Abstract
The complex nature of human cognition has resulted in cognitive genomics lagging behind many other fields in terms of gene discovery using genome-wide association study (GWAS) methods. In an attempt to overcome these barriers, the current study utilized GWAS meta-analysis to examine the association of common genetic variation (~8M single-nucleotide polymorphisms (SNP) with minor allele frequency ⩾1%) to general cognitive function in a sample of 35 298 healthy individuals of European ancestry across 24 cohorts in the Cognitive Genomics Consortium (COGENT). In addition, we utilized individual SNP lookups and polygenic score analyses to identify genetic overlap with other relevant neurobehavioral phenotypes. Our primary GWAS meta-analysis identified two novel SNP loci (top SNPs: rs76114856 in the CENPO gene on chromosome 2 and rs6669072 near LOC105378853 on chromosome 1) associated with cognitive performance at the genome-wide significance level (P<5 × 10−8). Gene-based analysis identified an additional three Bonferroni-corrected significant loci at chromosomes 17q21.31, 17p13.1 and 1p13.3. Altogether, common variation across the genome resulted in a conservatively estimated SNP heritability of 21.5% (s.e.=0.01%) for general cognitive function. Integration with prior GWAS of cognitive performance and educational attainment yielded several additional significant loci. Finally, we found robust polygenic correlations between cognitive performance and educational attainment, several psychiatric disorders, birth length/weight and smoking behavior, as well as a novel genetic association to the personality trait of openness. These data provide new insight into the genetics of neurocognitive function with relevance to understanding the pathophysiology of neuropsychiatric illness.
Introduction
Genome-wide association studies (GWAS) of complex quantitative phenotypes such as height1 and body mass index2 have successfully discovered and replicated hundreds of common variants meeting criteria for genome-wide significant association. By contrast, finding genetic loci associated with individual differences in cognitive ability using GWAS has proven challenging, despite considerable evidence from family and twin studies indicating that cognitive ability is highly heritable.3 For example, no genome-wide significant hits were detected in the earliest multi-cohort GWAS meta-analyses of general cognitive ability in ~3500 adults,4 or in ~5000 adults,5 or in ~18 000 youth.6 However, the first results attaining genome-wide significance, in three loci on chromosomes 6, 14 and 19, recently emerged in a GWAS meta-analysis of general cognitive function in 53 949 adults reported by the CHARGE Consortium.7 In addition, a recent study using data collected as part of the UK Biobank project reported three genomic regions significantly associated with performance on a test of verbal numerical reasoning (N=36 035), and two independent loci were significantly associated with performance on a reaction time task (N=111 483).8 However, in the same cohort, no genome-wide significant single-nucleotide polymorphisms (SNP)-based findings were detected for scores on a memory test, despite the large sample size (N=112 067), perhaps due to the low reliability of the very brief assay available.8
Recent GWAS meta-analyses of educational attainment, proposed as a proxy phenotype for cognition,8, 9, 10, 11, 12 have demonstrated that associations can be discovered with sufficient sample size, with the most recent analysis of 293 723 individuals yielding 74 independent SNPs that reached genome-wide significance.9 Nevertheless, this number of hits is an order of magnitude smaller than that reported for a similarly powered GWAS meta-analysis of height, which identified 697 variants that together explained ~16% of the variance for adult height in a sample of 253 288 individuals.1 Thus, the complex nature of human cognition, exacerbated by challenges of precise and reliable measurement, has rendered it a more difficult phenotype with which to gain traction in the era of GWAS discovery.
The importance of uncovering the molecular genetic basis of cognitive functioning is underscored by the fact that neurocognitive deficits represent a critical component of many neuropsychiatric disorders and disease states that can affect health outcomes across the lifespan. As examples, most early appearing neurodevelopmental disorders such as autism spectrum disorder13 and attention-deficit/hyperactivity disorder14 are associated with moderate to relatively impairing comorbid deficits in neurocognitive functioning. Longitudinally, lower cognitive ability scores in childhood have been linked to decreased rates of smoking cessation in adulthood.15 The major neuropsychiatric disorders that typically emerge in early adulthood, such as schizophrenia,16 bipolar disorder,17 anxiety disorders18 and depression,19 are also associated with a range of deficits in neurocognitive function. Human personality traits such as openness to new experiences20 and negative affect21 are, respectively, associated with better and worse neurocognitive performance. Individuals with debilitating neurological illnesses such as Parkinson's disease22, 23 and (by definition) the dementia spectrum including Alzheimer's disease24 also suffer from marked neurocognitive impairments. Further, early life cognitive performance can predict long-term development of illness,25 including mortality.26 From these findings, it has been suggested that general cognitive performance may index global bodily integrity, thereby permitting a potentially broad application of ‘cognitive epidemiology.'27
Deciphering the genetic overlap between cognition and risk for neuropsychiatric illness and other health-relevant traits can provide useful etiological insights and help prioritize likely causal relationships among complex human traits.28 Methods to estimate the genome-wide genetic correlation (rg) between two traits using summary GWAS statistics from published research studies utilizing linkage disequilibrium (LD) score regression procedures have recently become available.28, 29 LD score regression has been used recently to show significant genetic correlations between cognition-related phenotypes and cardiovascular disease,27 physical health30 and neuropsychiatric illness.31 However, the underlying causal variants and the genes through which they act have yet to be identified.32
There were two major aims of the current study: (1) conduct a large-scale (n=35 298) GWAS meta-analysis of general cognitive function in 24 independent cohorts, to identify SNP-based and gene-based loci associated with cognition; and (2) determine the extent of genetic correlation between general cognitive function and published neurobehavioral phenotypes of interest. These aims were executed within the context of the Cognitive Genomics Consortium (COGENT),5, 10 an international collaborative effort designed to study the molecular genetics of cognitive function.
Materials and methods
Participants
To date, COGENT has acquired individual-level neuropsychological, demographic, clinical and SNP array data from 24 studies (comprised of 35 sub-studies) enrolling 35 298 individuals (51.4% females, mean age of 45.6 (s.d.±8.6) years) of European ancestry drawn from the general population in North America, the United Kingdom and the European continent. Table 1 provides details of the individual study cohorts. A few of the cohorts overlap with those previously reported by the CHARGE consortium,7 so comparisons to the CHARGE report7 utilize 30 sub-studies comprising 27 888 fully independent subjects. All subjects provided written, informed consent to protocols approved by their institutional ethics boards in accordance with the Helsinki Declaration.
Table 1. Demographic characteristics of the consortium.
| Cohort | Study name | Country | N | Age mean | Age s.d. | Min age | Max age | N Male | % Male |
|---|---|---|---|---|---|---|---|---|---|
| ACPRC | Age and Cognitive Performance Research Cohort | UK | 1461 | 64.7 | 6.1 | 47 | 85 | 425 | 0.29 |
| ADNI | Alzheimer's Disease Neuroimaging Initiative | USA | 259 | 75.3 | 5.1 | 62 | 90 | 137 | 0.53 |
| ASPIS | Athens Study of Psychosis Proneness and Incidence of Schizophrenia | Greece | 919 | 20.7 | 1.9 | 18 | 25 | 919 | 1.00 |
| CAMH | Center for Addiction and Mental Health | Canada | 80 | 48.6 | 19.4 | 18 | 86 | 38 | 0.48 |
| CHS | Cardiovascular Health Study | USA | 2931 | 77.3 | 5.3 | 69 | 96 | 1208 | 0.41 |
| CNP | UCLA Consortium for Neuropsychiatric Phenomics | USA | 628 | 31.1 | 8.3 | 21 | 50 | 310 | 0.49 |
| DCC | Duke Cognition Cohort | USA | 1193 | 27.1 | 11.6 | 18 | 77 | 558 | 0.47 |
| DNS | Duke Neurogenetics Study | USA | 455 | 19.8 | 1.3 | 18 | 22 | 212 | 0.47 |
| DUBLIN | Galway and Dublin, Ireland | Ireland | 135 | 36.2 | 12.5 | 18 | 60 | 71 | 0.53 |
| FHS | Framingham Heart Study | USA | 5360 | 51.7 | 10.8 | 25 | 87 | 2460 | 0.46 |
| GCAP | NIMH Genes, Cognition and Psychosis Program | USA | 964 | 33.8 | 9.8 | 18 | 61 | 438 | 0.45 |
| GENADA | Genotype–Phenotype Associations in Alzheimer's Disease | Canada | 767 | 73.4 | 7.9 | 48 | 94 | 279 | 0.36 |
| HBCS | Helsinki Birth Cohort Study | Finland | 299 | 67.7 | 2.3 | 64 | 75 | 299 | 1.00 |
| IBG | Institute for Behavioral Genetics | USA | 260 | 15.9 | 1.5 | 12 | 19 | 235 | 0.90 |
| LBC1936 | Lothian Birth Cohort 1936 Study | UK | 951 | 69.6 | 0.8 | 68 | 71 | 509 | 0.54 |
| LLFS | Long Life Family Study | USA and Denmark | 4081 | 68.1 | 14.0 | 24 | 90 | 1861 | 0.46 |
| LOAD | Late Onset Alzheimer's Disease Family Study | USA | 1033 | 75.1 | 5.7 | 53 | 95 | 379 | 0.37 |
| LOGOS | Learning on Genetics of Schizophrenia Spectrum | Crete | 795 | 22.5 | 3.8 | 18 | 37 | 795 | 1.00 |
| MCTFR | Minnesota Center for Twin and Family Research | USA | 5448 | 32.5 | 14.2 | 17 | 65 | 2349 | 0.43 |
| MUNICH | Munich, Germany | Germany | 1095 | 47.8 | 15.3 | 19 | 76 | 540 | 0.49 |
| NCNG | Norwegian Cognitive NeuroGenetics Study | Norway | 625 | 47.6 | 18.3 | 18 | 79 | 214 | 0.34 |
| PNC | Philadelphia Neurodevelopmental Cohort | USA | 4711 | 13.8 | 3.6 | 8 | 21 | 2440 | 0.52 |
| TOP | Thematic Organized Psychosis Research Study | Norway | 661 | 31.9 | 8.9 | 16 | 55 | 359 | 0.54 |
| ZHH | Zucker Hillside Hospital | USA | 187 | 35.2 | 18.8 | 8 | 78 | 108 | 0.58 |
General cognitive function phenotype
We examined general cognitive function (‘g'), a statistically derived broadband index of within-person performance on a neuropsychological test battery. The g phenotype estimates overall performance and is relatively invariant to the battery used and specific cognitive abilities assessed.33, 34 As in our prior reports,5, 10 for each cohort, g was determined using the first unrotated component extracted from a principal components analysis of individual test scores. Details on phenotypic assessments are provided in the supplement. Briefly, each COGENT sub-study (n=35) administered an average of 8 (s.d.±4) neuropsychological tests. To be included as a participant in COGENT, data from at least one neuropsychological measure across at least three domains of cognitive performance (for example, digit span for working memory; logical memory for verbal declarative memory; and digit symbol coding for processing speed), or the use of a validated g-sensitive measure was required. Digit symbol coding, digit span, verbal memory for words, visual memory, word reading, semantic fluency, verbal memory for stories, vocabulary, phonemic fluency and the trail-making test were the most common tests administered across cohorts. All individual test scores were adjusted (using multiple regression) for age2 and sex, as well as age × sex and age2 × sex interaction terms. The average internal consistency across test batteries was 71% (s.d.±12%), and the first unrotated principal component accounted for 42% (s.d.±11%) of the variance in overall test performance, which was expected based on an extensive prior literature (Supplementary Table S1).35
Genotyping and imputation
All COGENT samples were genotyped on commercial Illumina or Affymetrix arrays, and a standardized GWAS quality control pipeline was developed and applied to the genetic data as described in detail in the Supplementary Information. Participants were of European ancestry, which was confirmed by analysis of genotype data using multidimensional scaling. Genetic clustering in each study was based on multidimensional scaling axis plotting versus four 1000 Genomes Project super populations (African, Admixed American, European and Asian), and non-European participants were removed. Genome-wide imputation was conducted using the largest available cosmopolitan reference cohort.36
GWAS meta-analysis
In each COGENT sample, allelic association analysis of general cognitive function was conducted using imputed allele dosages and the first 10 principal components from the genotyped data to additionally adjust for population stratification. Cohorts of unrelated individuals (27 sub-cohorts) were analyzed using Plink 1.9.37 Samples including related individuals (8 sub-cohorts) were analyzed with BOLT-LMM38 using a mixed linear model association function that corrects for population stratification and relatedness.39 GWAS results were combined for meta-analysis of all 35 sub-studies using the inverse variance-weighted Z-score method in METAL.40 SNPs were filtered according to the following quality control thresholds: (1) minimum imputation quality INFO score of 0.60; (2) minor allele frequency at least 1% and (3) minimum 10 000 samples successfully imputed. Application of these filters resulted in a total of 8 037 763 high-quality SNPs available for meta-analysis in up to 35 298 samples. The standard threshold for genome-wide significance (P<5 × 10−8) was applied to SNP results of the GWAS meta-analysis.
Gene-based analysis
Individual SNP results from the meta-analysis were aggregated to conduct a gene-based analysis using MAGMA.41 SNPs were mapped to genes based on NCBI build 37.3 and defined by the start and stop site ±5 kb, resulting in 18 164 autosomal genes. A genome-wide significance threshold for gene-based associations was calculated using the Bonferroni method (α=0.05/18 164; P=2.75 × 10−6).
Genetic correlation analysis
LD score regression28, 29 was used to derive genetic correlations among GWAS results for general cognitive function and publicly available GWAS results from multiple neurobehavioral phenotypes of potential relevance. LD score regression quantifies the extent to which two phenotypes share genetic etiology (at least with respect to common variation captured by GWAS). GWAS summary statistics for 29 phenotypes were downloaded and processed similar to the pipeline of Bulik-Sullivan et al.29 The following phenotypes were included—cognition: childhood intelligence, educational attainment and obtaining a college degree; neurodevelopmental: autism and attention-deficit/hyperactivity disorder; neuropsychiatric: schizophrenia, bipolar disorder, anxiety and major depression; tobacco use: ever smoked cigarettes, number of cigarettes per day, age of onset of smoking and being a former smoker; personality: neuroticism, extraversion, openness, agreeableness and conscientiousness; brain volume: intracranial volume, nucleus accumbens, caudate nucleus, putamen, globus pallidus, hippocampus and thalamus; early childhood growth and development: infant head circumference, birth length and birth weight. URLs and references for data sources are provided in the Supplementary Information.
Results
GWAS of general cognitive function
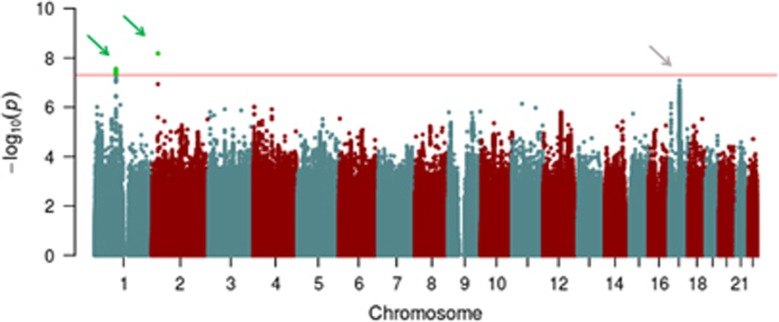
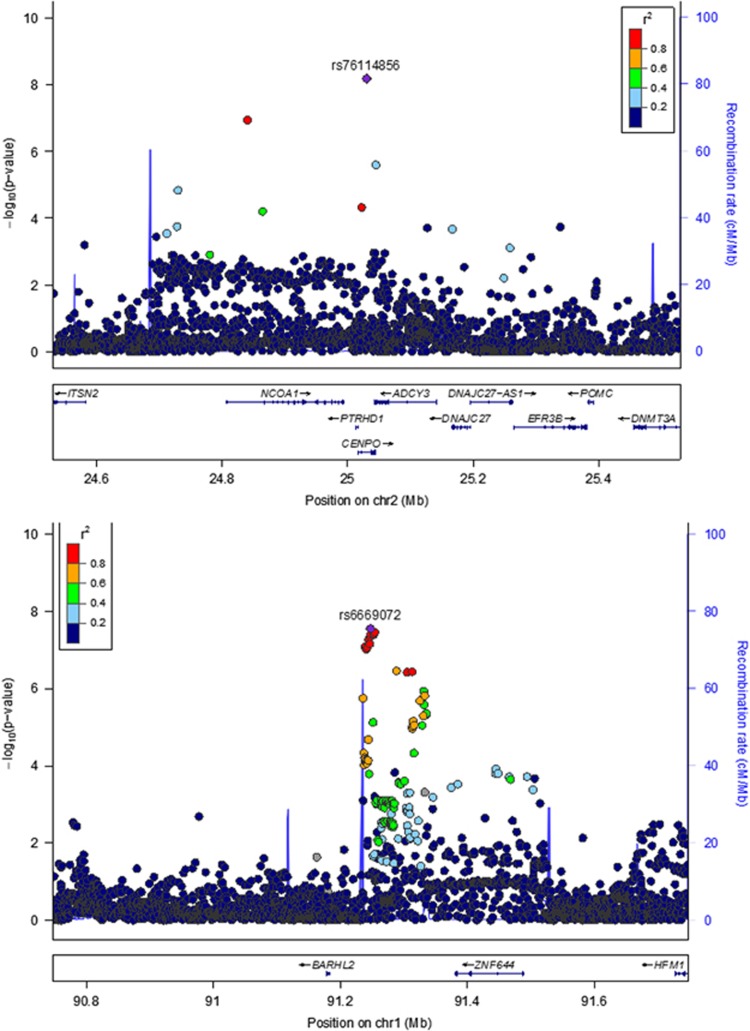
The QQ plot (Supplementary Figure 1) demonstrates λGC was 1.12, comparable to the value (1.14) observed in the recent CHARGE meta-analysis of cognitive ability.7 The LD score regression intercept of 1.04 indicates that polygenicity, rather than residual population stratification, accounted for most of the increase in the mean χ2 statistic.28, 29 As shown in the Manhattan plot (Figure 1), two loci surpassed the genome-wide threshold of P⩽5 × 10−8 in our GWAS meta-analysis (see Supplementary Table S2 for more details). On chromosome 2 (Figure 2, top), intronic SNP rs76114856 in the centromere protein O (CENPO) gene was genome-wide significant (P=6.58 × 10−9). On chromosome 1 (Figure 2, bottom), a cluster of six SNPs located in a lincRNA, RP4-665J23.1 (also known as LOC105378853), were also genome-wide significant (top SNP, rs6669072, P=2.77 × 10−8). Values of meta-analytic tests of heterogeneity were low and not statistically significant, indicating that outlier cohorts did not drive significant results. In addition, a large 1.4 Mb region at chromosome 17q21.31, coextensive with a known inversion polymorphism,42 harbored 101 nearly significant SNPs (all P's<10−6; top SNP, rs916888, P=8.18 × 10−8).
Figure 1.
Manhattan plot depicting results of genome-wide association study meta-analysis for general cognitive function. Green arrows indicate loci attaining genome-wide significance (red line, P<5 × 10−8). Gray arrow indicates locus at chromosome 17q21.31 approaching genome-wide significance.
Figure 2.
Region plots depicting genome-wide significant loci on Chromosome 2 (top) and Chromosome 1 (bottom). Local linkage disequilibrium (r2) is color-coded as shown in the legend, and local recombination rate is depicted by the bright blue peaks (magnitude indicated by the right-hand y axis).
Gene-based analysis of general cognitive function
Seven genes in three chromosomal regions (WNT3, PLEKHM1 and ARHGAP27 at chromosome 17q21.31; TP53 and WRAP53 at chromosome 17p13.1; and ATXN7L2 and CYB561D1 at chromosome 1p13.3) were significantly associated with cognitive function after Bonferroni correction (Table 2). Several genes at these loci, including NSF, STH, KANSL1, CRHR1 and MAPT in the 17q21.31 inversion region, demonstrate association just below the Bonferroni-corrected threshold.
Table 2. Results of gene analysis (top 20 genes).
| Symbol | Gene name | Chr | Start | Stop | SNPs | Parameters | N | Z stat | P-value |
|---|---|---|---|---|---|---|---|---|---|
| WNT3 | Wingless-type MMTV integration site family, member 3 | 17q21.31 | 44836686 | 44901082 | 127 | 46 | 29 063 | 5.4753 | 2.18E−08 |
| PLEKHM1 | Pleckstrin homology and RUN domain-containing M1 | 17q21.31 | 43508266 | 43573146 | 123 | 23 | 27 885 | 4.9724 | 3.31E−07 |
| TP53 | Tumor protein p53 | 17p13.1 | 7566720 | 7595863 | 94 | 32 | 31 813 | 4.9082 | 4.60E−07 |
| ARHGAP27 | Rho GTPase-activating protein 27 | 17q21.31 | 43466268 | 43515282 | 128 | 17 | 25 183 | 4.8923 | 4.98E−07 |
| CYB561D1 | Cytochrome b561 family, member D1 | 1p13.3 | 110031658 | 110048063 | 27 | 12 | 31 670 | 4.7687 | 9.27E−07 |
| WRAP53 | WD repeat containing, antisense to TP53 | 17p13.1 | 7584389 | 7611820 | 63 | 20 | 32 133 | 4.6221 | 1.90E−06 |
| ATXN7L2 | Ataxin 7-like 2 | 1p13.3 | 110021561 | 110040426 | 29 | 11 | 33 153 | 4.5842 | 2.28E−06 |
| PSMA5 | Proteasome subunit alpha 5 | 1p13.3 | 109936653 | 109974108 | 62 | 23 | 32 881 | 4.4835 | 3.67E−06 |
| NSF | N-ethylmaleimide sensitive factor | 17q21.31 | 44663035 | 44839830 | 89 | 20 | 29 574 | 4.4412 | 4.47E−06 |
| SORT1 | Sortilin 1 | 1p13.3 | 109847187 | 109945563 | 147 | 36 | 33 175 | 4.4403 | 4.49E−06 |
| SYPL2 | Synaptophysin-like 2 | 1p13.3 | 110004100 | 110029764 | 66 | 16 | 34 387 | 4.4171 | 5.00E−06 |
| CFAP99 | Cilia- and flagella-associated protein 99 | 4p16.3 | 2415701 | 2469690 | 171 | 43 | 33 195 | 4.3569 | 6.60E−06 |
| KANSL1 | KAT8 regulatory NSL complex subunit 1 | 17q21.31 | 44102282 | 44307740 | 775 | 22 | 27 788 | 4.3536 | 6.70E−06 |
| STH | Saitohin | 17q21.31 | 44071616 | 44082060 | 56 | 12 | 26 909 | 4.3028 | 8.43E−06 |
| SPPL2C | Signal peptide peptidase-like 2C | 17q21.31 | 43917256 | 43929438 | 69 | 8 | 30 906 | 4.2974 | 8.64E−06 |
| SP140L | SP140 nuclear body protein like | 2q37.1 | 231186894 | 231273445 | 365 | 51 | 34 250 | 4.1532 | 1.64E−05 |
| LRRC37A | Leucine-rich repeat containing 37A | 17q21.31 | 44367497 | 44420160 | 5 | 3 | 26 694 | 4.1414 | 1.73E−05 |
| MAPT | Microtubule-associated protein tau | 17q21.31 | 43966748 | 44110700 | 725 | 33 | 29 040 | 4.079 | 2.26E−05 |
| CRHR1 | Corticotropin-releasing hormone receptor 1 | 17q21.31 | 43692710 | 43918194 | 1024 | 75 | 30 191 | 3.7486 | 8.89E−05 |
| PCDH15 | Protocadherin-related 15 | 10q21.1 | 55557531 | 56566051 | 4082 | 531 | 33 480 | 3.218 | 6.46E−04 |
Abbreviation: SNP, single-nucleotide polymorphism. Genes that are in bold font were significant after genome-wide Bonferroni correction.
SNP lookups from published GWAS of cognition and educational attainment
We sought to expand the utility of our data by using it as a lookup table to confirm and extend associations previously reported in large-scale GWAS of cognition (from the CHARGE consortium7 and the UK Biobank8) and educational attainment (from the SSGAC consortium9). First, we looked up all cognitive SNPs nominally associated in the CHARGE study at P<10−5. Importantly, because of partial sample overlap between COGENT and CHARGE, we re-ran our cognitive GWAS excluding five overlapping cohorts (CHS, FHS, HBCS, LBC1936 and NCNG). A meta-analytic P-value was then generated across the two studies for those loci with nominal P<0.05 in COGENT. As shown in Supplementary Table S3, we found support for the three genome-wide significant loci reported in CHARGE, as well as support for an additional, novel locus at chromosome 3p22.3 that attained a meta-analytic P-value surpassing the genome-wide significance threshold (rs1523041, P=5.46 × 10−10). This SNP is intergenic; however, publicly available gene expression data (from GTEx43) have shown that rs1523041 is an expression quantitative trait locus for the ARPP21 gene (Supplementary Figure 1).
Next, we examined the genome-wide significant SNPs reported from the UK Biobank study of verbal numerical reasoning and reaction time to determine if these were also associated with general cognitive ability. As shown in Supplementary Table S4, we found nominally significant support for the chromosome 22 locus associated with verbal numerical reasoning (top local SNP in COGENT, rs12170228, P=0.0053, same direction of effect in COGENT and UK Biobank). We also showed a similar trend for the chromosome 7 locus associated with verbal numerical reasoning (rs9771228, P=0.074 in COGENT, same direction of effect in COGENT and UK Biobank). The lone SNP on chromosome 14 that attained genome-wide significance in the UK Biobank GWAS of verbal numerical reasoning was a rare variant (MAF=0.1%) that was not available in COGENT and has no known proxies. For the two loci reported to be associated with reaction time in UK Biobank, COGENT results demonstrated the same direction of allelic effects, but were not statistically significant (COGENT P's>0.6).
Finally, we looked up all SNPs that represented independent, genome-wide significant hits for educational attainment from the combined SSGAC+UK Biobank cohorts. Of a total of 164 SNPs meeting this criteria (as listed in Table 1.16 of that report9), 143 SNPs were directly available in COGENT, and an additional 12 SNPs were tested by proxy (9 SNPs were unavailable in COGENT even by proxy). There were 31 educational attainment SNPs that were nominally significant at P<0.05 in COGENT, all in the same direction of effect as for educational attainment, representing a highly significant enrichment (P=3.9 × 10−11, binomial test) of overlap between years of education and cognitive function (Supplementary Table S5). Further, two SNPs (rs7593947 and rs2568955) were Bonferroni-corrected significant (for 155 tests; P<3.23 × 10−4), suggesting two specific loci for cognition were discovered using this ‘proxy-phenotype' method.11 Notably, GTEx data reveal that rs2568955 is a strongly significant expression quantitative trait locus (Supplementary Figure 2) in brain tissue for RPL31P12, although this gene is annotated as a pseudogene; rs7593947 is an intronic variant in the BCL11A gene.
Genetic correlation of general cognitive function with related complex traits
SNP heritability (due to common variation) as calculated using LD score regression was 21.5% (s.e.=0.01%). This value for h2g is slightly lower than prior studies7, 8 which utilized the GCTA approach in single samples; this attenuation is expected based on the fact that LD score regression utilizes summary scores as opposed to full SNP data. The results of the LD score regression-based genetic correlations with other neurobehavioral phenotypes are detailed in Table 3. Note that our use of the LD score regression approach applied a stringent correction (unconstrained intercept) for potential sample overlap and population stratification, as well as a conservative Bonferroni correction for multiple phenotypes. Not surprisingly, strongly significant positive correlations were observed with the most closely related phenotypes: years of education (P=1.48 × 10−63), obtaining a college degree (P=1.88 × 10−23) and childhood intelligence (P=1.24 × 10−13). A significant negative correlation was observed with schizophrenia (P=4.09 × 10−4), such that genetic load for lower cognitive scores was associated with greater risk for schizophrenia, consistent with prior reports from COGENT5 and others.28, 30 Similar trends were observed for attention-deficit/hyperactivity disorder and anxiety, with nominal (but not Bonferroni-corrected) levels of statistical significance. A Bonferroni-corrected significant positive correlation was observed for autism (P=6.00 × 10−4), again consistent with prior reports.30, 44
Table 3. Results of genetic correlation using LD score regression.
| Genetic correlation with other traits using LD score regression | |||||
|---|---|---|---|---|---|
| Group | Phenotype | rg | s.e. | z | P-value |
| Cognition | Childhood IQ | 0.89 | 0.12 | 7.41 | 1.24E−13 |
| College degree | 0.66 | 0.07 | 9.98 | 1.88E−23 | |
| Years of education | 0.73 | 0.04 | 16.83 | 1.48E−63 | |
| Neuropsychiatric | ADHD | −0.35 | 0.16 | −2.14 | 3.22E−02 |
| Alzheimer's | −0.13 | 0.11 | −1.17 | 2.41E−01 | |
| Anorexia | −0.02 | 0.10 | −0.24 | 8.08E−01 | |
| Anxiety | −0.50 | 0.19 | −2.57 | 1.03E−02 | |
| Autism | 0.28 | 0.08 | 3.43 | 6.00E−04 | |
| Bipolar | 0.00 | 0.08 | −0.06 | 9.52E−01 | |
| Major depression | 0.10 | 0.10 | 0.96 | 3.35E−01 | |
| Schizophrenia | −0.17 | 0.05 | −3.53 | 4.09E−04 | |
| Personality | Extraversion | −0.13 | 0.10 | −1.36 | 1.74E−01 |
| Agreeableness | 1.24 | 1.24 | 1.00 | 3.17E−01 | |
| Conscietousness | 0.10 | 0.14 | 0.74 | 4.61E−01 | |
| Openness | 0.48 | 0.13 | 3.59 | 3.25E−04 | |
| Neuroticism | −0.18 | 0.12 | −1.49 | 1.35E−01 | |
| Smoking | Age of onset | 0.21 | 0.13 | 1.67 | 9.49E−02 |
| Cigarettes per day | 0.03 | 0.11 | 0.27 | 7.85E−01 | |
| Ever smoker | −0.24 | 0.08 | −3.07 | 2.13E−03 | |
| Former smoker | 0.29 | 0.10 | 2.80 | 5.09E−03 | |
| Brain volume | Accumbens | 0.26 | 0.15 | 1.74 | 8.18E−02 |
| Caudate | 0.08 | 0.10 | 0.79 | 4.30E−01 | |
| Hippocampus | 0.24 | 0.13 | 1.88 | 6.06E−02 | |
| Intracranial volume | 0.14 | 0.13 | 1.09 | 2.77E−01 | |
| Pallidum | 0.16 | 0.13 | 1.28 | 2.02E−01 | |
| Putamen | 0.13 | 0.09 | 1.44 | 1.50E−01 | |
| Thalamus | 0.13 | 0.12 | 1.07 | 2.83E−01 | |
| Early growth | Birth length | 0.20 | 0.09 | 2.13 | 3.33E−02 |
| Birth weight | 0.15 | 0.05 | 2.89 | 3.90E−03 | |
| Infant head Circumference | 0.19 | 0.11 | 1.69 | 9.04E−02 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; IQ, intelligence quotient; LD, linkage disequilibrium. Traits that are in bold font were nominally significant (P<0.05); traits that are italicized were significant after Bonferroni correction.
A novel observation is a significant, positive genetic correlation between general cognitive ability and the personality trait of openness (rg=0.48; P=3.25 × 10−4); no other correlations with personality traits were even nominally significant. (It should be noted that agreeableness was the only trait for which the SNP-based heritability did not significantly differ from zero (h2g=0.016; s.e.=0.029) and was, therefore, not included in correlational analyses). Higher cognitive ability was strongly genetically correlated with reduced rates of smoking (P=2.13 × 10−4) and greater rates of quitting smoking (P=5.09 × 10−3).
Nominally significant positive genetic correlations were observed with birth length and weight, with similar trends for infant head circumference. All genetic correlations with neuroanatomic measures trended in the positive direction (larger brain volumes associated with higher cognitive ability), consistent with a large literature revealing correlations at the phenotypic level,45 although none of the genetic correlations attained nominal significance under our conservative approach.
Discussion
Our GWAS meta-analysis of general cognitive function in a sample of 35 298 individuals of European ancestry revealed two novel associated SNP loci, three novel gene-based loci, and provided added support for several previously reported associations. Strengths of our study included access to individual-level genetic and neuropsychological data, which allowed us to run each sample through uniform genotype and phenotype quality control pipelines. Specifically, the general cognitive function phenotype was well characterized as a composite score derived from relatively large batteries of both verbal and nonverbal neuropsychological tests. Genotype data were processed with the latest imputation platforms and analytic procedures.
Our top GWAS hit was rs76114856, of which the minor T allele was associated with reduced cognitive performance. This SNP is an intronic variant in the CENPO gene, which encodes a component of the interphase centromere complex.46 This gene is highly expressed in the basal ganglia and thalamus of the human brain.65 CENPO is located at chromosome 2p23.3 and has prior GWAS associations to height.1, 47 The CENPO gene also had a nominal association to cognition in our gene-based analysis at P<0.05, as did neighboring genes NCOA1, PTRHD1 and ADCY3. The second strongest GWAS signal fell within a large intergenic non-coding RNA (lincRNA) of unknown function, RP4-665J23.1. Neighboring protein-coding genes are poorly annotated and do not provide strong clues as to the potential biological mechanism underlying the association.
We also found evidence that the chromosome 17q21.31 inversion region is associated with cognitive function. The chromosome 17q21.31 inversion consists of two haplotypes (H1 and H2), and the absence of recombination across the ~1.5 Mb region between the inverted (H2) and the noninverted (H1) chromosomes has resulted in two families of chromosomes.48 H1 chromosomes comprise the common (~80% frequency in European samples) noninverted gene order, whereas the H2 haplotype comprises the inverted gene order (~20% in European samples).48 There are several sources of evidence that variation at this locus is associated with neurobehavioral phenotypes. For example, the 17q21.31 microdeletion syndrome is associated with the H2 haplotype, which carries additional low-copy repeats susceptible to non-allelic homologous recombination. The syndrome is characterized clinically by developmental delay/intellectual disability, neonatal/childhood hypotonia, friendly behavior and specific facial dysmorphisms.49 Notably, KANSL1 gene disruption is associated with the full clinical spectrum of 17q21.31 microdeletion syndrome.49 In addition, the region harbors the MAPT gene, encoding microtubule-associated protein tau, a hallmark of multiple dementias.48, 50, 51, 52 The H1 family of haplotypes has been associated with increased risk for late-life tauopathies, diseases marked by the accumulation of MAPT neurofibrillary tangles in nerve cells, such as sporadic frontotemporal dementia,53 Alzheimer's disease,54 Parkinson's disease55 and progressive supranuclear palsy.48 By contrast, the H2 haplotype has been associated with developmental delay and learning difficulties,51, 56, 57, 58 as well as reduced intracranial volume.52 Consistent with these latter observations, our data suggest alleles corresponding to the H2 haplotype are associated with worse cognitive performance.
In addition to the loci attaining clear genome-wide significance through our primary SNP-based and gene-based analyses, our results confirmed and extended prior GWAS studies of cognitive and educational phenotypes. Although a prior COGENT report provided converging evidence for a role of a chromosome 6 locus (rs1906252),10 we now provide further support for the NPAS3/AKAP6 locus on chromosome 14 previously reported by the CHARGE consortium. NPAS3 is a promising candidate gene, as it has a role in neurodevelopment, and disruptions of this gene have been associated with psychiatric and intellectual disability phenotypes.59, 60
In the context of prior associations to cognitive and educational phenotypes, our data identified several loci with strong empirical support for a role in cognition. Of these, two are noteworthy for representing known expression quantitative trait locus, permitting inference of potential biological mechanisms underlying the statistical associations. Specifically, we found that the major (C) allele at rs1523041 was strongly (P=5.46 × 10−10) associated with better cognitive performance; this allele drives lower expression of the ARPP21 gene (Supplementary Figure S1). ARPP21 encodes a cAMP-regulated phosphoprotein, enriched in the basal ganglia and cerebellum, that has a central role in the integration of neurotransmitter inputs into striatal medium spiny neurons.61 Intriguingly, a deletion encompassing this gene segregated with syndromic intellectual disability in a multiply affected pedigree.62 Similarly, we found that the minor (T) allele of rs2568955 was associated with poorer cognitive performance, and this allele is associated with greater expression of RPL31P12 (Supplementary Figure S2). It should be noted that the strongest expression quantitative trait locus associations for these SNPs were observed in non-brain tissue in the GTEx database, perhaps due to smaller sample sizes available for neuronal phenotypes; these results should be tested in larger studies of brain expression that will soon be forthcoming. BCL11A is also a promising candidate gene for cognition. Haploinsufficiency of this gene has been associated with intellectual disability in a large clinical study, with the phenotype recapitulated in Bcl11a knockout mice, which was shown to be mediated through downstream transcriptional dysregulation in the hippocampus and cortex.63
Analysis of the genetic correlation between general cognitive function and various other phenotypes revealed that better cognitive performance was robustly genetically correlated with more years of schooling, decreased likelihood of smoking and decreased risk for several psychiatric disorders (as well as increased risk for autism). These results are generally consistent with recent genetic correlation studies of cognitive phenotypes30 and proxy phenotypes for cognition.9 The personality trait of openness, a core component of the ‘Big 5' model of personality, was positively correlated with cognitive ability at the genetic level. This novel finding is consistent with a prior literature in which moderate phenotypic correlations (values for r ranging between 0.25 and 0.5) between openness and cognitive ability have been repeatedly observed,20, 64 whereas cognition is generally uncorrelated with other personality dimensions. Moreover, phenotypic data from twin and family studies have suggested a specific genetic correlation between openness and general cognitive ability.65 Longitudinal studies have suggested a model in which openness may serve as a ‘buffer' against cognitive decline, as has been proposed in the Openness-Fluid-Crystallized-Intelligence model applied to late adulthood.66 Positive genetic correlations with birth length and weight suggest a critical role for prenatal developmental factors in the subsequent manifestation of cognitive ability throughout the lifespan.
One limitation of the current study is the wide age range of subjects, both across cohorts and within cohorts. Although we sought to control for confounding effects of age using covariates, genetic influence on cognitive ability is somewhat reduced in early childhood and adolescence relative to adulthood.67 In addition, late-life effects of cognitive decline may be mediated through somewhat disparate molecular pathways; this may explain the relatively weak effect of variation at APOE compared with prior GWAS meta-analysis.7 Nevertheless, cognitive abilities are remarkably stable across the entire lifespan,68 so we chose to include all available samples in order to maximize sample size and power.
Similarly, we chose to include cohorts with widely disparate neurocognitive batteries, which undoubtedly contributed to noise surrounding the estimates of g. Moreover, as demonstrated in Supplementary Table 1, the degree to which the first principal component captured the shared variance across tests was heterogeneous across cohorts. In general, cohorts with fewer available tests demonstrated greater loading onto the first factor, but with less reliability as determined by Cronbach's α. However, in each case, the scree plots clearly demonstrated a steep drop in variance accounted for beyond the first component, consistent with the known properties of g. Moreover, in a subset of subjects in one of the cohorts (TOP), we previously5 compared our computed g with estimated intelligence quotient from a 4-subscale composite from the WASI,69 and observed a strong correlation (r=0.67, P<10−46). Thus, we are confident that our computed index for each cohort was primarily reflecting general cognitive ability, but it is equally certain that substantial heterogeneity existed across cohorts, thereby reducing power in comparison with more easily measured quantitative traits such as height. Notably, it has been empirically demonstrated that such noise is more than compensated by increases in statistical power.70 Given the expense in conducting comprehensive cognitive assessments, we chose to include all available cohorts meeting our basic criteria.
Despite the statistical significance of the novel GWAS loci identified in the current report, it is important to emphasize that the effect sizes for individual SNPs are very small; each of our top two SNPs individually account for ~0.1% of the variance in cognitive performance. For context, these effect sizes are considerably smaller than those observed for the top individual loci associated with other quantitative anthropometric traits such as height and weight,1, 2 This difference may reflect the complexity of the underlying genetic architecture of cognition, as >80% of all genes are expressed in brain;71 this complexity has also slowed progress in identifying genetic loci for neuropsychiatric disorders, given the potentially large mutational target.70 This challenge is exacerbated by the fact that general cognitive ability is a latent trait that is only indirectly captured by the available phenotypic measures, which are also quite heterogeneous across cohorts. Moreover, the well-known winners' curse phenomenon72 will likely result in even further reduction of our effect size estimates in future studies of independent cohorts. However, as described in the preceding paragraphs, results of the present study can provide important information about the molecular underpinnings of cognitive function as well as clues relevant to the etiology neuropsychiatric disorders and other conditions relevant to human health. Although it is striking that general cognitive ability remains the quantitative trait most challenging to GWAS methodology, the recent success of very large-scale GWAS in educational attainment9 provides optimism that cognition is now at the beginning of the slope of increasing GWAS discovery that has been observed for all heritable complex traits.73
Acknowledgments
This work has been supported by grants from the National Institutes of Health (R01MH079800 and P50 MH080173 to AKM; R01 MH080912 to DCG; K23 MH077807 to KEB; K01 MH085812 to MCK). Data collection for the TOP cohort was supported by the Research Council of Norway, South-East Norway Health Authority and KG Jebsen Foundation. The NCNG study was supported by Research Council of Norway Grants 154313/V50 and 177458/V50. The NCNG GWAS was financed by grants from the Bergen Research Foundation, the University of Bergen, the Research Council of Norway (FUGE, Psykisk Helse), Helse Vest RHF and Dr Einar Martens Fund. The Helsinki Birth Cohort Study has been supported by grants from the Academy of Finland, the Finnish Diabetes Research Society, Folkhälsan Research Foundation, Novo Nordisk Foundation, Finska Läkaresällskapet, Signe and Ane Gyllenberg Foundation, University of Helsinki, Ministry of Education, Ahokas Foundation, Emil Aaltonen Foundation. For the LBC1936 cohort, phenotype collection was supported by The Disconnected Mind project. Genotyping was funded by the UK Biotechnology and Biological Sciences Research Council (BBSRC grant no. BB/F019394/1). The work was undertaken by The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative, which is funded by the Medical Research Council and the Biotechnology and Biological Sciences Research Council (MR/K026992/1). The CAMH work was supported by the CAMH Foundation and the Canadian Institutes of Health Research. The Duke Cognition Cohort (DCC) acknowledges K Linney, JM McEvoy, P Hunt, V Dixon, T Pennuto, K Cornett, D Swilling, L Phillips, M Silver, J Covington, N Walley, J Dawson, H Onabanjo, P Nicoletti, A Wagoner, J Elmore, L Bevan, J Hunkin and R Wilson for recruitment and testing of subjects. DCC also acknowledges the Ellison Medical Foundation New Scholar award AG-NS-0441-08 for partial funding of this study as well as the National Institute of Mental Health of the National Institutes of Health under award number K01MH098126. The UCLA Consortium for Neuropsychiatric Phenomics (CNP) study acknowledges the following sources of funding from the NIH: Grants UL1DE019580 and PL1MH083271 (RMB), RL1MH083269 (TDC), RL1DA024853 (EL) and PL1NS062410. The ASPIS study was supported by National Institute of Mental Health research grants R01MH085018 and R01MH092515 to Dr Dimitrios Avramopoulos. Support for the Duke Neurogenetics Study was provided the National Institutes of Health (R01 DA033369 and R01 AG049789 to ARH) and by a National Science Foundation Graduate Research Fellowship to MAS. Recruitment, genotyping and analysis of the TCD healthy control samples were supported by Science Foundation Ireland (grants 12/IP/1670, 12/IP/1359 and 08/IN.1/B1916).
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
The authors declare no conflict of interest.
Supplementary Material
References
- Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet 2014; 46: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015; 518: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Johnson W, Houlihan LM. Genetic foundations of human intelligence. Hum Genet 2009; 126: 215–232. [DOI] [PubMed] [Google Scholar]
- Davies G, Tenesa A, Payton A, Yang J, Harris SE, Liewald D et al. Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Mol Psychiatry 2011; 16: 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Knowles E, Davies G, Guha S, Liewald DC, Starr JM et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT). Mol Psychiatry 2013; 19: 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamin B, Pourcain B, Davis OS, Davies G, Hansell NK, Brion M-JA et al. Childhood intelligence is heritable, highly polygenic and associated with FNBP1L. Mol Psychiatry 2014; 19: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Armstrong N, Bis JC, Bressler J, Chouraki V, Giddaluru S et al. Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N=53 949). Mol Psychiatry 2015; 20: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Marioni RE, Liewald DC, Hill WD, Hagenaars SP, Harris SE et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N=112 151). Mol Psychiatry 2016; 21: 758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature 2016; 533: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trampush JW, Lencz T, Knowles E, Davies G, Guha S, Pe'er I et al. Independent evidence for an association between general cognitive ability and a genetic locus for educational attainment. Am J Med Genet Part B Neuropsychiatr Genet 2015; 168B: 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld CA, Esko T, Davies G, Pers TH, Turley P, Benyamin B et al. Common genetic variants associated with cognitive performance identified using the proxy-phenotype method. Proc Natl Acad Sci USA 2014; 111: 13790–13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld CA, Medland SE, Derringer J, Yang J, Esko T, Martin NW et al. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science 2013; 340: 1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ SE, Kanne SM, Reiersen AM. Executive function in individuals with subthreshold autism traits. Neuropsychology 2010; 24: 590–598. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. J Child Psychol Psychiatry 2008; 49: 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Hart CL, Davey Smith G, Starr JM, Hole DJ, Whalley LJ et al. Childhood mental ability and smoking cessation in adulthood: prospective observational study linking the Scottish Mental Survey 1932 and the Midspan studies. J Epidemiol Community Health 2003; 57: 464–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Goldberg TE, Gold JM, Elvevåg B, Weinberger DR. Cognitive factor structure and invariance in people with schizophrenia, their unaffected siblings, and controls. Schizophr Bull 2011; 37: 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Cornblatt B a, Keefe RS, Gopin CB, Derosse P et al. The MATRICS consensus cognitive battery in patients with bipolar I disorder. Neuropsychopharmacology 2011; 36: 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri F, Lapp LK, Peretti C-S. Current research on cognitive aspects of anxiety disorders. Curr Opin Psychiatry 2011; 24: 49–54. [DOI] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull 2013; 139: 81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Peterson JB, Higgins DM. Sources of openness/intellect: cognitive and neuropsychological correlates of the fifth factor of personality. J Pers 2005; 73: 825–858. [DOI] [PubMed] [Google Scholar]
- Low L-F, Harrison F, Lackersteen SM. Does personality affect risk for dementia? A systematic review and meta-analysis. Am J Geriatr Psychiatry 2013; 21: 713–728. [DOI] [PubMed] [Google Scholar]
- Bonnet AM, Jutras MF, Czernecki V, Corvol JC, Vidailhet M. Nonmotor symptoms in Parkinsons disease in 2012: relevant clinical aspects. Parkinsons Dis 2012; 2012: 198316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Hurtig HI, Van Deerlin VM et al. APOEMAPT and SNCA genes and cognitive performance in Parkinson disease. JAMA Neurol 2014; 71: 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan KB, Wilson RS, Weuve J, Barnes LL, Evans DA. Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia. Neurology 2015; 85: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Weiss A, Batty GD. Intelligence and personality as predictors of illness and death: how researchers in differential psychology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychol Sci Public Interest 2010; 11: 53–79. [DOI] [PubMed] [Google Scholar]
- Calvin CM, Deary IJ, Fenton C, Roberts BA, Der G, Leckenby N et al. Intelligence in youth and all-cause-mortality: systematic review with meta-analysis. Int J Epidemiol 2011; 40: 626–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ. Looking for 'system integrity' in cognitive epidemiology. Gerontology 2012; 58: 545–553. [DOI] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R et al. An atlas of genetic correlations across human diseases and traits. Nat Genet 2015; 47: 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J et alConsortium SWG of the PG. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 2015; 47: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars SP, Harris SE, Davies G, Hill WD, Liewald DCM, Ritchie SJ et al. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112 151) and 24 GWAS consortia. Mol Psychiatry 2016; 21: 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WD, Davies G, Liewald DC, McIntosh AM, Deary IJ. Age-dependent pleiotropy between general cognitive function and major psychiatric disorders. Biol Psychiatry 2016; 80: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè A V, Steinthorsdottir V et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 2012; 44: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W, te Nijenhuis J, Bouchard TJ Jr. Still just 1 g: consistent results from five test batteries. Intelligence 2008; 36: 81–95. [Google Scholar]
- Panizzon MS, Vuoksimaa E, Spoon KM, Jacobson KC, Lyons MJ, Franz CE et al. Genetic and environmental influences on general cognitive ability: is g a valid latent construct? Intelligence 2014; 43: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JB. Human Cognitive Abilities: A Survey of Factor-Analytic Studies. Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet 2016; 48: 1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015; 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh P, Tucker G, Bulik-Sullivan BK, Vilhjálmsson BJ, Finucane HK, Salem RM et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet 2015; 47: 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zaitlen NA, Goddard ME, Visscher PM, Price AL. Advantages and pitfalls in the application of mixed-model association methods. Nat Genet 2014; 46: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010; 26: 2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol 2015; 11: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G, Barnard J et al. A common inversion under selection in Europeans. Nat Genet 2005; 37: 129–137. [DOI] [PubMed] [Google Scholar]
- GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet 2013; 45: 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke T, Lupton MK, Fernandez-Pujals AM, Starr J, Davies G, Cox S et al. Common polygenic risk for autism spectrum disorder (ASD) is associated with cognitive ability in the general population. Mol Psychiatry 2016; 21: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschnig J, Penke L, Wicherts JM, Zeiler M, Voracek M. Meta-analysis of associations between human brain volume and intelligence differences: how strong are they and what do they mean? Neurosci Biobehav Rev 2015; 57: 411–432. [DOI] [PubMed] [Google Scholar]
- Saito A, Muro Y, Sugiura K, Ikeno M, Yoda K, Tomita Y. CENP-O, a protein localized at the centromere throughout the cell cycle, is a novel target antigen in systemic sclerosis. J Rheumatol 2009; 36: 781–786. [DOI] [PubMed] [Google Scholar]
- Berndt SI, Gustafsson S, Mägi R, Ganna A, Wheeler E, Feitosa MF et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet 2013; 45: 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly MP, Paschou P, Grigorenko E, Gurwitz D, Mehdi SQ, Kajuna SLB et al. The distribution and most recent common ancestor of the 17q21 inversion in humans. Am J Hum Genet 2010; 86: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollino M, Orteschi D, Murdolo M, Lattante S, Battaglia D, Stefanini C et al. Mutations in KANSL1 cause the 17q21.31 microdeletion syndrome phenotype. Nat Genet 2012; 44: 636–638. [DOI] [PubMed] [Google Scholar]
- Rao PN, Li W, LELM Vissers, Veltman JA, Ophoff RA. Recurrent inversion events at 17q21.31 microdeletion locus are linked to the MAPT H2 haplotype. Cytogenet Genome Res 2010; 129: 275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zody MC, Jiang Z, Fung HC, Antonacci F, Hillier LW, Cardone MF et al. Evolutionary toggling of the MAPT 17q21.31 inversion region. Nat Genet 2008; 40: 1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram MA, Fornage M, Smith A V, Seshadri S, Schmidt R, Debette S et al. Common variants at 6q22 and 17q21 are associated with intracranial volume. Nat Genet 2012; 44: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Rademakers R, Gijselinck I, van der Zee J, Dermaut B, de Pooter T et al. Genomic architecture of human 17q21 linked to frontotemporal dementia uncovers a highly homologous family of low-copy repeats in the tau region. Hum Mol Genet 2005; 14: 1753–1762. [DOI] [PubMed] [Google Scholar]
- Myers AJ, Kaleem M, Marlowe L, Pittman AM, Lees AJ, Fung HC et al. The H1c haplotype at the MAPT locus is associated with Alzheimer's disease. Hum Mol Genet 2005; 14: 2399–2404. [DOI] [PubMed] [Google Scholar]
- Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat Genet 2014; 46: 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C et al. A copy number variation morbidity map of developmental delay. Nat Genet 2011; 43: 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsiou-Tzeli S, Frysira H, Giannikou K, Syrmou A, Kosma K, Kakourou G et al. Microdeletion and microduplication 17q21.31 plus an additional CNV, in patients with intellectual disability, identified by array-CGH. Gene 2012; 492: 319–324. [DOI] [PubMed] [Google Scholar]
- Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S et al. Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nat Genet 2006; 38: 1032–1037. [DOI] [PubMed] [Google Scholar]
- Sha L, MacIntyre L, Machell JA, Kelly MP, Porteous DJ, Brandon NJ et al. Transcriptional regulation of neurodevelopmental and metabolic pathways by NPAS3. Mol Psychiatry 2012; 17: 267–279. [DOI] [PubMed] [Google Scholar]
- Pickard BS, Pieper AA, Porteous DJ, Blackwood DH, Muir WJ. The NPAS3 gene—emerging evidence for a role in psychiatric illness. Ann Med 2006; 38: 439–448. [DOI] [PubMed] [Google Scholar]
- Rakhilin SV, Olson PA, Nishi A, Starkova NN, Fienberg AA, Nairn AC et al. A network of control mediated by regulator of calcium/calmodulin-dependent signaling. Science 2004; 306: 698–701. [DOI] [PubMed] [Google Scholar]
- Marangi G, Orteschi D, Milano V, Mancano G, Zollino M. Interstitial deletion of 3p22.3p22.2 encompassing ARPP21 and CLASP2 is a potential pathogenic factor for a syndromic form of intellectual disability: a co-morbidity model with additional copy number variations in a large family. Am J Med Genet A 2013; 161A: 2890–2893. [DOI] [PubMed] [Google Scholar]
- Dias C, Estruch SB, Graham SA, McRae J, Sawiak SJ, Hurst JA et al. BCL11A haploinsufficiency causes an intellectual disability syndrome and dysregulates transcription. Am J Hum Genet 2016; 99: 253–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich D, Allemand M, Dellenbach M. Openness to experience, fluid intelligence, and crystallized intelligence in middle-aged and old adults. J Res Pers 2009; 43: 444–454. [Google Scholar]
- Wainwright M, Wright MJ, Luciano M, Geffen GM, Martin NG. Genetic covariation among facets of openness to experience and general cognitive ability. Twin Res Hum Genet 2008; 11: 275–286. [DOI] [PubMed] [Google Scholar]
- Ziegler M, Cengia A, Mussel P, Gerstorf D. Openness as a buffer against cognitive decline: the Openness-Fluid-Crystallized-Intelligence (OFCI) model applied to late adulthood. Psychol Aging 2015; 30: 573–588. [DOI] [PubMed] [Google Scholar]
- Haworth CM, Wright MJ, Luciano M, Martin NG, de Geus EJ, van Beijsterveldt CE et al. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Mol Psychiatry 2010; 15: 1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Whalley LJ, Lemmon H, Crawford JR, Starr JM. The stability of individual differences in mental ability from childhood to old age: follow-up of the 1932 Scottish Mental Survey. Intelligence 2000; 28: 49–55. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). Norwegian Manual Supplement. Pearson Assessment: Stockholm, 2007. [Google Scholar]
- Chabris CF, Lee JJ, Benjamin DJ, Beauchamp JP, Glaeser EL, Borst G et al. Why it is hard to find genes associated with social science traits: theoretical and empirical considerations. Am J Public Health 2013; 103: S152–S166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 2012; 489: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner S, Pritchard JK. Overcoming the winner's curse: estimating penetrance parameters from case-control data. Am J Hum Genet 2007; 80: 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet 2012; 90: 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.