Abstract
Mammalian DHOase (S-dihydroorotate amidohydrolase, EC 3.5.2.3) is part of a large multifunctional protein called CAD, which also has a carbamoyl-phosphate synthetase [carbon-dioxide: L-glutamine amido-ligase (ADP-forming, carbamate-phosphorylating), EC 6.3.5.5] and aspartate transcarbamoylase (carbamoyl-phosphate: L-aspartate carbamoyltransferase, EC 2.1.3.2) activities. We sequenced selected restriction fragments of a Syrian hamster CAD cDNA. The deduced amino acid sequence agreed with the sequence of tryptic peptides and the amino acid composition of the DHOase domain isolated by controlled proteolysis of CAD. Escherichia coli transformed with a recombinant plasmid containing the cDNA segment 5' to the aspartate transcarbamoylase coding region expressed a polypeptide recognized by DHOase domain-specific antibodies. Thus, the order of domains within the polypeptide is NH2-carbamoyl-phosphate synthetase-DHO-aspartate transcarbamoylase-COOH. The 334-residue DHOase domain has a molecular weight of 36,733 and a pI of 6.1. A fragment of CAD having DHOase activity that was isolated after trypsin digestion has extensions on both the NH2 (18 residues) and COOH (47-65 residues) termini of this core domain. Three of five conserved histidines are within short, highly conserved regions that may participate in zinc binding. Phylogenetic analysis clustered the monofunctional and fused DHOases separately. Although these families may have arisen by convergent evolution, we favor a model involving DHOase gene duplication and insertion into an ancestral bifunctional locus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. L., Meek T. D., Mong S. M., Johnson R. K., Metcalf B. W. cis-4-Carboxy-6-(mercaptomethyl)-3,4,5,6-tetrahydropyrimidin-2(1 H)-one , a potent inhibitor of mammalian dihydroorotase. J Med Chem. 1988 Jul;31(7):1355–1359. doi: 10.1021/jm00402a018. [DOI] [PubMed] [Google Scholar]
- BRESNICK E., BLATCHFORD K. STUDIES ON DIHYDROOROTASE ACTIVITY IN PREPARATIONS FROM NOVIKOFF ASCITES HEPATOMA CELLS. Arch Biochem Biophys. 1964 Mar;104:381–386. doi: 10.1016/0003-9861(64)90479-5. [DOI] [PubMed] [Google Scholar]
- Brown D. C., Collins K. D. Dihydroorotase from Escherichia coli. Cloning the pyrC gene and production of tryptic peptide maps. J Biol Chem. 1986 May 5;261(13):5917–5919. [PubMed] [Google Scholar]
- Bäckström D., Sjöberg R. M., Lundberg L. G. Nucleotide sequence of the structural gene for dihydroorotase of Escherichia coli K12. Eur J Biochem. 1986 Oct 1;160(1):77–82. doi: 10.1111/j.1432-1033.1986.tb09942.x. [DOI] [PubMed] [Google Scholar]
- Carrey E. A., Campbell D. G., Hardie D. G. Phosphorylation and activation of hamster carbamyl phosphate synthetase II by cAMP-dependent protein kinase. A novel mechanism for regulation of pyrimidine nucleotide biosynthesis. EMBO J. 1985 Dec 30;4(13B):3735–3742. doi: 10.1002/j.1460-2075.1985.tb04142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson R. I., Jones M. E. Interconversion of carbamayl-L-aspartate and L-dihydroorotate by dihydroorotase from mouse Ehrlich ascites carcinoma. J Biol Chem. 1979 Dec 25;254(24):12506–12512. [PubMed] [Google Scholar]
- Christopherson R. I., Jones M. E. The effects of pH and inhibitors upon the catalytic activity of the dihydroorotase of multienzymatic protein pyr1-3 from mouse Ehrlich ascites carcinoma. J Biol Chem. 1980 Apr 25;255(8):3358–3370. [PubMed] [Google Scholar]
- Christopherson R. I., Jones M. E. The overall synthesis of L-5,6-dihydroorotate by multienzymatic protein pyr1-3 from hamster cells. Kinetic studies, substrate channeling, and the effects of inhibitors. J Biol Chem. 1980 Dec 10;255(23):11381–11395. [PubMed] [Google Scholar]
- Coleman P. F., Suttle D. P., Stark G. R. Purification from hamster cells of the multifunctional protein that initiates de novo synthesis of pyrimidine nucleotides. J Biol Chem. 1977 Sep 25;252(18):6379–6385. [PubMed] [Google Scholar]
- Davidson J. N., Rumsby P. C., Tamaren J. Organization of a multifunctional protein in pyrimidine biosynthesis. Analyses of active, tryptic fragments. J Biol Chem. 1981 May 25;256(10):5220–5225. [PubMed] [Google Scholar]
- Faure M., Camonis J. H., Jacquet M. Molecular characterization of a Dictyostelium discoideum gene encoding a multifunctional enzyme of the pyrimidine pathway. Eur J Biochem. 1989 Feb 1;179(2):345–358. doi: 10.1111/j.1432-1033.1989.tb14560.x. [DOI] [PubMed] [Google Scholar]
- Freund J. N., Jarry B. P. The rudimentary gene of Drosophila melanogaster encodes four enzymic functions. J Mol Biol. 1987 Jan 5;193(1):1–13. doi: 10.1016/0022-2836(87)90621-8. [DOI] [PubMed] [Google Scholar]
- Goodman M., Pedwaydon J., Czelusniak J., Suzuki T., Gotoh T., Moens L., Shishikura F., Walz D., Vinogradov S. An evolutionary tree for invertebrate globin sequences. J Mol Evol. 1988;27(3):236–249. doi: 10.1007/BF02100080. [DOI] [PubMed] [Google Scholar]
- Grayson D. R., Evans D. R. The isolation and characterization of the aspartate transcarbamylase domain of the multifunctional protein, CAD. J Biol Chem. 1983 Apr 10;258(7):4123–4129. [PubMed] [Google Scholar]
- Grayson D. R., Lee L., Evans D. R. Immunochemical analysis of the domain structure of CAD, the multifunctional protein that initiates pyrimidine biosynthesis in mammalian cells. J Biol Chem. 1985 Dec 15;260(29):15840–15849. [PubMed] [Google Scholar]
- Guyonvarch A., Nguyen-Juilleret M., Hubert J. C., Lacroute F. Structure of the Saccharomyces cerevisiae URA4 gene encoding dihydroorotase. Mol Gen Genet. 1988 Apr;212(1):134–141. doi: 10.1007/BF00322456. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Jarry B., Falk D. Functional diversity within the rudimentary locus of Drosophila melanogaster. Mol Gen Genet. 1974;135(2):113–122. doi: 10.1007/BF00264779. [DOI] [PubMed] [Google Scholar]
- Jarry B. Isolation of a multifunctional complex containing the first three enzymes of pyrimidine biosynthesis in drosophila melanogaster. FEBS Lett. 1976 Nov;70(1):71–75. doi: 10.1016/0014-5793(76)80728-4. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Bouvier J., Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989 Jan 2;242(2):211–214. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
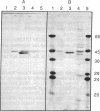
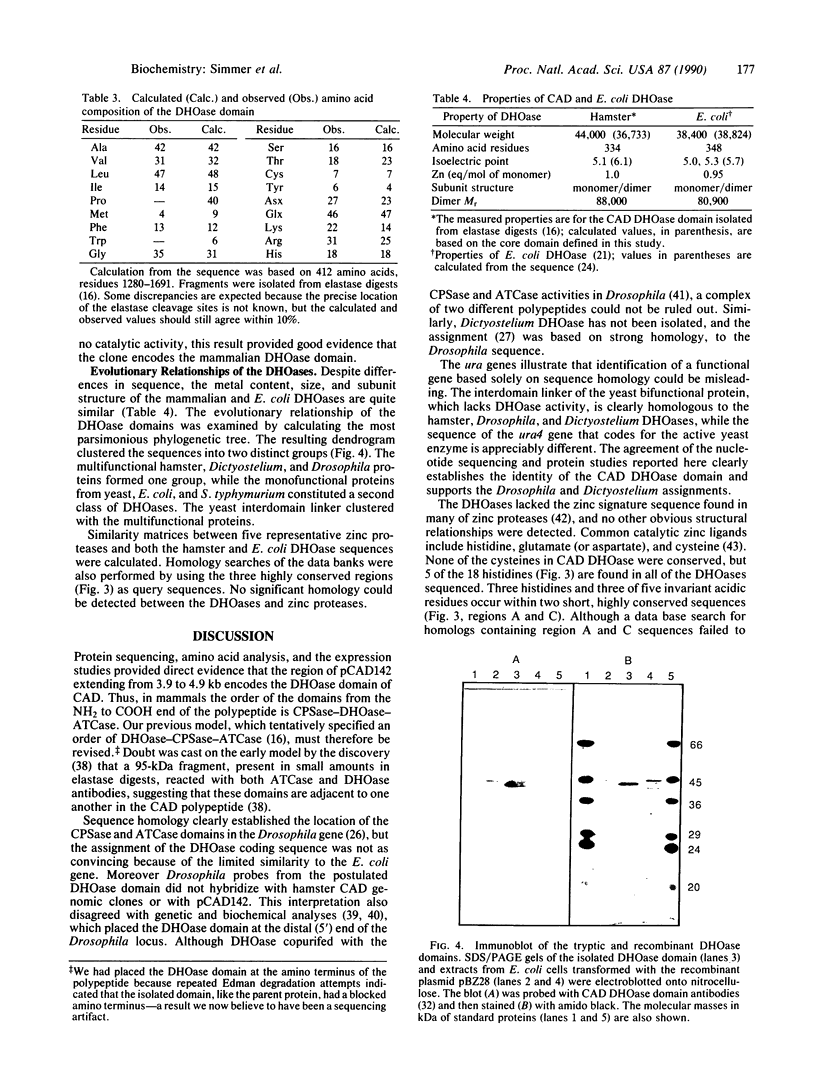
- Kelly R. E., Mally M. I., Evans D. R. The dihydroorotase domain of the multifunctional protein CAD. Subunit structure, zinc content, and kinetics. J Biol Chem. 1986 May 5;261(13):6073–6083. [PubMed] [Google Scholar]
- Kennedy J. Dihydroorotase from rat liver: purification, properties and regulatory role in pyrimidine biosynthesis. Arch Biochem Biophys. 1974 Feb;160(2):358–365. [PubMed] [Google Scholar]
- LIEBERMAN I., KORNBERG A. Enzymatic synthesis and breakdown of a pyrimidine, orotic acid. I. Dihydroortic acid, ureidosuccinic acid, and 5-carboxymethylhydantoin. J Biol Chem. 1954 Apr;207(2):911–924. [PubMed] [Google Scholar]
- Mally M. I., Grayson D. R., Evans D. R. Catalytic synergy in the multifunctional protein that initiates pyrimidine biosynthesis in Syrian hamster cells. J Biol Chem. 1980 Dec 10;255(23):11372–11380. [PubMed] [Google Scholar]
- Mally M. I., Grayson D. R., Evans D. R. Controlled proteolysis of the multifunctional protein that initiates pyrimidine biosynthesis in mammalian cells: evidence for discrete structural domains. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6647–6651. doi: 10.1073/pnas.78.11.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy M., Le Gouar M., Potier S., Souciet J. L., Hervé G. The primary structure of the aspartate transcarbamylase region of the URA2 gene product in Saccharomyces cerevisiae. Features involved in activity and nuclear localization. J Biol Chem. 1989 May 15;264(14):8366–8374. [PubMed] [Google Scholar]
- Neuhard J., Kelln R. A., Stauning E. Cloning and structural characterization of the Salmonella typhimurium pyrC gene encoding dihydroorotase. Eur J Biochem. 1986 Jun 2;157(2):335–342. doi: 10.1111/j.1432-1033.1986.tb09673.x. [DOI] [PubMed] [Google Scholar]
- Rawls J. M., Fristrom J. W. A complex genetic locus that controls of the first three steps of pyrimidine biosynthesis in Drosophila. Nature. 1975 Jun 26;255(5511):738–740. doi: 10.1038/255738a0. [DOI] [PubMed] [Google Scholar]
- SMITH L. H., Jr, BAKER F. A. Pyrimidine metabolism in man. I. The biosynthesis of orotic acid. J Clin Invest. 1959 May;38(5):798–809. doi: 10.1172/JCI103862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander E. G., Heeb M. J. Purification and properties of dihydroorotase from Escherichia coli B. Biochim Biophys Acta. 1971 Feb 10;227(2):442–452. doi: 10.1016/0005-2744(71)90075-1. [DOI] [PubMed] [Google Scholar]
- Sander E. G., Wright L. D., McCormick D. B. Evidence for function of a metal ion in the activity of dihydroorotase from Zymobacterium oroticum. J Biol Chem. 1965 Sep;240(9):3628–3630. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatzman A. R., Rosenberg M. Expression, identification, and characterization of recombinant gene products in Escherichia coli. Methods Enzymol. 1987;152:661–673. doi: 10.1016/0076-6879(87)52072-9. [DOI] [PubMed] [Google Scholar]
- Shoaf W. T., Jones M. E. Initial steps in pyrimidine synthesis in Ehrlich ascites carcinoma. Biochem Biophys Res Commun. 1971 Nov 5;45(3):796–802. doi: 10.1016/0006-291x(71)90487-6. [DOI] [PubMed] [Google Scholar]
- Shoaf W. T., Jones M. E. Uridylic acid synthesis in Ehrlich ascites carcinoma. Properties, subcellular distribution, and nature of enzyme complexes of the six biosynthetic enzymes. Biochemistry. 1973 Oct 9;12(21):4039–4051. doi: 10.1021/bi00745a004. [DOI] [PubMed] [Google Scholar]
- Simmer J. P., Kelly R. E., Scully J. L., Grayson D. R., Rinker A. G., Jr, Bergh S. T., Evans D. R. Mammalian aspartate transcarbamylase (ATCase): sequence of the ATCase domain and interdomain linker in the CAD multifunctional polypeptide and properties of the isolated domain. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4382–4386. doi: 10.1073/pnas.86.12.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. H., Taylor M. L., Balch W. E., Gilchrist P. S. Purification of properties of dihydroorotase, a zinc-containing metalloenzyme in Clostridium oroticum. J Bacteriol. 1976 Aug;127(2):863–873. doi: 10.1128/jb.127.2.863-873.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washabaugh M. W., Collins K. D. Dihydroorotase from Escherichia coli. Purification and characterization. J Biol Chem. 1984 Mar 10;259(5):3293–3298. [PubMed] [Google Scholar]