Abstract
Previous studies in late-life depression (LLD) have found that patients have altered intrinsic functional connectivity in the dorsal default mode network (DMN) and executive control network (ECN). We aimed to detect connectivity differences across a treatment trial among LLD patients as a function of remission status. LLD patients (N=37) were enrolled into a 12-week trial of venlafaxine and underwent five functional magnetic resonance imaging resting state scans during treatment. Patients had no history of drug abuse, psychosis, dementia/neurodegenerative diseases or medical conditions with known effects on mood. We investigated whether there were differences in three networks: DMN, ECN and anterior salience network connectivity, as well as a whole brain centrality measure (eigenvector centrality). We found that remitters showed increases in ECN connectivity in the right precentral gyrus and decreases in DMN connectivity in the right inferior frontal gyrus and supramarginal gyrus. The ECN and DMN had regions (middle temporal gyrus and bilateral middle/inferior temporal/fusiform gyrus, respectively) that showed reversed effects (decreased ECN and increased DMN, respectively). Early changes in functional connectivity can occur after initial medication exposure. This study offers new data, indicating that functional connectivity changes differ depending on treatment response and can occur shortly after exposure to antidepressant medication.
Introduction
Treatment of major depression often requires multiple trials of medications before identifying an effective regimen. Forty percent of patients drop from care within the first month of treatment1, 2 (an important risk of incomplete response3), and for those who remain in treatment over half do not respond.4 Although conventional methods of increasing dose and using augmentation strategies increase overall response rates,4 these trials require patients to endure prolonged episodes of depression. Failure to respond to treatment can increase suicide risk, contribute to worsening of medical co-morbidities, disability, cognitive impairment and death.5, 6, 7 As depressed older adults are at increased risk for all of these negative heath consequences, shortening the window from clinical presentation to effective treatment is particularly important.
Several prior functional magnetic resonance imaging (fMRI) studies have identified potential biological correlates, or markers of mid- and late-life depression (LLD).8 They suggest that depression is associated with changes spanning multiple resting state networks. Specifically, depression has been linked to changes within the executive control network (ECN), default mode network (DMN) and anterior salience network (ASN).8 We have defined these networks based on previous work by Greicius and colleagues.9 We used a region of interest (ROI)-based connectivity approach.
LLD has been associated with decreased functional connectivity in the ECN.10 The left dorsolateral prefrontal cortex (PFC) is highly correlated with emotion regulation and often used as the ROI for ECN.11, 12 The ECN is important for goal-directed behaviors and complex cognitive tasks such as working memory, cognitive control and decision-making.13 In LLD, poor cognitive control is often reported14, 15 and ECN connectivity has been associated with certain features of executive dysfunction, including rigidity in processing information/learning,16, 17 deficits in working memory and attention and cognitive inhibition.10, 18
Several studies in mid-life depression and LLD suggest that depression is associated with greater connectivity in the DMN.19, 20 The midline posterior cingulate cortex (PCC) has been used extensively as a central node of the DMN.21, 22, 23 Previous studies have shown that greater DMN activity is associated with negative bias, increased self-referential thoughts and rumination.10, 24, 25, 26, 27, 28 In mid-life depression, it has been shown that PCC and ventromedial PFC connectivity predicted rumination severity.29 Further, therapeutic effects of antidepressants are associated with decreased neural response to negative self-referential stimuli.30
Finally, greater functional connectivity in the ASN is associated with increased anxiety and somatization.31, 32 The right anterior insula is a central node of the ASN and has been shown to be more greatly activated (relative to the left) in studies of emotion reactivity and regulation.33, 34, 35 The ASN is extensively connected with regions involved in motivation, reward and salience (cognitive, homeostatic or emotional).13, 36 Increased ASN connectivity has also been associated with interoceptive hijacking, which may represent the neural basis of increased anxiety and somatization described in LLD.32, 37
Whole-brain networks were examined using eigenvector centrality (EVC), which identifies important nodes that are densely connected.38 These nodes may have an important compensatory role in damaged networks39 and they provide a measure of how central a node is within the brain (summarizing the number of connections and their relative strength). This metric is particularly responsive to acute exposure to selective serotonin reuptake inhibitor.40 Early changes in these networks might signal whether a treatment is likely to succeed.
By pairing fMRI scans with a pharmacological challenge, it is now possible to track whether/how brain activity changes in response to particular medications by looking at changes in functional connectivity after a single dose.41 It is possible that early markers of circuit engagement, in response to LLD treatment, will help identify remitters with greater accuracy than pretreatment imaging alone. This dynamic fMRI approach can help refine current hypotheses regarding the correlation between treatment response and activity in functional circuits. Furthermore, by using early changes in brain activity, this early change can help predict clinical outcomes for individual patients.
The feasibility of fMRI markers is supported by recent studies showing functional imaging changes as early as 1–7 days after starting a new medication.42, 43 Positron emission tomography studies have indicated similar potential: increases in monoaminergic occupancy rates are detectable after a single dose of an selective serotonin reuptake inhibitor.44, 45 However, no longitudinal study has examined dynamic functional connectivity changes that occur during an LLD treatment trial.
We investigated how changes in functional brain connectivity over a 12-week trial of venlafaxine differed between remitters and non-remitters. Patients underwent five resting state fMRI scans. We would expect early in the treatment trial that the DMN and ASN would decrease in connectivity, while the ECN would increase (decreased rumination and anxiety, and increased cognitive control, respectively). We hypothesized that these early changes would be sustained until the end of the treatment trial.
Materials and methods
Study design and subjects
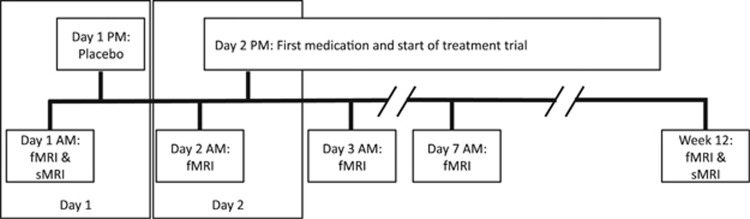
This project was part of a 5-year multi-site study of treatment of LLD, which used venlafaxine in the first phase and was then followed up with aripiprazole in non-remitters in the second phase. This was based on a study that found that augmentation of venlafaxine with aripiprazole improved treatment outcomes in treatment-resistant patients.46 It was also chosen due to its dual mechanism of action (at low versus high doses). Participants were included if they were >65 years of age, meeting DSM-IV criteria for major depressive episode (non-bipolar and non-psychotic), with Montgomery–Asberg Depression Rating Scale (MADRS) >15.47 Exclusion criteria were as follows: history of mania/psychosis, alcohol/substance abuse within the last 3 months, dementia/neurodegenerative disease and conditions with known effects on mood (for example, stroke, multiple sclerosis, vasculitis, significant head trauma and unstable hypertension and hypothyroidism). After informed consent, five MRI scans were performed: baseline, following the placebo lead-in (placebo), after first exposure to venlafaxine (day 1), a week after beginning treatment (week 1) and at the end (Figure 1).
Figure 1.
The study design protocol. Functional and structural magnetic resonance imaging (fMRI and sMRI, respectively) was performed throughout the treatment period. All scanning was done in the morning. On day 1, participants came in for an fMRI scan (Baseline) and then were given a placebo following the scan. On day 2 (~12 h after placebo), they returned for another fMRI scan (Placebo) and then were started on venlafaxine following the scan. They returned the next day (~12 h later) for another fMRI scan (Day 1, that is, day 1 of treatment). They continued on their medication as normal and came in for scans on week 1 (Week 1) and at the end of the trial (End).
A total of 37 participants signed consent but 4 were excluded due to venlafaxine side effects (N=2), non-adherence to protocol (N=1) and an inaccurate diagnosis of major depressive disorder (N=1). Thus, 33 subjects were included in this analysis. All subjects completed the first four scans but six failed to complete the fifth scan (but were included). Nine participants were on benzodiazepines (12 h exclusion period before scanning) during the study (mean lorazepam dose=0.61 mg). There were no significant differences (P=0.19) of lorazepam dose between remitters (N=4, 0.5 mg) and non-remitters (N=5, 0.7 mg). Four participants were on anti-hypertensive medications throughout the study.
Detailed dosage information has been published48 and are available in the Supplementary Information. Patients were designated as remitters at 12 weeks if they had a MADRS⩽10 for 2 consecutive weeks during the trial.48, 49
MRI data collection
Scanning was conducted using a 3T Siemens Trio TIM scanner (Munich, Germany) located at the MR Research Center at the University of Pittsburgh. A high-resolution T1-weighted sequence was collected (repetition time=2300 ms, inversion time=900 ms, flip angle=9°) with a field of view 256 × 224 with 176 slices. T2*-weighted blood oxygen-level dependent acquisition using gradient-echo echoplanar imaging was also collected (repetition time=2000 ms, echo time=34 ms, in-plane resolution=128 × 128, 28 slices, voxel size=2 × 2 × 4 mm).3 During resting scans, subjects (while awake and eyes open) observed a cross-hair.
Preprocessing
Data were preprocessed using statistical parametric mapping software (SPM12).50 Functional volumes were first slice-time corrected and then motion corrected. There were no significant differences between groups/time in mean relative motion and max absolute motion (see Supplementary Information for descriptive statistics). Manual skull stripping was done using ITK-SNAP,51 to improve functional to structural co-registration. The stripped structural image was then co-registered to the mean functional volume.
The structural image was segmented using six spatial priors (including gray/white matter). This generated a deformation field that was applied to the functional images.52 Smoothing was applied using a Gaussian kernel with full-width half-maximum of 8 mm.
EVC and ROI to voxel maps
Analyses were performed using in-house MatLab code.
Processing in both EVC and ROI to voxel analyses
We extracted a principal time series from the white matter (WM) and cerebrospinal fluid using singular value decomposition. We used these two signals and the motion parameters from the preprocessing in a multiple linear regression at each voxel. We extracted the residual time series from each voxel, which represents the time series not accounted for by WM, cerebrospinal fluid or motion. A band-pass filter (0.01–0.1 Hz Butterworth) was applied. This pipeline was adapted from Whitfield-Gabrieli and Nieto-Castanon.53
Eigenvector centrality
A whole-brain connectivity measure was calculated (EVC).38, 39, 54, 55, 56 The matrix of covariates was removed and band-pass-filtered residuals across all voxels was put through a singular value decomposition. The principal eigenvector is the EVC measure. The matrix was centered and then weighted by the inverse of the variance of each signal. In doing so, the singular value decomposition is done on the correlation rather than the covariance matrix.
Z-scores were generated (mean=0 and s.d.=1), smoothed and then masked for only gray matter.
ROI to voxel
The signal within the ROI was correlated to each voxel. A singular value decomposition was performed to generate a principal time series for the ROI. We computed the correlation between the ROI and all other voxels.
The Z-score map for these correlations was smoothed and then masked for only gray matter. This analysis was done for three separate ROIs. The PCC seed (DMN) was extracted from the posterior cingulate (eroded by hand in ITK-SNAP) from the automated anatomical labeling template.57 The right anterior insula seed (ASN) is extracted from the right insular cortex defined in the automated anatomical labeling atlas in the WFU Pick-Atlas. The left dorsolateral PFC (ECN) is defined as the left Brodmann area 46 in the Talairach Daemon database from the WFU Pick-Atlas. The network terminology used will reflect the terminology used in another study that performed an independent components analysis.9
Statistical and cluster analysis
Statistical analyses were performed using SPM12 for each ROI connectivity and EVC maps. A repeated-measures analysis of variance was performed containing the factors: group (response to treatment, 2 levels), time (5 levels, during treatment), an interaction between group and time, and a subject effect (models variability due to differences in average response of each subject).
In this study we assessed the significance of group, time and group-by-time interaction effects. Permutation methods for peak cluster level error correction (AlphaSim, http://afni.nimh.nih.gov/afni/) were applied for this whole-brain analysis by taking into account the significance of the peak voxel (P-value<0.005), thereby controlling for multiple comparisons (returning a minimum of 195 voxels). If the F-test was significant, we extracted the mean of each significant cluster (and 99% confidence intervals, CIs) and plotted that across the five time points for each group, to examine trends within these significant clusters.
To show regional changes in connectivity, we performed four change score analyses for each of the significant interactions. We subtracted baseline connectivity from placebo, day 1, week 1 and end connectivity, and performed a regression with two coefficients: a constant and a grouping variable. Parameter estimate means (tests whether there is a significant difference in group) and 99% CIs were extracted for each significant ROI and plotted.
Results
Table 1 shows the clinical and demographic characteristics by group (remitters (N=20 (16F)) and non-remitters (N=13 (7F)). We found no significant differences in any of the demographic or clinical measures (see Table 1), except for follow-up MADRS. We found no differences in WM hyperintensity burden by group either at baseline or follow-up (see Supplementary Information for information on WM hyperintensity segmentation/quantification58). The average venlafaxine dose (mean and 99% CI) in non-remitters was 263 mg (227.3 and 298.7, respectively), which was significantly greater (as expected; see Supplementary Information for titration information) than in remitters, for which it was 181.3 mg (153.9 and 208.7, respectively). There were no significant group/time or interaction effects in duration of depression and anxiety as measured by a single item in MADRS (see Supplementary Information).
Table 1. Clinical differences between groups.
| Non-remitters (N=13) | Remitters (N=20) | Group comparison (X/W,P) | |
|---|---|---|---|
| Age (median, IQR) | 65, 6 | 66, 11 | W=126.5, P=0.906 |
| Gender (F) | 7 | 16 | Fisher's exact P=0.139 |
| Education (median, IQR) | 15, 4 | 14, 5.25 | W=130.5, P=0.992 |
| Age at first MDE (median, IQR) | 29, 15.25 (N=12) | 29.5, 33.50 (N=18) | W=109, P=0.975 |
| CIRSG heart (0/1/2/3) | 9/2/1/1 | 14/2/0/4 | Fisher's exact P=0.518 |
| CIRSG vascular (0/1/2) | 4/0/9 | 4/1/15 | Fisher's exact P=0.810 |
| MMSE baseline (median, IQR) | 29, 1 | 30, 2 | W=101, P=0.273 |
| MADRS baseline (median, IQR) | 26, 9 | 22, 8.75 | W=181.5, P=0.058 |
| MADRS end (median, IQR) | 19.5, 10.5 (N=12) | 3, 5.5 (N=19) | W=211, P<0.05** |
| WMH baseline (median, IQR) | 0.0008, 0.0006 | 0.0011, 0.0015 | W=133, P=0.9277 |
| WMH end (median, IQR) | 0.0011, 0.0012 (N=12) | 0.0013, 0.0017 (N=19) | W=100, P=0.589 |
Abbreviations: CIRSG, Cumulative Illness Rating Scale for Geriatrics; F, female; IQR, interquartile range; MADRS, Montgomery–Asberg Depression Rating Scale; MDE, major depressive episode; MMSE, mini-mental state examination; WMH, white matter hyperintensity
As designed, MADRS at the end of the trial differed between remitters and non-remitters. If the number of subjects is fewer in the analysis than the total, it is listed in parentheses.
Only the ECN and DMN had significant group-by-time interaction effects. ASN and EVC had only significant group effects (remitters versus non-remitters). All brain imaging results are summarized in Table 2. These results are robust to Benzodiazepine use and baseline MADRS (see Supplementary Figure 1). We demonstrate the associations of connectivity and features of clinical response and medication (see Supplementary Figure 1). There were group differences independent of time (excluding areas with significant interactions) in DMN and ECN connectivity (see Supplementary Figure 2).
Table 2. Results summary table.
| Network/measure | Group × time interactions | Time | Group | X | Y | Z | F | Voxels |
|---|---|---|---|---|---|---|---|---|
| ECN | rPCG | NA | NA | 63 | 0 | 12 | 16.5 | 251 |
| rMTG/MOG | NA | NA | 48 | −80 | 24 | 16.3 | 246 | |
| DMN | NA | NA | 44 | 24 | 18 | 16.3 | 670 | |
| lITG/MTG/fusiform gyrii | NA | NA | −52 | −62 | −10 | 19.8 | 392 | |
| rITG/MTG/fusiform gyrii | NA | NA | 48 | −36 | −16 | 22 | 1407 | |
| rSMG | NA | NA | 60 | −58 | 36 | 22.3 | 297 | |
| ASN | NS | NS | lIFG | −38 | 6 | 24 | 15.9 | 240 |
| NS | NS | lMFG | −32 | 54 | 20 | 12.6 | 240 | |
| EVC | NS | NS | lIFG | −56 | 6 | 28 | 13.2 | 221 |
| NS | NS | rIFG | 52 | 34 | −12 | 16.1 | 203 | |
| NS | NS | MeFG/BA 10 | 2 | 64 | −8 | 15.5 | 713 |
Abbreviations: ASN, anterior salience network; BA, Brodmann area; DMN, default mode network; ECN, executive control network; EVC, eigenvector centrality; lIFG, left inferior frontal gyrus; lITG, left inferior temporal gyrus; lMFG, left middle frontal gyrus; MeFG, medial frontal gyrus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; NA, not applicable; NS, not significant; rIFG, right IFG; rITG, right ITG; rMTG, right MTG; rPCG, right precentral/postcentral gyrus; rSMG, right supramarginal gyrus.
X, Y, Z are the locations in Montreal Neurological Institute (MNI) space. F is the maximum F-statistic within the cluster. Voxels is the size of the cluster. If the Group × Time interaction is significant, then the main effects cannot be interpreted by themselves regardless of their significance. As an interaction term is present (reaching statistical significance), it means that the relationship between the outcome variable and time is not the same for both groups.
Executive control network
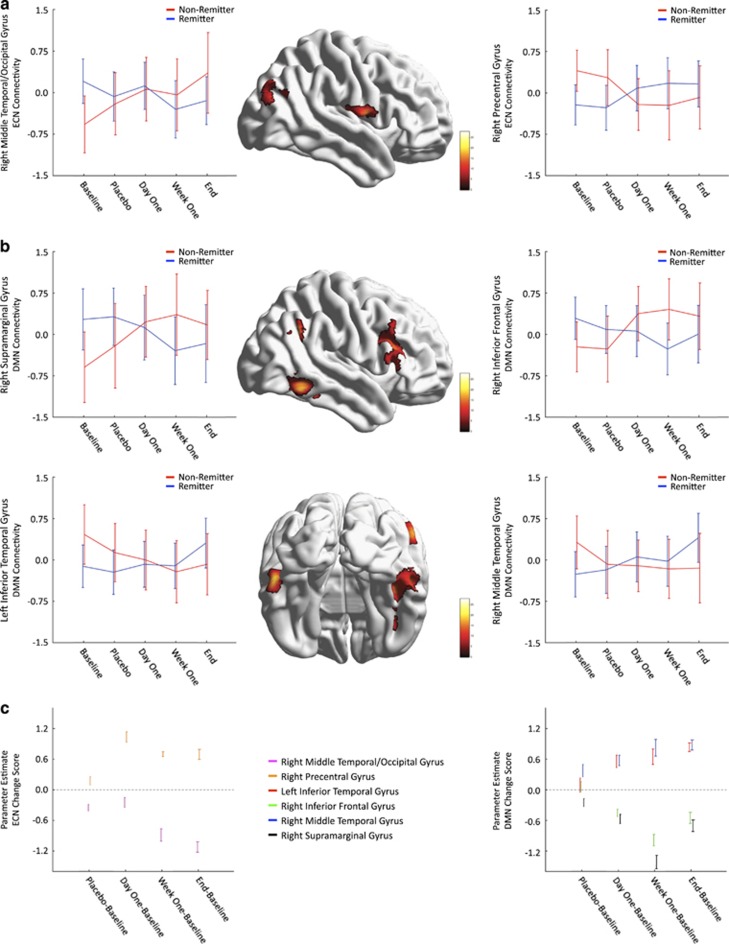
The regions with a significant group-by-time interaction (after multiple comparison correction) were the right precentral/postcentral gyrii (rPCG) and the right middle temporal gyrus (rMTG)/middle occipital gyrus, P<0.05 (corrected); see Table 2 and Figure 2a. The 99% CIs suggest no differences between remitters/non-remitters (Figure 2a). The change score analysis (Figure 2c, left) illustrates, relative to baseline, a larger change in connectivity following treatment than placebo. Across time, rPCG increased in connectivity while rMTG/middle occipital gyrus decreased.
Figure 2.
Connectivity changes where the interaction (group × time) was significant. (a) ECN connectivity changes that were significant. (b) DMN connectivity changes that were significant. For a and b, non-remitters are shown in red color and remitters are shown in blue color. The color bar indicates the value of the F-statistic. Error bars represent the 99% CIs. (c) Change score analysis results. Different regions are shown as different colors. The values represent mean and 99% CIs for the parameter estimate that tested whether there was a significant difference between remitters/non-remitters in the change scores (placebo/day 1/week 1/end−baseline). Dotted line represents β-estimate of zero. DMN, default mode network; ECN, executive control network.
Default mode network
Four clusters had significant interactions; they were the right inferior frontal gyrus (rIFG)/middle frontal gyrus, bilateral inferior temporal gyrus (ITG)/MTG/fusiform gyrii and right supramarginal gyrus (rSMG); P<0.05 (corrected), see Table 2 and Figure 2b. Much similar to the ECN, the 99% CI suggests no differences between remitters and non-remitters at any time point. The 99% CI suggests that relative to baseline, there is a larger change in connectivity following treatment than placebo (Figure 2c, right). Across time, bilateral inferior/middle temporal gyrus increased in connectivity while rIFG and rSMG decreased in connectivity in remitters.
Anterior salience network
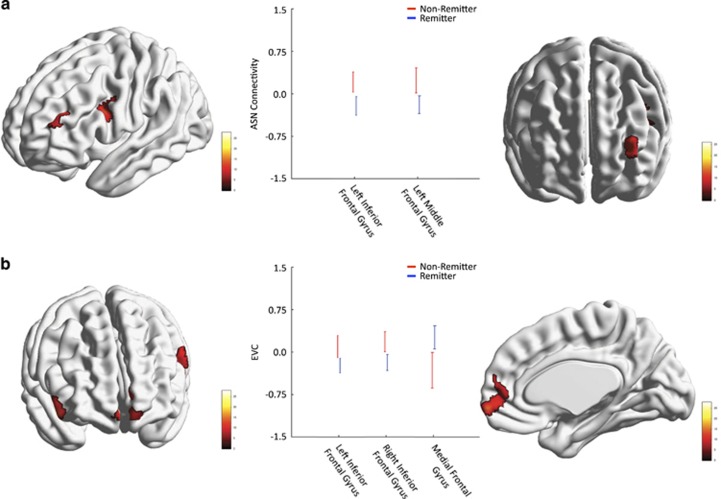
After applying the multiple comparison correction, no regions had significant group-by-time interaction effects. We then ran a model without the interaction effect and tested whether there were significant group and time effects. There was no significant time effect but there were significant group effects in the left IFG and left middle frontal gyrus; P<0.05 (corrected), Table 2 and Figure 3a. Non-remitters had greater ASN connectivity in both regions.
Figure 3.
Analyses where the interaction (group × time) was not significant, but where the group effect alone (not the time effect) was significant. (a) Regions where the ASN connectivity differed between remitters (blue) and non-remitters (red). (b) Regions where the EVC measure differed between groups. The color bar indicates the value of the F-statistic. Error bars represent the 99% CIs. ASN, anterior salience network; EVC, eigenvector centrality.
Eigenvector centrality
Eigenvector centrality is a summary measure of the influence of a node (voxel) in a network. No interaction between group and time was found for the EVC. However, there was a significant effect of group (but not time) in the bilateral IFG and the medial frontal gyrus (MeFG); P<0.05 (corrected), Table 2 and Figure 3b. Non-remitters had greater EVC in the bilateral IFG but lower EVC in the MeFG compared with remitters.
Discussion
To the best of our knowledge, this is the first study reporting early dynamic fMRI markers of treatment response variability in LLD. We evaluated changes in three functional networks and in EVC at five time points. Two networks (ECN and DMN) showed significant group-by-time effects (increased ECN–rPCG and DMN–bilateral MTG/ITG as well as decreased ECN–rMTG and DMN–rIFG, and rSMG in remitters across the trial compared with non-remitters). Only significant group (but not time) effects were found in the ASN (left IFG and middle frontal gyrus greater in non-remitters compared with remitters) and EVC (MeFG greater in remitters compared with non-remitters but lower in the bilateral IFG).
Previous LLD research suggests patients, compared with controls, have a hyperactive DMN and a hypoactive ECN.8, 10, 19 These may reflect clinical features of LLD such as increased rumination (hyperactive DMN) and cognitive impairment, indicating low cognitive control of limbic regions associated with emotional response (hypoactive ECN). A meta-analysis in mid-life depression found that DMN connectivity was predictive of treatment response.30, 59 Another study found that DMN connectivity was positively associated with treatment response, while dorsolateral PFC connectivity was negatively correlated.8 Other studies have found a normalization of task-based response following successful treatment.8 Our novel findings demonstrate, for the first time, that these effects are seen early following treatment and appeared larger in magnitude than placebo.
In remitters we observed increased ECN–rPCG connectivity and decreased ECN–rMTG connectivity relative to non-remitters. Although there is an effect of placebo, there appears to be an even greater effect following administration and continued treatment with venlafaxine. This suggests that the change in connectivity is related to the administration of venlafaxine and not to placebo. ECN–rPCG increases in remitters may reflect an improvement in cognitive control as a predictor of successful treatment. ECN–rMTG decreases did not show a large change following first exposure (day 1) to venlafaxine, rather this change is seen at week 1. ECN–MTG (outside the ECN) connectivity changes may indicate increased and dispersed effort in the non-remitters.
In remitters we observed decreased DMN–rSMG and rIFG connectivity, and an increased DMN–rMTG/left ITG/MTG and left fusiform gyrii connectivity relative to non-remitters. Similar to the ECN, the magnitude of the connectivity change appeared to be greater following treatment than following placebo. Decreased DMN–rSMG/rIFG connectivity may reflect an improvement in future ruminative thought processes in remitters, as suggested previously.8, 10 Increased DMN–rMTG/left MTG connectivity suggests that clinical correlates of neural changes (rumination–hyperactive DMN) are actually related to connectivity changes between specific nodes (PCC–PFC). Thus, we may witness a ‘rebalance' of the DMN in remitters, with a decrease in the ‘damaged' PCC–prefrontal connectivity and an increase in the connectivity between the other nodes.
Of note, the SMG has been involved (together with other sensory processing/associative brain regions, such as the fusiform gyrus) in the disrupted DMN connectivity in mid-life depression.60, 61 This may reflect disruptions in social interaction processes such as empathy62 and social engagement,63 which may ameliorate with improvement in depression symptoms. With regard to changes in PCC–IFG connectivity, we may speculate that given the recent reports regarding the role of rIFG in cognitive control and also in emotional appraisal, and alexithymia and verbalization of emotional responses/states,64 we may infer IFG, as a key region in the emotion–cognition interplay,65 becomes less involved during resting state, once depressive symptoms remits.
Alternatively, these results could be interpreted as increased intra-network coupling (increased ECN–rPCG and DMN–bilateral MTG/ITG) and decreased inter-network coupling (decreased ECN–rMTG and DMN–rIFG, and rSMG). In healthy individuals, ECN and DMN have inverse activations during tasks and this is disrupted in depression.13, 66, 67 This may reflect an important rebalancing of this association in remitters. These temporal regions are not nodes of the dorsal but rather ventral DMN and rSMG is part of the right ECN.
Recent evidence that shows that changes in DMN/ECN connectivity and other functional brain activation can be achieved through meditation, transcranial magnetic stimulation, cognitive behavioral therapy and psychotherapy.68, 69, 70, 71, 72, 73 These different therapies target different symptoms of depression and by targeting affected symptoms (for example, high rumination) it might be possible to achieve these changes through alternative means.
The early interaction may reflect a network engagement due to the increase in synaptic serotonin that seems to be consistently engaged (relative to the end scan). Thus, it seems that the network changes occur at a much earlier stage and these may be correlated with future changes in depression severity, rumination and cognitive control (although we do not demonstrate that here).
ASN–left IFG and middle frontal gyrus connectivity was higher in non-remitters than in remitters. Previous studies reported higher ASN connectivity in LLD participants compared with non-depressed elderly,8 a possible marker of increased anxiety and somatization.31 Given the lack of time differences, this may represent a trait rather than a state marker in LLD.
Using EVC (measures node importance), we found only group effects where EVC was higher in remitters than in non-remitters in the MeFG, but lower in the bilateral IFG. These findings suggest a potential neurobiological profile, indicating positive response to treatment. Thus, participants who start with high connectivity in the DMN (and increased EVC in the MeFG) are more likely to respond to treatment. This will require further empirical testing.
Several limitations should be noted. This study had a relatively small sample size, unequal group sizes and tested treatment response using only one medication. This result may not generalize well to other patient groups, including mid-life depression. Our definition of remitter, although established, has important limitations, especially in borderline cases. A well-known observation in LLD is that WM hyperintensity burden differs between remitters and non-remitters,74 which we failed to replicate, possibly due to the clinical and neurobiological heterogeneity of LLD.74 This study used ROI-based connectivity, whereas others have used data-driven approaches. Importantly, there is a strong correspondence between the two methods.75 We limited our analyses to three ROIs that represented core nodes of the default mode, executive control and salience networks; however, each of these networks has multiple nodes that we did not explore. All participants had similar dosages of venlafaxine at all measurements, except the final, where non-remitters had significantly greater mean dose than remitters. This was not controlled for in this analysis and may account for some differences at the final time point between remitters/non-remitters. Importantly, the dosage was equivalent over the course of the early changes (early interactions). Although there exists a literature that associates DMN/ECN connectivity with rumination/cognitive control measures, we did not specifically test this and thus future studies should perform these direct associations to validate these interpretations.
These group differences in trajectory of treatment may be important in predicting changes in depression symptoms; however, group differences do not necessarily give the ability to distinguish individual subjects.
Despite these limitations, we validate previous findings of pre- and posttreatment effects. Further, we found that there were early changes in the DMN and ECN, but not in ASN during the treatment trial, and that the treatment was associated with greater magnitude of change than placebo. Future studies should test whether an inter-network interaction between ECN and DMN exists, and investigate other nodes of each of these networks and also investigate the structural changes that may occur during the entire treatment trial.
Acknowledgments
This study was funded by NIMH R01 MH076079, 5R01 AG033575, K23 MH086686, P30 MH90333, 5R01 MH083660 and T32 MH019986. HTK had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. JK has received medication supplies from Reckitt Benckiser and Pfizer for investigator initiated trials. HA has received research support from Novartis Pharmaceuticals. CRIII reports receiving pharmaceutical support for NIH-sponsored research studies from Bristol-Myers Squibb, Forest, Pfizer and Lilly.
Author contributions
Study design: CA, DT, MAB, JFK, CR and HJA. Analysis, interpretation and drafting of the manuscript: HTK, CA, DT,SFS and HJA. Critical revision of manuscript: HTK, CA, DT, SFS, MAB, JFK, CR and HJA.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
The authors declare no conflict of interest.
Supplementary Material
References
- Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv 2009; 60: 1439–1445. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci 2011; 34: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D, Trivedi MH, Wisniewski SR, Davis L, Nierenberg AA, Gaynes BN et al. Predictors of attrition during initial (citalopram) treatment for depression: a STAR*D report. Am J Psychiatry 2007; 164: 1189–1197. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163: 28–40. [DOI] [PubMed] [Google Scholar]
- Katon W, Unutzer J, Russo J. Major depression: the importance of clinical characteristics and treatment response to prognosis. Depress Anxiety 2010; 27: 19–26. [DOI] [PubMed] [Google Scholar]
- Mulsant BH, Houck PR, Gildengers AG, Andreescu C, Dew MA, Pollock BG et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol 2006; 26: 113–120. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Delucchi KL, Schneider LS. Moderators of outcome in late-life depression: a patient-level meta-analysis. Am J Psychiatry 2013; 170: 651–659. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Khalaf A, Walker SE, Andreescu C. Magnetic resonance imaging predictors of treatment response in late-life depression. J Geriatr Psychiatry Neurol 2014; 27: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 2012; 22: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord 2012; 139: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 2012; 1251: E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2007; 2: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010; 214: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry 2009; 17: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry 2002; 10: 687–695. [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Figurski JL, Stenger VA, Reynolds CF 3rd, Carter CS. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry 2005; 58: 290–296. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Clark KA, Figurski JL, Andrew Stenger V, Nebes RD et al. Prefrontal and striatal activation in elderly subjects during concurrent implicit and explicit sequence learning. Neurobiol Aging 2006; 27: 741–751. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci 2007; 7: 367–379. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Tudorascu DL, Butters MA, Tamburo E, Patel M, Price J et al. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res 2013; 214: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RC et al. Resting-state functional connectivity in treatment-resistant depression. Am J Psychiatry 2011; 168: 642–648. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex 2008; 18: 1856–1864. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroImage 2008; 42: 1178–1184. [DOI] [PubMed] [Google Scholar]
- Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci 2012; 32: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007; 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 2001; 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry 2011; 70: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA 2010; 107: 11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol Rev 2012; 22: 229–251. [DOI] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci 2011; 6: 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci 2013; 7: 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Sheu LK, Tudorascu D, Gross JJ, Walker S, Banihashemi L et al. Emotion reactivity and regulation in late-life generalized anxiety disorder: functional connectivity at baseline and post-treatment. Am J Geriatr Psychiatry 2015; 23: 200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry 2006; 60: 383–387. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage 2003; 19: 1439–1448. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol 2012; 89: 273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Stein MB, Paulus MP. Anterior insula reactivity during certain decisions is associated with neuroticism. Soc Cogn Affect Neurosci 2006; 1: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 2009; 10: 59–70. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp 2013; 34: 2944–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O et al. Network centrality in the human functional connectome. Cereb Cortex 2012; 22: 1862–1875. [DOI] [PubMed] [Google Scholar]
- Binnewijzend MA, Adriaanse SM, Van der Flier WM, Teunissen CE, de Munck JC, Stam CJ et al. Brain network alterations in Alzheimer's disease measured by eigenvector centrality in fMRI are related to cognition and CSF biomarkers. Hum Brain Mapp 2014; 35: 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Burmann I, Regenthal R, Arelin K, Barth C, Pampel A et al. Serotonergic modulation of intrinsic functional connectivity. Curr Biol 2014; 24: 2314–2318. [DOI] [PubMed] [Google Scholar]
- Bourke JH, Wall MB. phMRI: methodological considerations for mitigating potential confounding factors. Front Neurosci 2015; 9: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska BR, Norbury R, Selvaraj S, Cowen PJ, Harmer CJ. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychol Med 2012; 42: 2609–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Takano A, Asai K, Suhara T et al. Effects of dopaminergic and serotonergic manipulation on emotional processing: a pharmacological fMRI study. NeuroImage 2005; 27: 991–1001. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry 2001; 158: 1843–1849. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Kent JM, Oquendo MA, Richards MC, Pratap M, Cooper TB et al. Acute occupancy of brain serotonin transporter by sertraline as measured by [11C]DASB and positron emission tomography. Biol Psychiatry 2006; 59: 821–828. [DOI] [PubMed] [Google Scholar]
- Rutherford B, Sneed J, Miyazaki M, Eisenstadt R, Devanand D, Sackeim H et al. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. Int J Geriatr Psychiatry 2007; 22: 986–991. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389. [DOI] [PubMed] [Google Scholar]
- Joel I, Begley AE, Mulsant BH, Lenze EJ, Mazumdar S, Dew MA et al. Dynamic prediction of treatment response in late-life depression. Am J Geriatr Psychiatry 2014; 22: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riso LP, Thase ME, Howland RH, Friedman ES, Simons AD, Tu XM. A prospective test of criteria for response, remission, relapse, recovery, and recurrence in depressed patients treated with cognitive behavior therapy. J Affect Disord 1997; 43: 131–142. [DOI] [PubMed] [Google Scholar]
- Penny W, Friston K, Ashburner J, Kiebel S. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press: Cambridge, MA, 1997.
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 2006; 31: 1116–1128. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage 2005; 26: 839–851. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012; 2: 125–141. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Margulies DS, Horstmann A, Pleger B, Lepsien J, Goldhahn D et al. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS ONE 2010; 5: e10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce KE, Laurienti PJ, Burdette JH, Hayasaka S. A new measure of centrality for brain networks. PLoS ONE 2010; 5: e12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink AM, de Munck JC, van der Werf YD, van den Heuvel OA, Barkhof F. Fast eigenvector centrality mapping of voxel-wise connectivity in functional magnetic resonance imaging: implementation, validation, and interpretation. Brain Connect 2012; 2: 265–274. [DOI] [PubMed] [Google Scholar]
- Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF 3rd, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res 2011; 194: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res 2006; 148: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology 2011; 36: 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang C, Zhu X, Tan Y, Zhong Y. Aberrant connectivity within the default mode network in first-episode, treatment-naive major depressive disorder. J Affect Disord 2015; 183: 49–56. [DOI] [PubMed] [Google Scholar]
- Peng D, Liddle EB, Iwabuchi SJ, Zhang C, Wu Z, Liu J et al. Dissociated large-scale functional connectivity networks of the precuneus in medication-naive first-episode depression. Psychiatry Res 2015; 232: 250–256. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist 2011; 17: 18–24. [DOI] [PubMed] [Google Scholar]
- Li W, Mai X, Liu C. The default mode network and social understanding of others: what do brain connectivity studies tell us. Front Hum Neurosci 2014; 8: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho NS, Wong MM, Lee TM. Neural connectivity of alexithymia: specific association with major depressive disorder. J Affect Disord 2016; 193: 362–372. [DOI] [PubMed] [Google Scholar]
- Okon-Singer H, Hendler T, Pessoa L, Shackman AJ. The neurobiology of emotion-cognition interactions: fundamental questions and strategies for future research. Front Hum Neurosci 2015; 9: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA 2008; 105: 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci USA 2013; 110: 19944–19949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci USA 2011; 108: 20254–20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH, Jung WH, Kang DH, Byun MS, Kwon SJ, Choi CH et al. Increased default mode network connectivity associated with meditation. Neurosci Lett 2011; 487: 358–362. [DOI] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 2014; 76: 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 2004; 61: 34–41. [DOI] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Segal ZV. The mindful brain and emotion regulation in mood disorders. Can J Psychiatry 2012; 57: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DE. How psychotherapy changes the brain—the contribution of functional neuroimaging. Mol Psychiatry 2006; 11: 528–538. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry 2013; 18: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosazza C, Minati L, Ghielmetti F, Mandelli ML, Bruzzone MG. Functional connectivity during resting-state functional MR imaging: study of the correspondence between independent component analysis and region-of-interest-based methods. Am J Neuroradiol 2012; 33: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.