Abstract
Protists are major predators of bacteria in soils. However, it remains unknown how protists sense their prey in this highly complex environment. Here, we investigated whether volatile organic compounds (VOCs) of six phylogenetic distinct soil bacteria affect the performance of three different soil protists and how that relates to direct feeding interactions. We observed that most bacteria affected protist activity by VOCs. However, the response of protists to the VOCs was strongly dependent on both the bacterial and protist interacting partner. Stimulation of protist activity by volatiles and in direct trophic interaction assays often coincided, suggesting that VOCs serve as signals for protists to sense suitable prey. Furthermore, bacterial terpene synthase mutants lost the ability to affect protists, indicating that terpenes represent key components of VOC-mediated communication. Overall, we demonstrate that volatiles are directly involved in protist−bacterial predator−prey interactions.
Soils are highly complex environments harbouring an enormous diversity of organisms. Besides fungi, bacteria and protists are the most abundant and diverse soil organisms (Fierer and Jackson, 2006; Geisen et al., 2015) that interact in many different ways. Protists are key predators of bacteria (de Ruiter et al., 1995; Bonkowski, 2004) and they shape bacterial communities through selective feeding (Bonkowski and Brandt, 2002; Rosenberg et al., 2009). Protists recognize prey quality when they are in direct contact through bacterial morphological differences and soluble compounds (Jousset, 2012). However, it remains unknown whether protists sense their prey over long distances in the porous soil environment.
Due to their physico-chemical properties including low molecular weight, lipophilicity, high vapour pressure and low boiling points, volatile organic compounds (VOCs) can diffuse through air- and water-filled pores (Effmert et al., 2012). Consequently, they play a key role in interactions between physically separated soil microbes (Kai et al., 2009; Garbeva et al., 2014; Schmidt et al., 2015; Schulz-Bohm et al., 2015). Most bacteria produce a broad spectrum of VOCs, which are of fundamental ecological importance in cross-kingdom interactions with, for example, plants, fungi and nematodes (Gu et al., 2007; Kai et al., 2009; Effmert et al., 2012). However, whether protists as the most important predators of bacteria can sense bacterial VOCs and whether this information translates to prey suitability in specific bacteria−protist interactions remain largely unknown.
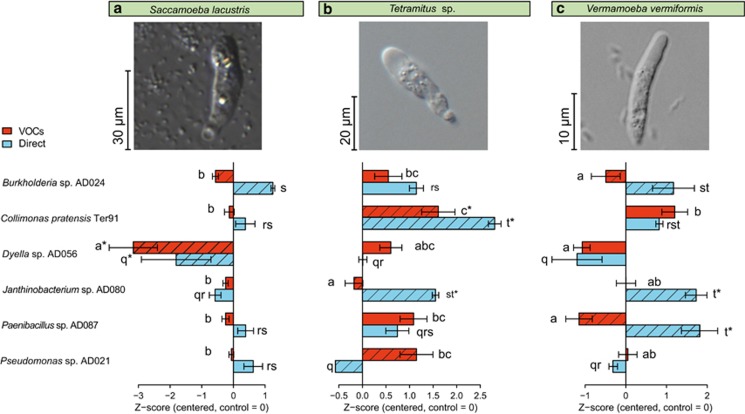
The responses of three different soil protists to VOCs emitted by six phylogenetically distinct soil bacteria were tested using a two-compartment Petri-dish system (Supplementary Material 1,Supplementary Figure S1, Supplementary Tables S1 and S2). Our results revealed that bacterial VOCs significantly altered protist activity (Figure 1), that is, higher relative abundance of trophozoites compared to cysts (Supplementary Figure S1), as well as motility and growth (Supplementary Figures S2 and S3), demonstrating that VOCs are key components in long-distance communication between protist predators and bacterial prey. To evaluate whether VOC-mediated responses reflect the outcomes of direct trophic interactions, we compared the impact of volatiles on protist activity with their responses in direct trophic interaction assays (Supplementary Material 1). In most cases, volatile-induced increases of protist activity were mirrored by an increase in activity in direct trophic interactions and vice versa (Figure 1). For example, the activity of Vermamoeba and Saccamoeba was reduced by Dyella, whereas Collimonas stimulated the activity of Vermamoeba and Tetramitus in VOC-mediated as well as in direct trophic interactions. This suggests that protists can sense suitable prey, based on bacterial species-specific VOCs. Specific long-distance diffusing bacterial VOCs can therefore provide early information about suitable prey and, consequently, more efficient predation.
Figure 1.
Effect (mean±s.e.) of six bacterial strains on the relative abundance of active protists (Supplementary Figure S1) in three species (a–c) in volatile-mediated (red bars) and direct trophic (blue bars) interactions. Relative abundances were standardized (using Z-scores) per protist species and interaction type (direct vs VOCs) and centred on the control treatment for each species. Positive values indicate more active protists relative to cysts than in the control (that is, no bacterial volatiles or no bacteria in trophic interaction assays), negative values vice versa. Different letters indicate significant differences among bars tested per type of interaction (that is, letters a−c for volatile-mediated interactions, and q−t for direct trophic interactions). Asterisks indicate significant differences from the control, while dashed bars indicate a significant difference between volatile-mediated and direct trophic interactions. For raw data see Supplementary Figures S2 and S3; for statistical analyses Supplementary Tables S3-S5.
However, opposing patterns were also observed. For instance, the presence of VOCs from Burkholderia and Paenibacillus reduced the activity of Vermamoeba and Saccamoeba, whereas these protists increased in activity and abundance when directly preying on Burkholderia and Paenibacillus (Figure 1 and Supplementary Figure S3). Similarly, VOCs of Pseudomonas stimulated the activity of Tetramitus, whereas the bacterium inhibited this protist species in direct trophic interactions (Figure 1 and Supplementary Figure S3). Opposing effects observed in VOC-mediated and direct trophic interactions support the idea that bacteria and protist predators engage in a complex chemical warfare (Mazzola et al., 2009; Jousset, 2012). Thereby, besides producing soluble toxic compounds in the presence of the predator (Mazzola et al., 2009, Pedersen et al., 2011; Jousset, 2012), bacteria can repel potential predators by volatiles, which is in line with a report revealing inhibition of the protist Acanthamoeba castellanii by bacterial VOCs (Kai et al., 2009).
Similarly to bacteria−bacteria and bacterial−fungal VOC-mediated interactions (Schmidt et al., 2015), the response of protists to bacterial volatiles was strongly dependent on the interacting partners (Figure 1, Supplementary Figures S2 and S3). VOCs of Burkholderia, Dyella and Paenibacillus inhibited Vermamoeba and Saccamoeba, whereas they stimulated the activity of Tetramitus. The bacteria tested in this study produce distinct blends of volatiles (Garbeva et al., 2014; Schulz-Bohm et al., 2015) that can explain the varying responses of the protist taxa. Species-specific bacteria−protist interactions are in line with differential feeding (Glücksman et al., 2010) and the sensitivity to soluble (toxic) bacterial secondary metabolites of protist taxa (Pedersen et al., 2011).
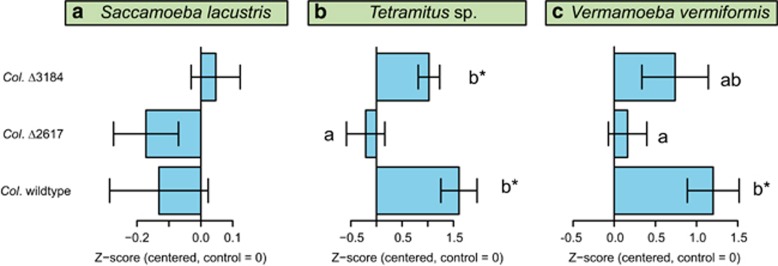
Bacterial volatiles belong to different chemical classes, including alkenes, ketones, sulphides and terpenes (Lemfack et al., 2014; Schmidt et al., 2015), the latter group being especially large and diverse. Recently, Song et al. (2015) showed that the Collimonas strain used in this study emits volatile monoterpenes (β-linalool and β-pinene) and sesquiterpenes (germacrene d-4-ol and δ-cadinene). To disentangle the contribution of specific groups of bacterial volatiles such as terpenes in bacteria−protist interactions, we created Collimonas strains mutated in the terpene synthase gene and phytoene synthase gene (Supplementary Material 1) and exposed protists to the VOCs of those mutants. For the terpene synthase mutant, the activities of Vermamoeba and Tetramitus were similar to the control level and significantly reduced compared to the wild type, demonstrating a loss of function in the mutant (Figure 2). Similar effects were observed for the motility of Vermamoeba and Tetramitus (Supplementary Figure S4). VOCs of the phytoene synthase mutant did not significantly change the activity of Vermamoeba and Tetramitus compared to the wild type (Figure 2). These results suggest that terpenes play a key role in VOC-mediated bacteria−protist interactions as catalysers of protist activity, which is in line with terpene-induced stimulations of nematodes (Rasmann et al., 2005). To date the ecological and the biological function of bacterial terpenes remains largely unknown. Most terpenes volatilize easily and, thus, can travel fast and over long distances through both, the liquid and gaseous phase of the soil (Hiltpold and Turlings, 2008). Hence, terpenes may be of great ecological importance for the interactions between spatially distant soil organisms.
Figure 2.
Effect (mean±s.e.) of volatiles of wild-type Collimonas pratensis Ter91 and two mutants (Δ2617: no terpene synthase activity; Δ3184: no phytoene synthase activity) on the activity of three protist species (a−c). Phytoene synthase mutant (Col. Δ3184) and terpene synthase mutant (Col. Δ2617) prevent production of non-volatile and volatile terpenes, respectively. Protist activity was standardized (using Z-scores) per protist species and centred on the control treatment for each species. Positive values indicate higher protist activity than in the control (that is, no bacterial volatiles), negative values vice versa. Different letters indicate significant differences among bars. Asterisks indicate significant differences from the control. For raw data see Supplementary Figure S4, for statistical analyses Supplementary Table S6.
In conclusion, we show that volatiles are key drivers of species-specific bacteria−protist interactions and that terpenes are among the informative compounds that enable protists to sense suitable prey bacteria. Species-specific responses of protists to bacterial VOCs suggest potential co-evolutionary dynamics in predator−prey interactions. Our study further suggests that specific volatiles can be used to activate protist predators of soil-borne disease agents, which might serve as a new method for biocontrol. Further work should aim at investigating the mechanisms and importance of volatile-mediated interactions in more complex settings, including natural conditions in soils.
Acknowledgments
Support for this study was provided by The Netherlands Organization for Scientific Research (NWO), VIDI personal grant (864.11.015). SG was supported by Wim van der Putten (ERC-Adv 260-55290 (SPECIALS)). This is publication 6157 of the NIOO-KNAW.
Author contributions
KS-B and SG designed and performed the experiments. CS constructed the Collimonas mutants. KS-B, SG and ERJW analysed and interpreted the data, KS-B and SG wrote the paper with contributions from all co-authors.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
The authors declare no conflict of interest.
Supplementary Material
References
- Bonkowski M. (2004). Protozoa and plant growth: the microbial loop in soil revisited. New Phytologist 162: 617–631. [DOI] [PubMed] [Google Scholar]
- Bonkowski M, Brandt F. (2002). Do soil protozoa enhance plant growth by hormonal effects? Soil Biol Biochem 34: 1709–1715. [Google Scholar]
- Effmert U, Kalderás J, Warnke R, Piechulla B. (2012). Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol 38: 665–703. [DOI] [PubMed] [Google Scholar]
- Fierer N, Jackson RB. (2006). The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103: 626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbeva P, Hordijk C, Gerards S, de Boer W. (2014). Volatiles produced by the mycophagous soil bacterium Collimonas. FEMS Microbiol Ecol 87: 639–649. [DOI] [PubMed] [Google Scholar]
- Geisen S, Tveit AT, Clark IM, Richter A, Svenning MM, Bonkowski M et al. (2015). Metatranscriptomic census of active protists in soils. ISME J 9: 2178–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glücksman E, Bell T, Griffiths RI, Bass D. (2010). Closely related protist strains have different grazing impacts on natural bacterial communities. Environ Microbiol 12: 3105–3113. [DOI] [PubMed] [Google Scholar]
- Gu Y-Q, Mo M-H, Zhou J-P, Zou C-S, Zhang K-Q. (2007). Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol Biochem 39: 2567–2575. [Google Scholar]
- Hiltpold I, Turlings TC. (2008). Belowground chemical signaling in maize: when simplicity rhymes with efficiency. J Chem Ecol 34: 628–635. [DOI] [PubMed] [Google Scholar]
- Jousset A. (2012). Ecological and evolutive implications of bacterial defences against predators. Environ Microbiol 14: 1830–1843. [DOI] [PubMed] [Google Scholar]
- Kai M, Haustein M, Molina F, Petri A, Scholz B, Piechulla B. (2009). Bacterial volatiles and their action potential. Appl Microbiol Biotechnol 81: 1001–1012. [DOI] [PubMed] [Google Scholar]
- Lemfack MC, Nickel J, Dunkel M, Preissner R, Piechulla B. (2014). mVOC: a database of microbial volatiles. Nucleic Acids Res 42: D744–D748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola M, de Bruijn I, Cohen MF, Raaijmakers JM. (2009). Protozoan-induced regulation of cyclic lipopeptide biosynthesis is an effective predation defense mechanism for Pseudomonas fluorescens. Appl Environ Microbiol 75: 6804–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AL, Winding A, Altenburger A, Ekelund F. (2011). Protozoan growth rates on secondary-metabolite-producing Pseudomonas spp. correlate with high-level protozoan taxonomy. FEMS Microbiol Lett 316: 16–22. [DOI] [PubMed] [Google Scholar]
- Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U et al. (2005). Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434: 732–737. [DOI] [PubMed] [Google Scholar]
- Rosenberg K, Bertaux J, Krome K, Hartmann A, Scheu S, Bonkowski M. (2009). Soil amoebae rapidly change bacterial community composition in the rhizosphere of Arabidopsis thaliana. ISME J 3: 675–684. [DOI] [PubMed] [Google Scholar]
- de Ruiter PC, Neutel AM, Moore JC. (1995). Energetics, patterns of interaction strengths, and stability in real ecosystems. Science 269: 1257–1260. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Cordovez V, de Boer W, Raaijmakers J, Garbeva P. (2015). Volatile affairs in microbial interactions. ISME J 9: 2329–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Bohm K, Zweers H, de Boer W, Garbeva P. (2015). A fragrant neighborhood: volatile mediated bacterial interactions in soil. Front Microbiol 6: 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CX, Schmidt R, de Jager V, Krzyzanowska D, Jongedijk E, Cankar K et al. (2015). Exploring the genomic traits of fungus-feeding bacterial genus Collimonas. BMC Genom 16: 1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.