Abstract
Quantifying the contribution of marine microorganisms to carbon and nitrogen cycles and their response to predicted ocean warming is one of the main challenges of microbial oceanography. Here we present a single-cell NanoSIMS isotope analysis to quantify C and N uptake by free-living and attached phytoplankton and heterotrophic bacteria, and their response to short-term experimental warming of 4 °C. Elevated temperature increased total C fixation by over 50%, a small but significant fraction of which was transferred to heterotrophs within 12 h. Cell-to-cell attachment doubled the secondary C uptake by heterotrophic bacteria and increased secondary N incorporation by autotrophs by 68%. Warming also increased the abundance of phytoplankton with attached heterotrophs by 80%, and promoted C transfer from phytoplankton to bacteria by 17% and N transfer from bacteria to phytoplankton by 50%. Our results indicate that phytoplankton-bacteria attachment provides an ecological advantage for nutrient incorporation, suggesting a mutualistic relationship that appears to be enhanced by temperature increases.
Introduction
The oceans cover 70% of our planet and have a key role in the global C cycle through CO2 exchanges with the atmosphere and organic C transformations within marine food webs. In the upper ocean, single-celled cyanobacteria and eukaryotic algae, collectively known as phytoplankton, fix inorganic C and partly release it as dissolved organic carbon (DOC), feeding heterotrophic bacteria through the microbial food web. A critical but often overlooked characteristic of these ecosystems is the interaction between these two major components that dominate the biogeochemical cycles of C and N in the upper oceans (Azam and Malfatti, 2007). In surface waters, heterotrophic bacterial biomass production (BP) depend on the availability of labile DOC, a large part of which is ultimately phytoplankton derived (Buchan et al., 2014). At the same time, bacteria sustain algal growth by providing substrates such as remineralized nutrients or vitamins (Croft et al., 2005; Amin et al., 2012). The transfer of C from phytoplankton to bacteria may occur through two different strategies: as free-living cells that incorporate DOC previously released by autotrophs to the surrounding water, and through physical attachment (Grossart and Simon, 2007a; Mével et al., 2008), where bacteria are in contact with their main sources of fresh DOC. Because of the physical, direct interaction between bacteria and phytoplankton cells, the attachment strategy could provide both components with an ecological advantage compared with their free-living counterparts (Rooney-Varga et al., 2005; Grossart and Simon, 2007a; Grossart et al., 2007b). Indeed, it has been recently observed that symbiotic relationships between cyanobacteria and microalgae through attachment are key strategies for C and N exchange between them (Thompson et al., 2012; Cornejo-Castillo et al., 2016).
According to climate models, a sea surface temperature increase as high as 6.4 °C may occur by the end of this century (Meehl et al., 2007), likely modifying oceanic C fluxes (Sarmiento et al., 2004). Raised temperature impacts on microbial food webs may include increases in bacterial abundances (Morán et al., 2015) and changes in DOC use (Sarmento et al., 2010; Duarte et al., 2013) ultimately resulting in increased bacterial production and respiration (López-Urrutia and Morán, 2007; Vázquez-Domínguez et al., 2007). It has been suggested that warming may also induce a stronger coupling between bacteria and phytoplankton (von Scheibner et al., 2014). Important questions include whether warming will promote higher bacterial incorporation of phytoplankton-derived DOC, and whether physical interactions through attachment may impact the magnitude of this coupling. As productivity of attached bacteria may be an order of magnitude higher than free-living cells (Azam and Long, 2001), distinguishing between these two ecological strategies is necessary to accurately quantify bacterial C fluxes. However, traditional methodologies such as bulk productivity measurements are problematic for resolving C incorporation by attached cells, and in general the study of attached bacteria in aquatic ecosystems remains a challenge (Simon et al., 2002). The rise of single-cell approaches (Wagner, 2009) now allows the study of microscale processes such as those that occur in the phycosphere, that is, the surface of algal cells and the thin layer that surrounds it (Bell and Mitchell, 1972), where compounds can be transported by diffusion allowing direct exchange between cells (Lazier and Mann, 1989). Although thus far bacteria–phytoplankton interactions through attachment have been described mostly between heterotrophic bacteria and microphytoplankton cells such as diatoms or dinoflagellates (Amin et al., 2012), the magnitude of bacterial attachment to smaller nano- and picophytoplankton, the most abundant autotrophs in the ocean (Stockner, 1988; Magazzù and Decembrini, 1995; Marañón et al., 2001), remains unclear. Even though picophytoplankton may be too small to be detectable by membrane chemosensory mechanism by heterotrophic bacteria (Jackson, 1987), some work has shown that pico and nanophytoplankton are also susceptible to colonization by heterotrophic bacteria (Salomon et al., 2003; Eiler et al., 2006).
Here we investigated the effect of warming (4 °C over ambient) in a temperate ecosystem on the cell-specific activities of marine heterotrophic and autotrophic cells and the corresponding transfer of C and N between them. Seawater samples were incubated at in situ and increased temperatures in the presence of stable isotope labeled substrates. Using nanoscale secondary ion mass spectrometry (NanoSIMS), we quantified the C and N uptake by free-living cells as well as phytoplankton-bacteria consortia, defined here as bacteria physically attached to phytoplankton.
Materials and methods
Sample collection and water incubations
Seawater samples were taken on April 2013 off the west coast of Gijón/Xixón, northern Spain (Southern Bay of Biscay; latitude 43° 40′ 12″/43.67°N longitude 5° 34′ 48″/5.58°W). Seawater was pre-filtered by 200 μm to remove mesozooplankton. Samples were collected at noon and transported to the laboratory in 20 l polycarbonate bottles (Nalgene, Rochester, NY, USA) within 6 h. Six transparent 4 l polycarbonate bottles (Nalgene) were filled with 2 l of the sampled water and placed in a controlled temperature incubator, 3 at 12.5 °C (ambient) and 3 at 16.5 °C (+4 °C). All bottles were incubated under saturating irradiance (~150 μmol photons m−2 s−1) (Morán and Scharek, 2015) with a 13:11 light:dark period. After the samples were placed in the incubators, the water was acclimated for 12 h until the next morning. After the acclimation period, 200 ml of each bottle were transferred to Pyrex glass bottles, where we added 0.1 mmol l−1 of 13C-bicarbonate (99 atom% 13C, Cambridge Isotopes Laboratories Inc., Cambridge, MA, USA, equivalent to 5% 13C after dilution by ambient CO2) and 50 nmol l−1 15N-leucine (Cambridge Isotopes Laboratories Inc.). The bottles were incubated under constant light conditions at the same temperatures. After 12 h, 5 ml of each sample were fixed with 4% formaldehyde for 3 h at room temperature and filtered through polycarbonate filters (type GTTP; pore size, 0.2 μm; diameter, 47 mm; Millipore, Eschborn, Germany). The collected samples were stored at −80 °C.
Samples for chlorophyll a and nutrients were collected once per day during 3 days (Supplementary Figure S1), even after the stable isotope incubation, in order to characterize the state of the autotrophic community. Both parameters were analyzed following Calvo-Díaz and Morán (2006).
Autotrophic cell abundance
The abundance of small autotrophic cells (Prochlorococcus, Synechococcus and picoeucaryotes) was determined by flow cytometry based on their orange (FL2, 585 nm) and red (FL3, 650 nm) fluorescence and light scatter at 90° (SSC, side scatter) signals following standard procedures (Gasol and Del Giorgio, 2008) with a BD FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA) flow cytometer, equipped with an argon 488 nm laser. To estimate Synechococcus and eukaryotes abundance at the end of the 12 h incubation, a linear interpolation was made using the abundances at T0 (0 h) and 24 h later. For enumeration of large phytoplankton cells, 15 mL of water was fixed with 2% lugol and cell abundance was determined by inverted microscopy.
Bulk bacterial and primary production
Bacterial production was estimated by the 3H-leucine incorporation method (Kirchman et al., 1985) modified by Smith and Azam (1992) using a final concentration of 50 nmol l−1. We used an empirical conversion factor measured for the same month in a previous survey (0.87 kg C mol per Leu, Calvo-Díaz et al., 2014). Total (particulate plus dissolved fractions) primary production was estimated using the 14C method in 2 h incubations (Morán et al., 2006), in the same experimental conditions used for the stable isotope substrate incubations.
NanoSIMS analysis
Filters pieces were mounted on aluminum stubs with conductive adhesives tabs and sputter coated with ~20 nm of gold. Filters were analyzed with a NanoSIMS 50 (Cameca, Gennevilliers, France), using a ~2 pA Cs+ primary beam focused to a nominal spot size of ~ 150 nm and stepped over the sample in a 256 × 256 pixel raster to generate secondary ions. Dwell time was 1 ms/pixel, and raster size was 30 μm2. The secondary mass spectrometer was tuned for ~6800 mass resolving power to resolve isobaric interferences. Four secondary ions (12C2−, 12C13C−, 12C14N− and 12C15N−) were detected in simultaneous collection mode by pulse counting to generate 20 serial quantitative secondary ion images (layers). Samples were sputtered to a depth of 100 nm before measurements to achieve sputtering equilibrium. The depth of analysis during a measurement was between 50 and 200 nm. Ion images for large areas were stitched with the LIMAGE software (LS Nittler, Carnegie Institution, Washington, DC, USA) and isotope ratio images (12C 13C−/12C2−, 12C15N−/12C14N−; note 12C 13C−/12C2−=2•13C/12C) were produced to identify isotopically enriched cells. Regions of interests were generated with LIMAGE using the automated particle finding algorithm for both ratio images (regions of interest parameters: maximum ratio for a cell must exceed 0.008 for 15N/14N and 0.035 for 12C13C−/12C2; minimum ratio of 0.007 for 15N/14N and 0.03 for 12C13C−/12C2; threshold set at 50% of maximum ratio). Regions of interests for heterotrophs attached to photoautotrophs were redrawn manually to avoid the area between the two cells that overlapped. For one replicate of the warm treatment, the attached cells were reanalyzed with a smaller raster (5 μm) to acquire more ion counts from the cells to more closely examine the relationship between isotope enrichment of the autotrophs and their attached heterotrophs. Isotope ratio data for regions of interests (12C13C−/12C2, 15N/14N) were normalized to the isotope abundances of the filter on which the cells were located and used to calculate permil (δ=[Rf/Ri−1]*1000‰) or atom percent excess (APE=[Ff−Fi]*100%) values, where Ri and Rf are the initial (0.0107 for 13C/12C and 0.00371 for 15N/14N) and final isotope ratios in the cells, respectively, and Fi and Ff are the corresponding fractions of the minor isotope (F=R/[R+1]).
Carbon and nitrogen assimilation rates (K) were calculated based on the Popa et al. (2007) net fixation calculation divided by incubation time:
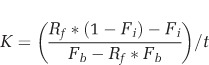 |
where Fi and Fb are the fraction of 13C or 15N initially in the cells and in the bulk, respectively, Rf is the final isotope ratio in the cells, and t is the incubation time.
To calculate the relative contribution of each compartment (heterotrophs/autotrophs and free-living/attached) to primary and secondary production, we used mean cell sizes from flow cytometry SSC and cell abundances from the NanoSIMS images to estimate phytoplankton and heterotrophic bacterioplankton biomass, assuming spherical cells and 50% C and 15% N cellular content (Bratbak and Dundas, 1984). These biomass values were multiplied by their respective K-values to obtain C and N assimilation rates by each population for each treatment. Percent contribution of free-living and attached autotrophs and heterotrophs in control and warm treatments were applied to bulk 14C- and 3H-derived primary and bacterial production rates to estimate net assimilation values in carbon and nitrogen units (Table 1).
Table 1. Total 13C and 15N assimilation by free-living and attached autotrophs and heterotrophs for both temperature treatments.
|
Net C assimilation (%, μg C L−1 h−1) |
Net N assimilation (%, μg N L−1 h−1) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Autotrophs |
Heterotrophs |
Autotrophs |
Heterotrophs |
|||||
| Free-living | Attached | Free-living | Attached | Free-living | Attached | Free-Living | Attached | |
| Control | 85.2±1.3 (3.802) | 13.2±1.5 (0.588) | 1.5±0.4 (0.068) | 0.05±0.02 (0.002) | 15.7±6.4 (0.009) | 3.2±1.8 (0.002) | 78.9±7.8 (0.047) | 2.3±0.3 (0.001) |
| Warm | 77.0±0.9 (5.355) | 20.7±1.1 (1.442) | 2.1±0.009 (0.146) | 0.12±0.03 (0.008) | 16.2±2.2 (0.012) | 7.2±1.4 (0.005) | 73.5±3.5 (0.056) | 3.1±0.7 (0.002) |
Results are shown as the percentage of contribution to total biomass assimilation (average±s.e.m.) and as estimation of C and N net assimilation (in parenthesis) based in PP and BP bulk values.
Results
Environmental properties
Chlorophyll a concentrations at the beginning of the stable isotope incubations were 2.44 and 2.54 μg l−1 in the control and warm treatments, respectively (Supplementary Figure S1). Within the 12 h of stable isotope incubation, chlorophyll values remained virtually unaltered in the control treatment, while they increased significantly by 15% in the warm treatment. Nitrate concentrations decreased moderately in both treatments, from 3.17 μmol l−1 to 2.9 and 2.8 μmol l−1 in control and warm treatments, respectively.
Phytoplankton and heterotrophic cell abundances
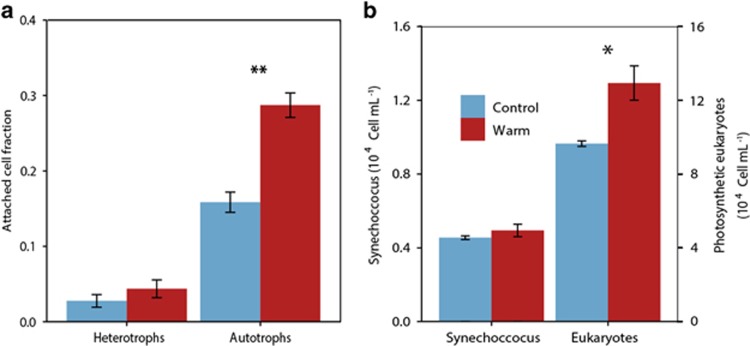
We identified both free-living and attached heterotrophic and autotrophic cells based on the isotope enrichment patterns detected by NanoSIMS. Heterotrophs were defined as cells isotopically enriched in 15N from leucine incorporation, and autotrophs as cells isotopically enriched in 13C from bicarbonate incorporation (Figure 1). In total, we analyzed more than 4800 single cells; 2003 heterotrophs and 301 autotrophs in control treatment and 2230 and 295 cells in the warm treatment). The high spatial resolution of NanoSIMS analysis, combined with the independent isotopic markers and the large number of cells analyzed, enabled us to identify cells in phytoplankton-bacteria consortia (totaling 132 autotrophs and 146 heterotrophs). In the incubations held at ambient temperature, we found a relatively low fraction of microbial cells forming algal–bacterial consortia (we did not consider bacteria attached to one another or phytoplankton attached to one another): 2.8% of heterotrophic bacteria and 16% of phytoplankton cells. Increasing temperature by 4 °C led to a statistically significant increase of the fraction of phytoplankton cells with at least one attached bacterium, comprising 29% of all autotrophs (Wilcoxon test, P<0.01; Figure 2a). The average fraction of bacteria attached to phytoplankton cells under warming conditions also increased (4.4%), but the increase was not statistically significant.
Figure 1.
Comparison between 13C and 15N isotopic enrichment for all analyzed microbial cells incubated with 13C bicarbonate and 15N leucine (dashed lines indicate isotope natural abundances). An example isotope image (inset) shows identified heterotrophs (left) and one autotroph (right, identified by the arrow).
Figure 2.
(a) Fraction of autotrophs attached to heterotrophs (left columns) and vice versa (right columns) in control versus warm incubations (mean ±s.e.m.). (b) Abundance of Synechococcus and photosynthetic eukaryotes in control and warm treatments (mean ±s.e.m.) (ANOVA *P-value<0.05 and **P-value<0.01). Note that the scale of the Y-axis of photosynthetic eukaryotes abundance is one order of magnitude higher than for Synechococcus.
Photosynthetic picoeukaryotes were the most abundant autotrophic cells in the incubations (Figure 2b), both at ambient temperature (9.6±0.15 × 104 cells ml−1) and in the warm treatment, which showed a significantly higher abundance of 12.9±0.9 × 104 cells ml−1 (t-test, P=0.02). Synechococcus cells were less abundant, and their concentration was similar in the control (4.5±0.1 × 103 cells ml−1) and the warm treatment (4.9±0.3 × 103 cells ml−1). Prochlorococcus were absent, as typical of this period of the year (Calvo-Díaz and Morán, 2006). Regarding microphytoplankton, we only detected the presence of Thalassiosira sp. cells in low abundance (<10 cells ml−1). In agreement with these observations, most of the autotrophic cells analyzed by NanoSIMS were also small (mean diameter 2.1±0.8 μm), at the nominal limit separating the pico- and nanoplankton size classes.
Primary production and bacterial production
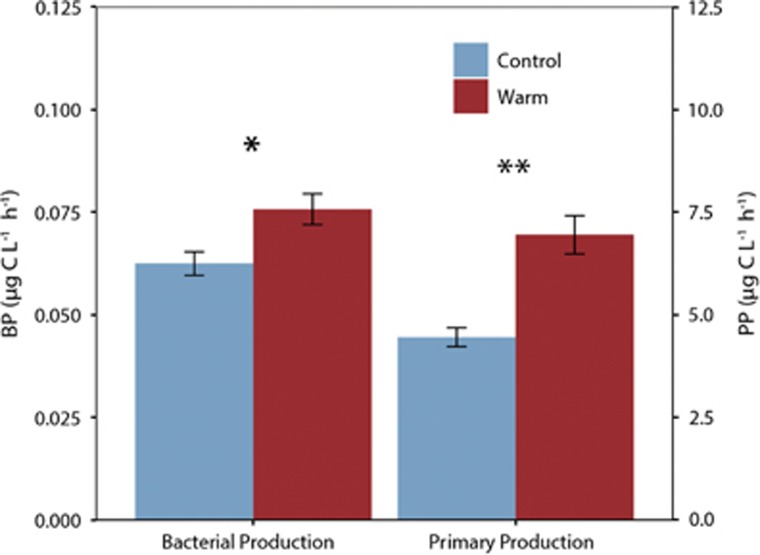
Total primary production (PP) values measured during the incubations (Figure 3) were 4.45±0.4 μg C L−1 h−1 for the ambient temperature treatment, while it increased significantly to 6.95±0.8 μg C L−1 h−1 in the warm treatment (t-test, P<0.01). Bulk BP values were 6.0±0.5 and 7.6±0.6 × 10−2 μg C L−1 h−1 in the ambient and warm treatments, respectively, a modest but significantly different increase (t-test, P=0.045).
Figure 3.
Bulk bacterial production and total primary production rates in each temperature treatment. Bars indicate s.e.m. Asterisks denote significant differences between treatments (ANOVA *P<0.05 and **P<0.01).
Phytoplankton and heterotrophic cell-specific stable isotope incorporation
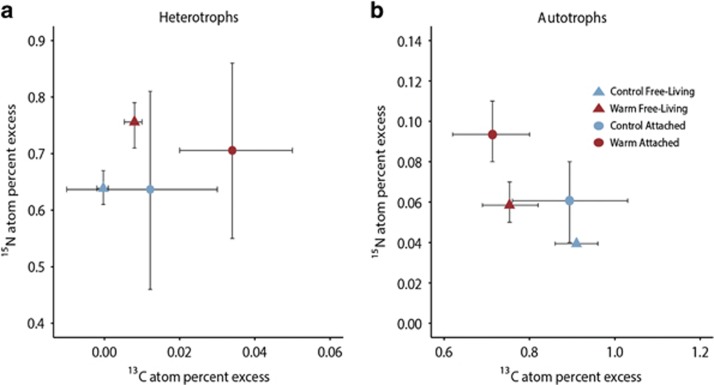
To investigate C and N incorporation at the cell-specific level, we report median values and used non-parametric statistics (Wilcoxon test) as the isotope data exhibited non-normal distributions. Comparing treatments, we detected significantly more 15N-leucine incorporation by heterotrophs (>95% of them being free-living) in the warm compared with the control treatment (P<0.01; Figure 4a). A small number of heterotrophic free-living cells had significant incorporation of 13C in the control treatment, but the median was not significantly enriched in 13C. In the warm treatment, the heterotrophs had significantly higher 13C incorporation (P<0.01; Figure 4a). Heterotrophs attached to phytoplankton in the warm treatment exhibited the highest 13C incorporation (significantly greater than their free-living counterparts, Wilcoxon test, P<0.01; median value=22% more enriched). In the case of phytoplankton, the median 13C incorporation per cell was significantly lower in the warm treatment (P<0.05; Figure 4b). Overall, phytoplankton exhibited low but significant 15N enrichment, with greater incorporation in the warm treatment (Figure 4b; P<0.01). We also observed that the 15N incorporation by phytoplankton was significantly higher in cells with attached heterotrophs, particularly in the warm treatment (Wilcoxon test, P<0.01).
Figure 4.
Cell-specific isotope incorporation from 13C bicarbonate and 15N leucine by attached (circle) and free-living (triangle) heterotrophs (a) and autotrophs (b) in response to a temperature increase of 4 °C over the control incubation. Data points are medians of all cells from three replicate incubations with ±95% confidence intervals.
To quantify how the increased temperature treatment influenced the flow of recently fixed C and leucine-derived N, we used the bulk PP and BP rates, the median stable isotope incorporation values, and the cell abundances to calculate the total incorporation for each of the two labeled pools (Popa et al., 2007; Adam et al., 2016; Supplementary Table S1,Table 1). In the control treatment, 84% of the autotropic cells were free-living and contained 85% of the newly fixed C, while the attached autotrophs contained 13% of the newly fixed C. Warming decreased the relative amount of recently fixed C in free-living autotrophs to 77%, but in absolute terms the net C-assimilation was still greater than the control treatment. As noted above, both the relative and absolute abundances of attached autotrophs increased substantially in the warm treatment, and their contribution to assimilation of newly fixed C rose to 21%, which was substantially more total mass C per liter compared with the control. Free-living and attached heterotrophic cell in the control experiment accounted for 1.5% and 0.05% of fixed C, respectively, and 2.0% and 0.12%, respectively, in the warming experiment. We carried out similar calculations for N derived from leucine incorporation. In the control treatment, free-living and attached heterotrophs contained 79% and 2% of the net N-incorporation, respectively, and free-living and attached autotrophs contained 16% and 3%, respectively. In the warm treatment, free-living and attached heterotrophs contained 74% and 3% of the net N-incorporation and free-living and attached autotrophs contained 16% and 7%, respectively. Warming particularly increased both the relative and absolute incorporation of leucine-derived N by attached autotrophs by greater than a factor of 2.
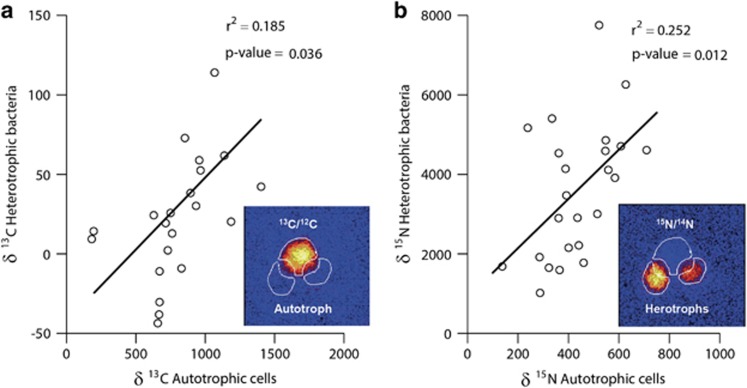
To further investigate possible isotope exchange between attached heterotrophs and phytoplankton, we identified 24 pairs of attached heterotrophs and phytoplankton from one of the warm replicates and re-analyzed them by NanoSIMS for a longer period of time (Figure 5), with a smaller raster to collect more precise isotope enrichment data (examples are shown in Figure 5 insets). These additional analyses showed that the 13C enrichment of the heterotrophic bacteria was positively correlated to the 13C enrichment of the phytoplankton cell to which they were attached (P<0.05; Figure 5a). Moreover, phytoplankton15N enrichment was positively correlated to the 15N enrichment of the heterotrophic cells attached to them (P<0.05, Figure 5b).
Figure 5.
(a) Positive correlation between the 13C incorporation rates of phytoplankton cells and those of their attached bacterial cells, analyzed with a smaller raster for one of the replicates of the warm incubations. (b) Similar positive correlation between the 15N incorporation rates of bacteria and those of the phytoplankton cells to which they were attached. Insets show an example of two heterotrophic bacteria attached to a picophytoplankton cell. Regions of interests (white outlines) for data extraction were drawn to ensure that overlapping areas were not used to measure isotope incorporation by the individual cells.
Discussion
We found that attachment between autotrophs and heterotrophs increased secondary C and N incorporation by the heterotrophs and autotrophs, respectively. Furthermore, a short-term 4 °C temperature increase led to higher secondary 13C incorporation by heterotrophs, suggesting that recently fixed photosynthate was being released more rapidly by phytoplankton and/or heterotrophs were incorporating it faster. Another possibility is that warming promoted autotrophy or increased anaplerotic C incorporation in these primarily heterotrophic cells. However, the highest 13C incorporation found in attached heterotrophs (Figure 4, Supplementary Table S1) supports the view that this 13C incorporation originated mainly from DOC recently fixed by phytoplankton, as attached heterotrophs would have had direct access to it before it became diluted in the bulk water. Moreover, we observed a positive correlation between 13C enrichment of the heterotrophic bacteria and 13C enrichment of the phytoplankton to which they were attached (Figure 5a), consistent with direct transfer of C from phytoplankton to attached bacteria. Thus, we infer that attachment promoted faster heterotrophic incorporation of phytoplankton-derived DOC, and that it was further enhanced with increased temperature. To determine the contribution of attached heterotrophs to the total phytoplankton-derived DOC incorporation, we combined the cell abundance and the cell-specific activity data (Table 1). These results showed that warmer temperatures increased DOC incorporation by free-living heterotrophs by two-fold, and this incorporation increased by almost four fold for attached heterotrophs, demonstrating the importance of attachment for the transfer of recently fixed C to secondary consumers.
In contrast to heterotrophs, phytoplankton 13C incorporation was not affected by attachment and in fact exhibited lower cell-specific incorporation at higher temperatures. However, as phytoplankton cell abundance increased under warming, total 13C incorporation (and therefore total C fixation) was significantly higher (increasing from 3.8 μg C L−1 h−1 to 5.35 μg C L−1 h−1, t-test, P<0.001; Table 1). The lower cell-specific 13C incorporation in the warm treatment could be explained by warming increasing the release of recently fixed (and 13C-labeled) C, as shown by experimental studies conducted in other oceanic regions (Morán et al., 2006; Engel et al., 2010). This process would have provided heterotrophic bacteria with more organic C and led to lower 13C enrichment in phytoplankton cells. Regardless of the mechanism, the bulk BP and PP measurements also showed an increase of heterotrophic and autotrophic activities with warming (Figure 3), similar to the stable isotope data.
In addition to the transfer of recently fixed DOC from phytoplankton to heterotrophic bacteria, the experimental design allowed us to consider the reverse process, that is, the transfer of compounds from heterotrophs to phytoplankton. The higher 15N incorporation by phytoplankton found in the warm treatment may indicate increased bacterial N transfer to autotrophic cells. Although some of this incorporation may have originated from the direct uptake of labeled leucine by phytoplankton, several lines of evidence indicate that this was not a major portion. First, most of the phytoplankton cells originally present in the samples were picoplanktonic eukaryotes. A number of studies have documented the ability of cyanobacteria to incorporate amino acids (Zubkov et al., 2003; Michelou et al., 2007), whereas uptake by eukaryotes has been observed less frequently (Kamjunke and Tittel, 2008; Znachor and Nedoma, 2009). In particular, mixotrophic activity by photosynthetic eukaryotes has been linked to low light conditions (Bouarab et al., 2004; Ruiz-González et al., 2012). Therefore, at the relatively high irradiance used in our incubations, leucine incorporation by phytoplankton was less likely to occur. Also, we observed that the 15N incorporation by phytoplankton was significantly higher in cells with attached heterotrophs, particularly in the warm treatment, and the positive correlation between the enrichment of autotrophs and bacteria attached to them (Figure 5b) suggests that a direct transfer of N from the 15N-leucine-labeled bacteria to the phytoplankton cells was taking place. Furthermore, the higher increase in total 15N assimilation by attached autotrophs compared with the free-living fraction (Table 1), highly supports this secondary uptake. The N transferred may have originated through leucine respiration by the bacterial cells and the subsequent production of ammonium (Kirchman et al., 1985). Although this process occurs in oligotrophic conditions (Alonso-Sáez et al., 2007; Del Giorgio et al., 2011), at saturating leucine concentrations, as used in this work, leucine respiration may increase up to threefold (Hill et al., 2013). Furthermore, the preference of picophytoplankton to incorporate ammonium rather than other nitrogen compounds such as nitrate (Le Bouteiller, 1986; Harrison et al., 1996; Rees et al., 1999), and the described inhibition of nitrate uptake by ammonium (L'Helguen et al., 2008), suggest that autotrophs may, more likely, have incorporated this newly remineralized ammonium as a N source.
Regardless of the exact mechanism of exchange, our results indicate a mutualistic relationship between autotrophs and heterotrophs through exchange of metabolites or nutrients mediated by attachment. Similar mutualistic partnerships have been previously observed between cyanobacteria and microalgae (Thompson et al., 2012; Adam et al., 2016), resulting in an obligate symbiosis (Cabello et al., 2016), or between heterotrophic bacteria and autotrophic organisms in co-cultures (Amin et al., 2015). Here, we reveal the occurrence of the latter in natural ecosystems. Our work further suggests that this mutualistic relationship in phytoplankton-bacteria consortia may increase its importance under warming, at least under the conditions tested (that is, short-term increased temperature of 4 °C). It should be noted that during the experiments, nutrient availability was not limiting, as suggested by the increase of Chl a concentration and the active uptake of nutrients (Supplementary Figure S1). The nutrient availability was key to observe the effect of temperature on microbial cell-specific activities, as resource limitation may mask the temperature dependency of heterotrophic and autotrophic metabolism (López-Urrutia and Morán, 2007).
In terms of C cycling, we found that short-term elevation of temperature induced higher rates of C fixation and cycling through heterotrophic bacteria as assessed at the single-cell level, in support to previous results analyzing bulk rates (Morán et al., 2006). Our results clearly show that attachment represents a key strategy promoting C and N exchange between the cells, while increased temperature stimulated this interaction (Figure 4). Even if during our experiments bacterial attachment was relatively low, this route of C transfer between phytoplankton and bacteria may intensify under conditions of phytoplankton blooms, where the contribution of particle-attached bacteria to total bacterial biomass can increase considerably (Becquevort et al., 1998; Mével et al., 2008), although their contribution to bulk production remains unclear (Riemann et al., 2000; Grossart et al., 2007b). Researchers have previously suggested the importance of examining the microscale habitats of marine bacteria (for example, Azam, 1998), and our results show that interactions occurring between photoautotrophs and heterotrophs in the phycosphere are important contributors to bulk element fluxes through the microbial food web. Interestingly, while most previous studies have focused exclusively on bacteria attached to large phototrophic cells such as diatoms (Gardes et al., 2011), our results show that this strategy is also significant for the smaller pico- and nanoeukaryotes. These phytoplanktonic groups are widely distributed in the ocean, contributing more than 40% of total primary production at low latitudes such as the tropical Atlantic (Jardillier et al., 2010), and their role is expected to increase in a warmer ocean (Agawin et al., 2000; Chen et al., 2014).
The observed increased metabolite transfer between attached cells with warming supports the view that the ecological strategy of attachment may be relevant in biogeochemical cycles, and that it should be taken into account when assessing nutrient fluxes among the planktonic components in warmer oceans. Other environmental conditions not specifically addressed in our experiments, and potentially affecting this interaction will have to be assessed in future work, including nutrient concentrations, the combined effects of temperature and inorganic C availability (as affected by ocean acidification), and the effect of climate change on microbial community structure. Our results exemplify the concept that quantitative, functional analyses carried out on a single cell level can uncover cryptic interactions of potential importance at the ecosystem level, and NanoSIMS and other single-cell enabled microbial activity tools (Samo et al., 2014) are essential to collect such data.
Acknowledgments
This work was partially supported by COMITE project by Spanish National Investigation+Development+Innovation (I+D+I). Financial support for NAG's PhD fellowship was provided by the Basque Government. LAS was supported by a ‘Juan de la Cierva' fellowship from the Spanish Ministry of Science and Education and a Marie Curie Reintegration Grant (FP7, Grant Agreement 268331). XM was partially supported by the Gordon and Betty Moore Foundation Marine Microbiology Initiative grant #3302, and method development at LLNL was funded by the Department of Energy's Genome Sciences Program grant SCW1039. Work at LLNL was performed under the auspices of the US Department of Energy at Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
Author contributions
NAG, XM, LAS and XACM designed the study, NAG carried out the incubation experiment and NanoSIMS sample preparation, XM, PKW and NAG performed NanoSIMS analyses, and NAG, PKW and XM analyzed the data. All authors discussed the results and contributed in the manuscript writing.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
The authors declare no conflict of interest.
Supplementary Material
References
- Adam B, Klawonn I, Sveden JB, Bergkvist J, Nahar N, Walve J et al. (2016). N2-fixation, ammonium release and N-transfer to the microbial and classical food web within a plankton community. ISME J 10: 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agawin NSR, Duarte CM, Agustí S. (2000). Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol Oceanogr 45: 1891–1891. [Google Scholar]
- Alonso-Sáez L, Gasol JM, Arístegui J, Vilas JC, Vaqué D, Duarte CM et al. (2007). Large-scale variability in surface bacterial carbon demand and growth efficiency in the subtropical northeast Atlantic Ocean. Limnol Oceanogr 52: 533–546. [Google Scholar]
- Amin SA, Parker MS, Armbrust EV. (2012). Interactions between diatoms and bacteria. Microbiol Molecular Biol Rev 76: 667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin SA, Hmelo LR, van Tol HM, Durham BP, Carlson LT, Heal KR et al. (2015). Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 522: 98–101. [DOI] [PubMed] [Google Scholar]
- Azam F. (1998). Oceanography: microbial control of oceanic carbon flux: the plot thickens. Science 280: 694–696. [Google Scholar]
- Azam F, Long RA. (2001). Sea snow microcosms. Nature 414: 497–498. [DOI] [PubMed] [Google Scholar]
- Azam F, Malfatti F. (2007). Microbial structuring of marine ecosystems. Nat Rev Microbiol 5: 782–791. [DOI] [PubMed] [Google Scholar]
- Becquevort S, Rousseau V, Lancelot C. (1998). Major and comparable roles for free-living and attached bacteria in the degradation of Phaeocystis-derived organic matter in Belgian coastal waters of the North Sea. Aquat Microb Ecol 14: 39–48. [Google Scholar]
- Bell W, Mitchell R. (1972). Chemotactic and growth responses of marine bacteria to algal extracellular products. Biol Bull 143: 265. [Google Scholar]
- Bouarab L, Dauta A, Loudiki M. (2004). Heterotrophic and mixotrophic growth of Micractinium pusillum Fresenius in the presence of acetate and glucose: effect of light and acetate gradient concentration. Water Res 38: 2706–2712. [DOI] [PubMed] [Google Scholar]
- Bratbak G, Dundas I. (1984). Bacterial dry matter content and biomass estimations. Appl Environ Microbiol 48: 755–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan A, LeCleir GR, Gulvik CA, Gonzalez JM. (2014). Master recyclers: features and functions of bacteria associated with phytoplankton blooms. Nat Rev Microbiol 12: 686–698. [DOI] [PubMed] [Google Scholar]
- Cabello AM, Cornejo-Castillo FM, Raho N, Blasco D, Vidal M, Audic S et al. (2016). Global distribution and vertical patterns of a prymnesiophyte-cyanobacteria obligate symbiosis. ISME J 10: 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Díaz A, Morán XAG. (2006). Seasonal dynamics of picoplankton in shelf waters of the southern Bay of Biscay. Aquat Microb Ecol 42: 159–174. [Google Scholar]
- Calvo-Diaz A, Franco-Vidal L, Morán XAG. (2014). Annual cycles of bacterioplankton biomass and production suggest a general switch between temperature and resource control in temperate coastal ecosystems. J Plankton Res 36: 859–865. [Google Scholar]
- Chen B, Liu H, Huang B, Wang J. (2014). Temperature effects on the growth rate of marine picoplankton. Mar Ecol Prog Ser 505: 37–47. [Google Scholar]
- Cornejo-Castillo FM, Cabello AM, Salazar G, Sanchez-Baracaldo P, Lima-Mendez G, Hingamp P et al. (2016). Cyanobacterial symbionts diverged in the late Cretaceous towards lineage-specific nitrogen fixation factories in single-celled phytoplankton. Nat Commun 7: 11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG. (2005). Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438: 90–93. [DOI] [PubMed] [Google Scholar]
- Del Giorgio PA, Condon R, Bouvier T, Longnecker K, Bouvier C, Sherr E et al. (2011). Coherent patterns in bacterial growth, growth efficiency, and leucine metabolism along a northeastern Pacific inshore-offshore transect. Limnol Oceanogr 56: 1–16. [Google Scholar]
- Duarte CM, Regaudie-de-Gioux A, Arrieta JM, Delgado-Huertas A, Agusti S. (2013). The oligotrophic ocean is heterotrophic. Annu Rev Mar Sci 5: 551–569. [DOI] [PubMed] [Google Scholar]
- Eiler A, Olsson JA, Bertilsson S. (2006). Diurnal variations in the auto- and heterotrophic activity of cyanobacterial phycospheres (Gloeotrichia echinulata and the identity of attached bacteria. Freshwater Biol 51: 298–311. [Google Scholar]
- Engel A, Handel N, Wohlers J, Lunau M, Grossart HP, Sommer U et al. (2010). Effects of sea surface warming on the production and composition of dissolved organic matter during phytoplankton blooms: results from a mesocosm study. J Plankton Res 33: 357–372. [Google Scholar]
- Gardes A, Iversen MH, Grossart HP, Passow U, Ullrich MS. (2011). Diatom-associated bacteria are required for aggregation of Thalassiosira weissflogii. ISME J 5: 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasol JM, Del Giorgio PA. (2008). Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Sci Mar 64: 197–224. [Google Scholar]
- Grossart HP, Simon M. (2007. a). Interactions of planktonic algae and bacteria: effects on algal growth and organic matter dynamics. Aquat Microb Ecol 47: 163–176. [Google Scholar]
- Grossart HP, Tang KW, Kiorboe T, Ploug H. (2007. b). Comparison of cell-specific activity between free-living and attached bacteria using isolates and natural assemblages. FEMS Microbiol Lett 266: 194–200. [DOI] [PubMed] [Google Scholar]
- Harrison WG, Harris LR, Irwin BD. (1996). The kinetics of nitrogen utilization in the oceanic mixed layer: Nitrate and ammonium interactions at nanomolar concentrations. Limnol Oceanogr 41: 16–32. [Google Scholar]
- Hill PG, Warwick PE, Zubkov MV. (2013). Low microbial respiration of leucine at ambient oceanic concentration in the mixed layer of the central Atlantic Ocean. Limnol Oceanogr 58: 1597–1604. [Google Scholar]
- Jackson GA. (1987). Simulating chemosensory responses of marine microorganisms. Limnol Oceanogr 32: 1253–1266. [Google Scholar]
- Jardillier L, Zubkov MV, Pearman J, Scanlan DJ. (2010). Significant CO2 fixation by small prymnesiophytes in the subtropical and tropical northeast Atlantic Ocean. ISME J 4: 1180–1192. [DOI] [PubMed] [Google Scholar]
- Kamjunke N, Tittel J. (2008). Utilisation of leucine by several phytoplankton species. Limnologica 38: 360–366. [Google Scholar]
- Kirchman DL, K'nees E, Hodson R. (1985). Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol 49: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazier JRN, Mann KH. (1989). Turbulence and the diffusive layers around small organisms. Deep-Sea Res 36: 1721–1733. [Google Scholar]
- Le Bouteiller A. (1986). Environmental control of nitrate and ammonium uptake by phytoplankton in the Equatorial Atlantic Ocean. Mar Ecol Prog Ser 30: 167–179. [Google Scholar]
- L'Helguen S, Maguer JF, Caradec J. (2008). Inhibition kinetics of nitrate uptake by ammonium in size-fractionated oceanic phytoplankton communities: implications for new production and f-ratio estimates. J Plankton Res 30: 1179–1188. [Google Scholar]
- López-Urrutia Á, Morán XAG. (2007). Resource limitation of bacterial production distorts the temperature dependence of oceanic carbon cycling. Ecology 88: 817–822. [DOI] [PubMed] [Google Scholar]
- Magazzù G, Decembrini F. (1995). Primary production, biomass and abundance of phototrophic picoplankton in the Mediterranean Sea: a review. Aquat Microb Ecol 9: 97–104. [Google Scholar]
- Marañón E, Holligan PM, Barciela R, González N, Mouriño B, Pazó MJ et al. (2001). Patterns of phytoplankton size structure and productivity in contrasting open- ocean environments. Mar Ecol Prog Ser 216: 43–56. [Google Scholar]
- Meehl GA et al. (2007) The physical science basisClimate Change 2007 IPCC Report. Cambridge University Press: Cambridge, UK, pp 748–845. [Google Scholar]
- Mével G, Vernet M, Goutx M, Ghiglione JF. (2008). Seasonal to hour variation scales in abundance and production of total and particle-attached bacteria in the open NW Mediterranean Sea (0–1000 m). Biogeosciences 5: 1573–1586. [Google Scholar]
- Michelou VK, Cottrell MT, Kirchman DL. (2007). Light-stimulated bacterial production and amino acid assimilation by cyanobacteria and other microbes in the North Atlantic Ocean. Appl Environ Microbiol 73: 5539–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán XAG, Sebastián M, Pedrós-Alió C, Estrada M. (2006). Response of Southern Ocean phytoplankton and bacterioplankton production to short-term experimental warming. Limnol Oceanogr 51: 1791–1800. [Google Scholar]
- Morán XAG, Scharek R. (2015). Photosynthetic parameters and primary production, with focus on large phytoplankton, in a temperate mid-shelf ecosystem. Estuar Coast Shelf Sci 154: 255–263. [Google Scholar]
- Morán XAG, Alonso-Sáez L, Nogueira E, Ducklow HW, Gonzalez N, Lopez-Urrutia A et al. (2015). More, smaller bacteria in response to ocean's warming? Proc R Soc B 282: 20150371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa R, Weber PK, Pett-Ridge J, Finzi JA, Fallon SJ, Hutcheon ID et al. (2007). Carbon and nitrogen fixation and metabolite exchange in and between individual cells of Anabaena oscillarioides. ISME J 1: 354–360. [DOI] [PubMed] [Google Scholar]
- Rees A, Woodward M, Joint I. (1999). Measurement of nitrate and ammonium uptake at ambient concentrations in oligotrophic waters of the North-East Atlantic Ocean. Mar Ecol Prog Ser 187: 295–300. [Google Scholar]
- Riemann L, Steward GF, Azam F. (2000). Dynamics of bacterial community composition and activity during a mesocosm diatom bloom. Appl Environ Microbiol 66: 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney-Varga JN, Giewat MW, Savin MC, Sood S, LeGresley M, Martin JL. (2005). Links between phytoplankton and bacterial community dynamics in a coastal marine environment. Microb Ecol 49: 163–175. [DOI] [PubMed] [Google Scholar]
- Ruiz-González C, Gali M, Sintes E, Herndl GJ, Gasol JM, Simó R. (2012). Sunlight effects on the Osmotrophic uptake of DMSP-sulfur and leucine by polar phytoplankton. PLoS ONE 7: e45545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon PS, Janson S, Granéli E. (2003). Molecular identification of bacteria associated with filaments of Nodulariaspumigena and their effect on the cyanobacterial growth. Harmful Algae 2: 261–272. [Google Scholar]
- Samo TJ, Smriga S, Malfatti F, Sherwood BP, Azam F. (2014). Broad distribution and high proportion of protein synthesis active marine bacteria revealed by click chemistry at the single cell level. Front Mar Sci 1: 48. [Google Scholar]
- Sarmento H, Montoya JM, Vázquez-Domínguez E, Vaque D, Gasol JM. (2010). Warming effects on marine microbial food web processes: how far can we go when it comes to predictions? Philos T R Soc B 365: 2137–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento JL, Slater R, Barber R, Bopp L, Doney SC, Hirst AC et al. (2004). Response of ocean ecosystems to climate warming. Global Biogeochem Cy 18: GB3003. [Google Scholar]
- Simon M, Grossart HP, Schweitzer B, Ploug H. (2002). Microbial ecology of organic aggregates in aquatic ecosystems. Aquat Microb Ecol 28: 175–211. [Google Scholar]
- Smith DC, Azam F. (1992). A simple, economical method for measuring bacterial protein synthesis rates in seawater using 3H-leucine. Mar Microb Food Webs 6: 107–111. [Google Scholar]
- Stockner JG. (1988). Phototrophic picoplankton: an overview from marine and freshwater ecosystems. Limnol Oceanogr 33: 765–775. [Google Scholar]
- Thompson AW, Foster RA, Krupke A, Carter BJ, Musat N, Vaulot D et al. (2012). Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science 337: 1546–1550. [DOI] [PubMed] [Google Scholar]
- Vázquez-Domínguez E, Vaqué D, Gasol JM. (2007). Ocean warming enhances respiration and carbon demand of coastal microbial plankton. Global Change Biol 13: 1327–1334. [Google Scholar]
- von Scheibner M, Dorge P, Biermann A, Sommer U, Hoppe HG, Jurgens K. (2014). Impact of warming on phyto-bacterioplankton coupling and bacterial community composition in experimental mesocosms. Environ Microbiol 16: 718–733. [DOI] [PubMed] [Google Scholar]
- Wagner M. (2009). Single-cell ecophysiology of microbes as revealed by Raman microspectroscopy or secondary ion mass spectrometry imaging. Annu Rev Microbiol 63: 411–429. [DOI] [PubMed] [Google Scholar]
- Znachor P, Nedoma J. (2009). Importance of dissolved organic carbon for phytoplankton nutrition in a eutrophic reservoir. J Plankton Res 32: 367–376. [Google Scholar]
- Zubkov MV, Fuchs BM, Tarran GA, Burkill PH, Amann R. (2003). High rate of uptake of organic nitrogen compounds by prochlorococcus cyanobacteria as a key to their dominance in oligotrophic oceanic waters. Appl Environ Microbiol 69: 1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.