Abstract
Background
The ability of a simple self-assessment tool for estimated functional capacity to predict long-term prognosis in patients with established peripheral artery disease (PAD) is unknown. We investigate whether subjective measurement of functional capacity estimated by using of the Duke Activity Status Index (DASI) questionnaire predicts long-term prognosis in patients with established PAD.
Methods
We administered the DASI questionnaire to 771 stable patients with established PAD, who underwent elective diagnostic coronary angiography with 5-year follow-up all-cause mortality.
Results
Two hundred ten patients (27%) died over 5-year follow-up. The lowest DASI score was associated with a 3.2-fold increased risk of 5-year all-cause mortality (unadjusted HR 3.23, 95%CI 2.19–4.75, P<0.001). For overall PAD patients, after adjustments for traditional risk factors, estimated glomerular filtration rate (eGFR), high-sensitivity C-reactive protein (hsCRP), and lowest DASI score remained predictive of 5-year all-cause mortality (adjusted HR 2.09, 95%CI 1.36– 3.23, P<0.001). Interestingly, the lowest DASI score remained predict 5-year allcause mortality regardless of each PAD diagnosis subtype (including lower extremity, non-lower extremity, or carotid artery PAD), although the mortality risk was attenuated when incorporating heart disease severity in the non-lower extremity group.
Conclusions
A simple self-assessment tool of functional capacity provides independent and incremental prognosis value for long-term adverse clinical events in stable patients with established PAD beyond each PAD diagnostic subtype.
Keywords: peripheral artery disease, functional capacity, prognosis
INTRODUCTION
Peripheral artery disease (PAD) is a common manifestation of systemic atherosclerosis associated with worse prognosis1, 2. It affects >27 million people across Europe and America3, 4. There are a number of proven therapies to reduce mortality among patients with PAD5. However, mortality remained high and risk stratification has received little attention, when compared to patients with CAD3, 6. The awareness among at-risk patients and the medical community of PAD remain relatively low3, 7. Therefore, predictors of mortality and identifying poor prognosis markers in patients with PAD are valuable.
The Duke Activity Status Index (DASI) is a simple 12-question self-assessment tool for estimating functional capacity8. DASI score correlated well with peak oxygen uptake on exercise treadmill stress testing (Spearman r=0.81, P<0.0001)8 and are validated measures of treadmill functional capacity measured in metabolic equivalent tasks (METs) (Spearman r=0.31, P<0.01)9. The DASI score predicted adverse prognosis in cohort of patients with various types of cardiac disease9–14.
Remarkably, the long-term prognosis of DASI measurements in stable patients with lower extremity peripheral artery disease (LEAD) as well as the other PAD subtypes has not been elucidated. Here, we sought to determine the long-term prognostic value of estimating functional capacity using the DASI questionnaire in stable patients with PAD. If the DASI questionnaire predicts long-term prognosis, then clinicians could potentially use the DASI to identify patients with PAD who are at risk for adverse prognosis either in the office setting or primary care clinics.
METHODS
Study Population
The Cleveland Clinic GeneBank study prospectively enrolled patients with a history of PAD who underwent elective coronary angiography in the absence of emergency conditions. All-cause mortality was prospectively tracked over 5 years using the Social Security Death Index and medical chart review, confirmed by follow-up contact. All participants gave written informed consent, and the study was approved by the Institutional Review Board.
Diagnosis Validation of Types of PAD
Peripheral artery diseases have been defined by the American College of Cardiology/American Heart Association and the European Society of Cardiology (Supplemental Table 1)2, 4. In our cohort, the term of PAD is used to encompass the majority of non-coronary arterial territories including extracranial carotid artery stenosis (CAS), upper extremity artery stenosis (UES), renal artery stenosis (RAS) and LEAD. Diseases of the aorta are not included. We indicated non-LEAD if primarily diagnosis were not LEAD, which were included CAS, RAS and UES.
Laboratory testing and Assessment of The Duke Activity Status Index Questionnaire
After informed consent was obtained from all patients, fasting blood samples were collected using EDTA tubes at the time of coronary angiography after arterial sheath access, but before the catheterization procedure or any drug administration (including heparin). The samples were then immediately processed and frozen at −80 °C until analysis. Routine laboratory tests were performed, and samples were measured on the Abbott Architect platform (Abbott Laboratories). Myeloperoxidase (MPO) was measured using the cardioMPO assay kit. Complete blood count was measured using the ADVIA 120 Hematology System (Siemens Medical Systems). The DASI questionnaire (Supplemental Table 2) was completed assessment by well trained research personnel at the time of coronary angiography as previously described12, and this questionnaire has been validated in similar population9, 15.
Statistical Analysis
Continuous data are presented as mean (standard deviation) or median (interquartile range [IQR]) and compared with student’s t-test or non-parametric test when appropriate. Categorical variables are presented as number (%) and compared between groups with chi-square tests. We divided DASI into quartiles. Kaplan-Meier analysis with Cox proportional hazards regression was used for the time-to-event analysis to determine hazard ratios (HR), and 95% confidence intervals (95% CI) for 5-year all-cause mortality was stratified according to DASI as a continuous variable (log-transformed per SD increase), as well as according to quartiles. Adjustments were made for individual traditional cardiovascular risk factors (age, sex, systolic blood pressure, diabetes mellitus, low-density and high-density lipoprotein cholesterol levels, and smoking status) and for log-transformed high-sensitivity C-reactive protein (hs-CRP) levels, log-transformed estimated glomerular filtration rate (eGFR), log-transformed MPO, log-transformed white blood cell (WBC), and apolipoprotein A-1 (ApoA1) and apolipoprotein B (ApoB), to predict all-cause mortality. Net reclassification index (NRI) and area under the receiver-operating characteristic (AUC) curve were calculated according to mortality risk estimated using Cox models adjusted for above-mentioned traditional risk factors with versus without DASI score as previously described16. Subgroups were stratified according to diagnosis subtype of PAD (LEAD and non-LEAD) as well as other baseline clinical and laboratory subgroups that might be affected for mortality risks. All analyses were performed used R 2.15.1 (Vienna, Austria). A P value < 0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
The 935 patients with medical history of PAD were directly questioned by research personnel about past medical problems of non-CAD and/or history of or repair of aortic dissection/aneurysm. Importantly, we carefully reviewed the electronic medical record (including angiographic data) for validation of PAD diagnostic subtypes (all patients were seen by a cardiologist at Cleveland Clinic before the left heart catheterization). A confirmed diagnosis of PAD was based primarily on the type of PAD, based on reporting evidence of stenosis at the corresponding vasculature (Supplemental Table 1). Of these, confirmed diagnosis data were not available for 14 patients, 50 patients did not have DASI data, and 100 patients with a diagnosis of aortic aneurysm were excluded. Consequently, 771 consecutive patients were included in this study. The baseline characteristics of our study cohort according to DASI score quartile are shown in Table 1. Patients with a lower DASI score were significantly associated with an underlying history of diabetes, previous stroke, COPD, or HF and having high levels of inflammatory biomarkers (hsCRP, or MPO). There were no differences in medication use across DASI quartiles.
Table 1.
Baseline Characteristics According to DASI Score Quartile
| DASI Score Quartile | ||||||
|---|---|---|---|---|---|---|
| Variable | All (n=771) |
Quartile 1 <18.95 |
Quartile 2 18.95–29.44 |
Quartile 3 29.45–42.6 |
Quartile 4 ≥ 42.7 |
P Value |
| Age, (years) | 66±10 | 68±10 | 68±11 | 67±11 | 63±9 | <0.001 |
| Male, (%) | 66 | 45 | 57 | 72 | 85 | <0.001 |
| Diabetes mellitus, (%) | 43 | 62 | 41 | 37 | 32 | <0.001 |
| Hypertension, (%) | 83 | 85 | 81 | 85 | 81 | 0.552 |
| Former/Current smokers, (%) | 74 | 68 | 71 | 75 | 82 | 0.009 |
| History of HF, (%) | 31 | 49 | 39 | 25 | 19 | <0.001 |
| History of Stroke/TIA, (%) | 29 | 56.5 | 44 | 45.5 | 27 | 0.01 |
| History of COPD, (%) | 23 | 48 | 35 | 23 | 19 | <0.001 |
| History of CAD | 90 | 91 | 93 | 92 | 85 | 0.012 |
| Number of CAD vessels (%) | 0.147 | |||||
| 0 | 10 | 8 | 8 | 8 | 15 | |
| 1 | 14 | 13 | 16 | 12 | 16 | |
| 2 | 21 | 22 | 19 | 22 | 22 | |
| 3 | 54 | 58 | 57 | 57 | 46 | |
| Framingham ATP III Risk Score | 10 (8–12) |
11 (9–14) |
10 (8–12) |
9 (7–12) |
9 (7–11) |
<0.001 |
| LDL cholesterol, (mg/dL) | 92 (75–111) |
87 (71–105) |
96 (75–115) |
93 (76–112) |
94 (77–113) |
<0.001 |
| HDL cholesterol, (mg/dL) | 32 (26–39) |
30 (25–37) |
33 (27–41) |
33 (26–40) |
33 (27–38) |
<0.001 |
| Triglycerides, (mg/dL) | 122 (90–172) |
132 (94–194) |
126 (88–179) |
119 (90–160) |
115 (89–162) |
<0.001 |
| hsCRP, mg/L | 3.1 (1.3–7.9) |
5.1 (2.3–11.5) |
3.7 (1.5–8.7) |
2.5 (1.2–6.2) |
2 (0.9–5.3) |
<0.001 |
| B-type natriuretic peptide (pg/mL) |
156 (72–413) |
254 (117–746) |
189 (85–488) |
142 (74–408) |
105 (41–187) |
<0.001 |
| Left ventricular ejection fraction (%-units) |
50 (40–60) |
50 (31–60) |
45 (35–60) |
53 (41–59) |
55 (45–60) |
<0.001 |
| eGFR, ml/min/1.73 m2 | 78.8 (59.5–91.1) |
72.2 (52.3–85.6) |
77.9 (61.3–91.1) |
78.1 (60.8–89.5) |
86.1 (70.6– 96.6) |
<0.001 |
| Apolipoprotein B, (mg/dL) | 80 (68–93) |
79 (67–91) |
82 (70–98.2) |
80 (68–91) |
81 (68–94) |
<0.001 |
| Apolipoprotein A1, (mg/dL) | 112 (100–128) |
108 (96.2–124) |
114 (101–129.2) |
115 (101–130) |
111 (98.8–128) |
<0.001 |
| TG/HDL | 3.9 (2.6–5.9) |
4.5 (2.9–6.2) |
3.9 (2.2–6.1) |
3.7 (2.5–5.4) |
3.6 (2.5–5.5) |
<0.001 |
| MPO, mg/L | 118.2 (80.3–261) |
132.8 (87–311.5) |
126.8 (85.6–268) |
122.3 (81.2– 246.6) |
107.5 (72.2–253) |
<0.001 |
| WBC, (per mm3) | 6.4 (5.2–7.9) |
7 (5.6–8.8) |
6.2 (5.3–7.4) |
6.2 (5–7.7) |
6.3 (5.1–7.9) |
<0.001 |
| Medication: | ||||||
| ACE or ARB, (%) | 60 | 60 | 58 | 61 | 61 | 0.935 |
| Beta-blocker, (%) | 69 | 69 | 65 | 73 | 67 | 0.413 |
| Statin, (%) | 71 | 68 | 68 | 73 | 74 | 0.434 |
| Aspirin, (%) | 77 | 78 | 77 | 72 | 80 | 0.316 |
Values expressed in mean ± SD, % or median (interquartile range). eGFR=estimated glomerular filtration rate, ACEI=angiotensin converting enzyme inhibitors, ARB=angiotensin-receptor blocker, hsCRP=high-sensitivity C-reactive protein, LDL=low-density lipoprotein, HDL=high-density lipoprotein, TG=triglyceride, MPO=myeloperoxidase, WBC=white blood cell, COPD=chronic obstructive pulmonary disease, TIA=transient ischemic attack, CAD=coronary artery disease, HF=heart failure.
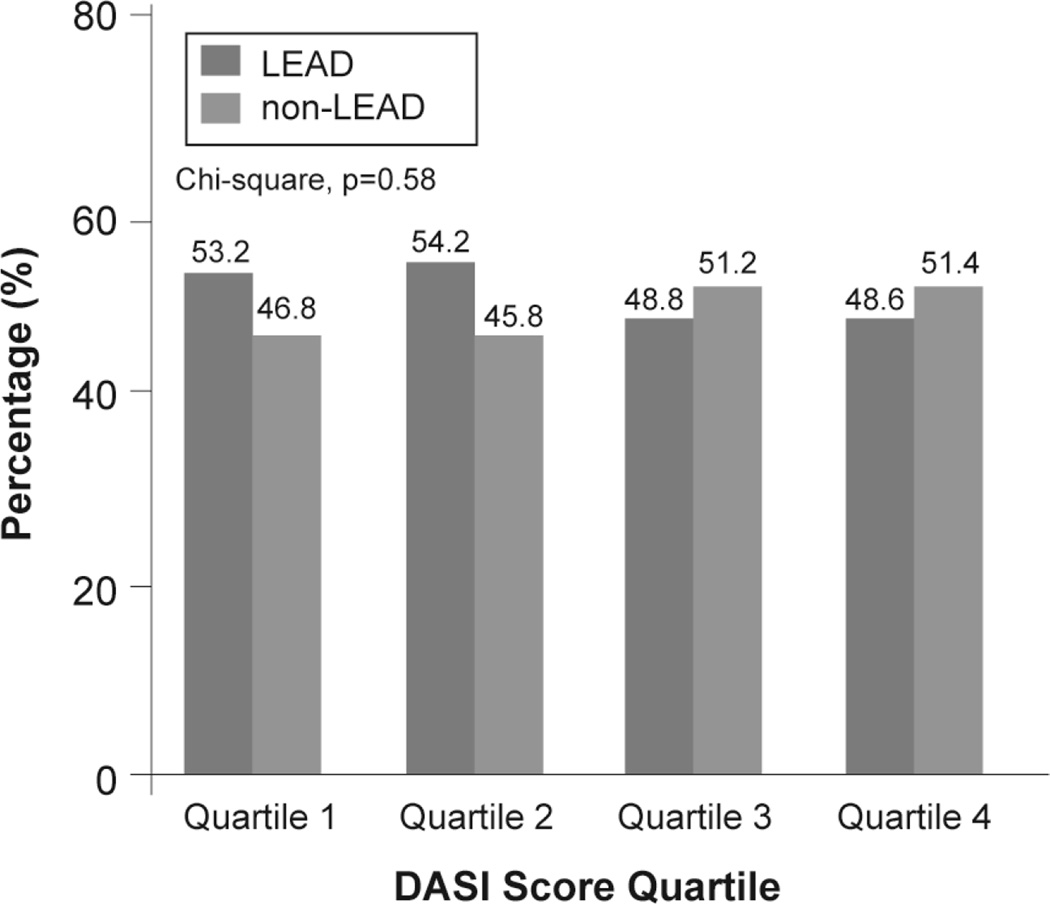
The baseline characteristics according to PAD diagnosis subtype are shown in Table 2. Patients with non-LEAD were more likely to have older age and history of stroke, but the other variables were no difference. The median DASI score was 29.45 (IQR 18.95–42.7). Interestingly, an unexpected, the median DASI score in patients with LEAD were not significant different to patients with Non-LEAD (30.2 [18.9–42.7] versus 34.7 [30.2–36.7], p=0.27) (Table 2). Moreover, the distribution of patients with LEAD and non-LEAD were not significant different across DASI score quartile (Figure 4).
Table 2.
Baseline Characteristics According to PAD Diagnosis Subtype
| Variable | All (n=771) |
LEAD (n=393) |
Non–LEAD (n=378) |
P Value | ||
|---|---|---|---|---|---|---|
| Age, (years) | 66±10 | 65±11 | 68±10 | <0.001 | ||
| Male, (%) | 66 | 64 | 68 | 0.19 | ||
| Diabetes mellitus, (%) | 43 | 45 | 40 | 0.22 | ||
| Hypertension, (%) | 83 | 84 | 82 | 0.7 | ||
| Former/Current smokers, (%) | 74 | 76 | 72 | 0.25 | ||
| History of HF, (%) | 31 | 32 | 33 | 0.88 | ||
| History of Stroke/TIA, (%) | 29 | 65 | 78 | 0.014 | ||
| History of COPD, (%) | 23 | 25 | 21 | 0.22 | ||
| History of CAD | 90 | 91 | 90 | 0.81 | ||
| Number of diseased vessels (%) | 0.009 | |||||
| 0 | 10 | 10 | 11 | |||
| 1 | 14 | 17 | 11 | |||
| 2 | 21 | 24 | 19 | |||
| 3 | 54 | 49 | 60 | |||
| Framingham ATP III Risk Score | 10 (8–12) |
10 (7–12) |
10 (8–13) |
0.96 | ||
| LDL cholesterol, (mg/dL) | 92 (75–111) |
94 (74–113) |
92 (76–108) |
0.68 | ||
| HDL cholesterol, (mg/dL) | 32 (26–39) |
33 (26–39) |
32 (27–38) |
0.61 | ||
| Triglycerides, (mg/dL) | 122 (90–172) |
123 (90–180) |
121 (90–167) |
0.46 | ||
| hsCRP, mg/L | 3.1 (1.3–7.9) |
3.3 (1.3–8.5) |
2.8 (1.3–6.9) |
0.17 | ||
| B-type natriuretic peptide (pg/mL) |
159 (72–413) |
153 (67–388) |
159 (79–430) |
0.287 | ||
| Left ventricular ejection fraction (%-units) |
50 (40–60) |
50 (40–60) |
50 (40–60) |
0.864 | ||
| eGFR, ml/min/1.73 m2 | 78.8 (59.5–91.1) |
79.6 (61.5–92) |
78.3 (58.2–90.2) |
0.27 | ||
| Apolipoprotein B, (mg/dL) | 80 (68–93) |
80 (68–96) |
80 (68–91) |
0.47 | ||
| Apolipoprotein A1, (mg/dL) | 112 (100–128) |
111 (100–128) |
112 (100–128) |
0.54 | ||
| TG/HDL | 3.9 (2.6–5.9) |
3.9 (2.6–6.2) |
3.9 (2.5–5.6) |
0.33 | ||
| MPO, mg/L | 118.2 (80.3–261) |
115.3 (80.3–262) |
120.6 (80.8–259.5) |
0.99 | ||
| WBC, (per mm3) | 6.4 (5.2–7.9) |
6.4 (5.2–8) |
6.4 (5.2–7.9) |
0.77 | ||
| Medication: | ||||||
| ACE or ARB, (%) | 60 | 57 | 63 | 0.09 | ||
| Beta-blocker, (%) | 69 | 70 | 67 | 0.28 | ||
| Statin, (%) | 71 | 70 | 72 | 0.58 | ||
| Aspirin, (%) | 77 | 78 | 76 | 0.55 | ||
| DASI Score | 29.45 (18.9–42.7) |
30.2 (18.9–42.7) |
34.7 (30.2–36.7) |
0.27 | ||
LEAD=lower extremity peripheral artery disease, DASI=Duke Activity Status Index, Other abbreviation as in Table 1
Figure 4. Distribution of Peripheral Artery Disease Diagnosis Subtype Across Duke Activity Status Index (DASI) Score Quartile.
LEAD=lower extremity peripheral artery disease
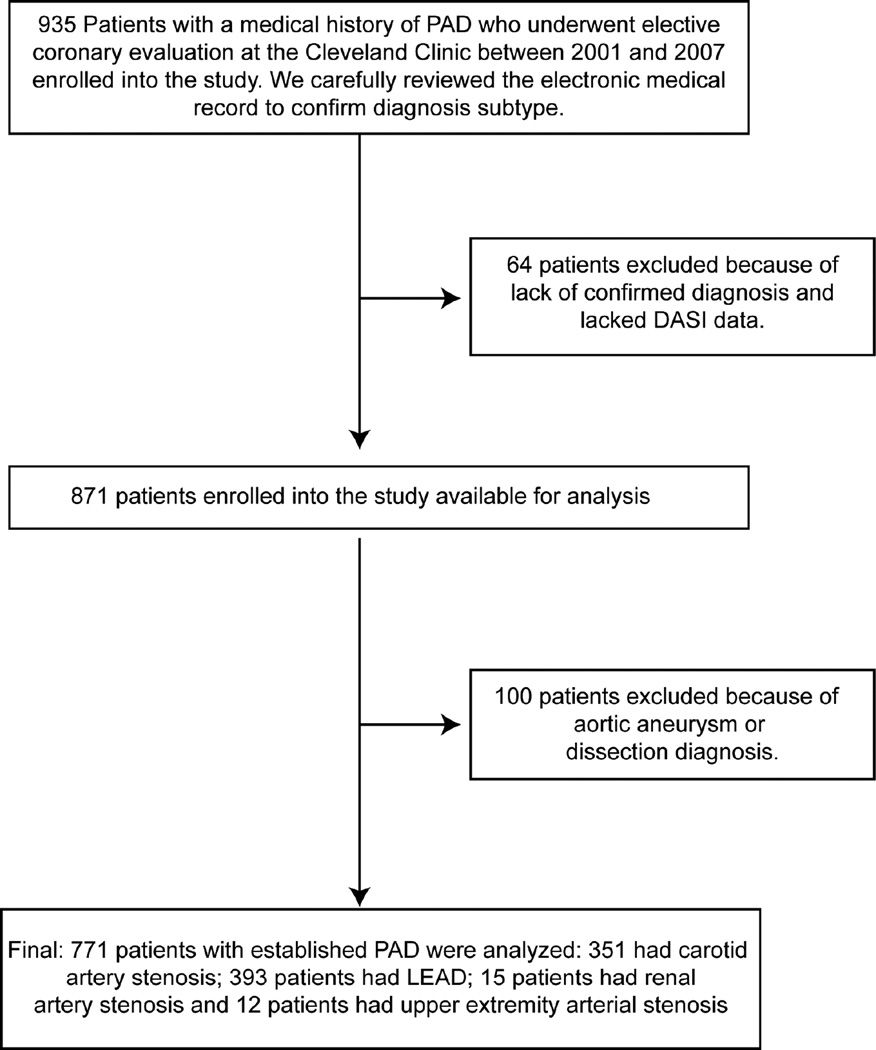
Of the 771 patients, 393 patients had diagnosis of LEAD confirmed by: an ankle-brachial index <0.9 (68.7%), duplex ultrasonography (DUS) (7.1%), computed tomographic angiography (CTA) (0.5%), magnetic resonance angiography (MRA) (0.5%), catheter-based radiocontrast angiography (CATH) (6.4%), prior angioplasty or stenting (8.4%), and prior surgical bypass graft (8.4%); 351 patients had diagnosis of CAS confirmed by DUS (83.8%), MRA (2.6%), CATH (3.4%), prior stenting (4%), and open carotid endarterectomy (6.3%); 15 patients had diagnosis with RAS confirmed by MRA (20%), CATH (53.3%) and prior stenting (26.7%); and 12 patients had upper extremity artery stenosis confirmed by CATH (41.7%) and prior angioplasty or stenting (58.3%) (Figure 1).
Figure 1. Consort Diagram.
PAD=peripheral artery disease, DASI=duke activity status index, LEAD=lower extremity peripheral artery disease
Associations of DASI score and All-Cause Mortality
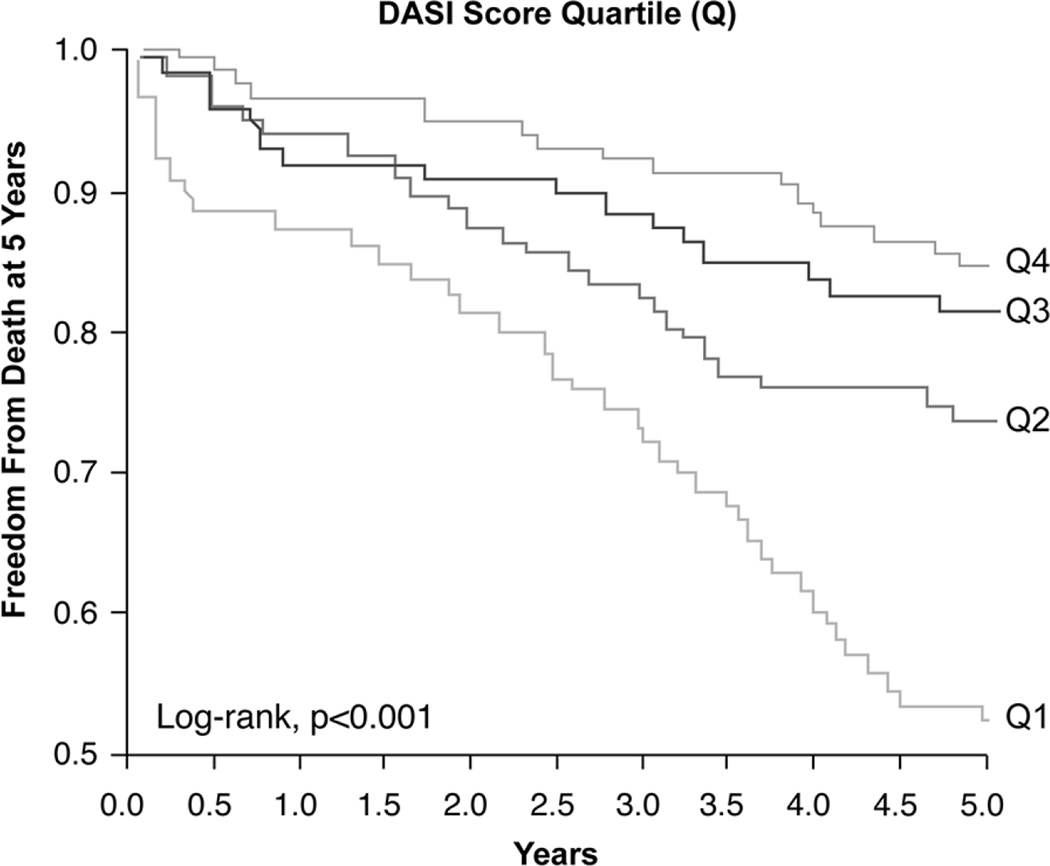
Over the 5-year follow up, 208 (27%) deaths occurred in our cohort. DASI score quartiles 1–4 had 83, 41, 48 and 36 deaths, respectively, by the end of follow-up. Figure 2 represents the Kaplan-Meier analysis of the DASI score stratified by quartiles, which illustrated a graded increase in risk of all-cause mortality observed with decreasing DASI score with log-rank; P<0.001. Importantly, the lowest DASI score quartile predicted a 3.2- fold increase in risk for all-cause mortality compared with the highest DASI score quartile (Quartiles 1st vs 4th, unadjusted HR 3.23, 95% CI 2.19–4.75, P<0.001) (Table 3). The prognosis value of the DASI score was preserved when adjusted for traditional risk factors (adjusted HR 2.62, 95% CI 1.72–3.98, P<0.001) or even plus eGFR and hsCRP (adjusted HR 2.09, 95% CI 1.36–3.23, P<0.001), as well as after adjusting for traditional risk factor, ApoA1, ApoB, MPO and WBC (adjusted HR 2.84, 95% CI 1.79–4.53, P<0.001) (Table 3). As a continuous variable in increments of 1 standard deviation (SD), an increased DASI score was associated with lower mortality risk at 5 years after adjustment for traditional risk factors (adjusted HR 0.67, 95% CI 0.56–0.79 per SD, P<0.001). The inclusion of the DASI score to a model of traditional cardiovascular risk factors showed that a lower DASI score significantly incremental prognosis value (integrated discrimination improvement 33.51%, P<0.001, NRI 33.51%, P<0.001; and differences in AUC 66.02 versus 69.34, P=0.032).
Figure 2. Kaplan-Meier Estimates of 5-Year All-Cause Mortality According to the Quartiles (Q) of Duke Activity Status Index Score.
Table 3.
Hazard Ratio and 95% Confidence Interval (95%CI) of DASI score for Risk of 5-Year All-Cause Mortality Stratified According to All Subjects and Each Diagnosis Subtype of PAD
| Model | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| All Subjects (n=771) | ||
| Unadjusted | 3.23 (2.19–4.75) | <0.001 |
| Adjusted model 1 | 2.62 (1.72–3.98) | <0.001 |
| Adjusted model 2 | 2.09 (1.36–3.23) | <0.001 |
| Adjusted model 3 | 2.84 (1.79–4.53) | <0.001 |
| Adjusted model 4 | 2.60 (1.40–4.81) | 0.0024 |
| Lower Extremity Peripheral Artery Disease (n=393) | ||
| Unadjusted | 2.71 (1.58–4.65) | <0.001 |
| Adjusted model 1 | 2.42 (1.36–4.3) | 0.003 |
| Adjusted model 2 | 2.01 (1.11–3.66) | 0.022 |
| Adjusted model 3 | 2.70 (1.45–5.06) | 0.002 |
| Adjusted model 4 | 2.30 (1.04–5.07) | 0.040 |
| Non-Lower Extremity Peripheral Artery Disease (n=378) | ||
| Unadjusted | 3.83 (2.19–6.68) | <0.001 |
| Adjusted model 1 | 2.72 (1.46–5.06) | 0.0016 |
| Adjusted model 2 | 2.15 (1.13–4.09) | 0.019 |
| Adjusted model 3 | 2.76 (1.38–5.51) | 0.004 |
| Adjusted model 4 | 2.50 (0.92–6.76) | 0.071 |
Quartile 1 (worst) versus Quartile 4 (best). Model 1: adjusted for traditional risk factors include age, gender, systolic blood pressure, LDL cholesterol, HDL cholesterol, smoking and diabetes mellitus. Model 2: adjusted for model 1 plus hsCRP (log-transformed) and eGFR (log-transformed). Model 3: adjusted for model 1 plus ApoA1, ApoB, MPO (log-transformed) and WBC (log-transformed). Model 4: adjusted for model 1 plus number of diseased vessels, B-type natriuretic peptide (log-transformed), and left ventricular ejection fraction. DASI=Duke Activity Status Index, other abbreviations as in Table 1.
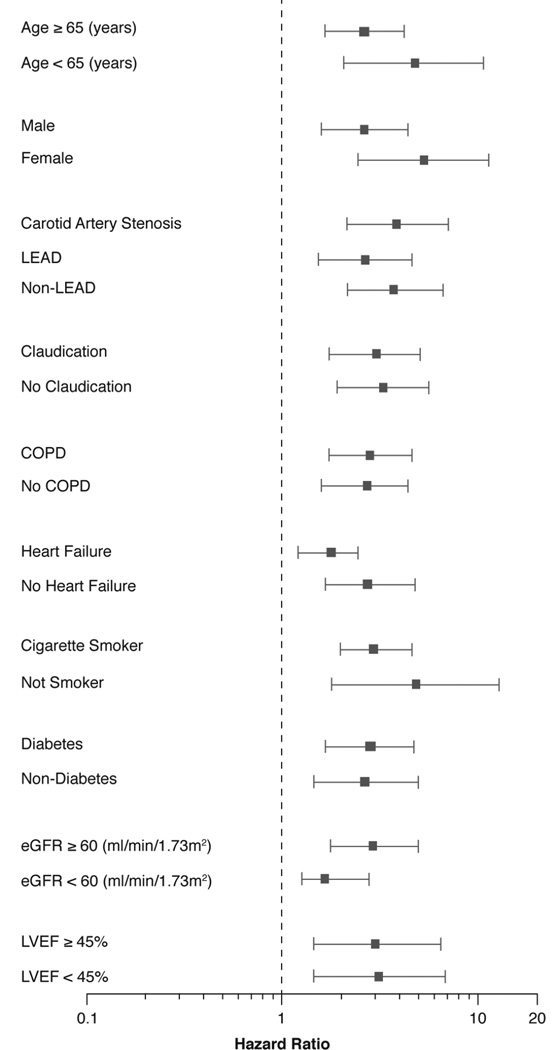
Interestingly, when we did the survival analyses separately according to diagnostic subtype of PAD, the lowest DASI score remained associated with increased 5-year mortality risk in each subtype of PAD, even after adjustment for traditional cardiac risk factors, eGFR and hsCRP: for LEAD (adjusted HR 2.01, 95% CI 1.11–3.66, P=0.02), and non-LEAD (adjusted HR 2.15, 95% CI 1.13–4.09, P=0.019), as well as after adjusting for traditional risk factor, ApoA1, ApoB, MPO and WBC: for LEAD (adjusted HR 2.70, 95% CI 1.45–5.06, P=0.002), and non-LEAD (adjusted HR 2.76, 95% CI 1.38–5.51, P=0.004) (Table 3). Furthermore, in the subgroup of patients with CAS, lowest DASI score was still independently associated with increased 5-year mortality risk even after adjustment for traditional cardiac risk factors, eGFR and hsCRP (adjusted HR 2.13, 95% CI 1.1–4.12, P=0.025), as well as after adjusting for traditional risk factor, ApoA1, ApoB, MPO and WBC (adjusted HR 2.58, 95% CI 1.27–5.25, P=0.009). However, when adjusted for BNP and LVEF, the mortality risk remained statistically significant only in the LEAD subset, while the non-LEAD group did not (Table 3). Importantly, subgroup analyses reveal that lowest DASI score predict 5-year all-cause mortality regardless of each diagnosis subtype of PAD (between LEAD, non-LEAD and CAS), age, sex, history of chronic obstructive lung disease, history of heart failure, present or absence of claudication symptoms, diabetes, smoking status, eGFR, and left ventricular ejection fraction (LVEF) (Figure 3).
Figure 3. Relationship between Duke Activity Status Index (DASI) Score and Mortality Risk Stratified According to Each Diagnostic PAD Subtype and Baseline Characteristics.
Forest plot of hazard ratio (HR) of 5-year all-cause mortality comparing first and fourth quartiles (Q) of DASI score.
eGFR=estimated glomerular filtration rate, LEAD=lower extremity peripheral artery disease, COPD=chronic obstructive pulmonary disease, LVEF=left ventricular ejection fraction.
DISCUSSION
There are several key findings in our study. First, results reported here show, for the first time, that the lowest DASI score is associated with greater 5-year mortality in patients with established diagnosis of PAD. Second, this association remained robust after adjustment for confounders including traditional cardiac risk factor, eGFR, ApoA1 and ApoB and marker of systemic inflammation (hsCRP, MPO and WBC). Meanwhile, the mortality risk was attenuated by confounding factors such as extent of coronary disease and cardiac dysfunction (Table 3). Third, when we did the survival analyses separately according to diagnostic subtype of PAD, the lowest DASI score remained associated with long-term mortality risk in each subtype of PAD. Fourth, the prognosis value for lowest DASI score and mortality remained significant regardless of other comorbid conditions and laboratory subgroups.
Patients with PAD have significantly increased risk of adverse prognosis2, 17. As indicated in recently published clinical studies and clinical practice guideline in patients with PAD, few data are available to document the role of functional capacity and natural history among patients with non-LEAD2, 4. Additionally, patients with LEAD are likely to have lower DASI score than non-LEAD. Interestingly, based on our findings, the prognostic value of the DASI score remained preserved in the subgroup of patients with either diagnosis of LEAD or non-LEAD, and the presence of equivalent baseline DASI score between patients with LEAD and non-LEAD. To our knowledge, this is the first study that assessed the association between lower baseline functional capacity, estimated by DASI score, and all-cause mortality, specifically in PAD patients with covered multiple vascular beds.
Previous studies have demonstrated poorer baseline functional capacity, estimated by a 6-minute walk test and 4-meter walking velocity, and were associated with higher mortality and mobility loss in patients with LEAD,18, 19. Although both functional capacity assessment tools are useful in mortality risk prediction, they are objective measures that require dedicated time and space in the clinical setting. The lower baseline values of Walking Impairment Questionnaire (WIQ) stair-climbing score were associated with higher mortality in LEAD20. Unfortunately, the WIQ is quite complex, patients need to answer 14 questions and each with 5-possible answers (5 different “difficulty graded scales”)21 and associated with a high rate of errors22. Whereas, the DASI is a short, easily administered questionnaire with simple yes/no rather than direct recall of prior physical activities and/or limitations, and is not time consuming or labor intensive.
There have been no studies that directly examined the potential use of DASI to predict long-term prognosis specifically in patients with PAD. However, the DASI has been validated in cohorts of patients with various types of cardiac disease. The Woman’s Ischemia Syndrome Evaluation (WISE) study showed that the DASI correlates with indeterminate exercise testing results and is associated with an adverse prognosis among women with suspected myocardial ischemia14. Our group has previously reported that among patients with stable chronic HF and stable cardiac patients undergoing elective diagnosis cardiac evaluation, DASI provides independent and incremental prognostic value for mortality prediction10, 12. In the setting of cardiac surgery, Koch et al. demonstrated that lower DASI score at perioperative baseline and postoperative follow-up identifies patients who are at risk for reduced long-term survival, and those who achieved a maximum baseline DASI were associated with better risk-adjusted long-term survival13. Recent data from the Trial of Intensified versus Standard medical therapy in Elderly patients With Congestive heart Failure (TIME-CHF) further implied that those with changes in DASI score over 1 year demonstrated stronger association with long-term outcomes than an objective assessment (6-min walking distance)23. Previous studies from the Walking and Leg Circulation Study (WALCS) cohort have reported that, greater than 2-year declines in functional performance and WIQ scores were associated with higher all-cause mortality in patients with LEAD24, 25. Importantly, home-based walking exercise program improve WIQ and functional performance in patients with LEAD26.These findings are supportive of the ability to prevent decline, or improving the DASI score as a potential therapeutic target in patients with PAD. Based on our findings, it seems reasonable to administer the estimated self-reported physical capacity by using the DASI in the daily clinical setting, either in the office or primary care clinics. This would provide an effective tool for identification of those patients at highest long-term adverse event risk who should be targeted for more intensive follow-up and treatment. Further study is needed to determine whether interventions that improve the DASI score also improve prognosis among patients with PAD.
Study Limitations
First, this was a single tertiary care center study that recruited patients at the point of cardiac catheterization; therefore, there was a higher proportion of patients with CAD. Second, our population consisted of a heterogeneous subgroup of patients with PAD; we addressed this issue by electronic record review to confirm diagnosis carefully captured individually, and the presence of equivalent baseline DASI score between LEAD and non-LEAD. Also, we separately performed survival analyses according to diagnosis subtype, as we excluded patients with LEAD, the lowest DASI score still associated with adverse prognosis. And we believe they may be generalized to all patients within the PAD cohort. Third, we lacked complete information regarding the severity of intermittent claudication, and disease severity of each diagnosis subtype of PAD, but we addressed this issue by enrolling patients in stable condition. Fourth, although we adjusted for confounders, including comorbidities, we cannot exclude other reasons that affect an individual’s functional limitation. We incorporated many disease severity measures that were available, but not all patients have LVEF measured or reported at the time of enrollment. Finally, DASI score were measured at only a single time-point, and we were unable to determine if improvement in DASI score can be associated with improvement in short- and long-term prognosis or the impact of intervention can improving DASI score. Despite these limitations, our results demonstrate that simple subjective measures for estimating functional capacity using the DASI questionnaire can be used to identify patients with PAD who are at highest risk for long-term adverse event risk.
CONCLUSIONS
The DASI, a simple self-assessment tool of functional capacity, provides strong independent and incremental prognosis value for long-term adverse event risk in patients with PAD.
Supplementary Material
Acknowledgments
Funding source: This research was supported by grants from the National Institutes of Health and the Office of Dietary Supplements (R01HL103866, P20HL113452, R01DK106000, P01HL076491, P01HL098055, R01HL103931, and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439).
Dr. Hazen reports receiving consultant fees/honoraria from Abbott Diagnostics, Cleveland Heart Lab,Eli Lilly and Co., Esperion, Frantz Biomarkers, Liposciences Inc., Merck, Nestle Holdings, Inc, Pfizer, Procter & Gamble, Siemens Healthcare Diagnostics, Inc, Takeda Global Research & Development Center, Inc.; Ownership interest/partnership/principal-Cleveland Heart Lab, and partially supported by a gift from the Leonard Krieger endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: All other authors have no disclosure to report.
REFERENCES
- 1.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003;163(8):884–892. doi: 10.1001/archinte.163.8.884. [DOI] [PubMed] [Google Scholar]
- 4.Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clement D, Collet JP, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2011;32(22):2851–2906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- 5.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss L, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(14):1555–1570. doi: 10.1016/j.jacc.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114(7):688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. Jama. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 8.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64(10):651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 9.Bairey Merz CN, Olson M, McGorray S, Pakstis DL, Zell K, Rickens CR, et al. Physical activity and functional capacity measurement in women: a report from the NHLBI-sponsored WISE study. J Womens Health Gend Based Med. 2000;9(7):769–777. doi: 10.1089/15246090050147745. [DOI] [PubMed] [Google Scholar]
- 10.Grodin JL, Hammadah M, Fan Y, Hazen SL, Tang WH. Prognostic value of estimating functional capacity with the use of the duke activity status index in stable patients with chronic heart failure. J Card Fail. 2015;21(1):44–50. doi: 10.1016/j.cardfail.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parissis JT, Nikolaou M, Birmpa D, Farmakis D, Paraskevaidis I, Bistola V, et al. Clinical and prognostic value of Duke's Activity Status Index along with plasma B-type natriuretic peptide levels in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2009;103(1):73–75. doi: 10.1016/j.amjcard.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 12.Tang WH, Topol EJ, Fan Y, Wu Y, Cho L, Stevenson C, et al. Prognostic value of estimated functional capacity incremental to cardiac biomarkers in stable cardiac patients. J Am Heart Assoc. 2014;3(5):e000960. doi: 10.1161/JAHA.114.000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch CG, Li L, Lauer M, Sabik J, Starr NJ, Blackstone EH. Effect of functional health-related quality of life on long-term survival after cardiac surgery. Circulation. 2007;115(6):692–699. doi: 10.1161/CIRCULATIONAHA.106.640573. [DOI] [PubMed] [Google Scholar]
- 14.Shaw LJ, Olson MB, Kip K, Kelsey SF, Johnson BD, Mark DB, et al. The value of estimated functional capacity in estimating outcome: results from the NHBLI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol. 2006;47(3 Suppl):S36–S43. doi: 10.1016/j.jacc.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 15.Nelson CL, Herndon JE, Mark DB, Pryor DB, Califf RM, Hlatky MA. Relation of clinical and angiographic factors to functional capacity as measured by the Duke Activity Status Index. Am J Cardiol. 1991;68(9):973–975. doi: 10.1016/0002-9149(91)90423-i. [DOI] [PubMed] [Google Scholar]
- 16.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott MM, Guralnik JM, Tian L, Ferrucci L, Liu K, Liao Y, et al. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;50(10):974–982. doi: 10.1016/j.jacc.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott MM, Tian L, Liu K, Guralnik JM, Ferrucci L, Tan J, et al. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008;51(15):1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain A, Liu K, Ferrucci L, Criqui MH, Tian L, Guralnik JM, et al. The Walking Impairment Questionnaire stair-climbing score predicts mortality in men and women with peripheral arterial disease. J Vasc Surg. 2012;55(6):1662.e2–1673.e2. doi: 10.1016/j.jvs.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg. 1996;23(1):104–115. doi: 10.1016/s0741-5214(05)80040-0. [DOI] [PubMed] [Google Scholar]
- 22.Mahe G, Ouedraogo N, Vasseur M, Faligant C, Saidi K, Leftheriotis G, et al. Limitations of self-reported estimates of functional capacity using the Walking Impairment Questionnaire. Eur J Vasc Endovasc Surg. 2011;41(1):104–109. doi: 10.1016/j.ejvs.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Spruit MA, Maeder MT, Knackstedt C, Ammann P, Jeker U, Uszko-Lencer NH, et al. Prognostic value of self-reported versus objectively measured functional capacity in patients with heart failure: results from the TIME-CHF (Trial of Intensified Versus Standard Medical Therapy in Elderly Patients with Congestive Heart Failure) J Am Coll Cardiol. 2012;60(20):2125–2126. doi: 10.1016/j.jacc.2012.08.968. [DOI] [PubMed] [Google Scholar]
- 24.McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Liao Y, et al. Decline in functional performance predicts later increased mobility loss and mortality in peripheral arterial disease. J Am Coll Cardiol. 2011;57(8):962–970. doi: 10.1016/j.jacc.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain A, Liu K, Ferrucci L, Criqui MH, Tian L, Guralnik JM, et al. Declining walking impairment questionnaire scores are associated with subsequent increased mortality in peripheral artery disease. J Am Coll Cardiol. 2013;61(17):1820–1829. doi: 10.1016/j.jacc.2013.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. Jama. 2013;310(1):57–65. doi: 10.1001/jama.2013.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol. 2011;57(8):1002–1044. doi: 10.1016/j.jacc.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 29.Creager MA, Belkin M, Bluth EI, Casey DE, Jr, Chaturvedi S, Dake MD, et al. 2012 ACCF/AHA/ACR/SCAI/SIR/STS/SVM/SVN/SVS key data elements and definitions for peripheral atherosclerotic vascular disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Peripheral Atherosclerotic Vascular Disease) Circulation. 2012;125(2):395–467. doi: 10.1161/CIR.0b013e31823299a1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.