Abstract
Objective
This study explored whether age moderated cognitive, symptom, and functional changes over a 12-week Compensatory Cognitive Training (CCT) intervention for participants with severe mental illnesses. CCT focused on the cognitive domains of attention, learning, prospective memory, and executive functioning, often impaired in this population.
Methods
Seventy-seven unemployed individuals (46 participants with severe mood disorders and 31 participants with schizophrenia/schizoaffective disorder; mean age=44 years) received CCT for 12 weeks in the context of a supported employment program. Participants were administered cognitive, symptom severity, and functional measures at baseline and 3-, 6-, and 12-month follow-ups, as well as at 18- and 24-months for symptom/functional measures. Mixed effects models, controlling for diagnosis, examined whether age impacted the trajectories of change following CCT.
Results
Analyses showed several significant time by age interactions; younger participants improved more over time on category fluency [β=−.280, t(42.10)=−2.76, p=.008] and financial capacity [UCSD Performance-Based Skills Assessment; β=−.194, t(54.02)=−2.21, p=.031], whereas older participants showed greater reduction in positive symptom severity [Positive and Negative Syndrome Scale; β=−.109, t(78.35)=−2.34, p=.022] and less functional decline on the Independent Living Skills Survey [β=.118, t(109.77)=2.05, p=.043].
Conclusions and Implications for Practice
Age moderated the effects of CCT over time on measures of cognition, symptom severity, and functioning. Younger participants improved on objective measures of verbal processing speed and financial capacity, whereas older participants showed reduced positive symptom severity and less decline in self-reported daily functioning. These findings suggest that CCT may differentially benefit persons with severe mental illnesses depending on age.
Keywords: cognitive remediation, depression, schizophrenia, bipolar disorder
Cognitive impairment is common for individuals with severe mental illnesses and significantly affects real-world functioning (Millan et al., 2012). There is evidence for impaired processing speed, attention/vigilance, working memory, learning, memory, and executive functions in individuals with schizophrenia (Fioravanti et al., 2012, Mesholam-Gately et al., 2009), bipolar disorder (Lee et al., 2013, Bora & Pantelis, 2015), and major depression (Rock et al., 2014). Cognitive impairment is persistent and strongly related to functional disability in individuals living with a severe mental illness (Millan et al., 2012). Even following symptom remission, cognitive impairment compromises real-world functioning and vocational outcomes (Green, Kern, & Heaton, 2004; Bowie et al., 2008; Mora et al., 2013; Depp et al., 2012; Jaeger, Berns, Uzelac, & Davis-Conway,2006; Baune et al., 2010). Due to the prevalence of cognitive impairment in this population as well as the significant impact of cognitive dysfunction on functional outcomes (Millan et al., 2012), there has been an increased number of cognitive intervention trials over the last several years (Anaya et al., 2012; Bowie et al., 2013, 2014a; Fisher et al., 2014; Lee et al., 2013; McGurk, Twamley, Sitzer, McHugo, & Mueser, 2007; Twamley, Jeste, & Bellack, 2003; Wykes et al., 2011).
Meta-analyses of cognitive training with samples of individuals with schizophrenia have found small-to-moderate durable training effects in cognition as well as functioning (McGurk et al., 2007; Wykes et al., 2011). In terms of work outcomes, it has also been shown that cognitive training could enhance the effects of other treatments such as supported employment (SE; Bell et al., 2005, 2008; McGurk, Mueser, DeRosa, & Wolfe, 2009), although some negative results have also been found (Au et al., 2015). The delivery and targets for cognitive training interventions for people living with severe mental illnesses range from computer-based training of a specific cognitive domain (e.g., Fisher et al., 2014) to in-person delivery of compensatory strategies that target multiple cognitive domains and aid the transfer of cognitive gains into the everyday functioning (Twamley et al., 2012; Mendella et al., 2015; Wykes & Reeder, 2005). The current study used Compensatory Cognitive Training (CCT; Twamley et al., 2012), which included four modules of training to address: 1) prospective memory (i.e., remembering to do things in the future), 2) conversational and task vigilance, 3) learning and memory, and 4) cognitive flexibility and problem-solving (i.e., executive functioning). CCT has been shown to improve measures of attention, verbal memory, functional capacity, subjective quality of life, and negative symptom severity in people with primary psychotic disorders (Twamley et al., 2012; Mendella et al., 2015).
Some previous studies have examined the role of age and the relationship with changes following cognitive training (e.g., Kontis et al., 2013; McGurk & Mueser, 2008; Wykes et al., 2009; Twamley, Burton, & Vella, 2011). In a large meta-analysis, age was not found to moderate the effects of cognitive training in schizophrenia but studies included in this meta-analysis were mostly focused in a narrow age range (Wykes et al., 2011). Studies that have directly examined the effects of age on cognitive training outcomes have found largely consistent support for the findings that younger participants (often those below 40 or 45 years old) show greater cognitive improvement after cognitive training than older participants (Kontis et al., 2013; McGurk & Mueser, 2008). One study found that both younger (<40 years old) and older (40+ years old) participants with schizophrenia improved on a measure of memory following cognitive training, but only younger participants showed improvement on measures of cognitive flexibility and planning (Wykes et al., 2009). Conversely, another study that examined who benefits from cognitive training found that older participants with psychosis showed greater improvement in prospective memory at a 6-month follow-up (Twamley et al., 2011). Although younger adults more often show greater improvement on measures of cognition, there is some evidence that older adults can benefit from cognitive training through a reduction in symptom severity (e.g., PANSS Negative scale; McGurk & Mueser, 2008), although, others have not replicated this finding (Wykes et al., 2009). Despite evidence that age may be a key moderator of intervention effects, there is still a scarcity of studies evaluating the relationship between age and response to cognitive training.
The current study examined whether age moderates the effect of time on cognition, symptom severity, and functioning within a sample that has received CCT in the context of supported employment. This study extends the current cognitive training literature related to severe mental illnesses by using age as a continuous variable in hierarchical linear modeling to examine the multiple follow-up visits that extend to two years after study enrollment. Although previous work has examined age as a moderator of response to cognitive training in samples that are largely comprised of participants with a diagnosis of schizophrenia, the current study included a wide range of diagnoses that include schizophrenia, schizoaffective disorder, major depression, and bipolar disorder. Based on previous literature (e.g., McGurk & Mueser, 2008; Twamley et al., 2011; Wykes et al., 2009), we hypothesized that younger participants would show greater cognitive benefit over time following CCT+SE, whereas older participants would report greater reduction in symptom severity. These hypotheses reflect not only previous work in the area of psychiatric illnesses, but also work in normal aging that suggests that younger adults may benefit more from cognitive training and the results may be more readily transferred to other tasks (e.g., Brehmer, Westerberg, & Bachman, 2008).
Methods
Participants
The current study included 77 participants with severe mental illnesses who received 12-week Compensatory Cognitive Training (CCT) in the context of supported employment (SE). These participants were randomized to this condition (CCT+SE) as part of a larger clinical trial (manuscript in preparation). The larger trial also included a condition where participants received SE without CCT; however, only participants who received CCT+SE were included in the current study due to the primary aim examining whether age impacted the long-term change in cognition, symptoms, and functioning within participants who received CCT. The inclusion/exclusion criteria for the larger trial were: (1) DSM-IV diagnosis of a severe mental illness, which were categorized as either schizophrenia-spectrum disorder (schizophrenia and schizoaffective disorders) or severe mood disorders (bipolar and major depressive disorder), (2) unemployed in the previous 30 days but stating a goal of work, (3) age 18 or older, (4) fluency and literacy in English, and (5) no presence of dementia or intellectual disability. The study was approved by the UC San Diego Institutional Review Board and all participants provided informed, written consent prior to beginning the study. Table 1 shows the baseline demographic characteristics of the participants.
Table 1.
Descriptive characteristics (n=77)
| Mean (SD) or % | Range | |
|---|---|---|
| Age, years | 44.43 (11.27) | 24–66 |
|
| ||
| 20–29 years | 11.7% | |
|
| ||
| 30–39 years | 23.4% | |
|
| ||
| 40–49 years | 23.4% | |
|
| ||
| 50–59 years | 39.0% | |
|
| ||
| 60+ years | 2.6% | |
| Education, years | 13.25 (2.70) | 6–18 |
| Male | 55.8% | |
| Racial/Ethnic minority status | 40.3% | |
| Diagnosis | 59.7% Mood disorder 40.3% Schizophrenia-spectrum disorder |
|
| Duration of illness, years | 23.68 (12.96) | 0–58 |
| Substance use in past year | 28.4% | |
| Premorbid IQ | 104.43 (8.10) | 72–118 |
| CCT sessions | 8.23 (4.88) | 0–12 (median=12) |
| Total weeks worked | 18.62 (30.03) | 0–100 |
| Completed study | 76.6% | |
IQ=Intelligence quotient; CCT=Compensatory Cognitive Training; Total weeks worked=number of weeks worked at a competitive job throughout the entire study (104 weeks).
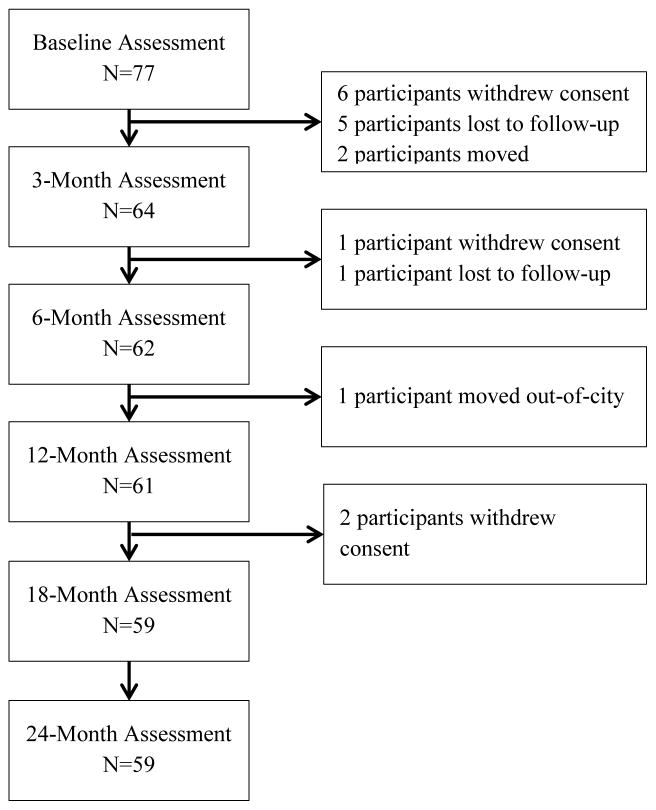
Figure 1 shows the numbers of participants who dropped out and were retained at each assessment. To characterize the selectivity of attrition, study participants with data through the conclusion of the study (retained; n =59) were compared with those participants who dropped out of the study (dropout; n=18). There were no significant differences between those participants who dropped out on measures of age, education, duration of illness, age of onset, premorbid IQ, global neuropsychological deficit score, depressive symptoms, or positive or negative symptom severity. However, schizophrenia-spectrum participants were more likely to drop out than were mood disorder participants (t(75)=2.09, p=0.04). For this reason, diagnosis group was included as a covariate in all analyses.
Figure 1.
Number of CCT+SE participants retained at each study assessment.
Procedure and Measures
Compensatory Cognitive Training (CCT)
CCT is a 12-week, manualized intervention designed to target cognitive domains of prospective memory, conversational and task vigilance, learning and memory, and executive functioning/cognitive flexibility. It focuses on teaching and implementing strategies to work around the cognitive difficulties that are often observed in psychiatric illnesses (Twamley et al., 2012). Consistent with the individualized nature of supported employment, CCT was delivered individually to participants by their employment specialist during the first 12 weeks of the study, whereas the prior study investigated group-based CCT (Twamley et al., 2008; 2012). Each of the 12 CCT sessions is approximately 1 hour. One master’s-level employment specialist administered CCT under supervision of the principal investigator and author. All CCT sessions were audio-recorded and a random 20% of the sessions each month were coded for fidelity.
Supported Employment (SE)
SE is an evidence-based practice for people with psychiatric illnesses who are interested in working. The participants worked individually with an employment specialist for the duration of the study (up to 24 months) with the goal of attaining competitive employment; frequency, duration, and content of all sessions were individualized based on the needs of the participant.
Assessments and Measures
Participants were assessed at Baseline, 3-months (i.e., following completion of the CCT portion of the study), 6-months, 12-months, 18-months, and 24-months. Measures of cognition, functional skills, symptom severity, and quality of life were examined. Cognitive measures and two performance-based measures of functional skills [i.e., University of California, San Diego Performance-Based Skills Assessment (UPSA; Patterson, Goldman, McKibbin, Hughs, & Jeste, 2001a) and Social Skills Performance Assessment (SSPA; Patterson, Moscona, McKibbin, Davidson, & Jeste, 2001b)] were administered at baseline through 12-months, while the symptom severity, Quality of Life Interview (QOLI; Lehman, 1988), and the Independent Living Skills Survey (ILSS; Wallace, Liberman, Tauber, & Wallace, 2000) were administered at every assessment occasion. Table 2 shows all measures that were administered and examined as outcomes as part of this study, baseline means (SD), and baseline to post-intervention (3-month assessment) change scores of the study sample. Cohen’s d is used to show the effect size of pre-to-post CCT+SE intervention change (small=0.2, medium=0.50, larger=0.80; Cohen, 1992).
Table 2.
Baseline scores and pre-to-post intervention change
| Test | Mean (SD) (n=77) | Range | Post-pretest change (n=42) | Cohen’s d |
|---|---|---|---|---|
| Cognitive | ||||
| Trail Making Test, Part Aa | 34.68 (13.81) | 16–85 | −3.78 | −0.57 |
| Trail Making Test, Part Ba | 87.60 (50.64) | 35–300 | −7.71 | −0.50 |
| BACS Symbol-Coding | 45.84 (11.10) | 14–71 | 0.74 | 0.22 |
| Letter Fluency | 36.99 (12.21) | 12–72 | −0.76 | −0.25 |
| Category Fluency | 20.70 (5.10) | 11–36 | −1.39 | −0.64* |
| CPT-IP Mean | 2.65 (0.81) | 0.04–4.15 | 0.08 | 0.40 |
| UM Letter Number Span | 13.67 (3.71) | 5–22 | 1.07 | 0.81* |
| WMS-III Spatial Span | 15.38 (2.97) | 9–22 | −0.09 | −0.09 |
| BVMT-R Immediate recall | 21.58 (7.15) | 2–34 | 3.15 | 1.21* |
| HVLT-R Immediate recall | 26.00 (4.90) | 16–36 | 1.59 | 0.68* |
| MIST | 35.57 (8.27) | 9–48 | 1.71 | 0.46 |
| NAB Mazes | 15.71 (6.62) | 2–26 | 0.93 | 0.42 |
| WCST-64 Total Errorsa | 20.27 (11.14) | 6–49 | −1.85 | −0.44 |
| Global Deficit Scorea | 0.64 (0.65) | 0–3.42 | −0.04 | −0.07 |
| Functional Capacity | ||||
| UPSA Total | 79.16 (10.44) | 43.94–100 | 2.38 | 0.51 |
| Financial subscale | 44.10 (4.61) | 27.27–50 | 0.89 | 0.43 |
| Communication subscale | 35.07 (7.77) | 5.56–50 | 1.49 | 0.37 |
| SSPA Mean | 4.41 (0.63) | 2.19–4.94 | −0.04 | −0.17 |
| ILSS Mean | 0.78 (0.07) | 0.55–0.92 | 0.03 | 0.83* |
| Quality of Life | ||||
| QOLI-Life in General | 4.00 (1.62) | 1–7 | 0.37 | 0.50 |
| Symptom Severity | ||||
| HAM-Da | 12.86 (6.94) | 0–28 | −1.27 | −0.43 |
| PANSS Positivea | 12.29 (5.04) | 7–27 | −0.02 | −0.01 |
| PANSS Negativea | 13.45 (5.12) | 7–26 | 1.29 | 0.55 |
Denotes measures in which lower scores are better. Cohen’s d=the effect size of post-intervention (3-months) minus baseline change (small=0.20, medium=0.50, large=0.80; Cohen, 1992). The sample size of the change score (n=42) differs from that of the HLM analyses (n=77) due to list-wise deletion of the change score approach.
denotes significant change from baseline (p<.05).
BACS=Brief Assessment of Cognition in Schizophrenia; CPT-IP=Continuous Performance Test—Identical Pairs; UM=University of Maryland; WMS-III=Wechsler Memory Scale-3rd edition; BVMT-R=Brief Visual Memory Test-Revised; HVLT-R=Hopkins Verbal Learning Test-Revised; MIST=Memory for Intentions Screening Test; NAB=Neuropsychological Assessment Battery; WCST-64=Wisconsin Card Sorting Test 64-item version; UPSA=University of California, San Diego Performance-Based Skills Assessment; SSPA=Social Skills Performance Assessment; ILSS=Independent Living Skills Survey; QOLI=Quality of Life Interview; HAM-D=Hamilton Depression Rating Scale; PANSS=Positive and Negative Syndrome Scale
Cognitive performance was measured by an extended MATRICS Consensus Cognitive Battery (Nuechterlein et al., 2008). Measures of processing speed included: Trail Making Test (TMT), Part A, Brief Assessment of Cognition in Schizophrenia (BACS) Symbol-Coding, Category Fluency. Measures attention/vigilance included: Continuous Performance Test—Identical Pairs (CPT-IP) and WAIS-III Digit Span Test. Measures of working memory included: WMS-III Spatial Span and University of Maryland (UM) Letter-Number Span. Measures of learning included: Hopkins Verbal Learning Test—Revised (HVLT-R) and Brief Visual Memory Test—Revised (BVMT-R). Measures of executive functioning included: Neuropsychological Assessment Battery (NAB) Mazes, Wisconsin Card Sorting Test 64-item version (WCST-64; Kongs et al., 2000), Part B of the Trail Making Test (TMT), and Letter Fluency (FAS). The Memory for Intentions Screening Test (MIST; Raskin, 2004) examined prospective memory.
Global Deficit Score
The Global Deficit score for each participant was calculated by converting the demographically-corrected T-score on each individual neuropsychological measure into a deficit score based on a five-point scale [T-score ≥40 = 0, no impairment; T-score 35–39=1, mild impairment; T-score 30–34=2, mild-to-moderate impairment; T-score 25–29=3, moderate impairment; T-score 20–24=4, moderate-to-severe impairment; T-score ≤19=5, severe impairment (Blackstone et al., 2012; Carey et al., 2004; Heaton, Miller, Taylor,& Grant, 2004)]. The individual test deficit scores were then averaged to create a Global Deficit Score (GDS) for each individual. Previous studies have found that a GDS cutoff of ≥0.5 to indicate abnormal neuropsychological functioning yields the most optimal balance between sensitivity and specificity (Heaton et al., 2004).
Functional skills were measured by the UPSA (Patterson et al., 2001a), which is a performance-based assessment of everyday functioning capacity. It measures skill performance utilizing props and standardized role-play situations and allowed for the examination of subscales in the domains of communication and financial functioning. The SSPA (Patterson et al., 2001b) is a performance-based measure of social skills requiring role-plays of a neutral and adversarial social situation (i.e., introducing oneself to a new neighbor and calling a landlord regarding a leak that has gone unrepaired after a previous complaint). The Independent Living Skills Survey (ILSS; Wallace et al., 2000) was used to assess self-reported community integration and independence in activities (e.g., hygiene, finances, living situation, and social interactions). Finally, the Quality of Life Interview (QOLI; Lehman, 1988) was used to assess objective indicators and subjective judgments of quality of life in several domains, including living situation, daily activities, family and social relationships, finances, employment, safety, and health. The question asking about satisfaction with life in general was used in these analyses. In addition to functional measures, the total number of weeks worked at a competitive job (i.e., job that makes at least minimum wage and is not set aside for someone with disabilities) was recorded for the duration of the study (up to 104 weeks).
Severity of positive and negative symptoms was assessed using the Positive and Negative Syndrome Scale (PANSS; Kay, Fiszbein, & Opler,1987), which included 30 items, each rated from 1 (for “absent”) to 7 (for “extremely severe”) designed to assess positive and negative symptoms and general psychopathology. Severity of depressive symptoms was measured with the 17-item Hamilton Depression Rating Scale (HAM-D; Hamilton, 1967).
Data Analyses
Hierarchical linear models (HLM) were conducted to examine whether age moderated the changes over time in cognitive, functional, and symptom severity measures for participants who received CCT+SE. Full information maximum likelihood estimation was used to account for missing data, allowing for all available data to be used for parameter estimates (Singer & Willet, 2003). The random effect of intercept for individuals was included in all models. The dummy-coded diagnostic group variable (mood disorder vs. schizophrenia-spectrum disorder) was included as a covariate in all analyses. The use of HLM, full information maximum likelihood, and the inclusion of the diagnosis covariate represents best practice for estimating longitudinal relationships in a sample with selective attrition (Schafer & Graham, 2002), in that results will be less biased than other methods (e.g., listwise deletion or mean replacement).
The age and time variables were modeled as continuous parameters. The time variable included four time-points (baseline, and 3-month, 6-month, and 12-month follow-ups) for the cognitive variables and the UPSA and SSPA measures. For the QOLI, HAM-D, ILSS, and PANSS variables, the time variable included data at 18- and 24-month follow-ups in addition to the baseline through 12-month data. Prior to analyses, the data were transformed into a z-score metric so the resulting effect estimates were comparable.
Given the cumulative nature of the total weeks worked variable, a separate linear regression analysis was used to determine whether age, while controlling for diagnosis, predicted the total number of weeks worked while in the study. Given the non-normality of the total weeks worked variable, this variable was blom-transformed prior to analyses (Blom, 1958).
Results
Table 3 shows the estimates (standard errors) and r-values (small=0.10, medium=0.30, large=0.50; Cohen, 1992) for the main effects of time and age as well as the age by time interactions. There were significant main effects of time for TMT Part A and B, BVMT-R, HVLT-R, NAB Mazes, UPSA, and the Global Deficit Score such that on average, participants improved over time (p<.05). There were significant main effects of age for TMT Parts A and B, BACS Symbol-Coding, Letter Fluency, Category Fluency, CPT-II, Letter-Number Span, Spatial Span, BVMT, MIST, NAB Mazes, WCST-64, UPSA Financial subscale, and Global Deficit Score such that younger participants performed better than older participants (p<.05).
Table 3.
Estimates for effects of time, age, and time x age interactions
| Test | Time | Age | Time x Age | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| β (SE) | p | r | β (SE) | p | r | β (SE) | p | r | |
|
|
|||||||||
| Cognitive | |||||||||
| Trail Making Test, Part Aa | −.272 (.085) | .002 | −.409 | .269 (.096) | .006 | .288 | −.119 (.088) | .183 | −.187 |
| Trail Making Test, Part Ba | −.124 (.057) | .034 | −.334 | .413 (.090) | <.001 | .483 | −.101 (.059) | .096 | −.264 |
| BACS Symbol-Coding | .085 (.068) | .213 | .169 | −.286 (.105) | .008 | −.255 | −.020 (.071) | .780 | −.039 |
| Letter Fluency | .084 (.066) | .205 | .183 | −.240 (.108) | .029 | −.214 | −.024 (.069) | .731 | .051 |
| Category Fluency | .067 (.097) | .492 | .109 | −.318 (.120) | .009 | −.271 | −.280 (.101) | .008 | −.392 |
| CPT-IP | .050 (.062) | .428 | .122 | −.125 (.106) | .244 | −.129 | .103 (.066) | .128 | .239 |
| UM Letter Number Span | .037 (.071) | .600 | .085 | −.298 (.107) | .007 | −.280 | .085 (.073) | .255 | .185 |
| WMS-III Spatial Span | .141 (.087) | .113 | .206 | −.390 (.114) | .001 | −.322 | .020 (.091) | .824 | .030 |
| BVMT-R Immediate recall | .341 (.076) | <.001 | .564 | −.505 (.098) | <.001 | −.493 | −.053 (.079) | .505 | −.103 |
| HVLT-R Immediate recall | .324 (.078) | <.001 | .467 | −.104 (.110) | .349 | −.096 | .149 (.082) | .079 | .231 |
| MIST Summary score | .148 (.078) | .062 | .258 | −.259 (.098) | .009 | −.260 | .043 (.081) | .600 | .075 |
| NAB Mazes | .166 (.068) | .018 | .330 | −.521 (.098) | <.001 | −.490 | −.016 (.071) | .823 | −.033 |
| WCST-64 Total Errorsa | .012 (.076) | .879 | .021 | .254 (.111) | .025 | .231 | .001 (.079) | .992 | .000 |
| Global Deficit Scorea | −.111 (.046) | .020 | −.358 | .350 (.101) | .001 | .345 | −.075 (.048) | .125 | −.241 |
| Functional Capacity | |||||||||
| UPSA Total | .200 (.098) | .047 | .255 | −.180 (.105) | .090 | −.195 | −.106 (.103) | .307 | −.136 |
| Financial subscale | .086 (.084) | .314 | .135 | −.209 (.089) | .022 | −.276 | −.194 (.087) | .031 | −.288 |
| Communication subscale | .204 (.115) | .083 | .214 | −.108 (.108) | .319 | −.119 | −.003 (.120) | .981 | −.003 |
| SSPA Mean | −.037 (.074) | .627 | −.067 | −.057 (.100) | .574 | −.058 | .048 (.077) | .539 | .085 |
| ILSS Mean | −.105 (.054) | .056 | −.185 | −.071 (.082) | .394 | −.103 | .118 (.058) | .043 | .192 |
| Quality of Life | |||||||||
| QOLI-Life in General | .009 (.046) | .843 | .020 | −.060 (.107) | .579 | −.062 | .021 (.050) | .672 | .042 |
| Symptom Severity | |||||||||
| HAM-Da | −.075 (.053) | .161 | −.132 | .031 (.108) | .773 | .032 | −.111 (.058) | .056 | −.178 |
| PANSS Positivea | −.031 (.043) | .478 | −.082 | −.115 (.108) | .290 | −.116 | −.109 (.047) | .022 | −.255 |
| PANSS Negativea | .053 (.050) | .289 | .109 | .062 (.091) | .500 | .077 | −.073 (.054) | .180 | −.135 |
Denotes measures in which lower scores are better.
BACS=Brief Assessment of Cognition in Schizophrenia; CPT-IP=Continuous Performance Test—Identical Pairs; UM=University of Maryland; WMS-III=Wechsler Memory Scale-3rd edition; BVMT-R=Brief Visual Memory Test-Revised; HVLT-R=Hopkins Verbal Learning Test-Revised; MIST=Memory for Intentions Screening Test; NAB=Neuropsychological Assessment Battery; WCST-64=Wisconsin Card Sorting Test 64-item version; UPSA=University of California, San Diego Performance-Based Skills Assessment; SSPA=Social Skills Performance Assessment; ILSS=Independent Living Skills Survey; QOLI=Quality of Life Interview; HAM-D=Hamilton Depression Rating Scale; PANSS=Positive and Negative Syndrome Scale
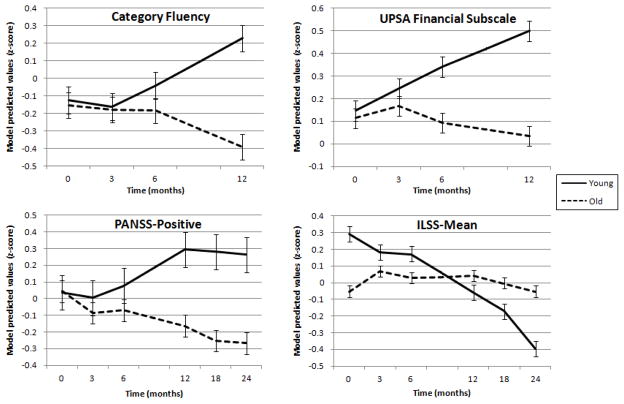
To examine whether age moderated changes over time, the age by time interactions were analyzed. Significant age by time interaction results are shown in Figure 2 using a median split of age (median age = 45 years old) to best display the differential trajectories. Category Fluency had a significant age by time interaction, β=−.280, t(42.10)=−2.764, p=.008, such that younger participants improved more over time, while older participants stayed the same or had slight decline. Similarly, on the Financial subscale of the UPSA, younger participants also improved over time while older participants were stable or had minimal decline, β=−.194, t(54.02)=−2.214, p=.031. On the other hand, for the ILSS, older participants were relatively stable over time in self-reported daily functioning, while younger participants reported more decline, β=.118, t(109.77)=2.046, p=.043. On the PANSS Positive measure, older participants reported fewer positive symptoms and younger participants reported more positive symptoms over time, β=−.109, t(78.35)=−2.336, p=.022. There were also trends for older participants improving more on TMT, Part B (p=.096) and at a faster rate on the HVLT (p=.073) and reporting greater depressive symptom reduction on the HAM-D (p=.056), particularly from baseline to the 3-month follow up (post-test). The other cognitive, functional, and symptoms measures did not show differential change over time by age (p>.05).
Figure 2.
Age trajectories, using median split (young=less than 45 years old; old= 45 years old or older), of significant age x time interaction results. UPSA=University of California, San Diego Performance-Based Skills Assessment; PANSS=Positive and Negative Syndrome Scale; ILSS=Independent Living Skills Survey. Error bars=standard error of mean.
The results of the linear regression examining whether age predicted total weeks worked showed that after controlling for diagnosis, age did not significantly predict the number of weeks worked in participants who had compensatory cognitive training, β=−.167, t(63)=−1.35, p=.181.
Discussion
Determining the characteristics of who is most likely to benefit from cognitive training is important to being able to adapt training programs to the individual in order to optimize cognitive performance, reduce symptom severity, and improve functional outcomes. The current study examined how age may impact the 12-month trajectories of cognitive change and 24-month trajectories of symptom and functional change following CCT in the context of supported employment.
While, as expected, there were a number of significant main effects of time and age, there were relatively few measures where age modified changes over time. In general, the results were mixed, such that younger adults showed statistically significant improvement on objective measures of verbal processing speed and financial capacity, whereas older participants demonstrated less severe positive symptoms and reported less decline in daily functioning. Older participants had trend level improvements on verbal learning and cognitive switching relative to younger participants and reported greater depressive symptom reduction.
Specifically, regarding objective, performance-based cognitive and functional capacity measures, the trajectories of Category Fluency and UPSA financial performance were significantly impacted by age, with younger participants performing better over time. Of note, the Global Deficit Score had significant main effects of time and age such that over time, participants had fewer deficits and, on average, older participants had more deficits. However, age did not significantly impact the magnitude of reduction in global cognitive deficits following CCT. The finding for largely minimal effects of age on changes in cognition after cognitive training are in line with previous a meta-analysis, which did not show age to be a significant moderator of cognitive training benefit in schizophrenia (Wykes et al., 2011). The significant finding of greater benefit in younger participants on a verbal processing speed task is consistent with individual studies that show greater cognitive improvement in younger participants following cognitive training (McGurk & Mueser, 2008; Wykes et al., 2009). However, on a measure of verbal learning (HVLT-R) and cognitive switching (TMT, Part B), there were trend-level interactions (p<.1) such that older participants showed greater improvement over time. Regarding objective financial capacity, it is possible that younger participants had less experience with managing money, so by learning skills that are potentially important for financial capacity (e.g., planning, attention strategies) as well as gaining experience handling money (if they obtained work during the course of the study) they were able to improve more in the objective financial capacity domain while older participants remained fairly stable over time.
It is important to consider whether the age variable in this analysis is a proxy-variable for duration of illness. Previous work has shown that even after controlling for age, shorter duration of illness predicts greater improvement on several cognitive measures after cognitive remediation for schizophrenia, including measures of processing speed (Bowie et al., 2014b). On the other hand, the current findings are consistent with the work of Mueser and colleagues (2010), which demonstrated the importance of age and diagnosis to the cognitive and social functioning in a psychiatric population. Thus, there may be a neurobiological explanation for greater cognitive improvement in younger participants. Younger participants may benefit more from cognitive training as a result of greater neural plasticity potential relative to older participants (Fisher et al., 2014), and older participants may benefit less as a result of normal age-related cognitive slowing or poorer learning potential (Fernandez-Ballesteros et al., 2012; Wykes et al., 2009). However, it is important to note that age did not affect response to CCT for the majority of cognitive and functional capacity outcomes. Therefore, compensatory approaches to cognitive training may benefit both younger and older individuals, particularly on aspects of cognition that had a significant main effect of time (e.g., processing speed, learning, reasoning and cognitive set shifting).
Regarding self-reported functioning and symptom severity outcome measures, older participants reported fewer declines in daily functioning on the ILSS and greater reduction in positive symptom severity on the PANSS following CCT, and a trend toward greater reduction in depressive symptom severity. Although the current study used age as a continuous variable, the results are consistent with the findings of McGurk & Mueser (2008), who dichotomized age and demonstrated that younger participants with severe mental illnesses have more cognitive improvement following cognitive training, whereas older participants show greater symptom reduction. Further research is needed to determine the mechanism of greater positive symptom reduction and trend toward depressive symptom reduction in older participants. However, it is possible that CCT provided strategies to improve prospective memory, which in turn, may improve real-world medication management and adherence. Thus, older participants who may have been more at-risk for forgetting their medications may remember to take their psychiatric medications more consistently after learning compensatory strategies (e.g., setting alarms, linking tasks, putting a pill box in an obvious place). Additionally, it is possible that older adults used cognitive flexibility skills to challenge and work through unusual experiences. Given the cognitive symptoms of depression, the trend for reduced depressive symptoms may be at least partially driven by the trend for improved cognition (verbal learning, switching). Future work should examine whether the changes in symptom severity following CCT+SE in older participants are the mechanism driving the greater improvement in self-reported daily functioning (ILSS).
Our study had the advantage of following participants who received CCT in the context of supported employment for up to two years post-study enrollment, which allowed for the modeling of longitudinal trajectories using hierarchical linear models. Thus, although implicit in these analyses are the baseline to post-intervention effects, our goal was to examine the long-term outcomes, or what happened after the intervention ends, and how age impacted these outcomes. Additionally, the current sample expanded on previous cognitive training literature by including a diverse range of diagnoses that was well-balanced between schizophrenia-spectrum and mood disorder participants.
Although there are a number of strengths of the study, there are also key limitations. Specifically, this analysis did not include a control group that did not receive CCT; therefore, it is difficult to discern what changes are directly related to the CCT and what changes may be better explained by practice effects. All participants also received supported employment, which may limit the interpretation of the findings as the additional support of the employment specialist may have contributed to improved cognition and/or symptoms. Additionally, because this was an exploratory study, a large number of dependent variables was examined in order to best understand how age impacts trajectories of these outcomes over 12- and 24-months, and we did not apply an alpha correction. Thus, our results should be considered preliminary until replicated.
The results of this study suggest that age had a relatively minor role in how it impacted the changes following CCT+SE. However, younger participants showed greater improvement over 12-months on objective measures of verbal processing speed and financial capacity. On the other hand, older participants showed greater positive symptom reduction and less decline in self-reported daily function over 24-months, with trends toward improved verbal learning, cognitive switching and depressive symptoms. Thus, while all participants improved on measures of processing speed, learning, reasoning and cognitive switching, there may be differential mechanisms for improvement for younger and older participants. Another question that remains is how compensatory cognitive training designed to target real-world functioning differs from other cognitive training platforms that use extensive practice of specific cognitive domains. The overall beneficial effects of CCT on older participants, especially in symptoms and functioning, raises the question of whether a compensatory skills training approach might be more effective for older participants than one focusing on extensive practice of cognitive skills. Future work should examine the mechanisms of improvement and how duration of illness/neurobiological changes, reduction in symptom severity, and type of cognitive intervention (e.g., compensatory vs. restorative) may differentially affect cognitive outcomes for younger and older participants with severe mental illnesses.
Acknowledgments
This work was funded by the National Institute of Mental Health (R01 MH080150 to EWT). The authors also thank the Alicia Koplowitz Foundation (FAK).
Footnotes
Disclosures: none
References
- Anaya C, Aran AM, Ayuso-Mateos JL, Wykes T, Vieta E, Scott J. A systematic review of cognitive remediation for schizo-affective and affective disorders. Journal of affective disorders. 2012;142(1):13–21. doi: 10.1016/j.jad.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Au DW, Tsang HW, So WW, Bell MD, Cheung V, Yiu MG, … Lee GTH. Effects of integrated supported employment plus cognitive remediation training for people with schizophrenia and schizoaffective disorders. Schizophrenia research. 2015;166(1):297–303. doi: 10.1016/j.schres.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Baune BT, Miller R, McAfoose J, Johnson M, Quirk F, Mitchell D. The role of cognitive impairment in general functioning in major depression. Psychiatry Research. 2010;176(2):183–189. doi: 10.1016/j.psychres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Bell MD, Bryson GJ, Greig TC, Fiszdon JM, Wexler BE. Neurocognitive enhancement therapy with work therapy: productivity outcomes at 6-and 12-month follow-ups. Journal of Rehabilitation Research & Development. 2005;42(6):829–838. doi: 10.1682/jrrd.2005.03.0061. [DOI] [PubMed] [Google Scholar]
- Bell MD, Zito W, Greig T, Wexler BE. Neurocognitive enhancement therapy with vocational services: work outcomes at two-year follow-up. Schizophrenia research. 2008;105(1):18–29. doi: 10.1016/j.schres.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, … Heaton RK. Defining Neurocognitive Impairment in HIV: Deficit Scores versus Clinical Ratings. The Clinical Neuropsychologist. 2012;26(6) doi: 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom G. Statistical estimates and transformed beta variables. New York, NY: John Wiley & Associates; 1958. [Google Scholar]
- Bora E, Pantelis C. Meta-analysis of cognitive impairment in first-episode bipolar disorder: Comparison with first-episode schizophrenia and healthy controls. Schizophrenia bulletin. 2015;41(5):1095–1104. doi: 10.1093/schbul/sbu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Grossman M, Gupta M, Oyewumi L, Harvey PD. Cognitive remediation in schizophrenia: efficacy and effectiveness in patients with early versus long-term course of illness. Early intervention in psychiatry. 2014b;8(1):32–38. doi: 10.1111/eip.12029. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Gupta M, Holshausen K, Jokic R, Best M, Milev R. Cognitive remediation for treatment-resistant depression: effects on cognition and functioning and the role of online homework. The Journal of nervous and mental disease. 2013;201(8):680–685. doi: 10.1097/NMD.0b013e31829c5030. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biological psychiatry. 2008;63(5):505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for schizophrenia: effects on cognition, functional competence, and real-world behavior. The American Journal of Psychiatry. 2014a;169(7):710–718. doi: 10.1176/appi.ajp.2012.11091337. [DOI] [PubMed] [Google Scholar]
- Brehmer Y, Westerberg H, Bäckman L. Working-memory training in younger and older adults: training gains, transfer, and maintenance. Frontiers in human neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26(3):307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Depp CA, Mausbach BT, Harmell AL, Savla GN, Bowie CR, Harvey PD, Patterson TL. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar disorders. 2012;14(3):217–226. doi: 10.1111/j.1399-5618.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ballesteros R, Botella J, Zamarrón MD, Molina MÁ, Cabras E, Schettini R, Tárraga L. Cognitive plasticity in normal and pathological aging. Clinical Interventions in Aging. 2012;7:15–25. doi: 10.2147/CIA.S27008. http://doi.org/10.2147/CIA.S27008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC psychiatry. 2012;12(1):64. doi: 10.1186/1471-244X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Loewy R, Carter C, Lee A, Ragland JD, Niendam T, … Vinogradov S. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophrenia bulletin. 2014;41(1):250–258. doi: 10.1093/schbul/sbt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia research. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. The British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically adjusted for neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- Jaeger J, Berns S, Uzelac S, Davis-Conway S. Neurocognitive deficits and disability in major depressive disorder. Psychiatry research. 2006;145(1):39–48. doi: 10.1016/j.psychres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test-64 Card Version. Lutz, FL: Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- Kontis D, Huddy V, Reeder C, Landau S, Wykes T. Effects of age and cognitive reserve on cognitive remediation therapy outcome in patients with schizophrenia. The American Journal of Geriatric Psychiatry. 2013;21(3):218–230. doi: 10.1016/j.jagp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. The American Journal of Psychiatry. 2013;170(3):334–341. doi: 10.1176/appi.ajp.2012.12040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RSC, Redoblado-Hodge MA, Naismith SL, Hermens DF, Porter MA, Hickie IB. Cognitive remediation improves memory and psychosocial functioning in first-episode psychiatric out-patients. Psychological medicine. 2013;43(06):1161–1173. doi: 10.1017/S0033291712002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman AF. A quality of life interview for the chronically mentally ill. Evaluation and program planning. 1988;11(1):51–62. [Google Scholar]
- Mendella PD, Burton CZ, Tasca GA, Roy P, Louis LS, Twamley EW. Compensatory cognitive training for people with first-episode schizophrenia: Results from a pilot randomized controlled trial. Schizophrenia research. 2015;162(1):108–111. doi: 10.1016/j.schres.2015.01.016. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Mueser KT. Response to cognitive rehabilitation in older versus younger persons with severe mental illness. American Journal of Psychiatric Rehabilitation. 2008;11(1):90–105. [Google Scholar]
- McGurk SR, Mueser KT, DeRosa T, Wolfe R. Work, recovery, and comorbidity in schizophrenia: A randomized controlled trial of cognitive remediation. Schizophrenia Bulletin. 2009;35(2):319–335. doi: 10.1093/schbul/sbn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. The American journal of psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, … Young LJ. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nature reviews Drug discovery. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Mora E, Portella MJ, Forcada I, Vieta E, Mur M. Persistence of cognitive impairment and its negative impact on psychosocial functioning in lithium-treated, euthymic bipolar patients: a 6-year follow-up study. Psychological medicine. 2013;43(06):1187–1196. doi: 10.1017/S0033291712001948. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Pratt SI, Bartels SJ, Forester B, Wolfe R, Cather C. Neurocognition and social skill in older persons with schizophrenia and major mood disorders: An analysis of gender and diagnosis effects. Journal of Neurolinguistics. 2010;23:297–317. doi: 10.1016/j.jneuroling.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, … Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. The American Journal of Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophrenia bulletin. 2001a;27(2):235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV. Social skills performance assessment among older patients with schizophrenia. Schizophrenia research. 2001b;48(2):351–360. doi: 10.1016/s0920-9964(00)00109-2. [DOI] [PubMed] [Google Scholar]
- Raskin S. Memory for intentions screening test. Journal of the International Neuropsychological Society. 2004;10(Suppl 1):110. [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychological medicine. 2014;44(10):2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. doi: 10.1037/1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Twamley EW, Burton CZ, Vella L. Compensatory cognitive training for psychosis: who benefits? who stays in treatment? Schizophrenia bulletin. 2011;37(suppl 2):S55–S62. doi: 10.1093/schbul/sbr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Jeste DV, Bellack AS. A review of cognitive training in schizophrenia. Schizophrenia bulletin. 2003;29(2):359–382. doi: 10.1093/oxfordjournals.schbul.a007011. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Savla GN, Zurhellen CH, Heaton RK, Jeste DV. Development and pilot testing of a novel compensatory cognitive training intervention for people with psychosis. American journal of psychiatric rehabilitation. 2008;11(2):144–163. doi: 10.1080/15487760801963678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Vella L, Burton CZ, Heaton RK, Jeste DV. Compensatory Cognitive Training for Psychosis: Effects in a Randomized Controlled Trial. The Journal of Clinical Psychiatry. 2012;73(9):1212–1219. doi: 10.4088/JCP.12m07686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace CJ, Liberman RP, Tauber R, Wallace J. The Independent Living Skills Survey: A comprehensive measure of the community functioning of severely and persistently mentally ill individuals. Schizophrenia bulletin. 2000;26(3):631–658. doi: 10.1093/oxfordjournals.schbul.a033483. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. American Journal of Psychiatry. 2011;68(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C. Cognitive remediation therapy for schizophrenia: Theory and practice. Taylor & Francis; Sussex, UK: Routledge; 2005. [Google Scholar]
- Wykes T, Reeder C, Landau S, Matthiasson P, Haworth E, Hutchinson C. Does age matter? Effects of cognitive rehabilitation across the age span. Schizophrenia research. 2009;113(2):252–258. doi: 10.1016/j.schres.2009.05.025. [DOI] [PubMed] [Google Scholar]