Abstract
Liver kinase B1 (LKB1) is mutationally inactivated in Peutz-Jeghers syndrome and in a variety of cancers including human papillomavirus (HPV)-caused cervical cancer. However, the significance of LKB1 mutations in cervical cancer initiation and progress has not been examined. Herein, we demonstrated that, in mouse embryonic fibroblasts, loss of LKB1 and transduction of HPV16 E6/E7 had an additive effect on constraining cell senescence while promoting cell proliferation and increasing glucose consumption, lactate production, and ATP generation. Knock-down of LKB1 increased and ectopic expression of LKB1 decreased glycolysis, anchorage-independent cell growth, and cell migration and invasion in HPV transformed cells. In the tumorigenesis and lung metastasis model in syngeneic mice, depletion of LKB1 markedly increased tumor metastatic colonies in lungs without affecting subcutaneous tumor growth. We showed that HPV16 E6/E7 enhanced the expression of hexokinase-ll (HK-II) in the glycolytic pathway through elevated c-MYC. Ectopic LKB1 reduced HK-II along with glycolysis. The inverse relationship between HK-II and LKB1 was also observed in normal and HPV-associated cervical lesions. We propose that LKB1 acts as a safeguard against HPV-stimulated aerobic glycolysis and tumor progression. These findings may eventually aid in the development of therapeutic strategy for HPV-associated malignancies by targeting cell metabolism.
Keywords: human papillomavirus (HPV), liver kinase B1 (LKB1), hexokinase-ll (HK-II), glycolysis, c-MYC, cervical cancer
Introduction
Human papillomaviruse (HPV) infection is a causative factor for cervical cancer.1 The molecular basis for HPV carcinogenesis has been extensively investigated.2 The “high-risk (HR)” HPV E7 and E6 target tumor suppressor pRB, p53, and many other host proteins for inactivation or degradation, resulting in the abrogation of cell cycle regulation and apoptosis and in the induction of genome instability.2-4 However, infection by HPV alone is not sufficient to cause cervical cancer, as HPV oncogenes could immortalize, but do not transform human epithelial cells, the natural virus host.2 Additional factors must contribute to the progression of HPV-infected lesions to cancer. Indeed, current data indicate that a variety of cofactors, such as immune status, hormones, recurring genetic alterations, nutritional status, environmental carcinogens, or co-infection with other sexually-transmitted agents, were involved in HPV-induced transformation and carcinogenesis.2,5,6
Liver kinase B1 (LKB1), also known as serine/threonine kinase 11 (STK11), is a tumor suppressor. The gene is mutationally inactivated in Peutz-Jeghers syndrome and in sporadic cancers, such as non-small cell lung cancer (NSCLC), intestinal, ovarian, breast, pancreatic cancer and melanoma.7-10 A recent report showed that LKB1 was somatically mutated in 20% of cervical carcinomas.11 However, the significance of LKB1 mutations in cervical cancer is not always understood.
Strong evidence suggests that an increased dependence on glycolysis provides ATP as well as metabolic intermediates for cancer cell proliferation and tumor development although it remains inconclusive whether the metabolic alterations drive tumorigenesis or are an outcome of transformation.12,13 Several groups have reported glucose metabolism alterations in cervical carcinoma with an increase in lactate dehydrogenase (LDH).14-16 High lactate levels predict the likelihood of metastases, tumor recurrence, and decreased survival in human cervical cancer patients17. These observations strongly suggest that cervical cancer is glycolytic. On the other hand, it remains unknown how HPV initiates the metabolic shift from oxidative phosphorylation to glycolysis, nor is it clear whether this shift contributes to the transformation and cancer progression induced by HPV.
In this study, we demonstrated that E6/E7 expression induced aerobic glycolysis. This induction was mediated by c-MYC, which elevated hexokinase-II (HK-II), the rate-limiting enzyme responsible for the first step in the glycolytic pathway. We also showed that LKB1 inhibited the expression of c-MYC and hexokinase-II, thereby suppressing aerobic glycolysis induced by HPV16 E6/E7. Furthermore, our results indicate LKB1 curtails the activities of HPV oncogenes through the inhibition of aerobic glycolysis.
Results
Loss of LKB1 collaborates with HPV16 E6/E7 to reduce senescence
To evaluate the effect of LKB1 on the growth properties of HPV-expressing cells, we deleted LKB1 in MEFs (passage 2) harboring a conditional knockout in the Stk11 gene (LKB1fl/fl) by infection with lentiviruses expressing the Cre recombinase (Supplementary Figure 1). The MEFs were then additionally infected with lentiviruses expressing HPV16 E6/E7. Senescence is a mechanism used by the cells to prevent transformation and over-growth.18,19 Up to 70% of the wild type MEFs entered into senescence at passage 12 (Supplementary Figure 2). In agreement with our recent study,20 HPV oncogene expression reduced senescent cells. Consistent with a previous report that deletion of LKB1 alone could not immortalize MEFs,21 LKB1 knockout had only a marginal impact on cellular senescence. However, HPV16 E6/E7-transduced LKB1-null MEFs continued to grow beyond crisis, which normally occurred at passages 9-11, and had substantially reduced senescent cell population as compared with MEFs lacking LKB1 or expressing HPV16 E6/E7 (Supplementary Figure 2). Thus, the loss of LKB1 acts synthetically with HPV oncogenes to overcome senescence.
Loss of LKB1 promotes migration, invasion and anchorage-independent growth of HPV16-transformed cells in vitro
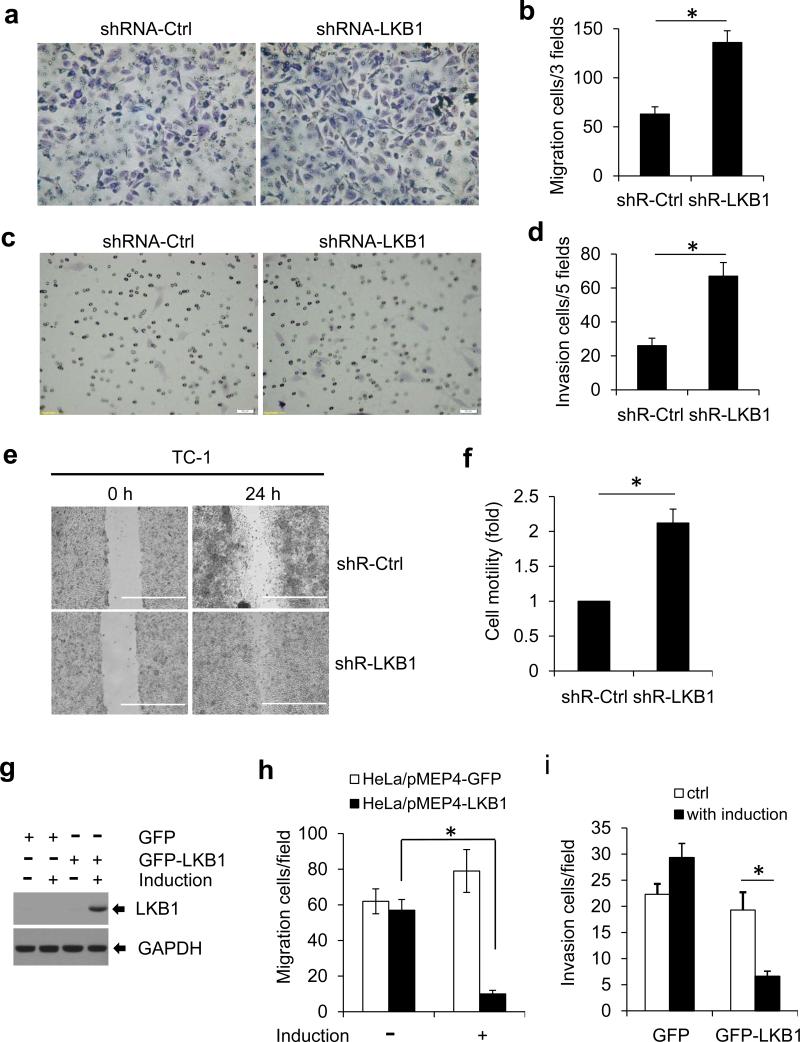
To determine whether LKB1 bears a direct impact on HPV-induced transformed phenotypes, we measured the migration and invasion of TC-1 cells22 after LKB1 knock-down with shRNA. Depletion of LKB1 markedly increased cell migration in the trans-well assay (Figure 1a). The migrated cell number was twice as many as the control cells (shRNA-Ctrl) (Figure 1b). Similarly, LKB1 knock-down increased the ability of TC-1 cells to pass through the Matrigel membrane as shown in Figure 1c and d. In an alternative wound healing (scratch) assay, LKB1-depleted TC-1 cells migrated more rapidly to close the wound than LKB1-intact cells did (Figures 1e and f).
Figure 1.
LKB1 inhibits cell migration and invasion. (a-d) 2×104 TC-1/shR-Ctrl or TC-1/shRLKB1 cells were seeded for migration assay using the Transwell Membrane Inserts (a and b) and for invasion assay with the pre-coated Matrigel Chambers (c and d). The cells were stained with 1% crystal violet and counted after 24 hours. (e) Wound-healing (scratch) assay was performed with TC-1/shR-Ctrl and TC-1/shR-LKB1 cells. Cells were photographed at 0 h and 24 h. (f) Quantification was carried out by measuring the migration distance compared with the controls. Data are mean ± S.D. from 3 independent experiments (*P < 0.01). (g-I) Ectopic expression of LKB1 inhibits cervical cancer cell migration and invasion. HeLa cells with inducible expression of LKB1 or GFP were established and designated as HeLa/pMEP4-GFP (control) or HeLa/pMEP4-LKB1. (g) Expression of LKB1 after induction with 100 μM zinc sulfate was confirmed by Western blot. (h and i) Migration and invasion assays were conducted as described above except that 100 μM zinc sulfate were added to the bottom well as a chemoattractant. The cells were stained with 1% crystal violet and counted after 24 hours. The data in panels b, d, h, and i represent mean ± S.D. of 3 independent experiments, each performed in duplicate (*P < 0.01).
The ability of transformed cells to grow in the absence of anchorage to the extracellular matrix correlates with their invasive and metastatic potential.23 Thus, we asked if LKB1 deficiency contributes to the transformed properties of cells expressing the HPV16 oncogenes. C33A cells, an LKB1-positive cervical cancer cell line devoid of HPV,24 were transduced with shRNA-LKB1 and then infected with lentiviruses expressing HPV16 E6/E7 or GFP (control). The cells were seeded in soft agar for anchorage-independent growth. The results showed that C33A cells expressing HPV16 E6/E7 or with LKB1 knock-down formed more and larger colonies as compared with the parental cells (Supplementary Figure 3). Cells with both LKB1 knock-down and HPV16 E6/E7 expression displayed a further increase in colony numbers and sizes (Supplementary Figure 3). Together, these results suggest that LKB1 dampens HPV-induced transformed phenotypes.
Ectopic expression of LKB1 suppresses the migration and invasion of HPV18-positive HeLa cells
HeLa is an HPV18 positive cervical cancer cell line and is deficient in endogenous LKB1.11,25,26 To validate the role of LKB1 in cervical cancer cells, we stably transduced HeLa cells with pMEP4/GFP-LKB1 or pMEP4/GFP. The expression of the transgenes was induced by zinc sulfate (Figure 1g).24 Upon induction, HeLa/pMEP4/GFP-LKB1 cells that migrated through the trans-well or invaded into Matrigel were both substantially reduced relative to HeLa/pMEP4/GFP cells (Figures 1h and i). In the absence of induction, the two derivative cell lines exhibited no appreciable differences in these properties. Thus, re-introduction of LKB1 suppresses HPV-induced transformed properties. These results corroborate the above observations (Supplementary Figures 2 and 3) that reduction of LKB1 in HPV-containing cells contributed to their transformed phenotypes.
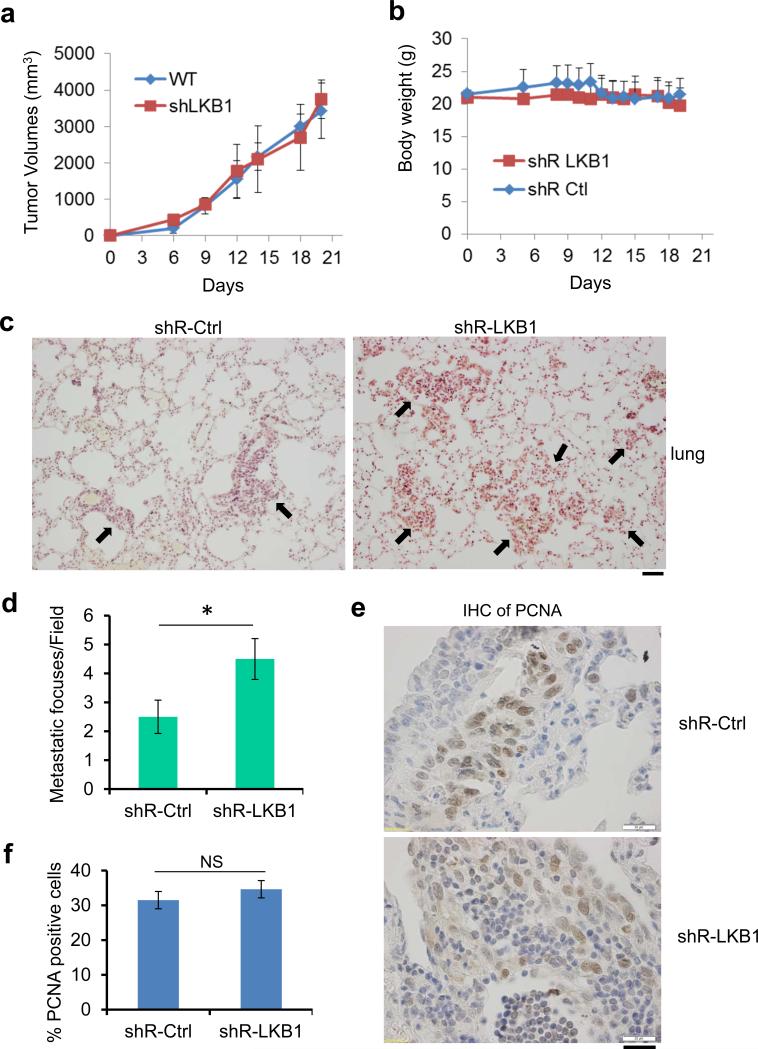
Loss of LKB1 promotes HPV16 E6/E7-mediated tumor metastasis
To examine the influence of LKB1 on HPV-positive tumors in vivo, we derived TC-1 cells in which LKB1 gene was knocked down with shRNA (Supplementary Figure 4) and compared their tumorigenesis and metastasis in syngeneic mice relative to the parental cells transduced with control shRNA. Interestingly, the LKB1 status did not influence subcutaneous tumor growth (Figure 2a), body weight (Figure 2b), nor eating habit (data not shown). However, knock-down of LKB1 markedly promoted lung metastasis upon injection of the cells into the tail vein (Figure 2c). The average number of metastatic foci was increased by 80% in LKB1-deficient group within a 3-week observation period (Figure 2d). 30-35% of the tumor cells were positive for the proliferating cell nuclear antigen (PCNA) in LKB1-intact or LKB1-compromised tumors (Figures 2e and f). Thus, tumor cell proliferative rates in the lung metastatic foci were similar regardless the LKB1 status. The larger foci of LKB1 knockdown cells relative to LKB1-intact cells would suggest earlier seeding and initiation of proliferation.
Figure 2.
Lack of LKB1 promotes lung metastasis of TC-1 cells. (a and b) 2×106 TC-1/shR-Ctrl or TC-1/shR-LKB1 cells were inoculated subcutaneously into the right flank of 6 to 8 week-old female C57BL/6 mice (n=10 each). Tumor size (a) and body weight (b) of the mice were measured every three days for 3 weeks. (c and d) 2×105 TC-1/shR-Ctrl or TC-1/shR-LKB1 cells were injected into each C57BL/6 mouse via tail vein (n = 10 each). Lung tumor colonies were analyzed 3 weeks post-injection. (c) Representative histological images revealed by hematoxylin and eosin staining are shown. Arrows indicate the metastatic foci. Scale bar = 50 μm. (d) Average metastatic foci in each field, at least 5 fields from each mouse were calculated (*P < 0.01, n = 10). (e) Cell proliferation marker, PCNA, was detected in the lung tumor foci with an antibody. Representative images were shown. Scale bar = 20 μm. (f) Percentage of PCNA positive cells in lung tumors with or without LKB1 expression. NS, not significant (n = 200).
LKB1 suppresses HPV-induced glycolysis
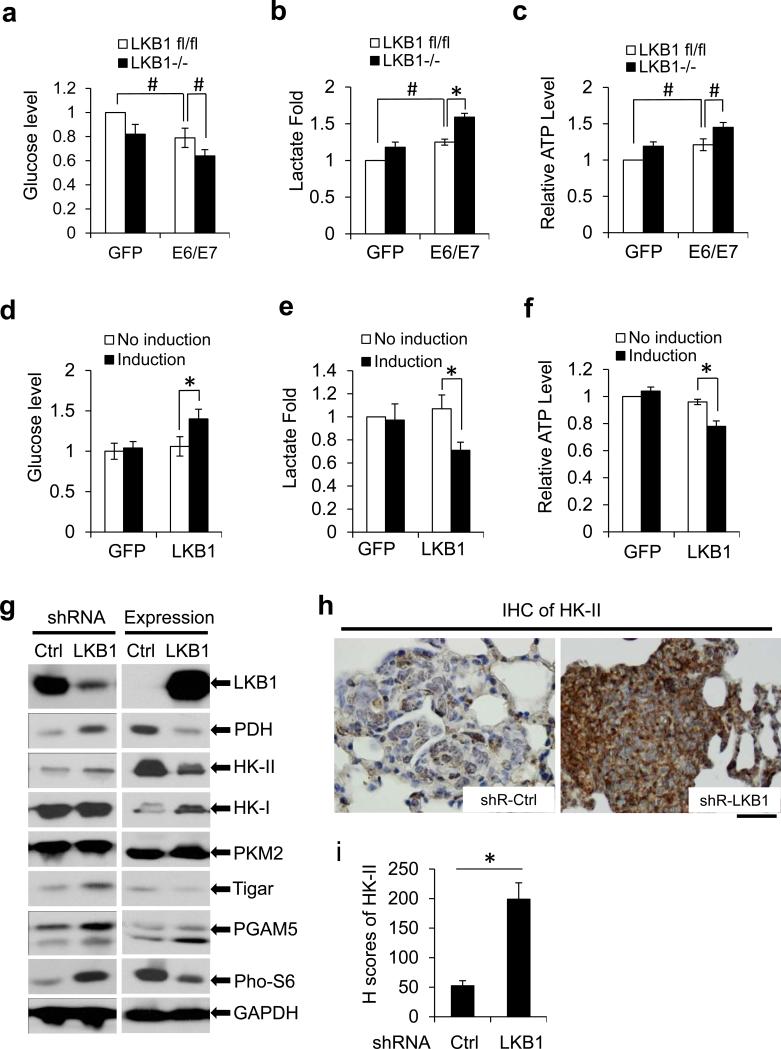
Previous studies showed that glucose metabolism is altered in cervical carcinomas and that HPV transformed cells have increased LDH.14-17 To determine whether LKB1 might counter HPV-mediated oncogenic activity through the reduction of glycolytic metabolism, we measured several standard indicators in MEF cells with different statuses in LKB1 and HPV16 E6/E7. Relative to GFP-transduced MEFs, HPV16 E6/E7-transduced MEFs possessed a reduced level of glucose in culture medium, indicative of increased glucose consumption (Figure 3a). Furthermore, the E6/E7-expressing MEFs had an elevated lactate production and ATP level, consistent with an increase of glycolysis (Figures 3b and c). Interestingly, LKB1−/− MEFs also exhibited an increase in glucose consumption (Figure 3a), and in lactate and ATP levels (Figures 3b and c). The combination of LKB1 deletion and HPV16 oncogene expression had an additive effect on glucose consumption and on these cellular metabolites (Figures 3a-c). To validate the action of LKB1 on HPV-mediated cellular glycolysis, we examined metabolites in HeLa cells with ectopic, inducible GFP-LKB1 or GFP. Induction of LKB1 resulted in an increase in glucose in the culture medium, and an appreciable reduction in lactate and ATP production relative to HeLa (GFP) cells (Figures 3d-f). These data show that LKB1 suppresses HPV oncogene-induced glycolysis.
Figure 3.
Deletion of LKB1 promotes the expression of HK-II and aerobic glycolysis. (a-c) Metabolites in primary MEFs (p5) with or without LKB1. MEFs were transduced with lentiviruses expressing GFP (control) or HPV16 E6/E7 for 48 h. Cell culture media were collected for measuring glucose (a) and lactate (b). (c) Cellular ATP level was measured. Measurements of the metabolites were normalized to cell number. The value in control group was set as “1”. The means ± SD for 3 independent experiments were presented (*P < 0.01 and #P < 0.05). (d-f) Metabolites in HeLa cells with ectopic expression of LKB1. HeLa/pMEP4-GFP and HeLa/pMEP4-LKB1 cells were induced with 100 μM zinc for 48 h. Levels of the glucose (d) and the lactate (e) in the cell culture media and intracellular ATP (f) were measured and presented as above described. The data represent the means ± SD for 3 independent experiments (*P < 0.01). (g) Immunoblot detection of metabolic enzymes in cells with or without LKB1. Thirty μg whole cell extracts (WCEs) from TC-1 cells with shR-Ctrl or shR-LKB1 (left panel) and HeLa cells with inducible expression of LKB1 (right panel) were assayed. GAPDH serves as a loading control. (h) IHC staining of HK-II in lung tumor tissues from the TC-1/shR-Ctrl and TC-1/shR-LKB1 mice. Scale bar = 20 μM. (i) the staining intensities were determined by calculating the H-score of the staining. At least 200 cells in each mouse tissue were calculated (*P < 0.01, n = 10).
Loss of LKB1 promotes the expression of HK-II
To determine how LKB1 affects HPV-induced glycolysis, we analyzed the expression of metabolic regulators in cervical cancer cells with genetically manipulated LKB1. mTOR is activated by HR HPV oncogene,27-29 but inactivated by LKB1.24 Consistent with these reports, our results showed that the level of phosphorylated S6, a downstream substrate of mTOR, was markedly increased in LKB1 knocked-down TC-1 cells (Figure 3g, left panel), whereas it was decreased in HeLa cells with ectopic LKB1 (Figure 3g, right panel). Unexpectedly, in our experimental settings, LKB1 status had no strong and consistent influence on metabolic enzymes, such as PKM2, PGAM5, Tigar, HK-I, and GAPDH in these two cell lines (Figure 3g). However, HK-II and pyruvate dehydrogenase (PDH) were markedly elevated in TC-1 cells with LKB1 depletion, whereas both were reduced upon ectopic expression of LKB1 in HeLa cells (Figure 3g).
HK-II is the first rate-limiting enzyme in the glycolytic pathway and plays a critical role in directing glucose entry into the cell.30,31 Thus, we focused on the regulation of HK-II by LKB1 in the presence or absence of HPV oncogenes. Expression of HPV16 oncoproteins or Cre-mediated deletion of LKB1 increased HK-II in MEFs (Supplementary Figure 5). HK-II was further elevated in HPV16 E6/E7-expressing LKB−/− MEFs. In contrast, the level of HK-I was unchanged (Supplementary Figure 5).
We also examined HK-II expression in LKB1-intact and LKB1-deficient tumor tissues generated from TC-1 cell lung metastatic model by immunohistochemistry (IHC). The signals of HK-II in LKB1-intact tumors were weak, similar to those in the adjacent normal cells; strongly-positive cells were sparse (Figure 3h, left panel). In contrast, LKB1-depleted tumor cells displayed uniformly strong HK-II signals (Figure 3h, right panel). The H score, a semi-quantitative method to determine staining intensities in conjunction with the percentage of cells stained at each level,32 was four-fold higher in LKB1-depleted tumors as compared to that in LKB1-intact tumors (Figure 3i). Collectively, our data demonstrate that LKB1 downregulated HK-II in vitro and in vivo.
Inhibition of HK-II reduces glycolysis in HPV16-infected cells
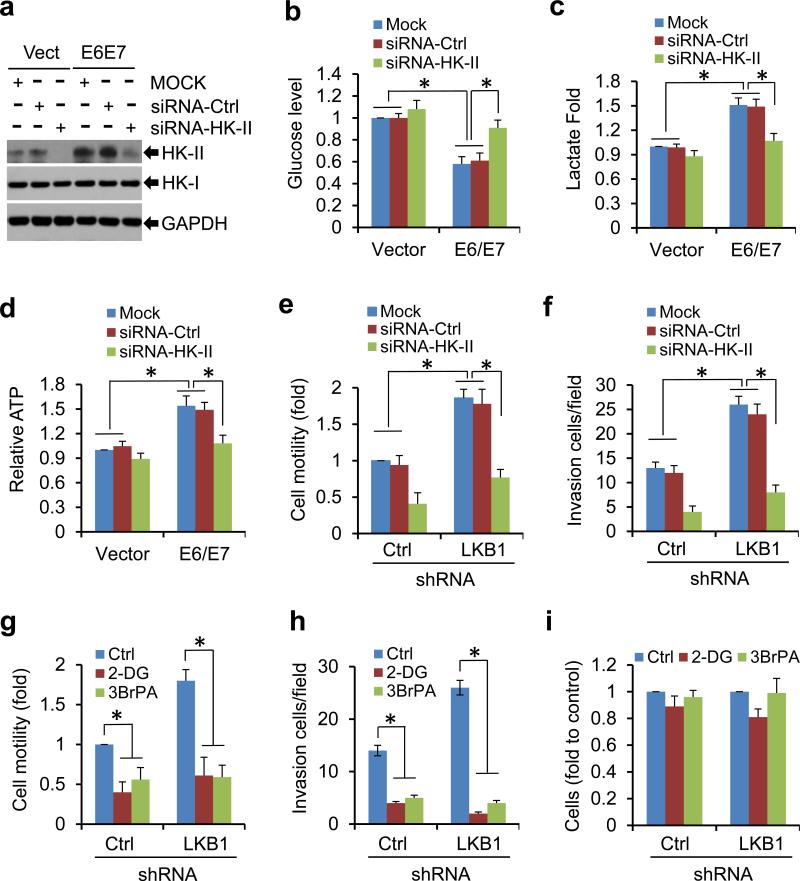
To assess the role of HK-II induction in HPV16 oncogene-induced metabolic reprogramming, we transduced MEF/HPV16 E6/E7 and MEF/GFP cells with control small interfering RNAs (siRNA-Ctrl) or siRNAs against HK-II (siRNA-HK-II). siRNA-Ctrl did not markedly impact the level of HK-II, nor the metabolites in the cells (Figures 4a-d). HK-II was markedly increased in HPV16 E6/E7 expressing cells (Figure 4a). As described in Figures 3a-c, HPV oncogene expressing led to a markedly increase in glycolysis (Figures 4b-d). Knockdown of HK-II substantially reduced the level of HK-II, but not HK-I (Figure 4a). More importantly, depletion of HK-II largely abrogated the viral effects on metabolites, reversing glycolysis to that exhibited by normal cells (Figures 4b-d), whereas knock-down of HK-II only marginally modulated the metabolites in cells without viral genes (Figures 4b-d). We also treated MEF/HPV16 E6/E7 and control MEF/GFP cells with 2-deoxy-D-glucose (2-DG) or 3-bromopyruvate (3-BrPA),33 two inhibitors of HK-II. Applications of 2-DG or 3-BrPA strikingly abolished the viral effects on glycolysis. In contrast, the effects of either agent on metabolites were marginal in control MEFs (Supplementary Figure 6).
Figure 4.
Suppression of HK-II reduces cell metabolic aberrance and oncogenic activities caused by HPV16 E6/E7 or LKB1-depletion. (a-d) MEFs/Vector or MEFs/HPV16 E6/E7 were transduced with mock, siRNA-Ctrl, or siRNA-HK-II for 48 h. The cell culture media were changed. Another 24 h later, WCEs were used for immunoblots (a). Extracellular glucose (b) and lactate (c) and intracellular ATP (d) were measured. Measurements of the metabolites were normalized to cell number. The value in control group was set as “1”. The means ± SD for 3 independent experiments were presented (*P < 0.01). (e, f) TC-1/shR-Ctrl and TC-1/shR-LKB1 cells were transduced with mock, siRNA-Ctrl, or siRNA-HK-II for 48 h. Cell migration (e) and invasion (f) were determined. (g-i) TC-1/shR-Ctrl and TC-1/shR-LKB1 cells were exposed to 10 mM 2-DG or 200 μM 3-BrPA for 24 h. Cell migration (g) and invasion (h) were determined. Cell viability was determined by MTT assay (i). All data were the average ± S.D. from 3 independent experiments (*P < 0.01).
To determine whether the suppression of HPV oncogenic activities by LKB1 is mediated through HK-II-regulated glycolysis, we knocked down HK-II in TC-1/shR-Ctrl and TC-1/shRLKB1 cells and examined cell invasion and migration in these cells. Depletion of LKB1 markedly increased cell migration and invasion (Figures 4e and f). These effects were largely abrogated by the knockdown of HK-II (Figures 4e and f). Similar effects were observed in TC-1/shR-Ctrl and TC-1/shR-LKB1 cells treated with 2-DG or 3-BrPA (Figures 4g and h). Cell viability assay showed that the inhibitors only slightly decreased the growth of either control or LKB1-knocked-down TC-1 cells within the exposure time period (Figure 4i). Taken together, these results suggest that HK-II plays a crucial role in the oncogenic activities of HPV-infected, LKB1-depleted transformed cells.
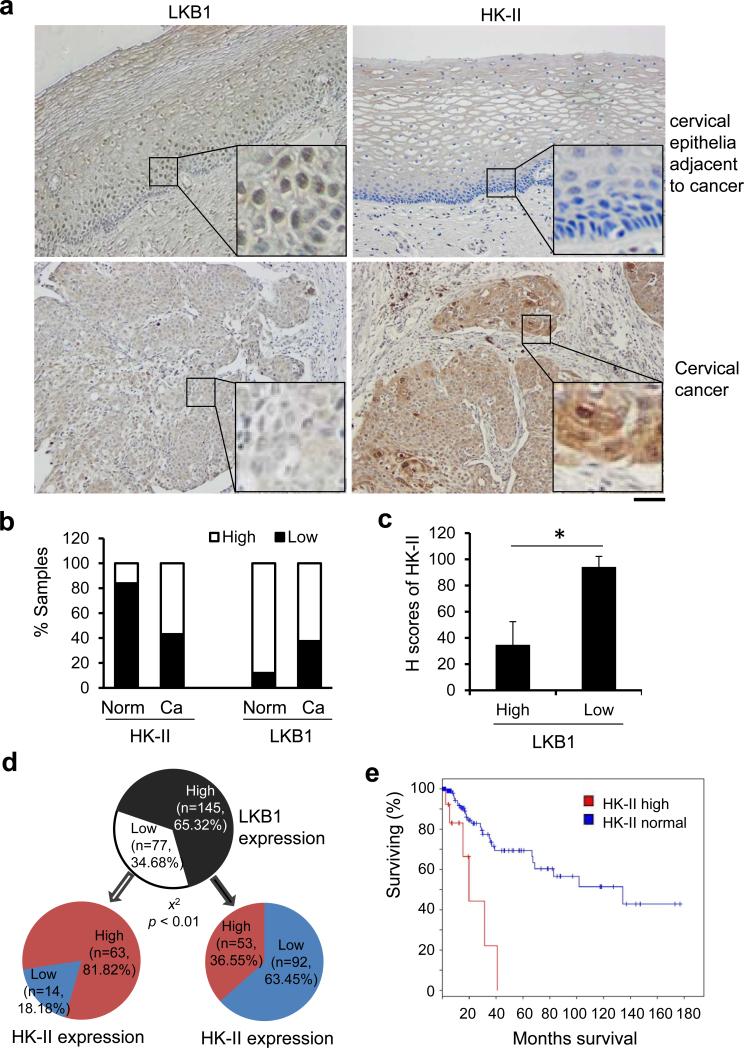
HK-II is inversely correlated with the expression of LKB1 in normal and cervical cancers
To investigate the pathological correlation between LKB1 and HK-II, we assessed their expression in human cervical tissues by using IHC. Tissues from normal (n = 25) and carcinoma (n = 197) were analyzed. Healthy cervical tissues generally displayed moderate LKB1 signals with little or no HK-II signals (Figures 5a and b). In contrast, nearly 40% cervical cancer tissues exhibited a reduced expression of LKB1 and about 60% cervical cancer cases were positive for HK-II (Figures 5a and b). The H score32 also demonstrated an intense signal of HK-II in cancerous tissues with low LKB1 expression (Figure 5c). Figure 5d summarized all 222 tissues examined. In general, a high proportion of tissues with high LKB1 had low HK-II. Among tissues with low LKB1, most had a high HK-II. We also searched the Cancer Genome Atlas (TCGA) data base with a focus on the Study of Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma. There were 309 patients analyzed in the study. Among them, 12% possessed an increased HK-II mRNA (expression above normal level + 1 standard deviation). 27% bore a decreased LKB1 mRNA (expression below normal level - 1 standard deviation) (Supplementary Figure 7). Around 40% of the specimens with elevated HK-II occurred in tissues with reduced LKB1 (Supplementary Figure 7). Importantly, patients with an upregulated HK-II had a significant shorter survival period as compared with those with normal HK-II (p < 0.0003) (Figure 5e). Taken together, these data suggest that LKB1 levels are inversely linked to HK-II in a large fraction of cervical cancers.
Figure 5.
Low expression of LKB1 in cervical tissues correlates to elevated HK-II. (a-d) Cervical tissues from healthy (n = 25) and cervical cancer (n = 197) subjects were assessed for the expression of LKB1 and HK-II by IHC. (a) Representative IHC staining of LKB1 and HK-II in normal cervical tissues and in cervical cancer tissues. Scale bar = 100 μm. (b) Quantification of LKB1 and HK-II expression in human cervical tissues. Low: overall negative or weak staining; High: overall moderate or strong staining. The Pearson's chi-square test was used to analyze the distribution difference of LKB1 and HK-II between healthy and cervical cancer tissues (P < 0.01). (c) H-scores of HK-II in cervical tissues with a low or high level of LKB1 (*P < 0.01). (d) Correlation between LKB1 and HK-II in cervical tissues from healthy (n = 25) and cervical cancer (n = 197) subjects. The Pearson's chi-square test was used to analyze the significance of the correlation (P < 0.01). (e) Survival curve of patients with or without HK-II elevation. Patients’ data from the Study of Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma in the TCGA data base were analyzed. 309 patients in the database were included for the analysis. The level of HK-II mRNA above the average + S.D. was defined as “high HK-II” (HK-2: EXP > 1 as a search definition). The survival curve was adopted from the TCGA website (http://www.cbioportal.org). P = 2.975e-4 with the Logrank Test for the survival rate.
c-MYC mediates the cross-talk between HPV and LKB1 in regulating HK-II
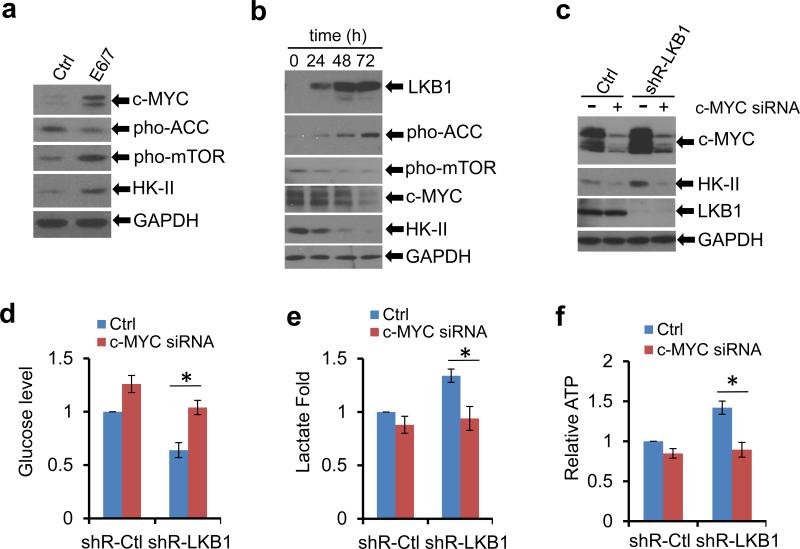
A previous report showed that loss of LKB1 synergizes with c-MYC to facilitate tumorigenesis in mammary glands.34 Being a proto-oncogene, c-MYC controls cell cycle in cultured cells and its over-expression induces tumors in mouse models.35,36 Interestingly, c-MYC also enhances the transcription of HK-II and thus promotes glycolysis37. Furthermore, our recent studies showed that expression of HPV16 E6/E7 stabilizes c-MYC20. Thus, we asked whether c-MYC could be a potential node involving in glycolysis regulated by the opposing activities of LKB1 and HPV oncogenes.
First, both c-MYC and HK-II were elevated in E6/E7 transduced MEFs, relative to the control cells (Figure 6a). Second, in HeLa/pMEP4-LKB1 inducible cells, induction of LKB1 decreased the activation of mTOR (phospho-mTOR, Figure 6b, Supplementary Figure 8) while enhancing the activation of AMP-activated protein kinase (AMPK) as reflected by an increase in the phosphorylation of acetyl-CoA carboxylase (ACC), a substrate of the LKB1-AMPK cascade (Figure 6b). These results confirm the activity of LKB1 in the cells.
Figure 6.
LKB1 inhibits HK-II and glycolysis via the suppression of c-MYC. (a) Whole cell extracts were isolated from MEFs transduced with GFP or HPV16 E6/7. Thirty microgram proteins from WCEs were used for immunoblots. GAPDH serves as a loading control. (b) HeLa/pMEP4-LKB1 cells were induced with 100 μM zinc for the indicated duration. WCEs were isolated for immunoblots. (c-f) TC-1/shR-Ctrl and TC-1/shR-LKB1 cells were transfected with a smart pool of c-MYC siRNA duplexes for 72 h. (c) Immunoblot detection; (d) extracellular glucose; (e) extracellular lactate; (f) intracellular ATP. The data in d-f were the average ± S.D. from 3 independent experiments (*P < 0.01).
Interestingly, upon LKB1 induction, the levels of both c-MYC and HK-II were reduced in a time-dependent manner (Figure 6b). Following up this observation, we modulated the levels of the c-MYC in LKB1-intact and LKB1-deficient TC-1 cells by using siRNAs. Knock-down of c-MYC dramatically reduced basal and LKB1-deficiency-induced HK-II (Figure 6c). Further, both the elevated glucose consumption and lactate production in TC-1/shR-LKB1 cells were strikingly reversed by the depletion of c-MYC (Figures 6d and e). Intracellular ATP levels also tended to return to normal as well (Figure 6f). In TC-1/shR-Ctrl cells, knock-down of c-MYC also reduced glucose consumption, lactate production and ATP production, but the effects were much more moderate (Figures 6d-f). Together, these results suggest that c-MYC mediates the elevation of HK-II and glycolysis in HPV transformed and LKB1-deficient cells.
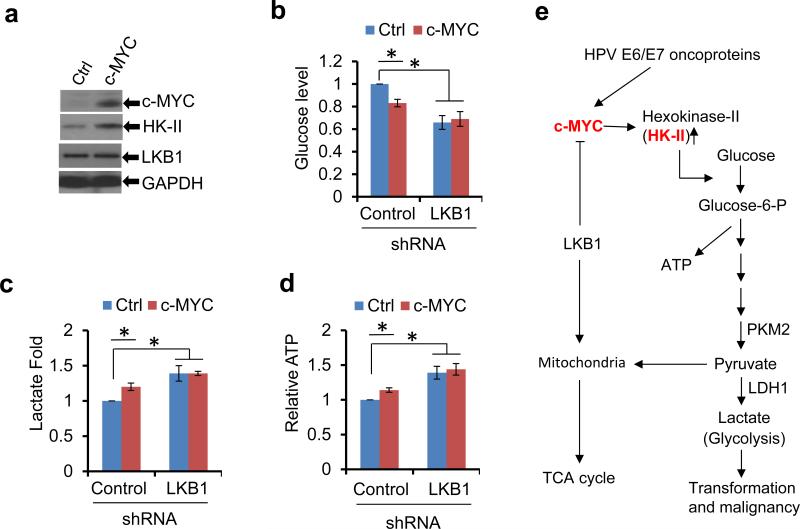
Over-expression of c-MYC leads to aerobic glycolysis in LKB1-intact cells
To validate the role of c-MYC in the cross-talk between HPV oncogenes and LKB1 in regulating HK-II and the resultant metabolic alterations, we transfected the c-MYC expression vector into TC-1 cells. Over-expression of c-MYC increased HK-II in the LKB1 intact cells, but to a less extent in LKB1 compromised cells (Figure 7a and data not shown), as these cells already had elevated HK-II (Figures 3g and h, 6c). Moreover, enforced expression of c-MYC reduced glucose level in culture medium and increased the production of lactate and ATP in LKB1-intact cells (Figures 7b-d). However, the effects were modest probably because c-MYC was already elevated by the HPV16 oncogenes. In contrast, ectopic c-MYC did not increase further the high level of glucose consumption, lactate production, and ATP levels in LKB1-compromised TC-1 cells (Figures 7b-d). Collectively, these results corroborate above conclusion that c-MYC mediates the upregulation of the LKB1-regulated HK-II and glycolysis in HPV-infected cells (Figure 7e).
Figure 7.
c-MYC mediates the cross-talk between HPV and LKB1 in the regulation of HK-II. (a-d) A c-MYC expression construct was transfected into TC-1/shR-Ctrl and TC-1/shR-LKB1 cells and selected with G418 for 2 weeks. (a) Immunoblot blot detection of c-MYC and cell metabolic enzymes in TC-1/shR-Ctrl cells with or without ectopic c-MYC. (b) Extracellular glucose; (c) Extracellular lactate; (d) Intracellular ATP. The data in b-d were the average ± S.D. from 3 independent experiments (*P < 0.01). (e) A model for LKB1-mediated suppression of HPV positive cancer progression by targeting cellular metabolism. The HR HPV infection and the loss of LKB1 collaborate to facilitate transformation of epithelial cells and progression to malignancy.
Discussion
Oncogenic HPV E6 and E7 inactivate tumor suppressive signals controlled by p53 and pRb, while activating oncogenic signals, including the AKT-, mTOR-, and survivin-associated pathways.3,27-29 However, more than 95% of HR HPV infections do not progress to malignancy.2 Factors or conditions that collaborate with HPV oncogenes and contribute to lesion progression and cancer development in HPV infection remain largely to be identified. In this study, we found that LKB1 deficiency enhanced HPV16 E6/E7 oncogenic activities and promoted lung metastasis of HPV16 E6/E7 and K-RAS transformed TC-1 cells (Figures 1 and 2). Previous reports showed that at least 20% of primary cervical cancers harbor somatically-acquired mutations in LKB1.11,25 Homozygous deletion of LKB1 locus or novel fusion transcripts involving LKB1 and its neighboring genes have been reported in cervical cancer cells.11,25,38 By using IHC analysis, we now found that some 40% of cervical cancer tissues bore a markedly reduced LKB1 protein (Figure 5). The higher percentage of patients with reduced LKB1 may be due to the particular patient population analyzed in this study. We suggest that LKB1 insufficiency may represent one of the genetic alterations that contribute to progression of HPV-induced dysplasias to invasive cancers.
Cervical carcinoma and HPV infection are glycolytic and have elevated LDH and pyruvate kinase M2 (PKM2).14-17 However, LDH and PKM2 are relatively late enzymes in the glycolysis pathway. Based on TCGA database, neither LDH nor PKM2 correlates with the survival rate of cervical cancer patients (data not shown). Our current data showed that early metabolic enzyme, HK-II, was upregulated in HPV infection (Figures 4a, 5a-d, Supplementary Figure 5). Hexokinases promote and sustain a concentration gradient that facilitates glucose entry into cells and the initiation of all major pathways of glucose utilization.30,31,39-44 Among the 4 isoforms (I-IV), only HK-II is primarily expressed in cancer cells30,41 and only HK-II upregulation correlated with a significant shorter survival period (p < 0.0003) (Figure 5e and data not shown), supporting the importance of HK-II in HPV-associated carcinogenesis. Notably, we characterized that both HK-II and glycolytic metabolites in HPV infection were further elevated by the depletion of LKB1 and reduced by ectopic expression of LKB1 (Figures 3a-i). These data implicate LKB1 in suppressing HPV-mediated oncogenic activities through the regulation of metabolic reprogramming.
Interestingly, our study showed that PDH was also elevated in TC-1 cells with LKB1 depletion, whereas it was reduced upon ectopic expression of LKB1 in HeLa cells (Figure 3g). These findings are consistent with a previous report showing that conditional knockout of LKB1 in mammary glands of mice genetically engineered to express activated Neu/HER2-MMTV-Cre (NIC) enhances the expression of LDH and PDH, leading to a glycolytic alteration and a high level of ATP.45 These findings may appear counterintuitive, as PDH promotes pyruvate flux to acetyl-CoA and entry into the tricarboxylic acid cycle (TCA). However, PDH is rapidly inactivated through the phosphorylation catalyzed by PDH kinases (PDKs), whereas, dephosphorylation of PDH increases the activity of the enzyme.46 Faubert et al reported that the expression level of PDK1 was markedly elevated in LKB1-null MEFs.47 Thus, the activity of PDH may in fact be reduced and the increased PDH expression in LKB1 deficient cells in this study and previous report45 may reflect a feedback mechanism for the inactivation of PDH.
We previously reported that expression of HPV oncogenes increase c-MYC.20 In the current study, we demonstrated that increased c-MYC in HPV positive cells elevated HK-II, which was reduced by ectopic expression of LKB1 (Figures 6a-c). The importance of c-MYC on mediating HK-II-promoted glycolysis is further illustrated by two reciprocal experiments. In one, over-expression of c-MYC increased glycolysis in LKB1-intact TC-1 cells but not in LKB1-compromised cells (Figures 7a-d). This is expected as the loss of LKB1 already elevated c-MYC and HK-II (Figure 6c). In the other, c-MYC knockdown in LKB1-intact or LKB1-deficient TC-1 cells reduced HK-II levels and glycolysis (Figures 6c-f). Taken together, our data characterized a new signaling pathway, in which LKB1 opposes HPV-induced metabolic reprogramming via a common pathway of c-MYC and HK-II (Figure 7e).
Metabolic switch toward elevated glycolysis is a fundamental event in tumorigenesis and tumor progression in multiple cancers.12 Pre-therapy lactate levels are inversely correlated with overall and disease-free patient survival.17 Our findings suggest that LKB1 acts as a safeguard against elevated glycolytic metabolism to suppress the oncogenic activities of HPVs, providing a plausible explanation for the observed LKB1 deficiency in a significant fraction of cervical cancer cases and other cancers. This interpretation is consistent with reports that, in lung cancer and melanoma, the absence of LKB1 activates K-RAS, SRC family kinase YES, SRC/FAK, HIF1α, and GTP-bound RAB7 pathways to promote the progression.10,48-55 Considering the important regulatory roles of LKB1 and HK-II in glycolysis, mutations in LKB1 or elevated HK-II might very well serve as markers for progression to cancer. Our results in TC-1 tumor model also suggest that targeting cellular energy homeostasis is a valid therapeutic strategy to reduce or eliminate tumor cells that rely heavily on glycolysis for growth and survival.
Materials and Methods
Cell culture and viability assays
MEFs harboring a conditional deletion in the stk11 gene (LKB1fl/fl) were maintained in the lab.56 The MEFs were infected with lentiviruses expressing Cre recombinase to generate isogenic MEFs expressing or lacking LKB1. Human cervical cancer cell lines HeLa and C33A are maintained in the lab.24 LKB1 inducible and knockdown cell lines were reported previously.24 TC-1 cells were obtained from Dr. T.C. Wu (John Hopkins University).22 TC-1 cells infected with lentiviruses expressing shR-Ctrl or shR-LKB1 were selected with 1 μg/ml puromycin for 2 weeks to derive stable clones. Cells were cultured in Dulbecco modified Eagle medium with L-glutamine and 10% fetal bovine serum. Cell viability was determined by 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide assay.24
Antibodies
Antibodies against HPV16 E6, HPV16 E7, GFP, LKB1, and PCNA were purchased from Santa Cruz (Santa Cruz, CA). Mouse monoclonal anti-HPV16 E7 antibody (716-281) purchased from Thermo Scientific (Rockford, IL) was also used for some immunoblots. Antibodies against c-MYC (ab69987), TIGAR (ab37910), LKB1 (ab15095), and HK-II (ab76959) were purchased from Abcam (Cambridge, MA). Antibody against phospho-ACC (S79) was purchased from Millipore Inc. (Lake Placid, NY). Anti- phospho-mTOR (Ser2448), phospho-S6 (S240/244), phospho-AKT (S473), LKB1 (#3050), HK-I (#2024), HK-II (#2867), PDH (#3205), and PKM2 (#3198) antibodies were purchased from Cell Signaling Technology, Inc. (Boston, MA). GAPDH polyclonal antibody was obtained from Bethyl Laboratories (Montgomery, TX).
Plasmids and DNA transfection
The pD40-His/V5-c-MYC WT construct was purchased from Addgene (Boston, MA). The HPV16 E6, E7, and E6/E7 in retroviral vectors were recloned into pEF1a lentiviral vector and packaged in 293T cells. Gene transfection was performed with Fugene 6 transfection reagent (Roche Diagnostics Corp., Indianapolis, IN.) in accordance with the manufacturer's instructions.
Trans-well migration and Matrigel invasion assays
HeLa cells with inducible LKB1 were placed in the upper chamber of the Transwell Membrane Inserts or pre-coated Matrigel Chambers from BD Biosciences (San Jose, CA). To induce LKB1, 100 μM zinc sulfate was added to the low chamber. After 24 h, migratory or invasive cells were stained with 1% crystal violet and quantified under a microscope. Assays with TC-1/shR-Ctrl and TC-1/shR-LKB1 cells were conducted similarly except the lower chambers contained regular culture medium.
Wound-healing migration assay
TC-1/shR-Ctrl and TC-1/shR-LKB1 cells were plated in a 6-well plate. After reaching confluence, a uniform scratch was made with a 10 μl-pipet tip to create a cell-free gap. After 24 h, plates were examined and photographed to assess the gap closure.
RNA interference and western blot
A pool of four small interfering RNA duplexes targeting mouse c-MYC or HK-II and a non-targeting siRNA pool were purchased from Dharmacon Inc. (Lafayette, CO, USA), and transfection was performed according to the manufacturer's protocol. The method for western blot was reported previously.56
Measurement of glucose, lactate, and ATP
The media from cultured cells were used for measuring glucose and lactate by a Flex Bioanalyzer (NOVA Biomedical). Amounts of glucose remained in the cell culture medium reflected the subtraction of the consumption by cells from total glucose level in uncultured medium. ATP levels were measured using Bioluminescence Assay Kit CLS II from Roche Scientific (Indianapolis, IN, USA), as per the manufacturer's protocol. Cell lysates were used for the determination. Measurements of the metabolites were normalized to cell number.
Subcutaneous tumorigenesis and lung metastasis in mouse models
TC-1/shR-Ctrl or TC-1/shR-LKB1 cells were harvested and mixed 2:1 in matrigel (BD Biosciences). Two million cells were inoculated subcutaneously at the right flank of C57BL/6 mice (n=10). Mice were housed in barrier facility on a 12 h light-dark cycle with food and water available ad libitum. Tumors developed in the syngeneic mice were measured twice a week with calipers. Tumor volumes were calculated by the formula: a2 × b × 0.4, where “a” is the smallest diameter and “b” is the diameter perpendicular to “a”. To assess metastasis to lungs, 2×105 TC-1/shR-Ctrl or TC-1/shR-LKB1 cells were injected through tail-vein into C57BL/6 mice (n=10). At the end of 3 weeks, the mice were euthanized and subcutaneous tumors and lungs were removed for histological and biochemical analyses. The animal study was approved by institutional IACUC.
Human cervical tissues and immunohistochemistry
Human cervical tissues were obtained from Department of Pathology at Jilin University (Changchun, China) and from UAB tissue bank. Cervical carcinoma tissue microarray slides (#CR2088, CR602, CR242) were ordered from US Biomax Inc. (Rockville, MD). Tissues from healthy controls (n = 25) and carcinoma (n = 197) were used for the analysis. IHC was performed as previously reported.20,32 The staining index was based on the staining intensity, which was graded as “−”, no staining; “+”, weak staining; “++”, moderate staining; and “+++”, strong staining. Samples that scored as “−” or “+” were considered as low expression and those scored as “++” or “+++” as high expression. All stained slides were observed and scored by 3 pathologists. If the staining interpretation differed among the investigators, the data for the slide were not included in the analysis. For determining the H score, stained tissues were scored by calculating the product of the percentage of cells staining at each intensity level and the intensity level (0, negative; 1, weak; 2, moderate; 3, strong). An H score was then calculated by summing the individual intensity level scores32.
Statistical analysis
All data presented are representative of 3 or more experiments, each with similar results. Quantitative data were shown as mean ± SD. Statistical significance was determined using Student t test if not specially indicated (*P < 0.01 and # P < 0.05).
Supplementary Material
Acknowledgments
This work was supported by grants from National Cancer Institute R01CA133053, R01CA83679, and the UAB Comprehensive Cancer Center Pilot Program Project IRG-60-001-50. The work was also supported by the National Natural Science Foundation of China No. 81271853, No. 81272243 and No. 81573087, and the National 863 Program of China No. 2004AA205020.
Sources of supports (grants): National Cancer Institute R01CA133053, R01CA83679, and the UAB Comprehensive Cancer Center Pilot Program Project IRG-60-001-50; and the National Natural Science Foundation of China No. 81271853, No. 81272243, and No. 81573087, and the National 863 Program of China No. 2004AA205020.
Footnotes
Conflict of interest
The authors state that there is no conflict of interest to report.
References
- 1.Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 2.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Scheffner M, Munger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA. 1991;88:5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galloway DA, Laimins LA. Human papillomaviruses: shared and distinct pathways for pathogenesis. Curr Opin Virol. 2015;14:87–92. doi: 10.1016/j.coviro.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Psyrri A, DiMaio D. Human papillomavirus in cervical and head-and-neck cancer. Nat Clin Pract Oncol. 2008;5:24–31. doi: 10.1038/ncponc0984. [DOI] [PubMed] [Google Scholar]
- 6.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nature Rev Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 7.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 8.Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link - ten years after. BMC Biol. 2013;11:36. doi: 10.1186/1741-7007-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 11.Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009;4:e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nature Cell Biol. 2015;17:351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayre HA, Goldberg DM. Enzymes of the human cervix uteri. Comparison of dehydrogenases of lactate, isocitrate, and phosphogluconate in malignant and non-malignant tissue samples. Br J Cancer. 1966;20:743–750. doi: 10.1038/bjc.1966.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazurek S, Zwerschke W, Jansen-Durr P, Eigenbrodt E. Effects of the human papilloma virus HPV-16 E7 oncoprotein on glycolysis and glutaminolysis: role of pyruvate kinase type M2 and the glycolytic-enzyme complex. Biochem J. 2001;356:247–256. doi: 10.1042/0264-6021:3560247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwerschke W, Mazurek S, Masssini P, Banks L, Eigenbrod E, Jansen-Dürr P, et al. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc Natl Acad Sci USA. 1999;96:1291–1296. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–921. [PubMed] [Google Scholar]
- 18.Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev. 2014;28:99–114. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dörr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Däbritz JH, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501:421–425. doi: 10.1038/nature12437. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Q, Zhao RX, Chen J, Li Y, Li XD, Liu XL, et al. O-linked GlcNAcylation elevated by HPV E6 mediates viral oncogenesis. Manuscript submitted. [DOI] [PMC free article] [PubMed]
- 21.Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 22.Ji H, Chang EY, Lin KY, Kurman RJ, Pardoll DM, Wu TC, et al. Antigen-specific immunotherapy for murine lung metastatic tumors expressing human papillomavirus type 16 E7 oncoprotein. Int J Cancer. 1998;78:41–45. doi: 10.1002/(sici)1097-0215(19980925)78:1<41::aid-ijc8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin-Drubin ME, Munger K. Oncogenic activities of human papillomaviruses. Virus Res. 2009;143:195–208. doi: 10.1016/j.virusres.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao X, He Q, Lu C, Werle KD, Zhao RX, Chen J, et al. Metformin impairs the growth of liver kinase B1-intact cervical cancer cells. Gynecol Oncol. 2012;127:249–255. doi: 10.1016/j.ygyno.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCabe MT, Powell DR, Zhou W, Vertino PM. Homozygous deletion of the STK11/LKB1 locus and the generation of novel fusion transcripts in cervical cancer cells. Cancer Genet Cytogenet. 2010;197:130–141. doi: 10.1016/j.cancergencyto.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mack HI, Munger K. The LKB1 tumor suppressor differentially affects anchorage independent growth of HPV positive cervical cancer cell lines. Virology. 2013;446:9–16. doi: 10.1016/j.virol.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Z, Hu X, Li Y, Zheng L, Zhou Y, Jiang H, et al. Human papillomavirus 16 E6 oncoprotein interferences with insulin signaling pathway by binding to tuberin. J Biol Chem. 2004;279:35664–35670. doi: 10.1074/jbc.M403385200. [DOI] [PubMed] [Google Scholar]
- 28.Zheng L, Ding H, Lu Z, Li Y, Pan Y, Ning T, et al. E3 ubiquitin ligase E6AP-mediated TSC2 turnover in the presence and absence of HPV16 E6. Genes Cells. 2008;13:285–294. doi: 10.1111/j.1365-2443.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- 29.Spangle JM, Münger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol. 2010;84:9398–9407. doi: 10.1128/JVI.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts DJ, Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22:248–257. doi: 10.1038/cdd.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balko JM, Cook RS, Vaught DB, Kuba MG, Miller TW, Bhola NE, et al. Profiling of residual breast cancers after neoadjuvant chemotherapy identifies DUSP4 deficiency as a mechanism of drug resistance. Nature Med. 2012;18:1052–1059. doi: 10.1038/nm.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J Biol Chem. 2001;276:43407–43412. doi: 10.1074/jbc.M108181200. [DOI] [PubMed] [Google Scholar]
- 34.Partanen JI, Nieminen AI, Mäkelä TP, Klefstrom J. Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc Natl Acad Sci USA. 2007;104:14694–14699. doi: 10.1073/pnas.0704677104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabay M, Li Y, Felsher DW. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med. 2014;4:a014241. doi: 10.1101/cshperspect.a014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altman BJ, Hsieh AL, Sengupta A, Krishnanaiah S, Stine ZE, Walton ZE, et al. MYC Disrupts the Circadian Clock and Metabolism in Cancer Cells. Cell Metab. 2015;22:1009–1019. doi: 10.1016/j.cmet.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dendele B, Tekpli X, Sergent O, Dimanche-Boitrel MT, Holme JA, Huc L, et al. Identification of the couple GSK3alpha/c-MYC as a new regulator of hexokinase II in benzo[a]pyrene-induced apoptosis. Toxicol In Vitro. 2012;26:94–101. doi: 10.1016/j.tiv.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Ojesina AI, Lichtenstein L, Freeman SS, Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 40.Cheung EC, Ludwig RL, Vousden KH. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci USA. 2012;109:20491–20496. doi: 10.1073/pnas.1206530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu R, Wyatt E, Chawla K, Tran M, Ghanefar M, Laakso M, et al. Hexokinas II knockdown results in exaggerated cardiac hypertrophy via increased ROS production. EMBO Mol Med. 2012;4:633–646. doi: 10.1002/emmm.201200240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anflous-Pharayra K, Cai ZJ, Craigen WJ. VDAC1 serves as a mitochondrial binding site for hexokinase in oxidative muscles. Biochim Biophys Acta. 2007;1767:136–142. doi: 10.1016/j.bbabio.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 44.John S, Weiss JN, Ribalet B. Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PLoS ONE. 2011;6:e17674. doi: 10.1371/journal.pone.0017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrade-Vieira R, Xu Z, Colp P, Marignani PA. Loss of lkb1 expression reduces the latency of ErbB2-mediated mammary gland tumorigenesis, promoting changes in metabolic pathways. PLoS ONE. 2013;8:e56567. doi: 10.1371/journal.pone.0056567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase inoncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- 47.Faubert B, Vincent EE, Griss T, Samborska B, Izreig S, Svensson RU, et al. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1α. Proc Natl Acad Sci U S A. 2014;111:2554–2559. doi: 10.1073/pnas.1312570111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, et al. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nature Cell Biol. 2014;16:108–117. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W, Monahan KB, Pfefferle AD, Shimamura T, Sorrentino J, Chan KT, et al. LKB1/STK11 inactivation leads to expansion of a prometastatic tumor subpopulation in melanoma. Cancer Cell. 2012;21:751–764. doi: 10.1016/j.ccr.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan P, Ito K, Perez-Lorenzo R, Del Guzzo C, Lee JH, Shen CH, et al. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc Natl Acad Sci USA. 2013;110:18226–18231. doi: 10.1073/pnas.1317577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carretero J, Shimamura T, Rikova K, Jackson AL, Wilkerson MD, Borgman CL, et al. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer Cell. 2010;17:547–559. doi: 10.1016/j.ccr.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shackelford DB, Vasquez DS, Corbeil J, Wu S, Leblanc M, Wu CL, et al. mTOR and HIF-1alpha-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc Natl Acad Sci USA. 2009;106:11137–11142. doi: 10.1073/pnas.0900465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hardie DG. AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J Intern Med. 2014;276:543–559. doi: 10.1111/joim.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fauber B, Vincent EE, Griss T, Samborska B, Izreig S, Svensson RU, et al. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1α. Proc Natl Acad Sci USA. 2014;111:2554–2559. doi: 10.1073/pnas.1312570111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dupuy F, Griss T, Blagih J, Bridon G, Avizonis D, Ling C, et al. LKB1 is a central regulator of tumor initiation and pro-growth metabolism in ErbB2-mediated breast cancer. Cancer Metab. 2013;1:18. doi: 10.1186/2049-3002-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu HG, Zhai YX, Chen J, Lu Y, Wang JW, Quan CS, et al. LKB1 reduces ROS-mediated cell damage via activation of p38. Oncogene. 2015;34:3848–3859. doi: 10.1038/onc.2014.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.