 cytokine IL-4, differentiate into an M2 phenotype with production of high levels of IL-10, IL-1RA and low expression of IL-12. M2 macrophages also express high levels of scavenger (CD163), mannose (CD206) and galactose receptors and help with parasite clearance, reduce inflammation, and also serve as immunoregulators by promoting tissue remodeling and tumor progression (14, 17–19). Little is known about the role of MΦ polarization in HCV infection.
During HCV infection TLR2 and TLR6–10 are up-regulated in monocytes (20, 21). HCV core and NS proteins are important PAMPs for TLR2 and -4, whereas TLR7 and -8 sense HCV ssRNA and TLR3, HCV dsRNA. HCV core and NS3 proteins stimulate TLR2 when associated with TLR1 and -6 in PBMCs, particularly monocytes and macrophages (22, 23). There is also increased TLR7 and -8 expressions on monocytes in HCV infection, thus underscoring the importance of TLR expression in monocytes/macrophages in HCV mediated pathogenesis (23, 24).
In the present study we investigated the viral factors responsible for monocyte differentiation and macrophage polarization in HCV infectionin vitro andin vivo. We evaluated changes in the phenotype and function of healthy monocytes cultured in the presence of cell-free HCV or exosome-packaged HCV or single-stranded viral RNA. We show that exosome-packaged HCV or single-stranded viral RNA can mediate changes in the monocytes similar to that of cell-free HCV. We also demonstrate that TLR7/TLR8 ligands independent of HCV can program monocytes to differentiate into M2 polarized macrophages with features of fibrocytes. Our results also indicate a role for TLR7/TLR8 in chronic HCV-infection in generation of polarized macrophages as well as fibrocytes.
MATERIALS AND METHODS
Biological Materials and cells
Huh7.5 cells (a gift from Dr. Charlie Rice, Rockefeller University, New York) were maintained in low-glucose Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 10 μg/ml ciprofloxacin, and supplemented with nonessential amino acids (NEAA) (Gibco, Grand Island, NY). Dr. Takaji Wakita (National Institute of Infectious Diseases, Tokyo, Japan) provided the JFH-1 (HCV) construct. HCV viral supernatants were collected from Huh7.5 cells highly infected with JFH1 HCVcc and determined by flow cytometry as previously described (25). The supernatant was passed through 0.22 μm filters and was concentrated 20–30 times using Amicon Ultra-15 100K centrifugal filter unit and virus stock was preserved at −80°C (Millipore, Billerica, MA) (25). HCV JFH-1 virus concentration in culture supernatants was determined using NanoSight LM10 (MOI of infectious viral particles or infectious exosomes) and by quantitative real-time PCR as previously described (26). Uninfected Huh7.5 cell supernatant concentrate was used as controls. For exosome isolation from the cell culture supernatant, the cells were cultured using exosome depleted FBS (System Bioscience, Mountain View, CA). Primary human hepatocytes were obtained from the National Institutes of Health (NIH) liver tissue cell distribution system (LTCDS; Minneapolis, MN, USA; Pittsburgh, PA; Richmond, VA, USA), which was funded by NIH contract #N01-DK-7-004/HHSN2670070004C. They were maintained in hepatocytes maintenance medium (HMM-hepatocyte maintenance medium from Lonza, Allendale, NJ). PHH were infected with concentrated JFH-1 virions and half of the medium was changed every 24 hours during the experiment. Cells were harvested at different time points, and HCV RNA levels were determined. PHH infected with HCV had detectable viral RNA as shown by Supplementary Fig 2A. Human peripheral blood was collected from healthy donors and chronic HCV-infected patients (Table 1) with approval from the Institutional Review Board for Protection of Human Subjects in Research at the UMASS Medical School with written informed consent.
Table 1.
Clinical Characteristics of the HCV-infected patients
| Sl.No | Genotype | Viral Load (IU/ml) | Fibrosis Stage |
|---|---|---|---|
| 1 | 1a | 23683 | NA |
| 2 | 4 | 193056 | Grade 4/4 |
| 3 | 1a | 3295884 | Grade 3/4 |
| 4 | 1a | NA | Grade 4/4 |
| 5 | 1a | 492468 | Grade 3/4 |
| 6 | 1a | 505346 | NA |
| 7 | 1a | 2508472 | Grade 2/4 |
| 8 | 1a | 275850 | Grade 1/4 |
Reagents
Poly I:C, Gardiquimod, ssRNA40, PolyI:C/Lyovec and Lyovec (vehicle control) were purchased from Invivogen (San Diego, CA). Human antibodies; CD16 APC, CD16 FITC, CD14 FITC, CD40 FITC and CD86 FITC were purchased from eBioscience (San Diego, CA). Antibodies CD14 APC, CD14 PE, CD40 PE-Cy7, CD163 PE, CD11c APC, CD68 PE, CD206 APC, DC-SIGN-FITC and isotype control antibodies were purchased from BD Pharmingen (Franklin Lakes, NJ). Human antibodies CD40 Alexa 700, CD68 PE-Cy7, Brilliant violet LAP (TGFβ), CD206-PE, CD16 Alexa-700 were purchased from Biolegend (San Diego, CA). Transwell-6 system with a 0.4-μm porous membrane was purchased from BD Biosciences, Franklin Lakes, NJ. TLR3, TLR7, TLR8 siRNA and scrambled siRNA were purchased from Ambion Life Technologies (Carlsbad, CA).
Human Monocyte isolation, co-culture and TLR stimulation experiments
Monocytes were isolated to more than 95% purity from PBMCs by using CD14 microbeads, an MS Column and a Mini MACS separator and were confirmed by flow cytometry as described previously (27). Cells were cultured in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, and NEAA. Huh7.5 or Huh7.5/JFH1 cells (48 hr post-infection) were plated in 12-well plates (2.5×105 cells/well) and co-cultured with monocytes (5×105 cells) in a 37°C, 5% CO2 incubator for 3–7 days. For transwell experiments, Huh7.5 or Huh7.5/JFH1 cells were cultured on transwell inserts and monocytes were seeded on the bottom chamber of 6-well plates. Monocytes were treated with HCV concentrate or Huh7.5 concentrate or exosomes for 7 days. Monocytes were stimulated with poly I:C (1μg/ml), Gardiquimod (1μg/ml), ssRNA40 (1μg/ml), PolyI:C/Lyovec (1μg/ml), Lyovec (1μg/ml) or M-CSF (50ng/ml). Supernatants, cells and RNA were collected from all these experiments at the indicated time points and surface markers, cytokines and gene expression were studied.
Exosome isolation and purification from cell lines and patient samples
Exosomes were isolated and purified from cell lines and patient samples as described previously (26, 28). Huh7.5 cells and Huh7.5/JFH-1 cells were maintained in DMEM (low glucose) supplemented with 10% exosome depleted FBS and 1% penicillin/streptomycin. Cell culture supernatants following cell infection or not, or patient serum samples were collected, centrifuged at 2500 rpm for 10 mins at 4°C to remove cell debris, then filtered through a 0.2 μm filter. For exosome isolation, 40 mL of filtered culture supernatant was concentrated to a final volume of 1ml using the Amicon Ultra-15 Centrifugal Filter Unit with Ultracel-100 membrane (Millipore, Billerica, MA). Concentrated culture supernatants or filtered patient serum (500 μL) were mixed with the appropriate volume of Exoquick-TC reagent or Exoquick respectively (System Bioscience, Mountain View, CA) respectively, for exosome isolation. Samples were gently mixed and incubated for 1 hr at 4°C and exosomes were precipitated by centrifugation at 1400 rpm for 10 mins at 4°C. The recovered exosomes were re-suspended in 1x PBS. Positive selection of exosomes was done using anti-CD63 immuno-magnetic capturing with primary anti-CD63 antibody (Abcam, Cambridge, MA) followed by corresponding secondary antibody coupled to magnetic beads (Miltenyi Biotec, Cambridge, MA). MidiMACS separator was used with LD columns for exosome isolation as previously described (26, 28).
siRNA experiment
Monocytes (5×106) were resuspended in 100μl (25°C) of Human Monocyte Nucleofector Solution (MN solution) (Amaxa Biosciences, Gaithersburg, MD). Either pmaxGFP (provided by Amaxa as a positive control) or TLR3, TLR7, TLR8 siRNA or scrambled siRNA (20 nM) was mixed with this 100μl cell suspension and transferred into an Amaxa-certified cuvette. Nucleofection was performed by using Y-01 program in a Nucleofector™ II Device (Amaxa Biosciences, Gaithersburg, MD). Following nucleofection the monocytes were transferred to 6-well plates and incubated for 48 hr and co-cultured in the presence of Huh and HCV concentrate for 5 days. The monocytes were harvested after 5 days and RNA was extracted for RT-PCR.
RNAse A treatment experiment
Viral concentrate were either permeabilized with 0.1% Saponin or left untreated for 15 min on ice. Further the viral concentrate was treated with either 1 U/ml of RNase A for 1 hr at 37°C. The viral concentrate was washed several times and viral RNA levels were measured by quantitative RT PCR (29).
ELISA
Cytokines, TNF-α and IL-1β were quantified in cell culture supernatant using commercially available ELISA kits from BD Biosciences (Franklin Lakes, NJ), whereas IL-10 and TGFβ were quantified by ELISA kits from eBioscience (San Diego, CA).
RNA Extraction and Quantification
RNA was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA) and 2-step real-time polymerase chain reaction (RT-PCR) as described previously (25) (Table 2). The level of the target gene expression was measured by Delta-Delta Ct values using the ratio of the fold change in target gene expression versus the fold change in reference gene expression (18S).
Table 2.
Primer sequences for Real time PCR
| Gene symbol | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| TLR-3 | GTGCCAGAAACTTCCCATGT | TCCAGCTGAACCTGAGTTCC |
| TLR-7 | AATGTCACAGCCGTCCCTAC | GCGCATCAAAAGCATTTACA |
| TLR-8 | TGTGATGGTGGTGCTTCAAT | ATGCCCCAGAGGCTATTTCT |
| COL1A | GGCGGCCAGGGCTCCGAC | AATTCCTGGTCTGGGGCACC |
| RIG-I | GCATATTGACTGGACGTGGCA | CAGTCATGGCTGCAGTTCTGTC |
| IL-28A | AGGGCCAAAGATGCCTTAGA | TCCAGAACCTTCCAGCGTCAG |
| IL-28B | TAAGAGGGCCAAAGATGCCTT | CTGGTCCAAGACATCCCCC |
| IL-29 | GCCCCCAAAAAGGAGTCCG | AGGTTCCCATCGGCCACATA |
| IFNβ | ATGACCAACAAGTGTCTCCTCC | GCTCATGGAAAGAGCTGTAGTG |
| 18s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Plasmids and Generation of HCV ssRNAs
Plasmids pBSII-core, pBSII-E1-p7, pBSII-HCVNS- 3′untranslated region (UTR), and pCMV-NS5A, containing cDNA inserts of HCV-core, E1/E2/p7, a 6.6-kb insert spanning partial NS2 through NS5B and the entire 3′ UTR sequences and NS5A sequences, respectively, were used (25) HCV ssRNAs were synthesized in vitro from linearized vectors using T7 (for + strand RNAs of core, E1-P7, NS-3′ UTR and - strand of NS5A RNA) and T3 (for - strand RNAs of core, E1-P7, NS-3′UTR and + strand of NS5A RNA) MegaScript kits (Ambion). The control RNA was generated from the pTRI-Xef control template which is a linearized TRIPLEscript plasmid containing the 1.85 kb Xenopus elongation factor 1α gene under the transcriptional control of tandem T7, and T3 promoters. 5 μg/ml of viral ssRNA was transfected in human monocytes by TransIT®-mRNA Transfection Kit (Mirus Bio LLC, Madison, WI, USA).
Flow Cytometry
To analyze cell surface marker expression, monocytes were immunostained for 30 min at 4°C by using appropriate antibody or isotype control as described previously (25). Monocytes were identified on the basis of CD14 expression and the expression of other cell surface markers were analyzed on CD14+ cells.
For fibrocytes detection, monocytes from both healthy controls and HCV-infected patient’s peripheral blood were immune stained with anti-CD206 APC and anti-CD14 FITC. The cells were washed, fixed and permeabilized with CytoFix/cytoperm kit (BD Pharmingen, Franklin Lakes, NJ). Cells were then incubated with rat-anti-human pro-Col-Iα or isotype control, washed and finally stained with PE conjugated secondary antibody. The samples were acquired on the BD LSR II (BD Bisciences, San Jose, CA) and analysis of the data was performed with Flowjo software (Flowjo LLC, Ashland, OR).
Statistical Analysis
All values are expressed as Mean±SEM obtained from 3 or more independent experiments. Student’st-test and one-way or two-way ANOVA test was used to compare means of multiple groups. AP value < 0.05 was considered statistically significant. GraphPad Prism software (La Jolla, CA) was used for statistical analysis.
RESULTS
HCV-infected primary human hepatocytes induce healthy monocyte differentiation into polarized macrophages
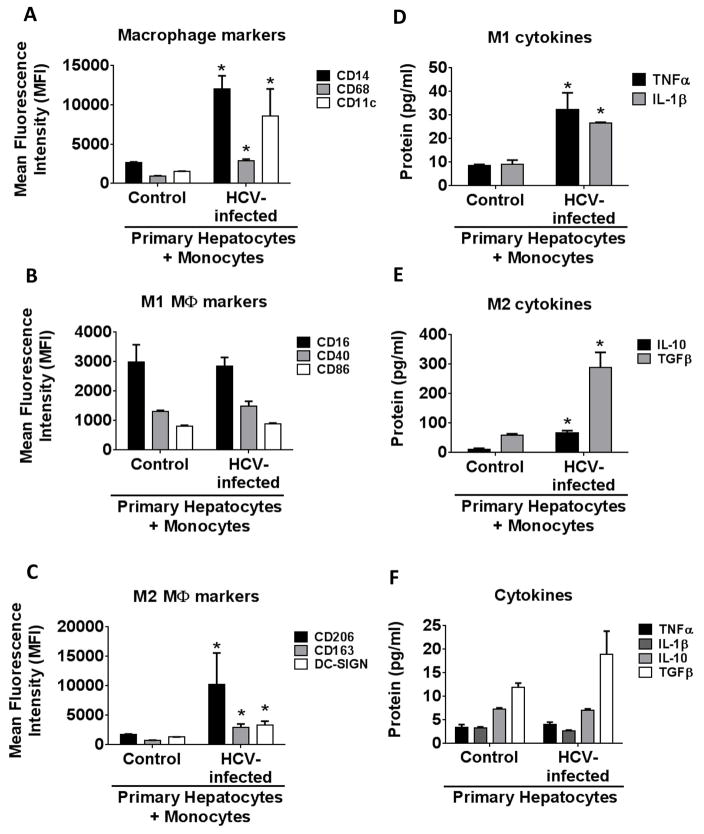
The liver tissue environment provides a close interaction between infected hepatocytes and immune cells. Here we evaluated the effect of co-culture of HCV-infected primary hepatocytes on healthy monocytes and found a significant increase in the expression of macrophage activation markers; CD14, CD68 and CD11c (Fig 1A and Supplementary Fig 1A). Furthermore, these macrophages also expressed M2 MΦs (Fig 1C and Supplementary Fig 1C) markers; CD206, CD163 and DC-SIGN but not a significant change in M1 MΦ markers; CD16, CD40 and CD86 (Fig 1B and Supplementary Fig 1B) (18, 19). As shown in the supplementary figure 1, there is no significant increase in M1 surface markers at earlier time points when co-cultured with infected hepatocytes as compared to the uninfected hepatocytes. This indicated that HCV-infected primary hepatocytes programmed monocytes to differentiate into macrophages with predominantly M2 polarization (Fig 1A–C). In order to demonstrate that the monocytes isolated from healthy controls are capable of differentiating into M1 or M2 macrophages, we treated the monocytes with M1 or M2 differentiating agents. As shown in Supplementary Fig 1D, we observed increased expression of M1 markers, CD16 and CD40, with LPS and IFNγ treatment. Increased expression of CD14 and CD68 was observed with both IFNγ plus LPS and IL-4 stimulation. IL-4 stimulation led to increased expression of M2 markers CD206 and CD163. Previous studies indicate that M2 macrophages produce TGFβ and promote tissue repair and fibrosis that are characteristics of liver pathology in chronic HCV infection (14, 16, 27). Thus we investigated the cytokine production profile of HCV exposed MΦ.
Figure 1. Monocytes co-cultured with HCV-infected primary human hepatocytes (PHH) differentiated into polarized macrophages.
Monocytes were isolated from healthy donors and were co-cultured with HCV-infected or uninfected PHH. Cells were harvested and immunophenotyped for macrophage markers by flow cytometry. The graphs represent expression of (A) macrophage markers CD14, CD68 and CD11c (B) M1 macrophage markers CD16, CD40 and CD86 and (C) M2 macrophage markers CD206, CD163 and DC-SIGN. The cell culture supernatant was measured for the various cytokines (D-E) The levels of TNFα, IL-1β, IL-10 and TGFβ in the cell-culture supernatants of infected or uninfected PHH with the healthy monocytes were measured. (F) The data shows the levels of TNFα, IL-1β, IL-10 and TGFβ in the HCV-infected or uninfected PHH. The data is representative of 3 independent experiments, *P ≤ 0.05.
Monocytes produced increased levels of TNFα and IL-1β when co-cultured with HCV-infected hepatocytes (Fig 1D). IL-10 and TGFβ levels were also significantly elevated (Fig 1E). Thus, the cytokine secretion by monocytes co-cultured in the presence of HCV-infected hepatocytes revealed both M1/M2 cytokine secretion patterns supporting a mixed M1/M2 cytokine profile with an M2 surface marker expression phenotype.
We isolated RNA from the PHH which were infected or non-infected and studied the expression of Type I IFN (IFN β) and Type III IFNs (IFN-lambda1 (IL-29), IFN-lambda2 (IL28A) and IFN-lambda3 (IL-28B). Consistent with previous publications, we observed an increased expression of Type I and Type III IFNs in the PHH infected with HCV (Supplementary Fig 2B). However, in the absence of monocytes, primary hepatocyte supernatants had low levels of M1/M2 cytokines (Fig. 1F).
Hepatocyte-derived exosomes or free HCV induce monocyte to macrophage polarization
Next to explore the mechanism of MΦ polarization we utilized the Huh/JFH1 system. To evaluate potential mechanisms by which HCV-infected hepatoma cells activate monocytes to differentiate into MΦs, we evaluated whether cell-cell contact was necessary. Separation of Huh7.5/JFH1 cells from monocytes with transwell failed to inhibit the process of HCV-induced macrophage differentiation and the surface marker expression or cytokine secretion profiles of MΦs indicating that the Huh7.5/JFH1 culture supernatant was sufficient for triggering monocyte to MΦs differentiation (Fig 2A).
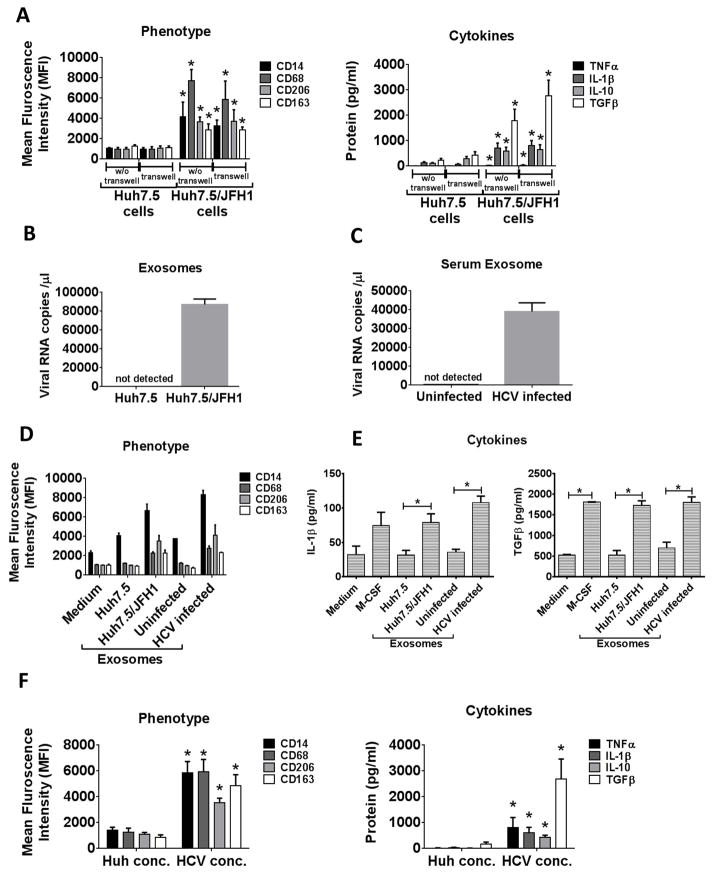
Figure 2. Exosomes packaged HCV RNA induce monocyte differentiation and macrophage polarization.
Monocytes were co-cultured with Huh7.5 or Huh7.5/JFH1 cells either with or without transwell for 7 days. (A) Expression levels of CD14, CD68, CD206 and CD163 were determined by flow cytometry. TNFα, IL-1β, IL-10 and TGFβ levels were measured in the culture supernatant. (B, C) Exosomes were isolated from Huh7.5 or Huh7.5/JFH1 culture supernatant, or serum from control or naïve HCV-infected patients. The viral RNA levels were determined by quantitative RT-PCR method is shown. (D) Monocytes were cultured in the presence of exosomes (exo) isolated from Huh7.5 or Huh7.5/JFH1 culture supernatant, or serum from control or naïve HCV-infected patients. Cells were harvested after 7 days and CD14, CD68, CD206 and CD163 levels were measured by flow cytometry. (E) IL-1β and TGFβ levels were measured in the culture supernatant. (F) Monocytes were cultured in the presence of Huh 7.5 culture supernatant concentrate or HCV concentrate for 7 days and immunostained for CD14, CD68, CD206 and CD163. TNFα, IL-1β, IL-10 and TGFβ levels were measured by ELISA from culture supernatant. The data is represented as mean±SEM, n=4–6, *P ≤ 0.05.
The transwell system that we used was with a 0.4-μm porous membrane (BD Biosciences, Franklin Lakes, NJ) and this prevented both the transfer of vesicles larger than exosomes and direct cell contact. Thus, we were interested in evaluating the contribution of HCV containing exosomes in monocyte differentiation. Recently, we have demonstrated that exosomes derived from HCV-infected hepatocytes contain HCV RNA and can transfer HCV infection into naïve hepatocytes (26). Here, we found that exosomes isolated from the culture supernatant of Huh7.5/JFH1 cells or from serum of HCV-infected patients contained HCV RNA (Fig 2B, C). Thus, we tested whether exosomes isolated from HCV-infected patients’ or normal controls’ serum, or from Huh7.5/JFH1 cell supernatant could mediate monocyte to MΦ differentiation. Exosomes isolated from HCV-infected patient’s serum or from Huh7.5/JFH1 cell supernatants led to increased expression of both macrophage (CD14, CD68) and M2 (CD206, CD163) markers (Fig 2D). We also observed significant IL-1β and TGFβ secretion from monocytes treated with the HCV RNA containing exosomes (Fig 2E). These results suggested that during HCV-infection exosomes carrying the viral RNA can induce monocyte differentiation and macrophage polarization.
In addition to exosome-packaged HCV, monocytes might also be exposed to free HCV viral particles in the circulation and in the liver. We found that HCV viral concentrate isolated from culture supernatants of infected hepatoma cells directly induced monocyte differentiation into MΦs with increased expression of macrophage markers CD14 and CD68. Further, M2 MΦ markers, CD206 and CD163 surface expression were also significantly higher as compared to the control (Fig 2F). Cell-free HCV induced M1/M2 mixed cytokine production indicated by increased secretion of both pro- (TNFα and IL-1β) and anti-inflammatory (IL-10 and TGFβ) cytokines from the HCV-exposed MΦs (Fig 2F). These results demonstrated that HCV viral isolates or hepatocyte-derived exosomes are mediators of monocyte differentiation and do not require cell-cell contact with HCV-infected hepatocytes.
HCV triggers monocyte differentiation via TLR7/8 activation
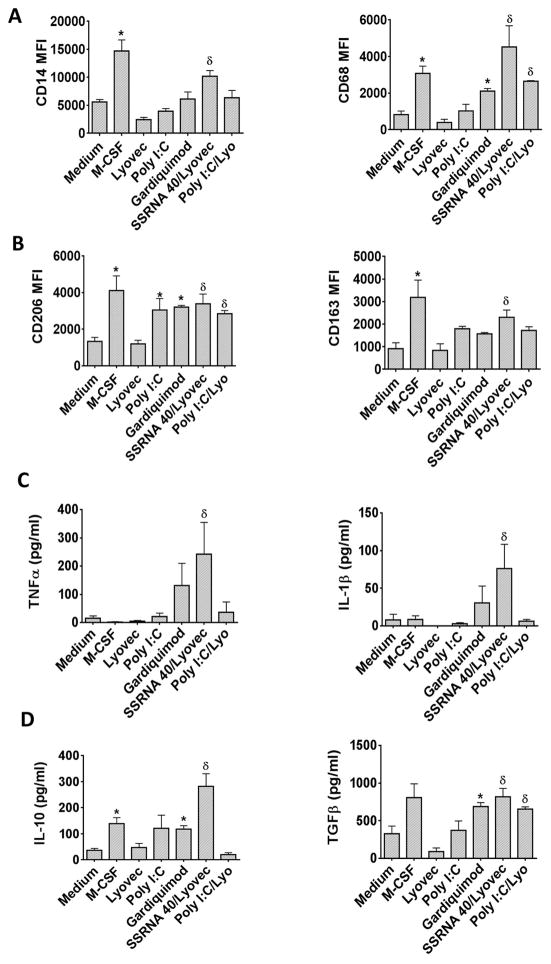
HCV is an RNA virus, thus, we hypothesized that HCV RNA, as a PAMP, can provide signals for monocytes to macrophage differentiation as ssRNA via TLR7 and TLR8 or as a dsRNA via TLR3 or RIG-I. Monocytes were treated with Lyovec (control), Poly I:C (TLR3 ligand), Gardiquimod (TLR7), ssRNA40/Lyovec (TLR8 ligand) or PolyI:C/Lyovec (RIG-I ligand) for 7 days to assess the effect of direct PAMP stimulation on monocyte differentiation. TLR7 and TLR8 activation with Gardiquimod and ssRNA40/Lyovec, respectively, increased the expression of CD14 and CD68 on monocytes along with M2 markers, CD206 and CD163 (Fig 3A, B). PolyI:C/Lyovec also increased the expression CD68 and CD206 on monocytes, whereas PolyI:C increased the expression of CD206 (Fig 3A, B).
Figure 3. TLR7 and TLR8 ligand mimics the effect of HCV on monocyte differentiation.
Monocytes were treated with M-CSF, Lyovec, Poly I:C, Gardiquimod, ssRNA 40/Lyovec or Poly I:C/Lyovec for 7 days. Differentiated monocytes were immunophenotyped for (A) CD14 and CD68, (B) CD206 and CD163. (C) TNFα, IL-1β, (D) IL-10 and TGFβ levels were measured in culture supernatants by ELISA. The data is shown as mean±SEM, n=3–5, *, δP ≤ 0.05, where *P is compared to the medium control and δP is compared to the Lyovec (vehicle control).
Secretion of TNFα and IL-1β were increased significantly with ssRNA40/Lyovec treatment and also with Gardiquimod (not significantly but showed an increasing trend) but not with other TLR stimulations (Fig 3C). IL-10 and TGFβ secretion was significantly elevated in the presence of Gardiquimod or ssRNA40/Lyovec (Fig 3D). TGFβ levels were also induced with PolyI:C stimulation but the levels were not significant. These results indicated that sustained and long-term TLR7 and TLR8 ligand activation leads to generation of MΦs independent of HCV.
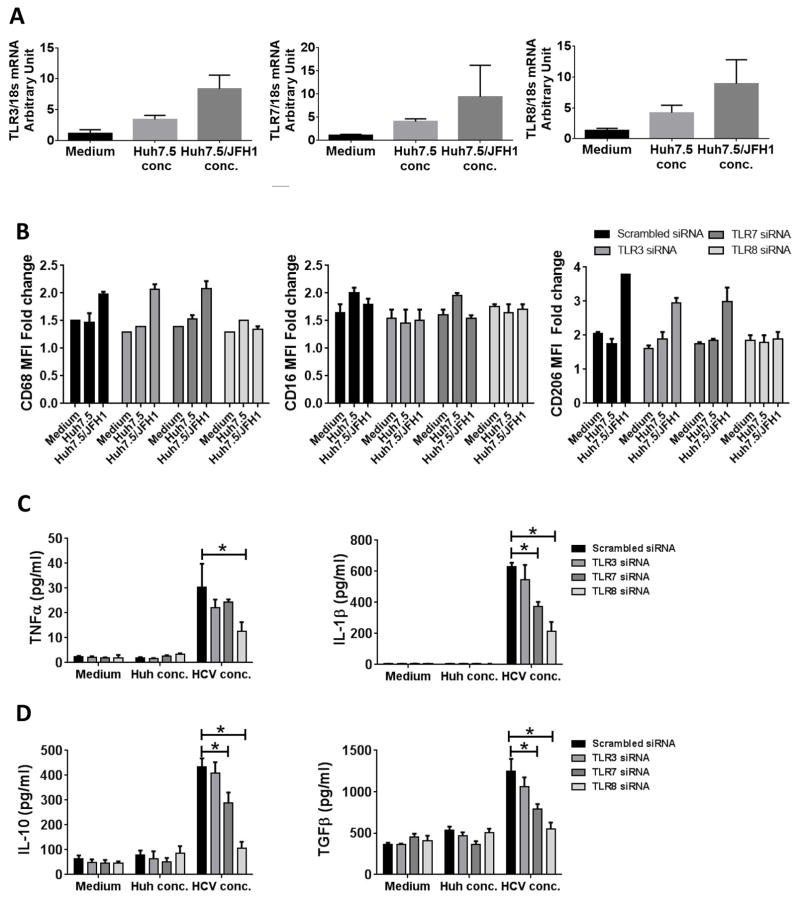
Chattergoon et al have demonstrated the role of TLR7 in inflammasome activation in monocytes during HCV infection (30). Another study has shown the role of TLR8 in recognizing HIV ssRNA by macrophages (31). Thus, we evaluated the functional role of TLR7 and TLR8 in HCV mediated monocyte differentiation. We studied the expression of TLR3, TLR7 and TLR8 mRNA levels in the monocytes cultured in the presence of HCV concentrate and observed increased expression of all 3 TLRs (Fig 4A). Next, we tested whether inhibiting TLR3, TLR7 or TLR8 could prevent the effect of HCV on monocyte differentiation. Knockdown of a particular TLR did not significantly affect the levels of other TLRs and transfection of TLR3-, TLR7- or TLR8-specific siRNA, respectively, knocked-down their expression in monocytes (Supplementary Fig 3). We found that TLR8, and not TLR3 or TLR7, siRNA transfection decreased the expression of CD68 and CD206 (Fig 4B). Monocyte transfection with TLR8 siRNA prevented HCV-induced increases in TNFα, IL-1β, IL-10 or TGFβ production (Fig 4C, D). TLR7 siRNA transfection led to partial but significant reduction in the cytokine secretion as compared to TLR8 knockdown (Fig 4C, D). However, TLR3 siRNA transfection had no significant effect on the HCV-induced cytokine secretion (Fig 4C, D). These data suggested first, that TLR7/8 activation is a central mechanism by which HCV triggers differentiation of monocytes into macrophages and, second, that TLR7/8 activation triggers monocytes to differentiate into a M2 macrophage surface phenotype with a mixed M1/M2 cytokine secretion profile.
Figure 4. Knockdown of TLR7 and TLR8 affects HCV-mediated monocyte differentiation and macrophage polarization.
Monocytes were treated with Huh7.5 or Huh7.5/JFH1 concentrate or were left untreated. After 7 days, the total RNA was isolated and cDNA synthesized. PCR was performed for TLR3, TLR7 and TLR8. (A) The graphs show the mRNA levels of TLR3, TLR7 and TLR8. (B–D) Monocytes were transfected with scrambled or TLR3, TLR7 or TLR8 siRNA for 48 hours and the transfected monocytes were stimulated for 5 days with Huh7.5 or Huh7.5/JFH1 concentrate and (B) cell surface expression of CD68, CD16 and CD206 were performed by flow cytometry. (C–D) Cell culture supernatant collected was assayed for TNFα, IL-1β, IL-10 and TGFβ by ELISA. The data is shown as mean±SEM, n=3–5, *P ≤ 0.05.
HCV ssRNA triggers monocyte differentiation
As ssRNA40, a synthetic TLR8 ligand mediated monocyte differentiation; we reasoned that HCV ssRNA would have the same effect. To test this hypothesis, we generated HCV ssRNA of various lengths and transfected those into healthy monocytes (Supplementary Fig 4A). We studied the expression level of TLR7, TLR8 and RIG-I in response to transfection of in-vitro generated ssRNA. As shown in Supplementary Fig. 4B, we observed the increased expression of TLR-7 and TLR-8 and not RIG-I in the presence of HCV RNA and not control RNA. In figure 4B we show that siRNA knockdown of TLR8 prevented HCV-induced phenotypic changes in monocytes. While this experiment does not rule out additional potential role of RIG-I, it does support our observations on the role of TLR8-mediated modulation of monocytes by HCV.
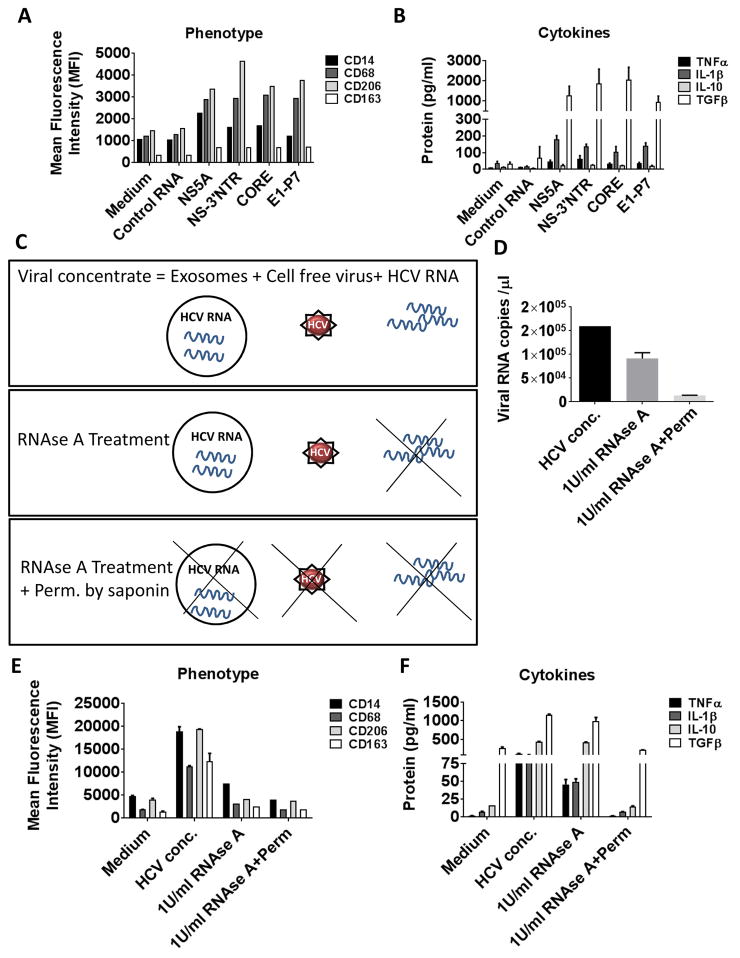
We also observed that HCV ssRNA induced the expression of CD14 and CD68 (Fig 5A). We also observed an increased expression of M2 markers CD206 and CD163 (Fig 5A). Further, monocytes responded to HCV ssRNA by producing increased levels of TNFα, IL-1β and TGFβ (Fig 5B). As shown in Fig 5A and 5B, we do not observe a significant change in the phenotype and cytokine secretion of monocytes transfected with control RNA compared to unstimulated non-transfected (medium) cells. These observations identified HCV ssRNA as the PAMP that leads to TLR7/8 activation and monocyte to macrophage differentiation.
Figure 5. HCV ssRNA mediates monocyte differentiation.
Healthy monocytes were transfected with 5μg/ml of different regions of HCV ssRNA or control RNA and cultured for 7 days. (A) Cell surface expression of CD14, CD68, CD206 and CD163 were measured by flow cytometry. (B) TNFα, IL-1β, IL-10 and TGFβ levels were measured in culture supernatants by ELISA. (C) Viral concentrate consists of free HCV, ssHCV RNA and exosome-containing HCV. RNAse treatment can degrade the ss viral RNA, but not the enveloped HCV RNA in a dose dependent manner. Permeabilization and RNAse treatment of viral concentrate degrades both enveloped and non-enveloped viral RNA. (D) Viral concentrate was treated with 1U/ml of RNAse in the presence or absence of permeabilization buffer. The viral RNA levels were determined by quantitative RT-PCR method. The graph is represented as viral RNA copies/μl. (E) Monocytes were cultured in the presence of viral concentrate with or without treatment. Cell surface expression of CD14, CD68, CD206 and CD163 were measured by flow cytometry. (F) TNFα, IL-1β, IL-10 and TGFβ levels were measured in culture supernatants by ELISA. The data is represented as mean±SEM, n=3.
In order to ascertain that the RNA from the viral concentrate was responsible for the monocyte differentiation and polarization, we co-cultured monocytes with viral concentrate that was treated with RNAse in the presence or absence of permeabilization buffer. RNase treatment destroys free RNA in the cell free virus concentrate and isolated exosomes from HCV infected Huh 7.5 cells, but RNAse along with permeabilization will lead to RNA degradation of exosome packaged and HCV virus packaged RNA also (Figure 5C). Permeabilization of the virus along with RNAse treatment resulted in undetectable levels of viral RNA as shown in the quantitation graph (Fig 5D) (26, 29). We evaluated the macrophage marker expression in the co-culture experiments and observed a significant reduction in the expression of CD14, CD68 and M2 markers CD163 and CD206 in the RNAse and permeabilized viral concentrate as compared to untreated HCV concentrate (Fig 5E). We also found a reduction in both the pro-inflammatory (TNFα, IL-1β) and anti-inflammatory (IL-10, TGFβ) secretion from the MΦ that were cultured in the presence of RNAse-treated and permeabilized viral concentrate as compared to HCV concentrate alone (Fig 5F). These results demonstrate that HCV ssRNA is the trigger for monocyte to macrophage differentiation and polarization.
TLR7 and TLR8 expression is increased in circulating monocytes with high collagen levels in HCV-infected patients
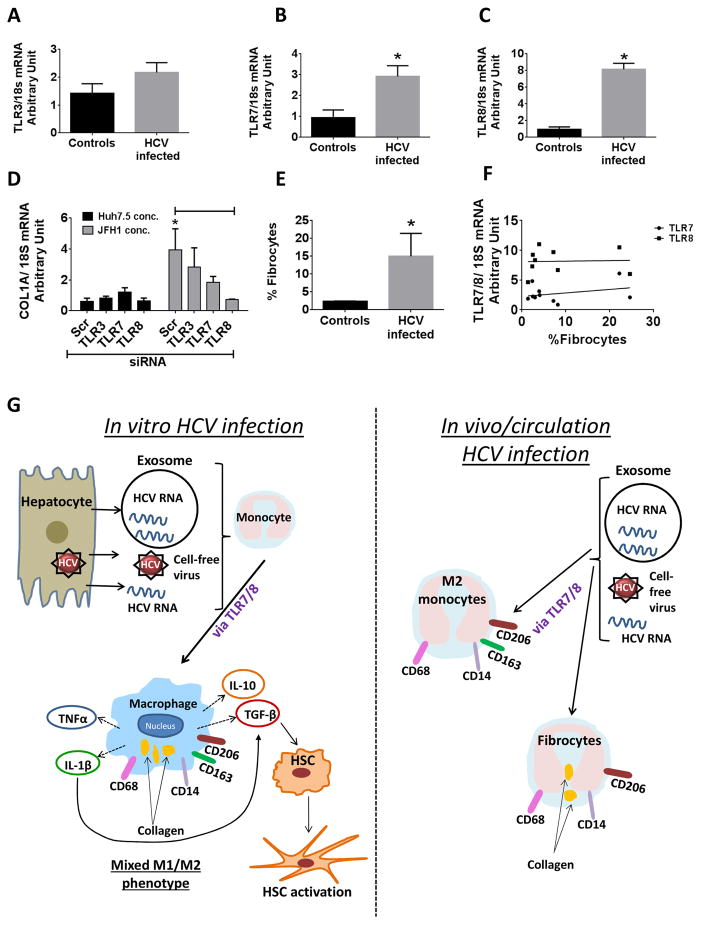
Ourin vitro results demonstrated the role of HCV ssRNA as a PAMP that mediated monocyte to MΦ differentiation and M2 marker expression via TLR7/8. Thus, we were interested in investigating whether TLR expression and M2 polarization also correlated with fibrocyte expression during HCV-infection. We observed that there was a significant increase in the expression of TLR7 and TLR8 levels and to a lesser extent of TLR3 in HCV patient monocytes as compared to that of healthy controls (Fig 6A-C).
Figure 6. TLR7 and TLR8 expression associated with collagen expression in monocytes during chronic HCV infection.
(A–C) CD14+ monocytes from controls and HCV-infected patients were isolated and the levels of TLR3, TLR7 and TLR8 were determined by PCR. The data is representative of n=5 (Controls) and n=8 (HCV). (D) Monocytes transfected with scrambled or TLR3, TLR7 or TLR8 siRNA for 48 hours and the transfected monocytes were stimulated for 5 days with Huh7.5 or Huh7.5/JFH1 concentrate. The levels of collagen mRNA was measured in the monocytes (E) PBMCs isolated from the controls and HCV-infected patients were immunophenotyped for the presence of fibrocytes (CD14+ pro-Collagen1α+). The data is representative of n=5 (Controls) and n=8 (HCV). (F) Correlation of TLR7 and TLR8 expression with circulating fibrocyte levels of HCV-infected patients (G) Working model of HCV mediated monocyte differentiation and polarization. HCV-infected hepatocytes release virus which interacts with monocytes to induce differentiation into MΦs. These M2-like macrophages secrete both pro- and anti-inflammatory cytokines. Early secretion of IL-1β facilitates the secretion of TGFβ, which leads to HSC activation. In vivo data reveals the presence of M2 marker expressing monocytes in circulation of HCV-infected patients and collagen expressing fibrocytes.
To investigate whether TLR activation plays a role in generation of fibrocytes we evaluated the levels of collagen-1α in monocytes which were knocked down for TLR3, TLR7 or TLR8 and co-cultured with HCV or Huh concentrate. We observed that TLR7 knockdown partially and TLR8 knockdown completely abrogated the expression of collagen in the monocytes which were exposed to HCV (Fig 6D). This suggested that TLR7/8 activation plays a role in generation of fibrocytes during HCV infection. Furthermore, we observed a significant increase in the percentage of fibrocytes in HCV-infected patients who had increased TLR7 and TLR8 expression (Fig 6E). TLR7 and TLR8 expression did not correlate with fibrocyte levels in chronic HCV patients (Fig 6F). Together these results demonstrate that during HCV-infection, viral RNA induces the generation of polarized macrophages and fibrocytes via TLR8.
DISCUSSION
Innate immune responses are important in the control and resolution of acute HCV infection and contribute to chronic inflammation (5, 32). Here we show that co-cultures of human monocytes and HCV infected hepatoma cells or cell-free HCV can differentiate monocytes to MΦs with mixed M1/M2 cytokine production and M2-polarized surface marker expression. We demonstrate that cell-free HCV and HCV containing exosomes induce macrophages that are characterized by high pro- and anti-inflammatory cytokine production and surface expression of M2 phenotypic markers. In our previous work we had shown that the M2 MΦ-derived TGFβ acts as a mediator of hepatic stellate cell activation leading to liver fibrosis (27). In the present study, we identified HCV ssRNA and TLR7/8 activation as triggers for differentiation of monocytes to polarized macrophages. Based on our results in the monocyte stimulation experiments with HCV and TLR7/8 agonist we conclude that the mixed M1/M2 phenotype may be due to activation of TLR7 and TLR8 by HCV. We showed that the absence of TLR7 or TLR8 is associated with inhibition of collagen expression in HCV-exposed monocytes. Further in chronic HCV-infected patients, high levels of TLR7/8 expression in monocytes were associated with the high percentage of collagen expressing fibrocytes suggesting that TLR7/8 may be involved in generation of fibrocytes (Fig 6F).
It is known that peripheral blood monocytes constantly enter the liver and interact with HCV-infected hepatocytes and also to replenish liver macrophages (33). We performed co-culture experiments to reveal the mechanism by which HCV-infected hepatocytes interact with monocytes. We reasoned that this would mimic thein vivo environment, where monocytes entering the liver come in contact with the HCV-infected hepatocytes, cell-free virus or virus containing exosomes. Consistent with previous studies, we showed that in the presence of HCV, monocytes are differentiating into M2 polarized macrophages with mixed M1/M2 cytokine profile (12, 27, 34). Our present study using primary human hepatocytes infected with HCV and co-cultured with the healthy monocytes also showed similar effects on monocyte phenotype and function demonstrating that this is a HCV-specific effect. It is also unlikely that interferons or cytokines produced by HCV-infected hepatocytes mediate the effects on monocytes, as we observe minimal secretion of these factors from the infected hepatocytes. Further, isolated HCV virus, exosome-packaged HCV and ssHCV RNA independently had similar phenotypic and functional effect on monocyte differentiation and macrophage polarization.
Pathogens such as HCV are recognized by innate immunity via their PAMPs. Given that cell-cell contact was not necessary for HCV to induce monocyte differentiation, we investigated the role of RNA sensing PRRs. We show that the TLR7 or TLR8 agonist, Gardiquimod or ssRNA40 induced monocyte differentiation to MΦs. This suggests that TLR7/8 ligands other than HCV can induce monocyte differentiation. The role of TLR7/8 was supported by the observation that the monocyte to macrophage differentiation by HCV was blocked by TLR7 or TLR8 knock-down and not by TLR3 knockdown in monocytes, indicating that TLR7/8 mediates the effect of HCV. We also demonstrated that HCV ssRNA induced monocyte differentiation, suggesting that HCV ssRNA plays an important signaling role in changing the monocyte/macrophage phenotype. This is important in HCV-host interaction because TLR induced differentiation of monocytes into either macrophages or DCs has been shown to crucially influence effective host defenses (35). Our in vivo results show that during HCV infection there is a significant increase in the expression of TLR7 and TLR8 levels in monocytes and also high expression of pro-collagen. Knockdown of the TLR7/8 also abrogated the increased expression of collagen in the monocytes exposed to HCV, demonstrating the role of TLR7/8 in fibrocytes generation.
Exosomes from HCV patients contain ssHCV RNA that can transfer HCV infection (26, 36). Exosomal transfer of HCV RNA from infected hepatoma cells can stimulate plasmacytoid DCs to secret IFN-α (37). Here we show that exosomes derived from infected hepatocytes can also modulate monocyte differentiation. Thus, the present study describes a new role of HCV containing exosomes in mediating immune effects during HCV infection. In a recent study we evaluated changes in the phenotype and function of healthy human monocytes cultured in the presence of HCV-infected hepatoma cells and cell-free HCV (27). We demonstrated that HCV induces circulating monocytes to differentiate into macrophages with a M2 surface phenotype and mixed M1/M2 cytokine production. We further showed that chronic presence of HCV programs monocytes that induce hepatic stellate cell activation via TGFβ in HCV infection (27). The present study and the previous study underscore that during human diseases rather than distinct M1 and M2 macrophage populations, macrophages often coexist and the resultant mixed phenotype depends on the balance of activatory and inhibitory factors and the tissue environment.
Studies have shown that CD14+ human monocytes can patrol blood vessels and exert specific effector functions in response to viruses and nucleic acids which is mediated by TLR7 and TLR8 (11). The difference between pDC activation and monocyte activation with viral infection has also been demonstrated by a study by Chattergoon et al, where they demonstrate that innate sensing of virus/viral particles leads to in type I interferon (IFN) production in pDCs and inflammasome activation in monocytes during HCV and HIV infection (30). This study shows that even though pDC and monocyte sense virus through TLRs, the TLRs involved are different and the responses are elicited by HCV in different cell types. Studies from our group demonstrate that exosomes secreted by HCV-infected hepatocytes contain HCV viral RNA along that is associated with miR-122 and Ago-2 and these exosomes are taken up by uninfected cells (26). We have also shown that the exosomes from hepatocytes are taken up by monocytes as demonstrated by the presence of miR-122 in monocytes upon exosome uptake of hepatocyte derived exosomes(38). Thus, our study shows that in the presence HCV virus and HCV-containing exosomes, monocytes differentiates into M2 macrophages.
Studies have shown that CD14+ peripheral blood monocytes can differentiate into cells that express collagen, called fibrocytes, which are attracted to the sites of tissue injury and mediate tissue repair and fibrosis (39, 40). Fibrocytes have features of both macrophages and fibroblasts and express both markers of hematopoietic cells and collagen I and III (39, 41). Increased number of fibrocytes are reported in diseases which are characterized by chronic inflammation and has been reported in animal models of liver, kidney and lung fibrosis (40, 42). Collagen producing monocytes are detected in the blood of the patients with systemic sclerosis and are important in progression of lung fibrosis (43). In our previous study we showed collagen expression in the monocytes co-cultured with HCV-infected hepatocytes. In addition to the presence of circulating fibrocytes in chronic HCV infected patients (27), we also identified a unique population of circulating monocytes with M2-marker and collagen expression that correlated with the presence of liver fibrosis in chronic HCV infected patients (27). In the present study we show the role of TLR7 and TLR8 in induction of fibrocytes in HCV-infection. Our study suggests that HCV infection can stimulate the generation of fibrocytes which in turn can be important in the process of liver fibrosis. However, further work needs to be done to directly link fibrocytes and liver fibrosis during HCV infection.
Inflammation is a major component of the pathogenesis during chronic HCV infection and macrophages are the prominent inflammatory cells in the liver. Increased TNFα and IL-1β expression is observed in the Kupffer cells in the liver during chronic HCV infection(6, 7, 12, 13). Proinflammatory cytokines have been implicated in the process of liver fibrosis (44, 45). We have shown that IL-1β promotes TGFβ secretion during chronic HCV infection leading to macrophage polarization to M2 phenotype. IL-6 and TNFα play significant role in chronic HCV infection as demonstrated by studies in HCV transgenic mice (46, 47). Further studies on HCV transgenic mice has shown that M2 macrophages increase in the liver and these M2 macrophages secrete pro-inflammatory cytokines, IL-6 and TNFα that leads to chronic inflammation in the liver (48). Based on our results it can be speculated that an increase in the M2 macrophages secreting pro-inflammatory cytokines, will increase in the inflammation in the liver and on the other hand, secretion of cytokines like TGFβ will lead to fibrosis.
In summary, our data illustrate the role of TLR7/8 activation in monocytes during HCV infection which leads to monocyte differentiation and macrophage polarization. These results identified a novel role of TLR7/8 in fibrocytes generation during HCV infection and underscore the importance of TLR8 activation in liver fibrosis in chronic hepatitis C infection.
Supplementary Material
Acknowledgments
We thank Dr. Takaji Wakita (National Institute of Infectious Diseases, Tokyo, Japan) and Dr. Charles M. Rice (Rockefeller University, New York) for kindly providing the infectious JFH-1 molecular clone and Huh7.5 cells. We thank Dr. Kui Li (University of Tennessee Health Science Centre, Memphis, Tennessee) for providing the HCV genome containing plasmids. We thank Donna Giansiracusa and Trang Vo from the GI research office for assistance with the clinical samples. The authors greatly appreciate the participation of patients and volunteers for this study. Primary human hepatocytes were obtained from the National Institutes of Health (NIH) liver tissue cell distribution system (LTCDS; Minneapolis, MN, USA; Pittsburgh, PA; Richmond, VA, USA).
Grant Support: The study was supported by NIH funded grant R37 AA014372 to Dr. Gyongyi Szabo. National Institutes of Health (NIH) liver tissue cell distribution system (LTCDS; Minneapolis, MN, USA; Pittsburgh, PA; Richmond, VA, USA) was funded by NIH contract #N01-DK-7-004/HHSN2670070004C.
Abbreviations used in this paper
- COL
Collagen
- dsRNA
double-stranded RNA
- FBS
Fetal Bovine Serum
- HCV
Hepatitis C Virus
- Huh7.5/JFH1
Huh7.5 cells infected with JFH1 (HCV)
- IL
interleukin
- JFH1
Japanese fulminant hepatitis1
- mRNA
messenger RNA
- MΦ
macrophage
- PAMPs
pathogen associated molecular patterns
- PBMC
peripheral blood mononuclear cell
- PHH
primary human hepatocytes
- PRR
pattern recognition receptor
- PBS
phosphate-buffered saline
- RT-PCR
Real-Time Polymerase Chain Reaction
- ssRNA
single-stranded RNA
- TGF
Transforming Growth factor
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology (Baltimore, Md) 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology (Baltimore, Md) 2015;61:77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015 doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rein DB, Wittenborn JS, Smith BD, Liffmann DK, Ward JW. The Cost-effectiveness, Health Benefits, and Financial Costs of New Antiviral Treatments for Hepatitis C Virus. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015 doi: 10.1093/cid/civ220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo G, Dolganiuc A. Hepatitis C and innate immunity: recent advances. Clin Liver Dis. 2008;12:92, x. doi: 10.1016/j.cld.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Bekeredjian-Ding I, Roth SI, Gilles S, Giese T, Ablasser A, Hornung V, Endres S, Hartmann G. T cell-independent, TLR-induced IL-12p70 production in primary human monocytes. Journal of immunology (Baltimore, Md: 1950) 2006;176:7438–7446. doi: 10.4049/jimmunol.176.12.7438. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science (New York, NY) 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 9.Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu BS, Groothuismink ZM, Janssen HL, Boonstra A. Role for IL-10 in inducing functional impairment of monocytes upon TLR4 ligation in patients with chronic HCV infections. J Leukoc Biol. 2011;89:981–988. doi: 10.1189/jlb.1210680. [DOI] [PubMed] [Google Scholar]
- 11.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, Mandrekar P, Szabo G. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627–1636. doi: 10.1053/j.gastro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH, Gale M., Jr IL-1beta production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavez-Galan L, Olleros ML, Vesin D, Garcia I. Much More than M1 and M2 Macrophages, There are also CD169(+) and TCR(+) Macrophages. Front Immunol. 2015;6:263. doi: 10.3389/fimmu.2015.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Rey-Giraud F, Hafner M, Ries CH. In vitro generation of monocyte-derived macrophages under serum-free conditions improves their tumor promoting functions. PLoS One. 2012;7:e42656. doi: 10.1371/journal.pone.0042656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, Clementi E, Bianchi ME, Cossu G, Manfredi AA, Brunelli S, Rovere-Querini P. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol. 2009;85:779–787. doi: 10.1189/jlb.0908579. [DOI] [PubMed] [Google Scholar]
- 20.Szabo G, Dolganiuc A. Hepatitis C core protein - the “core” of immune deception? J Hepatol. 2008;48:8–11. doi: 10.1016/j.jhep.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Ishikawa T, Okumura A, Yamauchi T, Sato S, Ayada M, Matsumoto E, Hotta N, Oohashi T, Fukuzawa Y, Kakumu S. Expression of Toll-like receptors in chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2007;22:1627–1632. doi: 10.1111/j.1440-1746.2006.04783.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YL, Guo YJ, Bin L, Sun SH. Hepatitis C virus single-stranded RNA induces innate immunity via Toll-like receptor 7. J Hepatol. 2009;51:29–38. doi: 10.1016/j.jhep.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Saha B, Szabo G. Innate immune cell networking in hepatitis C virus infection. J Leukoc Biol. 2014;96:757–766. doi: 10.1189/jlb.4MR0314-141R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolganiuc A, Garcia C, Kodys K, Szabo G. Distinct Toll-like receptor expression in monocytes and T cells in chronic HCV infection. World journal of gastroenterology: WJG. 2006;12:1198–1204. doi: 10.3748/wjg.v12.i8.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Kodys K, Li K, Szabo G. Human type 2 myeloid dendritic cells produce interferon-lambda and amplify interferon-alpha in response to hepatitis C virus infection. Gastroenterology. 2013;144:425.e427. doi: 10.1053/j.gastro.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G. Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10:e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha B, Kodys K, Szabo G. HCV-induced monocyte differentiation into polarized M2 macrophages promotes stellate cell activation via TGFβ. Cellular and Molecular Gastroenterology and Hepatology. 2016 doi: 10.1016/j.jcmgh.2015.12.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine: nanotechnology, biology, and medicine. 2014 doi: 10.1016/j.nano.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chattergoon MA, Latanich R, Quinn J, Winter ME, Buckheit RW, 3rd, Blankson JN, Pardoll D, Cox AL. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog. 2014;10:e1004082. doi: 10.1371/journal.ppat.1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han X, Li X, Yue SC, Anandaiah A, Hashem F, Reinach PS, Koziel H, Tachado SD. Epigenetic regulation of tumor necrosis factor alpha (TNFalpha) release in human macrophages by HIV-1 single-stranded RNA (ssRNA) is dependent on TLR8 signaling. The Journal of biological chemistry. 2012;287:13778–13786. doi: 10.1074/jbc.M112.342683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barth H, Rybczynska J, Patient R, Choi Y, Sapp RK, Baumert TF, Krawczynski K, Liang TJ. Both innate and adaptive immunity mediate protective immunity against hepatitis C virus infection in chimpanzees. Hepatology (Baltimore, Md) 2011;54:1135–1148. doi: 10.1002/hep.24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heydtmann M. Macrophages in hepatitis B and hepatitis C virus infections. J Virol. 2009;83:2796–2802. doi: 10.1128/JVI.00996-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosomura N, Kono H, Tsuchiya M, Ishii K, Ogiku M, Matsuda M, Fujii H. HCV-related proteins activate Kupffer cells isolated from human liver tissues. Dig Dis Sci. 2011;56:1057–1064. doi: 10.1007/s10620-010-1395-y. [DOI] [PubMed] [Google Scholar]
- 35.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, Bloom BR, Modlin RL. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JA, Stalin Raj V, Jenster G, Kwekkeboom J, Tilanus HW, Haagmans BL, Baumert TF, van der Laan LJ. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A. 2013;110:13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dreux M, Garaigorta U, Boyd B, Decembre E, Chung J, Whitten-Bauer C, Wieland S, Chisari FV. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell host & microbe. 2012;12:558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Momen-Heravi F, Bala S, Kodys K, Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep. 2015;5:9991. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nature reviews Immunology. 2011;11:427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Laboratory investigation; a journal of technical methods and pathology. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 42.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Tourkina E, Bonner M, Oates J, Hofbauer A, Richard M, Znoyko S, Visconti RP, Zhang J, Hatfield CM, Silver RM, Hoffman S. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1 scaffolding domain peptide. Fibrogenesis Tissue Repair. 2011;4:15. doi: 10.1186/1755-1536-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107:1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oikonomou N, Harokopos V, Zalevsky J, Valavanis C, Kotanidou A, Szymkowski DE, Kollias G, Aidinis V. Soluble TNF mediates the transition from pulmonary inflammation to fibrosis. PLoS One. 2006;1:e108. doi: 10.1371/journal.pone.0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sekiguchi S, Kimura K, Chiyo T, Ohtsuki T, Tobita Y, Tokunaga Y, Yasui F, Tsukiyama-Kohara K, Wakita T, Tanaka T, Miyasaka M, Mizuno K, Hayashi Y, Hishima T, Matsushima K, Kohara M. Immunization with a recombinant vaccinia virus that encodes nonstructural proteins of the hepatitis C virus suppresses viral protein levels in mouse liver. PLoS One. 2012;7:e51656. doi: 10.1371/journal.pone.0051656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi T, Burguiere-Slezak G, Van der Kemp PA, Boiteux S. Topoisomerase 1 provokes the formation of short deletions in repeated sequences upon high transcription in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2011;108:692–697. doi: 10.1073/pnas.1012582108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohtsuki T, Kimura K, Tokunaga Y, Tsukiyama-Kohara K, Tateno C, Hayashi Y, Hishima T, Kohara M. M2 Macrophages Play Critical Roles in Progression of Inflammatory Liver Disease in Hepatitis C Virus Transgenic Mice. J Virol. 2015;90:300–307. doi: 10.1128/JVI.02293-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.