Abstract
The blood-labyrinth barrier (BLB) in the stria vascularis is a highly specialized capillary network that controls exchanges between blood and the intrastitial space in the cochlea. The barrier shields the inner ear from blood-born toxic substances and selectively passes ions, fluids, and nutrients to the cochlea, playing an essential role in the maintenance of cochlear homeostasis. Anatomically, the BLB is comprised of endothelial cells (ECs) in the strial microvasculature, elaborated tight and adherens junctions, pericytes (PCs), basement membrane (BM), and perivascular resident macrophage-like melanocytes (PVM/Ms), which together form a complex “cochlear-vascular unit” in the stria vascularis. Physical interactions between the ECs, PCs, and PVM/Ms, as well as signaling between the cells, is critical for controlling vascular permeability and providing a proper environment for hearing function. Breakdown of normal interactions between components of the BLB is seen in a wide range of pathological conditions, including genetic defects and conditions engendered by inflammation, loud sound trauma, and ageing. In this review, we will discuss prevailing views of the structure and function of the strial cochlear-vascular unit (also referred to as the “intrastrial fluid-blood barrier”). We will also discuss the disrupted homeostasis seen in a variety of hearing disorders. Therapeutic targeting of the strial barrier may offer opportunities for improvement of hearing health and amelioration of auditory disorders.
1. Introduction
The inner ear is a remarkably stable homeostatic system controlled by a range of regulatory mechanisms, including control over ion, fluid, and nutrient transport (active and passive) by the blood-labyrinth barrier (BLB). Precise regulation of substrate transport into and out of the inner ear is essential for maintaining the stable composition of inner ear fluids for hearing (Juhn et al., 1981; Juhn et al., 2001). Normal function of the stria vascularis (referred to as the “intrastrial fluid-blood barrier”) is critical for maintaining the ionic gradients and endocochlear potential (EP) required for sensory hair cell transduction (Hibino et al., 2010; Marcus et al., 1983; Quraishi et al., 2008; Salt et al., 1987; Spicer et al., 1996; Wangemann, 2002; Zhang et al., 2012). Dysfunction of the stria, including the intrastrial fluid-blood barrier, is considered to be one of the etiologies in a number of hearing disorders, including autoimmune inner ear disease, noise-induced hearing loss, age-related hearing loss, and genetically linked hearing diseases (Lin et al., 1997; Neng et al., 2015; Shi, 2009; Yang et al., 2011; Zhang et al., 2015). Despite the importance of the intrastrial fluid-blood barrier, the physiology of the barrier is largely unknown. Recent progress has been made in detailing the structural complexity of the barrier points to the important role accessory cells, such as pericytes (PCs), perivascular resident macrophage-like melanocytes (PVM/Ms), and basement membrane (BM), play in the intrastrial fluid-blood barrier. This review provides a topical overview of intrastrial fluid-blood barrier structure and function as well as a synopsis of the dysfunction seen in particular barrier components in different hearing disorders. The review also introduces current methods used for studying the pathophysiology of the intrastrial fluid-blood barrier.
2. Major components and structure of the cochlear intrastrial fluid-blood barrier
The intrastrial fluid-blood barrier is a specialized capillary network characterized by a relative absence of endothelial cell (EC) fenestration (Juhn, 1988). Vascular ECs connected to each other by tight junctions (TJs) and an underlying BM form a diffusion barrier that prevents most blood-borne substances from entering the stria vascularis (Sakagami et al., 1999; Sakagami et al., 1987). Recent studies, however, have shown the intrastrial fluid-blood barrier to be more complex than the conventional view, as the barrier is shown to include a large number of PCs (Shi, 2009; Shi et al., 2008; Takeuchi et al., 2001) and PVM/Ms (Shi, 2010) in addition to ECs and the BM, as shown in Figure 1. Through close anatomical and chemical interactions, these cells monitor the state of ion, fluid, and nutrient flow into the stria vascularis from the circulation and trigger responses to changes in demand.
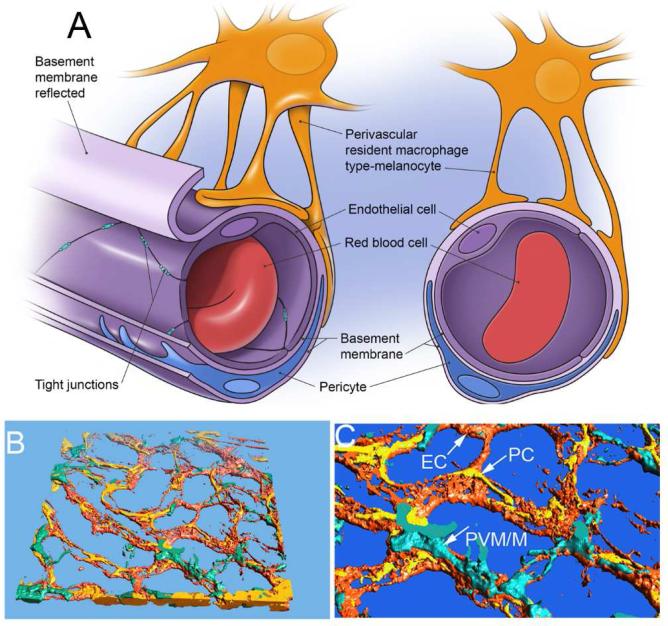
Figure 1.
(A) The illustration of a cochlear micro-vessel in cross-section shows the major components of the intrastrial fluid-blood barrier. The vessel lumen comprises ECs connected by TJs. ECs are ensheathed by a dense basement membrane shared with PCs. PVM/M end-feet cover a large portion of the capillary surface. (B) & (C) The reconstructed confocal image of the intrastrial fluid-blood barrier highlights the morphological complexity of interactions between ECs, PCs, and PVM/Ms. The PVM/Ms are immunolabeled for F4/80, PCs for desmin, and ECs with fluorescent Dil.
A population of around 1220 to 1300 PCs is found in the intrastrial fluid-blood barrier of the normal adult C57/6J mouse cochlea (Neng et al., 2015), as shown in Figure 2A. Extensively branched strial PCs tightly embrace the abluminal strial capillary wall and embed in the BM (Figure 2B). PCs are known to display a heterogeneous range of morphology, phenotype, and function in different tissues (Allt et al., 2001). PCs in the stria vascularis express platelet-derived growth factor receptor-β (PDGFR-β), desmin, neural/glial antigen 2 (NG2) and CD90 (Thy-1) (Shi et al., 2008). In particular, the strial PCs are notably rich in the structural protein desmin, an intermediate filament protein (Shi et al., 2008). The capillary network in the stria vascularis has a unique cellular architecture of polygonal loops. The rich desmin fibers in strial PCs are thought to give the loops mechanical strength and enhance the physical resilience of the vascular networks (Shi et al., 2008). By contrast, PCs of the spiral ligament express more contractile proteins such as α-SMA and tropomyosin (Franz et al., 2004; Shi et al., 2008). The PCs are known to be vital for vascular development, blood flow regulation, vascular integrity, angiogenesis, and tissue fibrogenesis (Allt et al., 2001; Balabanov et al., 1998; Betsholtz et al., 2005; Díaz-Flores et al., 1991; Dore-Duffy et al., 2006; Greenhalgh et al., 2013; Hall et al., 2014; Peppiatt et al., 2006; Quaegebeur et al., 2010; von Tell et al., 2006). We recently reported that strial PCs regulate TJ expression between ECs and are essential for intrastrial fluid-blood barrier integrity (Neng et al., 2013a). Strial PCs derived from neonatal day-10~15 mice dramatically promote spouting angiogenesis in an in vitro cell-line-based 3D co-culture, as shown in Figure 6 (Neng et al., 2015). Studies from non-cochlear tissues show that PCs also contribute to BM formation by directly synthesizing type IV collagen, glycosaminoglycan, fibronectin, nidogen-1, perlecan, and laminin (Allt et al., 2001; Fisher, 2009; Shepro et al., 1993), and inhibit the activity of destabilizing matrix metalloproteinases (MMP), such as MMP-2 and MMP-9 (Zozulya et al., 2008). How strial PCs contribute to the composition and formation of strial BM has not been specifically studied.
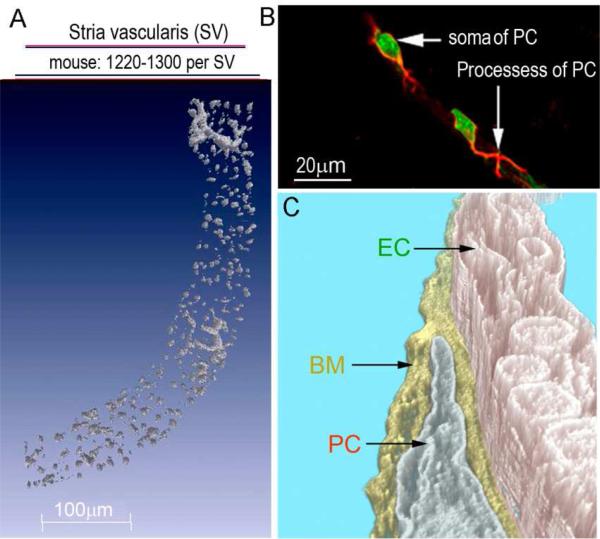
Figure 2.
(A) The super-resolution image shows the high density of strial PCs (labeled with NG2, gray) in the mouse SV (~1220-1300 PCs per SV). (B) The confocal projection shows the PC soma (stained with DAF-2DA, short arrow, green) and primary processes (labeled with desmin, long arrow, red). Two PCs are shown, each having a characteristic of a “bump on a log” shape, situated on the outer wall of a strial vessel (PC: pericyte, PVM/M: perivascular resident macrophage, V/SV: vessels of the stria vascularis, V/SL: vessels of the spiral ligament). (C) TEM tomography shows the cochlear PCs are embedded in the basement membrane (BM) and are closely associated with ECs. TEM tomography enables detection of the interactions between PCs, ECs, and the BM at high resolution (BM: basement membrane, EC: endothelial cell).
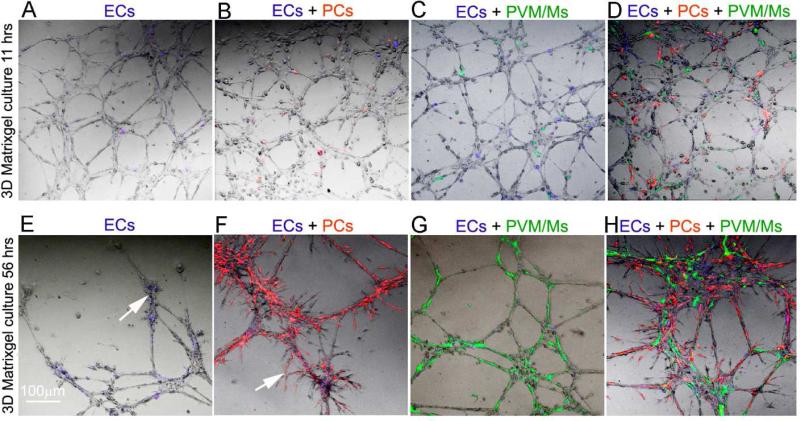
Figure 6.
Mono-culture of ECs and co-culture of ECs + PCs, ECs + PVM/ Ms, and ECs + PCs + PVM/Ms at various times in a three-dimensional (3-D) matrix gel. (A–H) Representative confocal images of a mono-culture of ECs and co-culture of ECs +PCs, ECs + PVM/Ms, and ECs + PCs + PVM/Ms at 11 h and 56 h in a matrix gel. No angiogenesis or EC tube regression is seen at 11 h in culture (A–D). Significant angiogenesis and EC tube formation is seen at 56 h (E–H). (E) Sparse branched networks and sprouting angiogenesis are seen in the EC alone group at 56 h (arrow). PCs promote sprouting angiogenesis of the tube structures (arrow). (G) PVM/Ms significantly delay regression of EC-formed capillary-like tubes. (H) Both PCs and PVM/Ms are required for capillary stability and angiogenesis. The PCs and PVM/Ms cooperate to promote sprouting angiogenesis.
A certain population of PVM/Ms reside in the intrastrial fluid-blood barrier of the normal adult C57/6J mouse cochlea (Neng et al., 2015). It is generally accepted the PVM/Ms originate from cochlear melanocytes derived from the neural crest which have migrated to the stria vascularis during development (Freyer et al., 2011; Steel et al., 1989; Steel et al., 1992; Wakaoka et al., 2013). The majority of PVM/Ms are capable of self-renewal and turn over within approximately a 10 month time frame from circulating blood cells (Shi, 2010). The PVM/Ms situate in close proximity beneath the subepithelial layer of marginal cells and are highly invested on the abluminal surface of capillaries with multiple thin membrane protrusions (Figure 3A-3C). PVM/Ms are a hybrid cell type, displaying characteristics of both macrophage and melanocyte (Zhang et al., 2012). Earlier studies have shown the strial PVM/Ms express a number of macrophage surface markers including F4/80, CD68, and CD11b as well as macrophagic scavenger receptor classes A (1) and B (1) (Shi, 2010). Later studies have also shown the PVM/Ms to exhibit melanocyte characteristics, including containing significant amounts of melanin and expression of melanocyte marker proteins such as glutathione S-transferase alpha 4 (Gstα4) and Kir 4.1, the latter being the fiduciary marker of intermediate cells (Zhang et al., 2012).
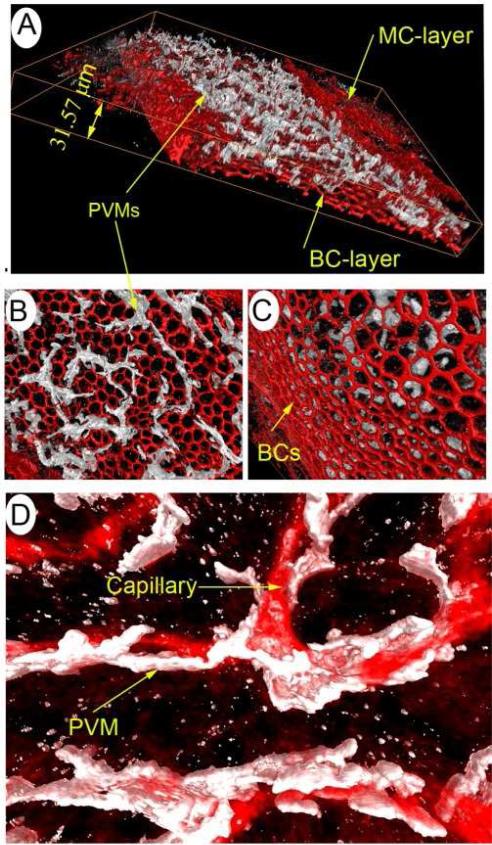
Figure 3.
PVM/Ms in the stria vascularis interface with capillaries. (A) PVM/Ms in the 3-D reconstruction are immunohistochemically labeled with antibody for F4/80 (white), the cytoskeleton labeled with Alexa Fluor® 568 phalloidin (red). The ramified processes of PVM/Ms are sandwiched between marginal and basal cell layers of the stria vascularis. (B) The 3-D reconstruction shows PVMs are situated in or under subepithelial marginal cells and have no basal cell contacts (C). (D) The ramified processes of PVM/Ms interface with the endothelial tube. Capillaries have been labeled with an antibody for IgG.
A recent study shows that physical contact and signal communication between ECs and PVM/Ms is necessary for controlling intrastrial fluid-blood barrier integrity (Zhang et al., 2012). PVM/Ms, as a type of melanocyte, produce melanin pigment in response to noxious factors in the local tissue environment (Soulas et al., 2009; Sulaimon et al., 2003). The melanin plays an essential immunological role in tissue homeostasis by buffering calcium, scavenging heavy metals, foreign protein, and lipids, and promoting antioxidant activity (Bush et al., 2007; Dräger, 1985; Murillo-Cuesta et al., 2010; Ohlemiller et al., 2009; Plonka et al., 2009; Slominski, 2009; Slominski et al., 2012). PVM/Ms, as tissue resident macrophages in the cochlea, may also have a role in immunological defense and repair (Cui et al., 2009; Ekdahl et al., 2009). For example, tissue resident macrophages can scavenge invading microorganisms and dead cells, and the macrophages act as immune or immuno-effector cells by producing superoxide anions, nitric oxide, and inflammatory cytokines during inflammation in non-cochlear tissue (Block et al., 2005; Block et al., 2007; Chéret et al., 2008; Mitrasinovic et al., 2002; Mitrasinovic et al., 2005; Nimmerjahn et al., 2005). Some studies have also reported that tissue resident macrophages are capable of differentiating into fibroblast/myofibroblast for repair of damaged tissue in other organs (Pilling et al., 2009; Pufe et al., 2008; Takagi et al., 2008).
The intrastrial fluid-blood barrier also consists of extensive BM. Major components of the BM are collagen IV, laminin, heparan sulphate proteoglycan (HSPG), entactin/nidogen, and fibronectin (Cosgrove et al., 1996; Gratton et al., 2002; Satoh et al., 1998). Beta 1 and alpha 1 integrin subunits, as well as usherin, are also found in the BM (Bhattacharya et al., 2002; Tsuprun et al., 2001). Electron microscopy studies of the chinchilla cochlea shows the BM uniquely organized, with different proteins in the BM interacting to form a homogeneous lamina densa (Tsuprun et al., 2001). At the ultrastructural level, the distribution of proteoglycans in the BM is heterogeneous and patterned. The strial BM is also found to be negatively charged (Suzuki et al., 1996). Anionic sites have been identified in the BM of the strial capillary wall in both mouse and guinea pig strains (Suzuki et al., 1996; Suzuki et al., 1997; Suzuki et al., 1991; Suzuki et al., 1995; Torihara et al., 1994).
3. Intrastrial fluid-blood barrier permeability
Information on the regulation of blood barrier permeability in the stria vascularis is very limited. Earlier studies have shown the strial blood barrier in immature animals is more permeable than in adult animals. For example, Suzuki et al., (1998) reported that the strial blood barrier in rats isn't well formed until ~14 days after birth.
Two major pathways including para-cellular and transcellular pathways have been proposed to control vascular permeability in the intrastrial blood barrier. The para-cellular pathway is related to the transient state of TJs between ECs. The trans-cellular pathway involves endocytotic activity and trans-endothelial channels. The major TJ proteins in the intrastrial fluid–blood barrier are occludin, claudins, zona occludens, and adherens junction proteins such as VE-cadherin (Kitajiri et al., 2004; Neng et al., 2013a; Yang et al., 2011). Recent experiments have shown that up-regulation of TJ and adherens junction protein improves barrier integrity (Zhang et al., 2012), while, conversely, down-regulation of TJ and adherens junction protein increases permeability (Neng et al., 2013a; Zhang et al., 2012). Transcellular permeability, on the other hand, is dependent on transporters and channels. The intrastrial fluid–blood barrier is found to be rich in transporters. For example, with mass spectrometry analysis of isolated strial capillaries, Yang et al., (2011) reported that about 40% of barrier proteins relate to transport activities. ATP1A1 is the most prevalent molecular transporter on a mass basis.
Permeability of the vascular barrier has also been shown to be related to basement membrane properties such as density and molecular conformation in other organs (Azzi et al., 2013; Qiu et al., 2012). However, how the change of BM structure in the intrastrial fluid-blood barrier influences the vascular permeability has not yet been studied.
4. Intrastrial fluid-blood barrier and hearing disorders
Recent experimental results from different laboratories demonstrate the potential link of dysfunction of the intrastrial fluid-blood barrier in different hearing disorders, including noise-induced hearing loss (Shi, 2009; Shi et al., 2003; Zhang et al., 2012), age-related hearing loss (Gratton et al., 1996; Gratton et al., 1997; Neng et al., 2015; Ohlemiller et al., 2009), autoimmune disease (Lin et al., 1997; Ruckenstein et al., 1999), genetic hearing disorders (Cohen-Salmon et al., 2007; Fujimura et al., 2005; Jabba et al., 2006; Kitamura et al., 1994), and inflammatory edema and hydrops (Hirose et al., 2014; Zhang et al., 2015). In addition, the barrier is also the point of entry for certain ototoxic drugs, such as cisplatin and gentamicin, which permeate the cochlea through the blood barrier and damage hearing function (Dai et al., 2008; Laurell et al., 2000; Wang et al., 2009). Although these pathological conditions have common features such as changes in vascular permeability and selectivity of the intrastrial fluid-blood barrier, each of them has distinct characteristics.
Noise-induced hearing loss (NIHL)
Acoustic trauma damages sensory cells, neurons, and support cells as well as disrupts the microcirculation in the cochlea (Canlon, 1987; Canlon, 1988; Hultcrantz et al., 1987; Kamogashira et al., 2015; Kujawa et al., 2015; Liberman et al., 2015; Ohlemiller et al., 2007; Shi, 2009; Shi et al., 2007; Wang et al., 2002; Yoshida et al., 1999). Increased vascular permeability, reduced circulation (ischemia), aggregation of leukocytes, and injury to endothelial cells are frequently seen in loud sound exposed animals (Goldwyn et al., 1997; Hultcrantz et al., 1987; Lamm et al., 1999; Quirk et al., 1992; Scheibe et al., 1993; Seidman et al., 1999; Shi et al., 2007; Suzuki et al., 2002). Further studies have revealed structural and molecular changes in the intrastrial fluid-blood barrier after acoustic trauma, including decreased expression of tight- and adherens-junction proteins, loosened TJs between ECs, and increased vascular permeability (Yang et al., 2011; Zhang et al., 2013). PCs are particularly vulnerable to acoustic trauma. Upon exposure to loud sound, some PCs undergo changes. They develop irregularities in their processes and can migrate from their normal locations attached to endothelial cells, resulting in destabilization of the intrastrial fluid-blood barrier (Shi, 2009). However, the specific signals underlying these changes in cochlear PCs have not been identified. Acoustic trauma also causes a proportion of PVM/Ms to activate, as shown in Figure 4 A-C. The traumatized PVM/Ms produce less PEDF, leading to down-regulation of tight junction-associated proteins and vascular leakage (Zhang et al., 2013). The PEDF produced by normal PVM/Ms is essential for stabilizing the intrastrial fluid–blood barrier, as the PEDF regulates expression of tight junction-associated proteins such as ZO-1 and VE-cadherin (Zhang et al., 2012).
Figure 4.
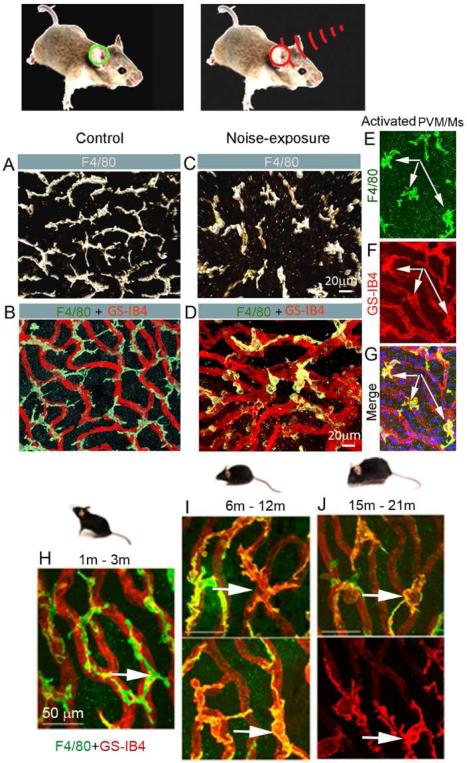
Noise exposure activates PVM/Ms. (A) & (B) Confocal images show the morphology of PVM/Ms on strial capillaries labeled with GS-IB4 (red) in a control animal. (C) & (D) Activated PVM/Ms in noise-exposed animals show reduced branching and withdrawal of ramifications, and display less physical contact with capillaries. (E–G) Double-labeling of whole-mounted stria vascularis shows activated PVM/Ms are positive for GS-IB4. (H–J) show PVM/M activation in C57/6J mice at age 6m (I), with progressively more activation by age 15-21m.
Age-related hearing loss
Capillary damage and regression in aged animals have long been observed (Gratton et al., 1996; Gratton et al., 1997; Neng et al., 2015; Ohlemiller et al., 2008; Ohlemiller et al., 2009; Prazma et al., 1990; Schulte et al., 1992). A degree of strial capillary loss is found in both aged C57/J (Neng et al., 2015) and genetically deficient NOD NON-H2nb1 mice (Ohlemiller et al., 2008). In a human temporal bone study, presbycusis patients showed atrophy of the stria vascularis (Sprinzl et al., 2010). Thickened basement membrane and increased immunoglobulin and laminin deposits are found in the aged strial capillaries (Gratton et al., 1996; Sakaguchi et al., 1997a; Sakaguchi et al., 1997b). For example, Thomopoulos et al., (1997) reported 65-85% of gerbils 33 months or older had strial capillaries with thickened basement membranes (BM), exceeding by several-fold that observed in young controls. Aged animals also exhibit a significant decrease in PC and PVM/M density, accompanied by marked morphological changes in all regions of the stria vascularis (Neng et al., 2015). For example, young C57/6BJ animals (<3 months) have an abundance of PCs with a flat and slender morphology, and are tightly associated with ECs. The PCs in older animals (>6 months) are less abundant and have a prominent round body in less physical contact with the endothelium, a morphology previously described as a sign of PC migration (Pfister et al., 2008). At the ultrastructural level, the PCs from aged animals show a loss of cytoplasmic organelles, have a vacuolated appearance, and are detached from ECs (Neng et al., 2015). PVM/Ms are also dramatically different in aged animals. For example, in younger C57/6J mice, PVM/Ms typically exhibit long and branched processes, and are closely associated with strial capillaries, as shown in Figure 4H-I. However, in animals at age 6, 9, and 12 months some of the PVM/Ms show retraction of branching. At age 21 months, some of the PVM/Ms are flat and amoeboid-shaped, as shown in Figure 4J, and exhibit less physical contact with the capillaries. Concurrent with the morphological changes, biochemical markers on the PVM/Ms also undergo a significant change. Terminal galactopyranosyl groups are exposed on membrane surfaces in the older animals, detected by binding to the lectin GS-IB4, as shown in Figure 4. This is the hallmark of macrophage activation (Maddox et al., 1982; Neng et al., 2015).
Autoimmune hearing disorder
Autoimmune disease in the inner ear often causes progressive sensorineural hearing loss and sometimes presents with vestibular symptoms (Meniere's disease) (Fujioka et al., 2014; Goodall, 2015; Greco et al., 2012; Hughes et al., 1983; Kim et al., 2014; Sara et al., 2014). There is strong evidence showing strial capillaries may be a target of autoimmune disease (Ågrup et al., 2006; Goodall, 2015; Lin et al., 1997; Takahashi et al., 1988). For example, deposition of immune-complexes and direct attack of the endothelium by autoantibodies are common features in these hearing and vestibular disorders (Ågrup et al., 2006; Goodall, 2015; Lin et al., 1997; Takahashi et al., 1988). Research using a C3H/lpr autoimmune mouse model has demonstrated the primary defect in hearing is breakdown of strial blood barrier integrity, IgG deposition in strial capillaries, and thickening of the basement membrane (Lin et al., 1997; Trune et al., 1989; Wong et al., 1992; Young et al., 1988). In clinical studies, blood drawn from patients with autoimmune hearing disorders show high levels of anti-endothelial and anti-phospholipid antibodies, including anti-choline transporter-like protein 2 and anti-heat shock protein (HSP70) (Cadoni et al., 2002; Mijovic et al., 2013; Mouadeb et al., 2005; Nair et al., 2004; Ottaviani et al., 1999; Toubi et al., 2004; Yehudai et al., 2006). Restoration of strial vascular function may be effective in treating autoimmune hearing loss.
Genetic hearing loss
Genetic defects in intrastrial fluid-blood barrier components have been identified in several genetically linked hearing loss pathologies, including Norrie Disease, Alport syndrome, Nr3b2(−/−) and Light (Blt) mutant, white spotting (Ws) and Varitint-waddler-J (VaJ) mouse mutants, and connexin 30 deficiency related hearing loss ((Rehm et al., 2002) (Cable et al., 1998; Cable et al., 1992; Cable et al., 1993; Chen et al., 2007; Cohen-Salmon et al., 2007; Fujimura et al., 2005; Gratton et al., 2005; Kitamura et al., 1994; Kruegel et al., 2013; Ruan et al., 2005; Zallocchi et al., 2013). Norrie Disease presents with profound sensorineural deafness. The primary cause of Norrie Disease is the strial avascularity associated with an Ndp gene defect (Rehm et al., 2002). Dominant white spotting [W/W(v) and W(v)/W(v)] mice are well-known mutants with profound sensorineural hearing loss (Cable et al., 1992; Fujimura et al., 2005). The mutants lack strial intermediate cells. The mice also display a marked thickening of the basement membrane on strial capillaries as well as IgG deposits, similar to that found in aged animals and animals with autoimmune sensorineural hearing loss (Cable et al., 1992; Fujimoto, 1995; Kitamura et al., 1994). Light (Blt) mutant mice lack melanocytes, resulting in strial atrophy and loss of the EP (Cable et al., 1993). Alport syndrome with high-frequency sensorineural hearing loss results from mutations in genes coding for collagen alpha3, alpha4, or alpha5 (Gratton et al., 2005; Kruegel et al., 2013; Zallocchi et al., 2013), and the syndrome exhibits as a thickened capillary basement membrane in the stria. Hearing loss in Nr3b2 mutant mice is associated with reduced density of strial capillaries (Chen et al., 2007) and deficiency in connexin 30, both of which disrupt the intrastrial fluid-blood barrier (Cohen-Salmon et al., 2007). Recent research shows Spinster homolog 2 (Spns2)-deficient mice to rapidly lose auditory sensitivity and EP at 2 to 3 weeks of age. The pathology presents with significant structural changes in strial capillary and marginal cell boundaries (Chen et al., 2014).
Inflammation
Inflammatory factor-induced hearing disorders are hypothesized to be associated with disrupted vascular integrity in the stria vascularis and disturbed endolymph ion homeostasis (Hilger, 1952; Trune et al., 2012). In support of the hypothesis, Zhang et al., (2015) recently showed lipopolysaccharide-induced middle ear inflammation to disrupt the cochlear intrastrial fluid-blood barrier by down-regulating tight junction protein expression. Correspondingly, Hirose et al., (2014) demonstrated that lipopolysaccharide increases entry of serum fluorescein into the perilymph via the blood barrier (Hirose et al., 2014). A study by Quintanilla-Diek, et al. (2013) showed lipopolysaccharide-induced inflammation to increase cytokine levels in the murine cochlea. Action by cytokines may be one of the causes for the increased permeability of the blood barrier. Previous studies have also shown that viral or bacterial infection induces anti-endothelial (anti-phospholipid) antibody attack on glycocalyx components in the barrier (Blank et al., 2007; Blank et al., 2004). These results indicate systemic or local inflammatory events can perturb the normal function of the blood barrier, resulting in homeostatic imbalance and hearing loss.
Intrastrial fluid–blood barrier as an ototoxic drug target
A variety of drugs, including antibacterial aminoglycoside antibiotics such as gentamicin and amikacin, anticancer agents such as cisplatin, carboplatin, nedaplatin, and oxaliplatin as well as loop diuretics such as furosemide, have side effects that damage the sense of hearing or balance in humans and animals (Ding et al., 2012; Kamogashira et al., 2015; Karasawa et al., 2011; Oishi et al., 2012; Rybak et al., 2007; Schacht et al., 2012). Recent research shows the intrastrial fluid–blood barrier might be a main port of entry for certain ototoxic drugs from the blood into cochlear fluids (Adamson, 2009; Dai et al., 2008; Laurell et al., 2000; Wang et al., 2009). This is further evidenced by the enhanced drug uptake and significantly increased hearing damage when the barrier is disrupted by diuretics or noise exposure (Ding et al., 2007; Li et al., 2015). Vasoactive peptides also augment cochlear uptake of ototoxic drugs such as gentamicin (Koo et al., 2011). The pathway of drug uptake from the stria vascularis is found to involve transporter systems as well as channels, including transient receptor potential cation channel subfamily V member 4 (TRPV4) in strial capillaries (Ishibashi et al., 2009; Karasawa et al., 2008). There is less evidence for paracellular transport of the drugs through the barrier (Laurell et al., 1997). Extensive experimental research shows that some ototoxic drugs, including cisplatin, cause structural damage to the stria vascularis (Campbell et al., 1999; Cardinaal et al., 2000; Kohn et al., 1991; Meech et al., 1998).
5. Experimental models for study of the intrastrial fluid-blood barrier
Direct measurement of the strial blood barrier function is challenging and techniques for assessing blood barrier function are still under development. However, recently developed in vitro and in vivo experimental methods are providing an opportunity for more intensive study of the intrastrial fluid-blood barrier than was previously possible. In particular, recently developed in vitro cell line-based models enable direct investigation of cell-cell interactions, which provides a window for better understanding of how intrastrial fluid–blood barrier integrity is maintained.
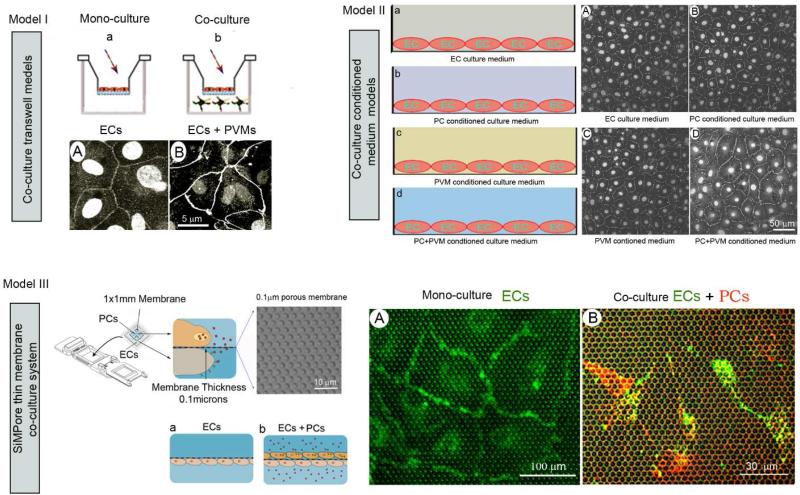
In vitro cell line based blood barrier models have been widely used in functional studies of the blood-brain barrier and blood-retina barrier for decades (Duport et al., 1998; Lai et al., 2005). In recent work on the inner ear, Neng et al., (2013b) demonstrated use of three different in vitro blood barrier 2D models to directly study the specific role of cochlear EC, PC, and PVM/M signaling on blood-labyrinth barrier integrity. The models included a co-cultivated Transwell model, shown in Figure 6a (Model I), conditioned medium co-culture model, shown in Figure 6b (Model II), and CytoVu/SiMPore thin membrane co-culture model, shown in Figure 6c (Model III). The purpose of the study at hand will determine which in vitro blood barrier model is most suitable. For example, the co-cultivated Transwell and conditioned medium co-culture models enable investigators to explore the effect of signaling in paracrine / juxtacrine pathways between vascular cells on TJ formation. The models are also useful for testing drug candidates (studies of drug permeability, toxicity, interaction with efflux transporters). Compared with models I and II, the CytoVu/SiMPore thin membrane co-culture model enables more intensive study of the vascular direct cell-cell interactions which underlie formation of the barrier.
In addition to cell culture–based 2D in vitro blood barrier models, newly developed cell culture–based 3D matrigel models provide an additional tool for understanding how individual vascular cell interactions affect the properties of the vessel. For example with this experimental model, , Neng et al., (2015) showed PVM/Ms play an essential role in stabilizing the artificial capillary networks formed in endothelial cell cultures (Figure 6). Likewise, the strial PCs were shown to play a role in the sprouting of new blood vessels characteristic of angiogenesis, as shown in Figure 6.
In vitro cell culture-based models are useful tools for determining the interaction between vascular blood barrier cells and testing potential drug candidates, although care should be exercised in selecting the model. Some models are better suited to the task than others and caveats apply to all. In addition, factors such as formulation of the medium and cell passage number can result in significant variation. It should also be kept in mind the information gained from the in vitro model may not directly reflect the functionality of the blood barrier under physiological conditions. The findings will always need to be validated in an in vivo animal model.
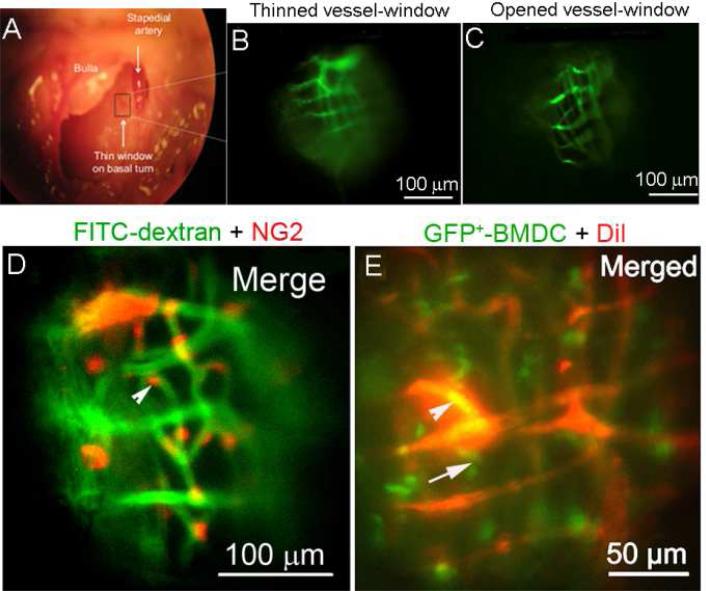
Newly developed in vivo mouse-based models, including intra-vital fluorescence microscopy and enhanced MRI techniques, give the larger picture of blood barrier function under relatively intact physiological conditions. The recently developed thin or open vessel-window approach, used in combination with fluorescence intra-vital microscopy, can be used to study barrier physiology in the cochlear lateral wall under normal and pathological conditions (Shi et al., 2014). The open vessel-window approach, if used in conjunction with a high spatial and temporal resolution imaging system and fluorescent tracers, can provide sufficient resolution for determination of vascular permeability and cell migration. The approach is also useful for examining changes in cell morphology and other pathophysiological parameters in mouse models. For example, genetic NG2 labeled PCs can be observed in NG2DsRedBAC transgenic mice in the open window preparation (as shown in Figure 7D) and PC contractility in response to agents such as CaCl2 in lateral wall capillaries can also be studied (Shi et al., 2014). The open vessel-window approach has also proved extremely useful for tracing the extravasation of GFP+-labeled cells (or other inflammatory cells) from the intrastrial blood barrier (shown in Figure 7E) and assessing vascular permeability under different conditions (Shi et al., 2014). However, experiments with the open vessel-window approach are challenging. Subtle animal movements, such as those from animal breathing, arterial pulse, pulsatile blood flow, and CSF communication with perilymph, cause translation of the imaging location, making correlation and interpretation of the results difficult. Minimization of animal motion and stabilization of image focus are critical for obtaining useable data.
Figure 7.
(A) The surgical view shows an opened bulla and location of a vessel-window on the basal turn of a murine cochlea (black rectangle in panel A). Cochlear blood vessels are visualized with intravenously administered FITC-dextran. (B) & (C) compare in vivo images of strial blood vessels though a thin vessel-window and open vessel-window. (D) An open vessel-window preparation was used to visualize PCs and determine PC contractility using a NG2 DsRed labeled-PC transgenic cell mouse model. (E) Migrated GFP+-bone marrow cells are shown extravasated through blood vessels (red, labeled by Dil). The arrowhead alone points to a GFP+ bone marrow cell within the vessel, the arrow to a GFP+ bone marrow cell outside the vessel.
Alternatively, a thinned vessel-window approach, recently adopted from the thin cranial window (Drew et al., 2010), minimizes disruption of the homeostatic balance in the lateral wall and enables study of barrier function under relatively intact physiological conditions. The thin vessel-window approach allows visualization of vascular structures in the lateral wall for longer periods of time. For example, a successful thin vessel-window remains clear for about a week (Shi et al., 2014). However, the main drawback with the thin window preparation is the increased optical scattering (relative to the open window preparation) and the bone auto-fluorescence which increases the background signal in the image. The increased background limits the image resolution. Another challenge is that an exceptionally steady hand with fine surgery skills (under the microscope) are needed to obtain a smooth bone surface which minimizes light scattering.
Magnetic resonance imaging (MRI)
Recent developments in MRI offer exciting opportunities for structural, functional, and metabolic investigation in an intact, living cochlea. In particular, dynamic contrast enhanced (DCE) MRI using gadolidium-chelate (GdC) or gadolidium based contrast agents (GBCA) is increasingly being used to assess changes in vascular permeability (Le Floc'h et al., 2014; Walton et al., 2015). Intense noise, inflammation, and immune reactions disrupt the blood-barrier and cause leakage of GdC. Using contrast enhanced-MRI and GBCA, lipopolysaccharide (LPS) induced vascular leakage can be assessed in guinea pig (Le Floc'h et al., 2014; Walton et al., 2015). In humans, the technique provides an opportunity to visualize the different cochlear compartments and directly evaluate the integrity of inner ear barriers. For example, using three-dimensional fluid attenuated inversion recovery (3D-FLAIR3D-FLAIR) MRI, Tanigawa et al., (2010) found changes in the composition of the inner ear fluid in patients with low-tone sudden deafness. MRI also holds considerable potential for clinical assessment of labyrinth disease (Manfrè et al., 1999) and Meniere's disease (Pyykkö et al., 2010; Zou et al., 2009).
Summary
The intrastrial fluid-blood barrier comprises a microvascular endothelium closely associated with a substantial population of accessory cells (PCs and PVM/Ms) and a specific matrix of extracellular BM proteins that, together, constitute a unique “cochlear vascular unit.” A functional intrastrial fluid-blood barrier is critical for maintaining solute and ion homeostasis in the inner ear and preventing the influx of toxic substances in the stria vascularis. Disruption of the intrastrial fluid-blood barrier is considered to be involved in various clinical hearing disorders, including autoimmune inner ear disease, Meniere's disease, drug-induced hearing loss, noise-induced hearing loss, sudden deafness, and genetically-linked hearing dysfunction. Despite its central importance, the physiology of the intrastrial fluid-blood barrier in hearing health and disease remains poorly understood. The current lack of knowledge is limiting development of effective drug therapies for blood barrier dysfunction-related hearing loss. A better understanding of intrastrial fluid blood barrier pathophysiology is the foundation needed for developing new medical interventions treating blood barrier related hearing loss and balance disorders.
Figure 5.
The schematic illustrates several variations of the in vitro blood-labyrinth barrier model. (a) Model I shows a schematic of cells co-cultivated on a Transwell layer. Images of an EC monolayer labeled with antibody for ZO-1 are shown in both a mono-culture and co-culture setup. (b) Model II shows an EC monolayer labeled with antibody for ZO-1 and treated with different conditioning growth media. (c) Model III is a CytoVu/SiMPore thin membrane co-culture system. (d) The accompanying confocal fluorescence images show direct visualization of BSI-B4-labeled ECs on the thin membrane (left) and a population of PE-PDGFRβ–labeled PCs to one side of the thin membrane and FITC-BSI-B4–labeled ECs on the other (right).
Highlights.
The blood-labyrinth-barrier (BLB) in the stria vascularis (“intra-strial fluid-blood barrier”) is comprised of endothelial cells in the strial microvasculature, elaborated tight and adherens junctions, basement membrane, pericytes, and perivascular resident macrophage-like melanocytes, which together form a complex “cochlear-vascular unit” in the stria vascularis.
A functional intra-strial fluid-blood barrier is critical for maintaining solute and ion homeostasis and preventing the influx of toxic substances in the inner ear.
Disruption of intra-strial fluid-blood barrier is associated with many clinical hearing disorders, including autoimmune inner ear disease, drug-induced hearing loss, noise-induced hearing loss, and genetically-linked hearing dysfunction. Therapeutic targeting of BLB function may lead to improved hearing health and the amelioration of hearing loss.
Acknowledgments
All the data presented in this review reflects the efforts of my colleagues and students at the Oregon Hearing Research Center. In particular, the author is deeply indebted to Dr. Alfred Nuttall for stimulating discussion and advice. The author also thanks Mr. Allan Kachelmeier and Ms. Janice Moore for editorial assistance, and Christine Casabar for assistance with the references.
This work was supported by the National Institutes of Health grants NIH NIDCD R01-DC010844 (XS), DC R21DC1239801 (XS), and NIH NIDCD P30-DC005983.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adamson R. Role of macrophages in normal wound healing: an overview. J Wound Care. 2009;18:349–351. doi: 10.12968/jowc.2009.18.8.43636. [DOI] [PubMed] [Google Scholar]
- Ågrup C, Luxon LM. Immune-mediated inner-ear disorders in neuro-otology. Curr Opin Neurol. 2006;19:26–32. doi: 10.1097/01.wco.0000194143.02171.46. [DOI] [PubMed] [Google Scholar]
- Allt G, Lawrenson J. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers. Front Oncol. 2013;3 doi: 10.3389/fonc.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53:637–644. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis, Mechanisms of Angiogenesis. Springer. pp; 2005. pp. 115–125. [DOI] [PubMed] [Google Scholar]
- Bhattacharya G, Miller C, Kimberling WJ, Jablonski MM, Cosgrove D. Localization and expression of usherin: a novel basement membrane protein defective in people with Usher's syndrome type IIa. Hear Res. 2002;163:1–11. doi: 10.1016/s0378-5955(01)00344-6. [DOI] [PubMed] [Google Scholar]
- Blank M, Barzilai O, Shoenfeld Y. Molecular mimicry and auto-immunity. Clin Rev Allergy Immunol. 2007;32:111–118. doi: 10.1007/BF02686087. [DOI] [PubMed] [Google Scholar]
- Blank M, Asherson R, Cervera R, Shoenfeld Y. Antiphospholipid syndrome infectious origin. J Clin Immunol. 2004;24:12–23. doi: 10.1023/B:JOCI.0000018058.28764.ce. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong J-S. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bush WD, Simon JD. Quantification of Ca2+ binding to melanin supports the hypothesis that melanosomes serve a functional role in regulating calcium homeostasis. Pigment Cell Res. 2007;20:134–139. doi: 10.1111/j.1600-0749.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- Cable J, Steel KP. Combined cochleo-saccular and neuroepithelial abnormalities in the Varitint-waddler-J (VaJ) mouse. Hear Res. 1998;123:125–136. doi: 10.1016/s0378-5955(98)00107-5. [DOI] [PubMed] [Google Scholar]
- Cable J, Barkway C, Steel K. Characteristics of stria vascularis melanocytes of viable dominantspotting (WvWv) mouse mutants. Hear Res. 1992;64:6–20. doi: 10.1016/0378-5955(92)90164-i. [DOI] [PubMed] [Google Scholar]
- Cable J, Jackson IJ, Steel KP. Light (Blt), a mutation that causes melanocyte death, affects stria vascularis function in the mouse inner ear. Pigment Cell Res. 1993;6:215–225. doi: 10.1111/j.1600-0749.1993.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Cadoni G, Fetoni AR, Agostino S, Santis AD, Manna R, Ottaviani F, Paludetti G. Autoimmunity in sudden sensorineural hearing loss: possible role of anti-endothelial cell autoantibodies. Acta Otolaryngol. 2002;122:30–33. doi: 10.1080/00016480260094947. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Rybak LP, Hughes LF. D-Methionine protects against cisplatin damage to the stria vascularis. Hear Res. 1999;138:13–28. doi: 10.1016/s0378-5955(99)00142-2. [DOI] [PubMed] [Google Scholar]
- Canlon B. Acoustic overstimulation alters the morphology of the tectorial membrane. Hear Res. 1987;30:127–134. doi: 10.1016/0378-5955(87)90130-4. [DOI] [PubMed] [Google Scholar]
- Canlon B. The effect of acoustic trauma on the tectorial membrane, stereocilia, and hearing sensitivity: possible mechanisms underlying damage, recovery, and protection. Scand Audiol Suppl. 1988;27:1–45. [PubMed] [Google Scholar]
- Cardinaal RM, de Groot JC, Huizing EH, Veldman JE, Smoorenburg GF. Dose-dependent effect of 8-day cisplatin administration upon the morphology of the albino guinea pig cochlea. Hear Res. 2000;144:135–146. doi: 10.1016/s0378-5955(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Chen J, Nathans J. Estrogen-related receptor β/NR3B2 controls epithelial cell fate and endolymph production by the stria vascularis. Dev Cell. 2007;13:325–337. doi: 10.1016/j.devcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Chen J, Ingham N, Kelly J, Jadeja S, Goulding D, Pass J, Mahajan VB, Tsang SH, Nijnik A, Jackson IJ. Spinster homolog 2 (spns2) deficiency causes early onset progressive hearing loss. PLoS Genet. 2014 doi: 10.1371/journal.pgen.1004688. DOI: 10.1371/journal.pgen.1004688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chéret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause K-H, Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Salmon M, Regnault B, Cayet N, Caille D, Demuth K, Hardelin JP, Janel N, Meda P, Petit C. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci U S A. 2007;104:6229–34. doi: 10.1073/pnas.0605108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D, Samuelson G, Pinnt J. Immunohistochemical localization of basement membrane collagens and associated proteins in the murine cochlea. Hear Res. 1996;97:54–65. [PubMed] [Google Scholar]
- Cui Q, Yin Y, Benowitz L. The role of macrophages in optic nerve regeneration. Neuroscience. 2009;158:1039–1048. doi: 10.1016/j.neuroscience.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai CF, Steyger PS. A systemic gentamicin pathway across the stria vascularis. Hear Res. 2008;235:114–124. doi: 10.1016/j.heares.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Flores L, Gutiérrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes, a review of their morphological and functional characteristics. Histol Histopath. 1991;6:269–286. [PubMed] [Google Scholar]
- Ding D, Allman BL, Salvi R. Review: ototoxic characteristics of platinum antitumor drugs. Anat Rec. 2012;295:1851–1867. doi: 10.1002/ar.22577. [DOI] [PubMed] [Google Scholar]
- Ding D, Jiang H, Wang P, Salvi R. Cell death after co-administration of cisplatin and ethacrynic acid. Hear Res. 2007;226:129–139. doi: 10.1016/j.heares.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Dräger U. Calcium binding in pigmented and albino eyes. Proc Natl Acad Sci U S A. 1985;82:6716–6720. doi: 10.1073/pnas.82.19.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PJS, A.Y., Driscoll JD, Knutsen PM, Blinder P, Davalos D, Akassoglou K, Tsai PS, Kleinfeld D. Chronic optical access through a polished and reinforced thinned skull. Nat Methods. 2010;7:981–4. doi: 10.1038/nmeth.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duport S, Robert F, Muller D, Grau G, Parisi L, Stoppini L. An in vitro blood–brain barrier model: Cocultures between endothelial cells and organotypic brain slice cultures. Proc Natl Acad Sci U S A. 1998;95:1840–1845. doi: 10.1073/pnas.95.4.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl C, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009;40:S13–S15. doi: 10.1161/STROKEAHA.108.533117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz P, Helmreich M, Stach M, Franz-Italon C, Böck P. Distribution of actin and myosin in the cochlear microvascular bed. Acta Otolaryngol. 2004;124:481–485. doi: 10.1080/00016480410017206. [DOI] [PubMed] [Google Scholar]
- Freyer L, Aggarwal V, Morrow BE. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development. 2011;138:5403–5414. doi: 10.1242/dev.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K. Pericyte-endothelial gap junctions in developing rat cerebral capillaries: A fine structural study. Anat Rec. 1995;242:562–565. doi: 10.1002/ar.1092420412. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Suzuki H, Shimizu T, Tokui N, Kitamura T, Udaka T, Doi Y. Pathological alterations of strial capillaries in dominant white spotting W/W v mice. Hear Res. 2005;209:53–59. doi: 10.1016/j.heares.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Okano H, Ogawa K. Inflammatory and immune responses in the cochlea: potential therapeutic targets for sensorineural hearing loss. Front Pharmacol. 2014;5 doi: 10.3389/fphar.2014.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwyn BG, Quirk WS. Calcium channel blockade reduces noise-induced vascular permeability in cochlear stria vascularis. Laryngoscope. 1997;107:1112–6. doi: 10.1097/00005537-199708000-00019. [DOI] [PubMed] [Google Scholar]
- Goodall AF. Current understanding of the pathogenesis of autoimmune inner ear disease: a review. Clin Otolaryngol. 2015 doi: 10.1111/coa.12432. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Schmiedt RA, Schulte BA. Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Hear Res. 1996;102:181–90. doi: 10.1016/s0378-5955(96)90017-9. [corrected and republished article originallly printed in Hear Res 1996 May;94(1-2):116-24].
- Gratton MA, Schulte BA, Smythe NM. Quantification of the stria vascularis and strial capillary areas in quiet-reared young and aged gerbils. Hear Res. 1997;114:1–9. doi: 10.1016/s0378-5955(97)00025-7. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Meehan DT, Smyth BJ, Cosgrove D. Strial marginal cells play a role in basement membrane homeostasis: in vitro and in vivo evidence. Hear Res. 2002;163:27–36. doi: 10.1016/s0378-5955(01)00358-6. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Rao VH, Meehan DT, Askew C, Cosgrove D. Matrix metalloproteinase dysregulation in the stria vascularis of mice with Alport syndrome: implications for capillary basement membrane pathology. Am J Pathol. 2005;166:1465–74. doi: 10.1016/S0002-9440(10)62363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco A, Gallo A, Fusconi M, Marinelli C, Macri G, De Vincentiis M. Meniere's disease might be an autoimmune condition? Autoimmun Rev. 2012;11:731–738. doi: 10.1016/j.autrev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Greenhalgh SN, Iredale JP, Henderson NC. Origins of fibrosis: pericytes take centre stage. F1000Prime Rep. 2013;5 doi: 10.12703/P5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O/'Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Nin F, Tsuzuki C, Kurachi Y. How is the highly positive endocochlear potential formed? The specific architecture of the stria vascularis and the roles of the ion-transport apparatus. Pflugers Arch. 2010;459:521–533. doi: 10.1007/s00424-009-0754-z. [DOI] [PubMed] [Google Scholar]
- Hilger JA. The common ground of allergy, autonomic dysfunction and endocrine imbalance. Trans Am Acad Ophthalmol Otolaryngol. 1952;57:443–446. [PubMed] [Google Scholar]
- Hirose K, Hartsock JJ, Johnson S, Santi P, Salt AN. Systemic lipopolysaccharide compromises the blood-labyrinth barrier and increases entry of serum fluorescein into the perilymph. J Assoc Res Otolaryngol. 2014;15:707–719. doi: 10.1007/s10162-014-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G, Kinney S, Barna B, Calabrese L. Autoimmune reactivity in Meniere's disease: a preliminary report. Laryngoscope. 1983;93:410–417. doi: 10.1002/lary.1983.93.4.410. [DOI] [PubMed] [Google Scholar]
- Hultcrantz E, Nuttall AL. Effect of hemodilution on cochlear blood flow measured by laser-Doppler flowmetry. Am J Otolaryngol. 1987;8:16–22. doi: 10.1016/s0196-0709(87)80014-5. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Takumida M, Akagi N, Hirakawa K, Anniko M. Changes in transient receptor potential vanilloid (TRPV) 1, 2, 3 and 4 expression in mouse inner ear following gentamicin challenge. Acta Otolaryngol. 2009;129:116–126. doi: 10.1080/00016480802032835. [DOI] [PubMed] [Google Scholar]
- Jabba SV, Oelke A, Singh R, Maganti RJ, Fleming S, Wall SM, Everett LA, Green ED, Wangemann P. Macrophage invasion contributes to degeneration of stria vascularis in Pendred syndrome mouse model. BMC Med. 2006;4:37. doi: 10.1186/1741-7015-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhn SK. Barrier systems in the inner ear. Acta Otolaryngol Suppl. 1988;458:79–83. doi: 10.3109/00016488809125107. [DOI] [PubMed] [Google Scholar]
- Juhn SK, Rybak LP. Labyrinthine barriers and cochlear homeostasis. Acta Otolaryngol. 1981;91:529–34. doi: 10.3109/00016488109138538. [DOI] [PubMed] [Google Scholar]
- Juhn SK, Hunter BA, Odland RM. Blood-labyrinth barrier and fluid dynamics of the inner ear. Int Tinnitus J. 2001;7:72–83. [PubMed] [Google Scholar]
- Kamogashira T, Fujimoto C, Yamasoba T. Reactive oxygen species, apoptosis, and mitochondrial D\dysfunction in hearing loss. Biomed Res Int. 2015;2015 doi: 10.1155/2015/617207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T, Steyger PS. Intracellular mechanisms of aminoglycoside-induced cytotoxicity. Integr Biol. 2011;3:879–886. doi: 10.1039/c1ib00034a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T, Wang Q, Fu Y, Cohen DM, Steyger PS. TRPV4 enhances the cellular uptake of aminoglycoside antibiotics. J Cell Sci. 2008;121:2871–2879. doi: 10.1242/jcs.023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim JY, Lee HJ, Gi M, Kim BG, Choi JY. Autoimmunity as a candidate for the etiopathogenesis of Meniere's disease: detection of autoimmune reactions and diagnostic biomarker candidate. PLoS One. 2014 doi: 10.1371/journal.pone.0111039. DOI: 10.1371/journal.pone.0111039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajiri S.-i., Furuse M, Morita K, Saishin-Kiuchi Y, Kido H, Ito J, Tsukita S. Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear Res. 2004;187:25–34. doi: 10.1016/s0378-5955(03)00338-1. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Sakagami M, Umemoto M, Takeda N, Doi K, Kasugai T, Kitamura Y. Strial dysfunction in a melanocyte deficient mutant rat (Ws/Ws rat). Acta Otolaryngol. 1994;114:177–181. doi: 10.3109/00016489409126038. [DOI] [PubMed] [Google Scholar]
- Kohn S, Nir I, Fradis M, Podoshin L, David YB, Zidan J, Robinson E. Toxic effects of cisplatin alone and in combination with gentamicin in stria vascularis of guinea pigs. Laryngoscope. 1991;101:709–716. doi: 10.1288/00005537-199107000-00004. [DOI] [PubMed] [Google Scholar]
- Koo J-W, Wang Q, Steyger PS. Infection-mediated vasoactive peptides modulate cochlear uptake of fluorescent gentamicin. Audiol Neurootol. 2011;16:347–358. doi: 10.1159/000322851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel J, Rubel D, Gross O. Alport syndrome—insights from basic and clinical research. Nat Rev Nephrol. 2013;9:170–178. doi: 10.1038/nrneph.2012.259. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330:191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-H, Kuo K-H. The critical component to establish in vitro BBB model: Pericyte. Brain Res Rev. 2005;50:258–265. doi: 10.1016/j.brainresrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Lamm K, Arnold W. Successful treatment of noise-induced cochlear ischemia, hypoxia, and hearing loss. Ann N Y Acad Sci. 1999;884:233–48. doi: 10.1111/j.1749-6632.1999.tb08645.x. [DOI] [PubMed] [Google Scholar]
- Laurell G, Teixeira M, Sterkers O, Ferrary E. Paracellular transport properties of inner ear barriers do not account for cisplatin toxicity in the rat. Hear Res. 1997;110:135–140. doi: 10.1016/s0378-5955(97)00067-1. [DOI] [PubMed] [Google Scholar]
- Laurell G, Viberg A, Teixeira M, Sterkers O, Ferrary E. Blood-perilymph barrier and ototoxicity: an in vivo study in the rat. Acta Otolaryngol. 2000;120:796–803. doi: 10.1080/000164800750061624. [DOI] [PubMed] [Google Scholar]
- Le Floc'h J, Tan W, Telang RS, Vlajkovic SM, Nuttall A, Rooney WD, Pontré B, Thorne PR. Markers of cochlear inflammation using MRI. J Magn Reson Imaging. 2014;39:150–161. doi: 10.1002/jmri.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kachelmeier A, Furness DN, Steyger PS. Local mechanisms for loud sound-enhanced aminoglycoside entry into outer hair cells. Front Cell Neurosci. 2015;9:130. doi: 10.3389/fncel.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Suzuki J, Liberman MC. Erratum to: Dynamics of cochlear synaptopathy after acoustic overexposure. J Assoc Res Otolaryngol. 2015;16:221. doi: 10.1007/s10162-015-0514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DW, Trune DR. Breakdown of stria vascularis blood-labyrinth barrier in C3H/lpr autoimmune disease mice. Otolaryngol Head Neck Surg. 1997;117:530–4. doi: 10.1016/S0194-59989770026-3. [DOI] [PubMed] [Google Scholar]
- Maddox DE, Shibata S, Goldstein IJ. Stimulated macrophages express a new glycoprotein receptor reactive with Griffonia simplicifolia I-B4 isolectin. Proc Natl Acad Sci U S A. 1982;79:166–170. doi: 10.1073/pnas.79.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfrè L, Bencivinni F, Caronia A, Angileri T, Manasia G, De Maria M. Changes in the blood-labyrinth barrier. Assessments by magnetic resonance. Radiol Med. 1999;98:472–476. [PubMed] [Google Scholar]
- Marcus DC, Rokugo M, Ge X-X, Thalmann R. Response of cochlear potentials to presumed alterations of ionic conductance: endolymphatic perfusion of barium, valinomycin and nystatin. Hear Res. 1983;12:17–30. doi: 10.1016/0378-5955(83)90116-8. [DOI] [PubMed] [Google Scholar]
- Meech RP, Campbell KC, Hughes LP, Rybak LP. A semiquantitative analysis of the effects of cisplatin on the rat stria vascularis. Hear Res. 1998;124:44–59. doi: 10.1016/s0378-5955(98)00116-6. [DOI] [PubMed] [Google Scholar]
- Mijovic T, Zeitouni A, Colmegna I. Autoimmune sensorineural hearing loss: the otology–rheumatology interface. Rheumatology. 2013:ket009. doi: 10.1093/rheumatology/ket009. [DOI] [PubMed] [Google Scholar]
- Mitrasinovic OM, Murphy GM. Accelerated phagocytosis of amyloid-β by mouse and human microglia overexpressing the macrophage colony-stimulating factor receptor. J Biol Chem. 2002;277:29889–29896. doi: 10.1074/jbc.M200868200. [DOI] [PubMed] [Google Scholar]
- Mitrasinovic OM, Grattan A, Robinson CC, Lapustea NB, Poon C, Ryan H, Phong C, Murphy GM. Microglia overexpressing the macrophage colony-stimulating factor receptor are neuroprotective in a microglial-hippocampal organotypic coculture system. J Neurosci. 2005;25:4442–4451. doi: 10.1523/JNEUROSCI.0514-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouadeb DA, Ruckenstein MJ. Antiphospholipid inner ear syndrome. Laryngoscope. 2005;115:879–883. doi: 10.1097/01.MLG.0000158666.15447.37. [DOI] [PubMed] [Google Scholar]
- Murillo-Cuesta S, Contreras J, Zurita E, Cediel R, Cantero M, Varela-Nieto I, Montoliu L. Melanin precursors prevent premature age-related and noise-induced hearing loss in albino mice. Pigment Cell Melanoma Res. 2010;23:72–83. doi: 10.1111/j.1755-148X.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- Nair TS, Kozma KE, Hoefling NL, Kommareddi PK, Ueda Y, Gong T-W, Lomax MI, Lansford CD, Telian SA, Satar B. Identification and characterization of choline transporter-like protein 2, an inner ear glycoprotein of 68 and 72 kDa that is the target of antibody-induced hearing loss. J Neurosci. 2004;24:1772–1779. doi: 10.1523/JNEUROSCI.5063-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neng L, Zhang F, Kachelmeier A, Shi X. Endothelial cell, pericyte, and perivascular resident macrophage-type melanocyte interactions regulate cochlear intrastrial fluid-blood barrier permeability. J Assoc Res Otolaryngol. 2013a;14:175–85. doi: 10.1007/s10162-012-0365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neng L, Zhang J, Yang J, Zhang F, Lopez IA, Dong M, Shi X. Structural changes in thestrial blood–labyrinth barrier of aged C57BL/6 mice. Cell Tissue Res. 2015:1–12. doi: 10.1007/s00441-015-2147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neng L, Zhang W, Hassan A, Zemla M, Kachelmeier A, Fridberger A, Auer M, Shi X. Isolation and culture of endothelial cells, pericytes and perivascular resident macrophage-like melanocytes from the young mouse ear. Nat Protoc. 2013b;8:709–20. doi: 10.1038/nprot.2013.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Genetic dependence of cochlear cells and structures injured by noise. Hear Res. 2007;224:34–50. doi: 10.1016/j.heares.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Rice MER, Gagnon PM. Strial microvascular pathology and age-associated endocochlear potential decline in NOD congenic mice. Hear Res. 2008;244:85–97. doi: 10.1016/j.heares.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Rybak Rice ME, Lett JM, Gagnon PM. Absence of strial melanin coincides with age-associated marginal cell loss and endocochlear potential decline. Hear Res. 2009;249:1–14. doi: 10.1016/j.heares.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Oishi N, Talaska AE, Schacht J. Ototoxicity in dogs and cats. Vet Clin North Am Small Anim Pract. 2012;42:1259–1271. doi: 10.1016/j.cvsm.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani F, Cadoni G, Marinelli L, Fetoni AR, De Santis A, Romito A, Vulpiani P, Manna R. Anti-endothelial autoantibodies in patients with sudden hearing loss. Laryngoscope. 1999;109:1084–1087. doi: 10.1097/00005537-199907000-00014. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands J-L, Shani M, Deutsch U, Hammes H-P. Pericyte migration a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008;57:2495–2502. doi: 10.2337/db08-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PloS One. 2009 doi: 10.1371/journal.pone.0007475. DOI: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plonka P, Passeron T, Brenner M, Tobin D, Shibahara S, Thomas A, Slominski A, Kadekaro A, Hershkovitz D, Peters E. What are melanocytes really doing all day long...? Exp Dermatol. 2009;18:799–819. doi: 10.1111/j.1600-0625.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prazma J, Carrasco VN, Butler B, Waters G, Anderson T, Pillsbury HC. Cochlear microcirculation in young and old gerbils. Arch Otolaryngol Head Neck Surg. 1990;116:932. doi: 10.1001/archotol.1990.01870080054015. [DOI] [PubMed] [Google Scholar]
- Pufe T, Petersen W, Fändrich F, Varoga D, Wruck CJ, Mentlein R, Helfenstein A, Hoseas D, Dressel S, Tillmann B. Programmable cells of monocytic origin (PCMO): A source of peripheral blood stem cells that generate collagen type II-producing chondrocytes. J Orthop Res. 2008;26:304–313. doi: 10.1002/jor.20516. [DOI] [PubMed] [Google Scholar]
- Pyykkö I, Zou J, Poe D, Nakashima T, Naganawa S. Magnetic resonance imaging of the inner ear in Meniere's disease. Otolaryngol Clin North Am. 2010;43:1059–1080. doi: 10.1016/j.otc.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Hu J, E Van den Steen P, Opdenakker G. Targeting matrix metalloproteinases in acute inflammatory shock syndromes. Comb Chem High Throughput Screen. 2012;15:555–570. doi: 10.2174/138620712801619159. [DOI] [PubMed] [Google Scholar]
- Quaegebeur A, Segura I, Carmeliet P. Pericytes: blood-brain barrier safeguards against neurodegeneration? Neuron. 2010;68:321–323. doi: 10.1016/j.neuron.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Quintanilla-Dieck L, Larrain B, Trune D, Steyger PS. Effect of systemic lipopolysaccharide-induced inflammation on cytokine levels in the murine cochlea a pilot study. Otolaryngol Head Neck Surg. 2013:0194599813491712. doi: 10.1177/0194599813491712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk WS, Laurikainen E, Avinash G, Nuttall AL, Miller JM. The role of endothelin on the regulation of cochlear blood flow (CBF). Assoc Res Otolaryngol. 1992;15:37. [Google Scholar]
- Quraishi IH, Raphael RM. Generation of the endocochlear potential: a biophysical model. Biophys J. 2008;94:L64–L66. doi: 10.1529/biophysj.107.128082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm HL, Zhang D-S, Brown MC, Burgess B, Halpin C, Berger W, Morton CC, Corey DP, Chen Z-Y. Vascular defects and sensorineural deafness in a mouse model of Norrie disease. J Neurosci. 2002;22:4286–4292. doi: 10.1523/JNEUROSCI.22-11-04286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H-B, Zhang N, Gao X. Identification of a novel point mutation of mouse proto oncogene c-kit through N-ethyl-N-nitrosourea mutagenesis. Genetics. 2005;169:819–831. doi: 10.1534/genetics.104.027177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckenstein MJ, Hu L. Antibody deposition in the stria vascularis of the MRL-Fas lpr mouse. Hear Res. 1999;127:137–142. doi: 10.1016/s0378-5955(98)00189-0. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Sakagami K, Wu DM, Puro DG. Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J Physiol. 1999;521:637–650. doi: 10.1111/j.1469-7793.1999.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Harada T, Sano M, Sakai S, Matsunaga T. Quantitative evaluation of pinocytosis of capillaries of the stria vascularis under normal and experimental conditions. Acta Otolaryngol. 1987;103:189–97. [PubMed] [Google Scholar]
- Sakaguchi N, Spicer SS, Thomopoulos GN, Schulte BA. Immunoglobulin deposition in thickened basement membranes of aging strial capillaries. Hear Res. 1997a;109:83–91. doi: 10.1016/s0378-5955(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Spicer SS, Thomopoulos GN, Schulte BA. Increased laminin deposition in capillaries of the stria vascularis of quiet-aged gerbils. Hear Res. 1997b;105:44–56. doi: 10.1016/s0378-5955(96)00180-3. [DOI] [PubMed] [Google Scholar]
- Salt AN, Mleichar I, Thalmann R. Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope. 1987;97:984–991. [PubMed] [Google Scholar]
- Sara S, Teh B, Friedland P. Bilateral sudden sensorineural hearing loss: review. J Laryngol Otol. 2014;128:S8–S15. doi: 10.1017/S002221511300306X. [DOI] [PubMed] [Google Scholar]
- Satoh H, Kawasaki K, Kihara I, Nakano Y. Importance of type IV collagen, laminin, and heparan sulfate proteoglycan in the regulation of labyrinthine fluid in the rat cochlear duct. Eur Arch Otorhinolaryngol. 1998;255:285–288. doi: 10.1007/s004050050060. [DOI] [PubMed] [Google Scholar]
- Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec. 2012;295:1837–1850. doi: 10.1002/ar.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe F, Haupt H, Ludwig C. Intensity-related changes in cochlear blood flow in the guinea pig during and following acoustic exposure. Eur Arch Otorhinolaryngol. 1993;250:281–5. doi: 10.1007/BF00186226. [DOI] [PubMed] [Google Scholar]
- Schulte BA, Schmiedt RA. Lateral wall Na, K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear Res. 1992;61:35–46. doi: 10.1016/0378-5955(92)90034-k. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Quirk WS, Shirwany NA. Mechanisms of alterations in the microcirculation of the cochlea. Ann N Y Acad Sci. 1999;884:226–232. doi: 10.1111/j.1749-6632.1999.tb08644.x. [DOI] [PubMed] [Google Scholar]
- Shepro D, Morel N. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Shi X. Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia inducible factor-1alpha and vascular endothelial growth factor. Am J Pathol. 2009;174:1692–704. doi: 10.2353/ajpath.2009.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell Tissue Res. 2010;342:21–30. doi: 10.1007/s00441-010-1040-2. [DOI] [PubMed] [Google Scholar]
- Shi X, Nuttall AL. Upregulated iNOS and oxidative damage to the cochlear stria vascularis due to noise stress. Brain Res. 2003;967:1–10. doi: 10.1016/s0006-8993(02)04090-8. [DOI] [PubMed] [Google Scholar]
- Shi X, Nuttall AL. Expression of adhesion molecular proteins in the cochlear lateral wall of normal and PARP-1 mutant mice. Hear Res. 2007;224:1–14. doi: 10.1016/j.heares.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Shi X, Han W, Yamamoto H, Tang W, Lin X, Xiu R, Trune DR, Nuttall AL. The cochlear pericytes. Microcirculation. 2008;15:515–29. doi: 10.1080/10739680802047445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Zhang F, Urdang Z, Dai M, Neng L, Zhang J, Chen S, Ramamoorthy S, Nuttall AL. Thin and open vessel windows for intra-vital fluorescence imaging of murine cochlear blood flow. Hear Res. 2014;313:38–46. doi: 10.1016/j.heares.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18:760–763. doi: 10.1111/j.1600-0625.2009.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25:14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulas C, Donahue RE, Dunbar CE, Persons DA, Alvarez X, Williams KC. Genetically modified CD34+ hematopoietic stem cells contribute to turnover of brain perivascular macrophages in long-term repopulated primates. Am J Path. 2009;174:1808–1817. doi: 10.2353/ajpath.2009.081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res. 1996;100:80–100. doi: 10.1016/0378-5955(96)00106-2. [DOI] [PubMed] [Google Scholar]
- Sprinzl G, Riechelmann H. Current trends in treating hearing loss in elderly people: a review of the technology and treatment options–a mini-review. Gerontology. 2010;56:351–358. doi: 10.1159/000275062. [DOI] [PubMed] [Google Scholar]
- Steel K, Barkway C. Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development. 1989;107:453–463. doi: 10.1242/dev.107.3.453. [DOI] [PubMed] [Google Scholar]
- Steel K, Davidson DR, Jackson I. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992;115:1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- Sulaimon SS, Kitchell BE. The biology of melanocytes. Vet Dermatol. 2003;14:57–65. doi: 10.1046/j.1365-3164.2003.00327.x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kaga K. Effect of cisplatin on the negative charge barrier in strial vessels of the guinea pig A transmission electron microscopic study using polyethyleneimine molecules. Eur Arch Otorhinolaryngol. 1996;253:351–355. doi: 10.1007/BF00178291. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kaga K. Effect of cisplatin on the basement membrane anionic sites in the ampulla, macula, and stria vascularis of guinea pigs. Ann Otol Rhinol Laryngol. 1997;106:971–5. doi: 10.1177/000348949710601114. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kitamura K, Nomura Y. Anionic sites of the basement membrane of the labyrinth. Acta Otolaryngol. 1991;111:112–115. doi: 10.3109/00016489109131360. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kitamura K, Nomura Y. Influence of changed blood pH on anionic sites in the labyrinth. Acta Otolaryngol. 1995;115:747–753. doi: 10.3109/00016489509139397. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yamasoba T, Kaga K. Development of the blood-labyrinth barrier in the rat. Hear Res. 1998;116:107–112. doi: 10.1016/s0378-5955(97)00208-6. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yamasoba T, Ishibashi T, Miller JM, Kaga K. Effect of noise exposure on blood–labyrinth barrier in guinea pigs. Hear Res. 2002;164:12–18. doi: 10.1016/s0378-5955(01)00397-5. [DOI] [PubMed] [Google Scholar]
- Takagi K, Kudo A. Bone marrow stromal cell lines having high potential for osteoclast-supporting activity express PPARγ1 and show high potential for differentiation into adipocytes. J Bone Miner Metab. 2008;26:13–23. doi: 10.1007/s00774-007-0787-3. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Harris JP. Anatomic distribution and localization of immunocompetent cells in normal mouse endolymphatic sac. Acta Otolaryngol. 1988;106:409–416. doi: 10.3109/00016488809122264. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Ando M, Sato T, Kakigi A. Three-dimensional and ultrastructural relationships between intermediate cells and capillaries in the gerbil stria vascularis. Hear Res. 2001;155:103–12. doi: 10.1016/s0378-5955(01)00252-0. [DOI] [PubMed] [Google Scholar]
- Tanigawa T, Tanaka H, Sato T, Nakao Y, Katahira N, Tsuchiya Y, Nonoyama H, Ueda H. 3D-FLAIR MRI findings in patients with low-tone sudden deafness. Acta Otolaryngol. 2010;130:1324–1328. doi: 10.3109/00016489.2010.496461. [DOI] [PubMed] [Google Scholar]
- Thomopoulos GN, Spicer SS, Gratton MA, Schulte BA. Age-related thickening of basement membrane in stria vascularis capillaries. Hear Res. 1997;111:31–41. doi: 10.1016/s0378-5955(97)00080-4. [DOI] [PubMed] [Google Scholar]
- Torihara K, Suganuma T, Ide S, Morimitsu T. Anionic sites in blood capillaries of the mouse cochlear duct. Hear Res. 1994;77:69–74. doi: 10.1016/0378-5955(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Toubi E, Halas K, Ben-David J, Sabo E, Kessel A, Luntz M. Immune-mediated disorders associated with idiopathic sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 2004;113:445–449. doi: 10.1177/000348940411300605. [DOI] [PubMed] [Google Scholar]
- Trune DR, Nguyen-Huynh A. Semin Hear. Vol. 33. Thieme Medical Publishers; 2012. Vascular pathophysiology in hearing disorders; pp. 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune DR, Craven JP, Morton JI, Mitchell C. Autoimmune disease and cochlear pathology in the C3H/lpr strain mouse. Hear Res. 1989;38:57–66. doi: 10.1016/0378-5955(89)90128-7. [DOI] [PubMed] [Google Scholar]
- Tsuprun V, Santi P. Proteoglycan arrays in the cochlear basement membrane. Hear Res. 2001;157:65–76. doi: 10.1016/s0378-5955(01)00278-7. [DOI] [PubMed] [Google Scholar]
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Wakaoka T, Motohashi T, Hayashi H, Kuze B, Aoki M, Mizuta K, Kunisada T, Ito Y. Tracing Sox10-expressing cells elucidates the dynamic development of the mouse inner ear. Hear Res. 2013;302:17–25. doi: 10.1016/j.heares.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Walton JH, Ng KF, Anderson SE, Rutledge JC. MRI measurement of blood-brain barrier transport with a rapid acquisition refocused echo (RARE) method. Biochem Biophys Res Commun. 2015;463:479–482. doi: 10.1016/j.bbrc.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Steyger PS. Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells. J Assoc Res Otolaryngol. 2009;10:205–219. doi: 10.1007/s10162-009-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165:1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- Wong ML, Young JS, Nilaver G, Morton JI, Trune DR. Cochlear IgG in the C3H/lpr autoimmune strain mouse. Hear Res. 1992;59:93–100. doi: 10.1016/0378-5955(92)90106-w. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dai M, Wilson TM, Omelchenko I, Klimek JE, Wilmarth PA, David LL, Nuttall AL, Gillespie PG, Shi X. Na+/K+-ATPase α1 identified as an abundant protein in the blood-labyrinth barrier that plays an essential role in the barrier integrity. PLoS One. 2011 doi: 10.1371/journal.pone.0016547. DOI: 10.1371/journal.pone.0016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehudai D, Shoenfeld Y, Toubi E. The autoimmune characteristics of progressive or sudden sensorineural hearing loss. Autoimmunity. 2006;39:153–158. doi: 10.1080/08916930500499599. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Kristiansen A, Liberman MC. Heat stress and protection from permanent acoustic injury in mice. J Neurosci. 1999;19:10116–24. doi: 10.1523/JNEUROSCI.19-22-10116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Morton J, Nilaver G, Trune D. Distribution of IgG in the inner ear of C3H/lpr autoimmune disease mice. Abst Assoc Res Otolaryngol. 1988:225. [Google Scholar]
- Zallocchi M, Johnson BM, Meehan DT, Delimont D, Cosgrove D. α1β1 integrin/Rac1-dependent mesangial invasion of glomerular capillaries in Alport syndrome. Am J Path. 2013;183:1269–1280. doi: 10.1016/j.ajpath.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Dai M, Neng L, Zhang JH, Zhi Z, Fridberger A, Shi X. Perivascular macrophage-like melanocyte responsiveness to acoustic trauma—a salient feature of strial barrier associated hearing loss. FASEB J. 2013;27:3730–3740. doi: 10.1096/fj.13-232892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen S, Hou Z, Cai J, Dong M, Shi X. Lipopolysaccharide-induced middle ear inflammation disrupts the cochlear intra-strial fluid-blood barrier through down-regulation of tight junction proteins. PloS One. 2015 doi: 10.1371/journal.pone.0122572. DOI: 10.1371/journal.pone.0122572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Dai M, Fridberger A, Hassan A, Degagne J, Neng L, Zhang F, He W, Ren T, Trune D, Auer M, Shi X. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc Natl Acad Sci U S A. 2012;109:10388–93. doi: 10.1073/pnas.1205210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Poe D, Bjelke B, Pyykkö I. Visualization of inner ear disorders with MRI in vivo: from animal models to human application. Acta Otolaryngol. 2009;129:22–31. doi: 10.1080/00016480902729850. [DOI] [PubMed] [Google Scholar]
- Zozulya A, Weidenfeller C, Galla H-J. Pericyte–endothelial cell interaction increases MMP-9 secretion at the blood–brain barrier in vitro. Brain Res. 2008;1189:1–11. doi: 10.1016/j.brainres.2007.10.099. [DOI] [PubMed] [Google Scholar]