Abstract
The emergence of insecticide resistant insect pests is of significant concern worldwide. The whitefly, Bemisia tabaci, is an important agricultural pest and has shown incredible resilience developing resistance to a number of chemical pesticides. Entomopathogenic fungi such as Isaria fumosorosea offer an attractive alternative to chemical pesticides for insect control, and this fungus has been shown to be an effective pathogen of B. tabaci. Little is known concerning the potential for the development of resistance to I. fumosorosea by B. tabaci. Five generations of successive survivors of B. tabaci infected by I. fumosorosea were assayed with I. fumosorosea. No significant differences in susceptibility to I. fumosorosea, number of ovarioles, or ovipostioning were seen between any of the generations tested. Effects of I. fumosorosea and cell-free ethyl acetate fractions derived from the fungus on the B. tabaci fat body, ovary, and vitellogenin were also investigated. These data revealed significant deformation and degradation of ovary tissues and associated vitellogenin by the fungal mycelium as well as by cell-free ethyl acetate fungal extracts. These data indicate the lack of the emergence of resistance to I. fumosorosea under the conditions tested and demonstrate invasion of the insect reproductive tissues during fungal infection.
The sweet potato whitefly, Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae), is one of the most destructive insect pests worldwide negatively affecting ornamental, vegetable, grain legume, and cotton production1,2,3. Damage to the host plant is caused by insect feeding on the phloem sap, with significant secondary effects resulting in infection of the plant by sooty molds that grow on the honeydew excreted by the insect as it feeds. B. tabaci can also act as a vector for the transmission of >600 different plant pathogenic viruses, infection by which can result in severe reductions in the photosynthetic ability of host plant, and hence stunt growth, decreasing crop yield and quality4,5,6,7. Chemical pesticides are the most commonly used method for suppressing B. tabaci populations8, however, the insect has been shown to rapidly develop insecticide resistance. This, coupled to the increasing environmental awareness of the hazards of chemical insecticides9,10,11, has promoted the development of alternative approaches. These include the use of biological control agents such as entomopathogenic fungi, that are more environmentally friendly and compatible with organic farming practices12,13,14,15. The use of fungal biological control agents has several advantages including: the low likelihood of resistance development, decreased non-target effects, and decreased human health and environmental impacts16,17,18,19. More than 20 species of entomopathogenic fungi are known to infect whiteflies20,21, including isolates of Beauveria bassiana22,23 and Isaria fumosorosea24,25,26.
Isaria fumosorosea (previously, Paecilomyces fumosoroseus27 is an important natural enemy of whiteflies including B. tabaci13,24,25,28. A number of studies have focused on the potential of I. fumosorosea to control adult and/or various larval stages of P. xylostella, Trialeurodes vaporariorum (Westwood), Diaphorina citri, and B. tabaci12,24,29,30,31,32,33. Although far more is known concerning the biochemical and molecular aspects of infection in the related Beauveria and Metarhizium species34,35, several studies have begun to examine these processes in Isaria sp28,36,37 including the isolation and characterization of several bioactive secondary metabolites38,39.
Aside from outright death of the host, sub-lethal effects of entomogenous fungi on insect fecundity and longevity has been explored24,40,41. Such effects can have important consequences for host population dynamics42,43, e.g. I. fumosorosea infection of B. tabaci and P. xylostella results in fewer eggs laid prior to death than control females of the same age24. An important consideration is the development of insect resistance to the agents employed for their control. Adaptation for resistance to chemical pesticides in a range of insect species, including B. tabaci is well documented11,44. Indeed, pesticide resistant B. tabaci can occur as rapidly as within few years, e.g. high resistance to pyriproxyfen (>1000-fold increase) has been reported within three years of use of the chemical pesticide11. Although some insects appear to be intrinsically more resistant to entomopathogenic fungi than others, e.g. amongst beetles, the red flour beetle Tribolium castaneum displays a relatively high level of resistance to B. bassiana in part due to the secretion of cuticular quinones45, there are no reports of the development of resistance to fungi used in biological control efforts in the field. This is likely due to the observation that infection of host is multi-modal, involving a combination of enzymes targeting degradation of the host, production of toxins and other secondary metabolites, and diverse mechanisms for overcoming the immune defenses of the host35,46,47. A report in which larvae of the greater waxmoth, Galleria mellonella, was under constant selective pressure from B. bassiana infection for 25 generations resulted in only a minor decrease in susceptibility (<10%), although changes in cuticular, humoral, and cellular defenses were noted between the 1st and 25th generations48. However, many insects that have been reported to have developed resistance to chemical pesticides continue to be susceptible to infection by entomopathogenic fungi, and in some cases have been reported to be more vulnerable than their wild type parents49,50,51,52,53.
Here, we investigated the potential of B. tabaci to develop resistance to I. fumosorosea under constant selective pressure from the fungus. In addition, insect fecundity and ovary development as well as reproductive tissue invasion by the fungus was monitored during infection. These data suggest that use of entomopathogenic fungi poses a low risk of resistance development and confirms their utility in pest control as part of Integrated Pest Management (IPM) practices.
Results
Susceptibility of B. tacabi selected under I. fumosorosea pressure to I. fumosorosea
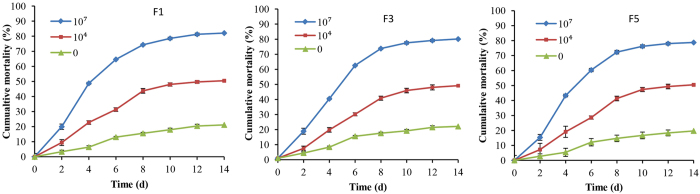
B. tabaci was selected under I. fumosorosea pressure for five generations, labeled F1 to F5, via iterative exposure of surviving insects to the fungal agent as detailed in the Methods section. The susceptibility of the F1, F3, and F5 generations to various concentrations of I. fumosorosea conidial suspensions was examined as described in the Methods section (Fig. 1). No significant differences were seen in the time course or mean cumulative mortalities of the different B. tabaci generations to either a low (104 conidia/ml) or high dose (107 conidia/ml) of the fungal agent (Table 1). The mean lethal time to kill (LT50) values for the high/low dose was 5.5 ± 0.5 d/28.1 ± 8.0 d, 6.3 ± 0.5 d/29.7 ± 7.8 d, and 6.4 ± 0.5 d/26.9 ± 6.8 d, for the F1, F3, and F5 B. tabaci generations, respectively, and were not significantly different.
Figure 1. The cumulative mortality of B. tabaci nymph against I. fumosorosea.
0, 4, 7 mean the concentration of fungal conidia with 0, 1 × 104, 1 × 107 conidia/ml, respectively. F1, F3 and F5 mean the No. 1, 3 and 5 generation of B. tabaci nymph. Experiments were performed in triplicate, error bars = ±SE.
Table 1. Analysis of mortality difference among different generations of B. tabaci nymph against I. fumosorosea.
| B. tabaci | 4d |
8d |
14d |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 0 | 4 | 7 | 0 | 4 | 7 | |
| F1 | 6.4 ± 1.1 a | 22.8 ± 1.9 a | 48.8 ± 1.8 a | 15.6 ± 0.5 a | 43.8 ± 1.2 a | 74.3 ± 0.3 a | 21.1 ± 1.2 a | 50.5 ± 0.9 a | 82.1 ± 0.9 a |
| F3 | 8.4 ± 0.7 a | 19.8 ± 1.6 a | 40.5 ± 2.0 a | 17.6 ± 0.6 a | 40.9 ± 1.0 a | 73.8 ± 0.5 a | 22.1 ± 1.1 a | 49.1 ± 1.7 a | 80.1 ± 0.8 a |
| F5 | 5.4 ± 1.7 a | 19.1 ± 4.2 a | 43.3 ± 1.9 a | 14.7 ± 2.4 a | 41.4 ± 1.1 a | 72.3 ± 1.1 a | 19.7 ± 1.9 a | 50.5 ± 1.5 a | 78.7 ± 0.9 a |
| F, df, P | 1.549, 2, 0.287 | 1.370, 2, 0.324 | 4.811. 2, 0.057 | 0.994, 2, 0.424 | 2.319, 2, 0.179 | 2.240, 2, 0.188 | 0.694, 2, 0.536 | 0.398, 2, 0.688 | 4.046, 2, 0.077 |
Note: Means ± SE in the same column followed by different letters are significant different (Tukey’s, a = 0.05). 0, 4, 7 mean the concentration of fungal conidia with 0, 1 × 104, 1 × 107 conidia/ml, respectively. F1, F3 and F5 mean the No. 1, 3 and 5 generation of B. tabaci nymph, respectively.
Effect of I. fumosorosea on B. tabaci ovipositioning
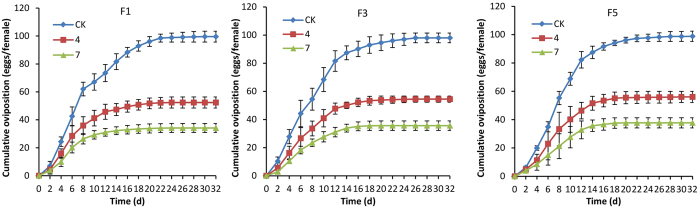
The effect of low (104 conidia/ml) and high (107 conidia/ml) doses of I. fumosorosea on the number of eggs laid by the F1, F3, and F5 generations of I. fumosorosea-selected B. tabaci generations was examined as detailed in the Methods section. A time course of the cumulative number of eggs laid by untreated, and low and high dose treared B. tabaci was performed (Fig. 2). Control (untreated) B. tabaci adult laid approximately 100 ± 4 eggs/female/30 d, with no significant differences seen between the F1, F3, and F5 generations. Infection by I. fumorososea even at the low dose (104 conidia/ml) resulted in a sharp decrease (~50%) in the total number of eggs laid to ~55 ± 4 eggs/female/30 d when assayed against the F1, F3, and F5 B. tabaci generations, with equal reductions, i.e. no significant differences seen between the different generations (Table 2). Infections using the higher dose (107 conidia/ml) resulted in a greater overall reduction in the number of eggs laid to ~35 ± 3 eggs/female/30 d, and similar to what was observed at the low dose, the reduction was similar across all of the B. tabaci selected generations, with no significant differences observed.
Figure 2. The cumulative oviposition of B. tabaci adult emergence from I. fumosorosea-selected B. tabaci nymph.
CK, 4, 7 mean the concentration of fungal conidia with 0, 1 × 104, 1 × 107 conidia/ml, respectively. F1, F3 and F5 mean the No. 1, 3 and 5 generation of B. tabaci adult. Experiments were performed in triplicate, error bars = ±SE.
Table 2. Analysis of oviposition difference among different generations of B. tabaci adult against I. fumosorosea.
| B. tabaci | 6d |
12d |
18d |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 0 | 4 | 7 | 0 | 4 | 7 | |
| F1 | 42.6 ± 6.6 a | 28.5 ± 7.3 a | 20.2 ± 3.9 a | 73.5 ± 6.1 a | 45.8 ± 5.4 a | 31.1 ± 2.7 a | 92.8 ± 3.7 a | 50.8 ± 3.8 a | 33.4 ± 3.1 a |
| F3 | 44.2 ± 9.4 a | 26.7 ± 7.5 a | 18.3 ± 2.7 a | 81.5 ± 7.3 a | 47.7 ± 3.9 a | 31.1 ± 3.4 a | 92.9 ± 5.9 a | 53.3 ± 3.1 a | 35.6 ± 3.3 a |
| F5 | 34.9 ± 3.6 a | 23.0 ± 11.3 a | 15.0 ± 7.1 a | 82.2 ± 5.8 a | 46.3 ± 5.8 a | 32.8 ± 6.3 a | 94.1 ± 1.9 a | 55.1 ± 4.4 a | 37.6 ± 3.7 a |
| F, df, P | 0.507, 2 0.646 | 0.101, 2, 0.907 | 0.294, 2 0.764 | 0.572, 2 0.616 | 0.040, 2 0.962 | 0.050, 2 0.952 | 0.031, 2 0.970 | 0.319, 2 0.749 | 0.377, 2 0.715 |
Note: Means ± SE in the same column followed by different letters are significant different (Turkey’s, a = 0.05). 0, 4, 7 mean the concentration of fungal conidia with 0, 1 × 104, 1 × 107 conidia/ml, respectively. F1, F3 and F5 mean the No. 1, 3 and 5 generation of B. tabaci adult, respectively.
Effect of I. fumosorosea on B. tabaci fat body, ovaries, and associated vitellogenin
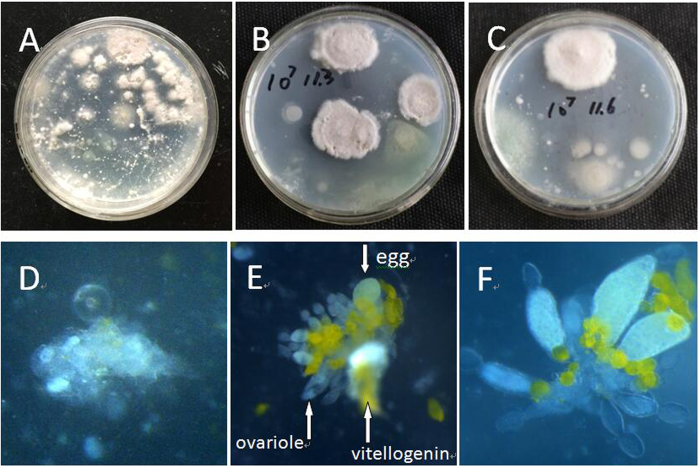
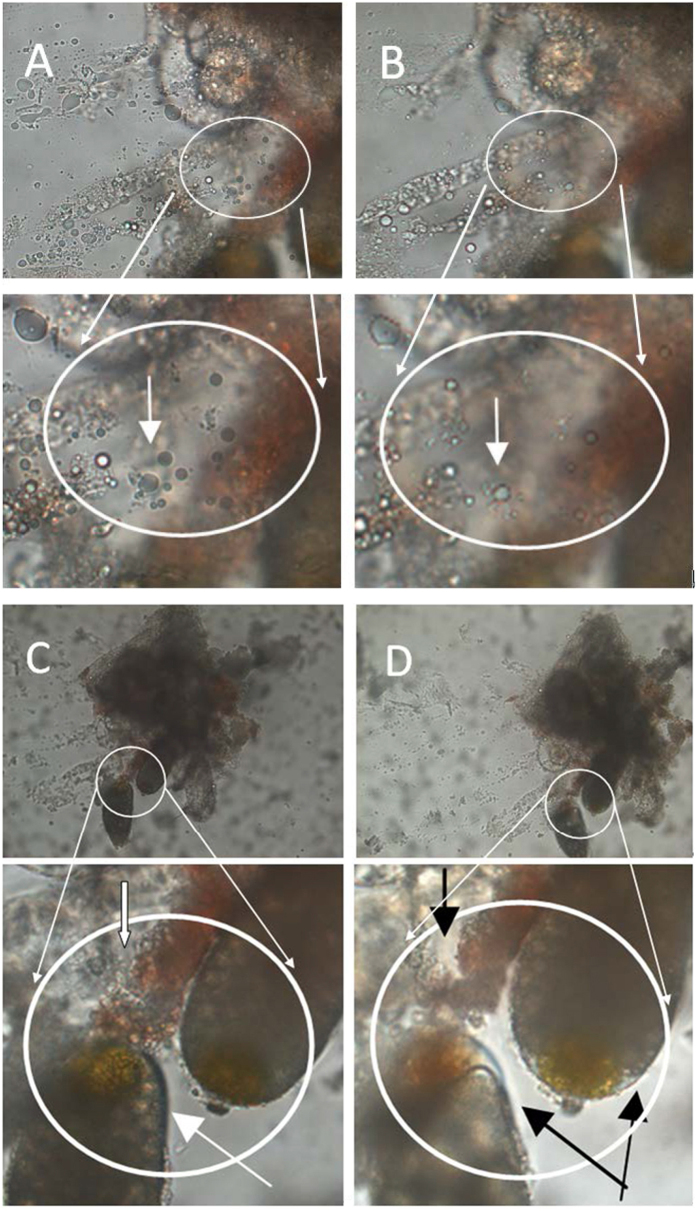
In order to examine whether infection of B. tabaci by I. fumosorosea reached the ovaries during the normal progress of mycosis in vivo, the ovaries of B. tabaci newly emerged adults from topically nymph infected by I. fumosorosea (1 × 104 and 1 × 107 conidia/ml) were dissected and cultured on PDA. Fungal colonies could readily and continuously be isolate from dissected ovaries of adult at different ages cultured on PDA (Fig. 3A,B and C, images for 1 × 107 conidia/ml infection shown). Microscopic observations revealed distortion and deformation of infected ovaries that was not seen in control B. tabaci ovaries (Fig. 3E and F). Degradation and almost complete loss of vitellogenin was also noted (Fig. 3D). Quantification of the number of ovarioles produced in the F1, F3, and F5 fungal-selected B. tabaci generations after infection with low (104 conidia/ml) and high doses (107 conidia/ml) of I. fumosorosea revealed (1) no significant effects with respect to infection, i.e. infection by either low or high doses of I. fumosorosea did not change the number of ovarioles seen as compared to control, and (2) no significant differences between the F1, F3, and F5 B. tabaci generations, the concentration of fungal inoculate used, or untreated controls (Table 3).
Figure 3. Fungal colony and ovary morphology of B. tabaci adult from different fungi treatment.
(A) representative images of fungal colonies derived from dissected ovaries of B. tabaci adult at 2 d age, with adult emergence from I. fumosorosea-selected B. tabaci nymph. Dissected ovary were cultured on PDA medium; (B) colony from dissected ovaries of B. tabaci adult at 5 d age, and (C) colony from dissected ovaries of B. tabaci adult at 8 d age; (D) Representative image of deformity ovary in infected insects; (E,F) images of normal B. tabaci (untreated) ovaries (ovariole, egg and vitellogenin are marked with arrows).
Table 3. Analysis of ovariole difference among different generations of B. tabaci adult against I. fumosorosea.
| B. tabaci | 2d |
5d |
8d |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 0 | 4 | 7 | 0 | 4 | 7 | |
| F1 | 12.1 ± 0.3 a | 12.5 ± 0.1 a | 12.7 ± 1.1 a | 13.5 ± 0.1 a | 13.3 ± 0.1 a | 12.6 ± 0.2 a | 13.4 ± 0.6 a | 13.3 ± 0.1 a | 13.0 ± 0.6 a |
| F3 | 12.8 ± 0.4 a | 11.1 ± 1.1 a | 11.9 ± 0.5 a | 12.3 ± 0.1 a | 13.0 ± 0.6 a | 12.3 ± 0.5 a | 14.0 ±0.8 a | 12.9 ± 0.7 a | 12.5 ± 1.1 a |
| F5 | 13.3 ± 0.7 a | 12.2 ± 0.4 a | 12.4 ± 0.2 a | 13.7 ± 1.1 a | 13.0 ± 1.0 a | 12.6 ± 0.4 a | 13.2 ± 1.0 a | 11.8 ± 0.6 a | 11.5 ± 1.1 a |
| F, df, P | 1.437, 2, 0.358 | 1.181, 2, 0.418 | 0.327, 2, 0.744 | 1.398, 2, 0.372 | 0.066, 2, 0.938 | 0.049, 2, 0.953 | 0.260, 2, 0.787 | 2.105, 2, 0.268 | 0.629, 2, 0.591 |
Note: Means ± SE in the same column followed by different letters are significant different (Turkey’s, a = 0.05). 0, 4, 7 mean the concentration of fungal conidia with 0, 1 × 104, 1 × 107 conidia/ml, respectively. F1, F3 and F5 mean the No. 1, 3 and 5 generation of B. tabaci adult, respectively.
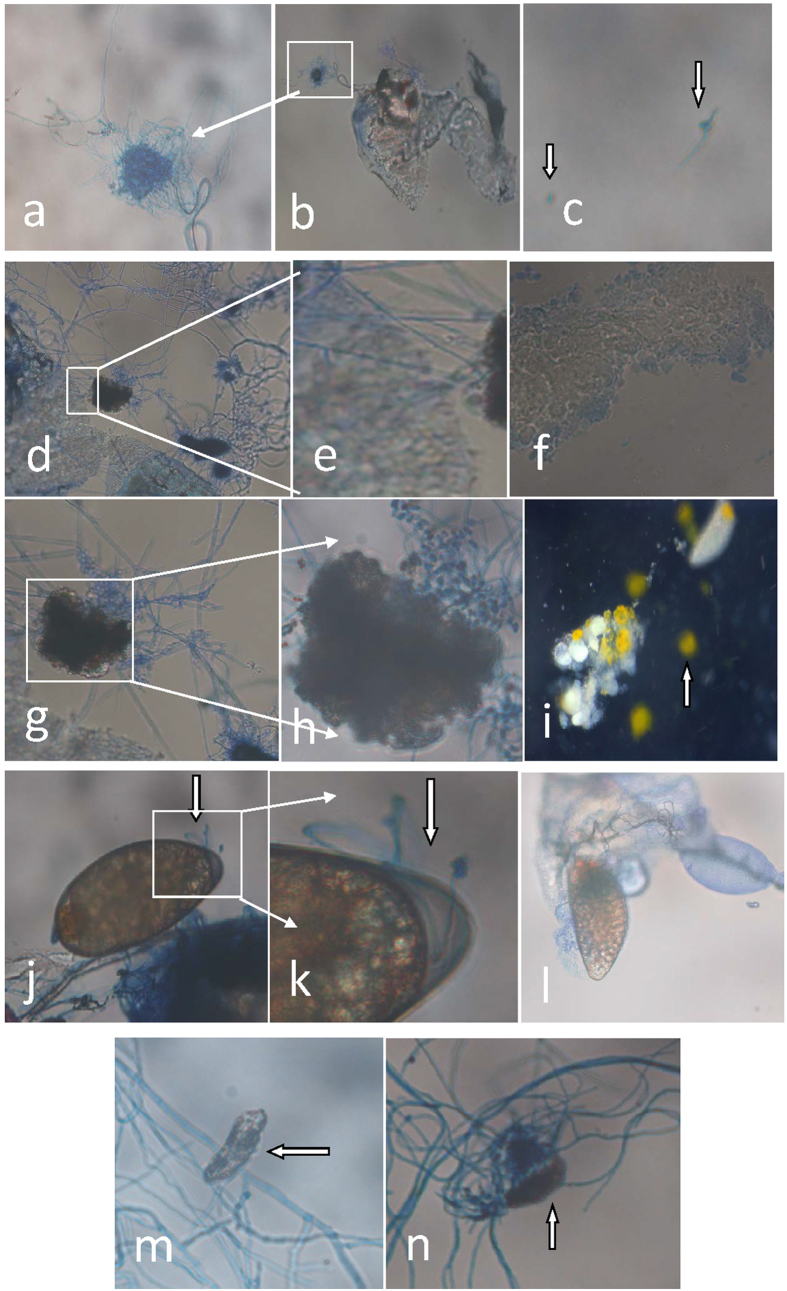
In order to further examine the effect of fungal growth on the host reproductive system, dissected adult reproductive tissues were treated with fungal conidial suspensions. Dissected fat bodies and ovaries from healthy B. tabaci adults could readily support growth of I. fumosorosea in vitro. In the presence of dissected B. tabaci ovaries, I. fumorosea rapidly grew forming extended hyphae and emerging mycelia within 12 h of co-incubation (Fig. 4a,b). In contrast, little to no growth was seen in fungi kept in the buffer solution alone (Fig. 4c), and no microbial growth was evident in untreated dissected ovaries (Fig. 4i,l). Similarly, dissected fat bodies provided a good growth substrate for the fungus (Fig. 4d,e). Degradation of vitellogenin was also evident (Fig. 4g,h) in in vitro incubations of dissected tissues incubated with I. fumosorosea, as well as infection of dissected eggs (Fig. 4j,k,m,n).
Figure 4. Representative images of I. fumosorosea growth on dissected B. tabaci fat body, egg and associated vitellogenin.
(a,b) I. fumosorosea growth on dissected B. tabaci ovaries (12 h post-incubation), (c) I. fumosorosea growth in buffer solution alone (12 h post-incubation), (d,e) I. fumosorosea growth on dissected B. tabaci fat bodies (42 h post-incubation), (f) untreated B. tabaci fat body (42 h); (g,h) effect of I. fumosorosea on associated vitellogenin (42 h post-incubation), (i) control egg/vitellogenin samples (vitellogenin marked with arrow), (j,k) I. fumosorosea growth on dissected B. tabaci eggs (42 h post-incubation), (m,n) deformation of eggs due to I. fumosorosea infection (fungal mycelia marked with arrow, 42 h post-incubation).
Effect of I. fumosorosea cell-free EthOAc extracts on dissected B. tabaci fat body, ovary, and associated vitellogenin
We have previously shown that I. fumosorosea EthOAc extracts can be toxic to B. tabaci54. In order to examine whether these extracts can directly affect B. tabaci immune and reproductive tissues, healthy dissected B. tabaci fat bodies and ovaries were incubated in the presence of the I. fumosorosea cell-free EthOAc extracts and examined visually over time. Degradation of dissected B. tabaci fat bodies was evident as early as 6 h post treatment with clear disruption of the tissue seen by 24 h (Fig. 5A,B), whereas control untreated dissected fat bodies remained intact. Similarly, eggs treated with the cell-free I. fumosorosea extracts were significantly damaged and loss of vitellogenin was evident at 24 h post treatment, whereas control eggs were unaffected (Fig. 5C,D).
Figure 5. Effect of EtOAc extract on insect fat droplet, egg, and associated vitellogenin.
(A) normal fat droplet, (B) fat droplet incubated in the presence of I. fumosorosea cell-free culture supernatant EtOAc extract (24 h post-incubation) showing changes in the overall morphology and number of droplets present (marked with arrows). Lower panels show enlargement of select regions. (C) Normal B. tabaci fat droplets, egg and associated vitellogenin, (D) B. tabaci fat droplet, egg and associated vitellogenin treated with I. fumosorosea EtOAc extract (24 h post-treatment) showing shrinkage/damage of the egg and reduction in fat droplets present (arrows). Representative images are shown.
Discussion
An important consideration is the development of insect resistance to the agents employed for their control. To date, there are no reports of the development of resistance to insect pathogenic fungi used in biological control efforts in the field, although it is well known that some insects (even between closely related species) display higher intrinsic resistance to fungal infection than others45. Greenhouse and field trials using B. bassiana to target the emerald ash borer, Agrilus planipennis indicated sublethal effects that included decreased longevity and egg laying, and increased larval development periods55. However, in several instances although mortality by these fungal agents can occur, little to no sublethal effects have been observed56, or else field conditions have been shown to have the potential to mask or eliminate any significant sublethal effects57.
A number of reports have examined the sublethal effects of entomopathogenic fungi on various insects under laboratory conditions. These can impact the insect behavior, i.e. result in reduced feeding58,59, behavioral fever60, or can affect developmental programs including molting and egg laying24,61,62. Exposure to entomopathogenic fungi can sometimes impact the subsequent generation, i.e. progeny that survive fungal exposure. Reduced reproductive fitness of the progeny of Frankliniella occidentalis exposed to B. bassiana has been noted63, and Aedes aegypti larvae surviving infection by the narrow host range fungal pathogen Leptolegnia chapmanii were disrupted in reproductive success, laying fewer eggs64. Reports concerning selection for resistance to entomopathogenic fungi have been mixed. Galleria mellonella larvae appeared to develop only minimal resistance to B. bassiana even after 25 generations of selection48. However, although significant sublethal effects were seen in surviving generations of B. tabaci exposed to B. bassiana, a gradual reduction in mortality rates was seen in 1st to 3rd generations41. Here we observed little to no changes in susceptibility of B. tabaci to either low or high doses of I fumosorosea after up to 5 generations of selection. In addition, similar numbers of eggs were laid by each respective generation, and they remained equally affected by fungal exposure, i.e. a significant decrease in egg laying was noted after I. fumosorosea exposure, however, the effect was similar across the various B. tabaci generations examined. These data suggest that in our experimental set-up, no significant resistance development was detected in B. tabaci to I. fumosorosea infection after several generations under fungal selection. These observations may also be linked to the observed lack of resistance development in that although these tissues were affected during fungal infection, no significant differences were seen across the fungal-selected whitefly generations, suggesting no carry-over effects on fitness with respect to reproduction that might lead to the development of resistance.
Although subtle developmental effects on resistance were not examined in this study, fungal infection was shown to reach the fat body and ovaries of insect adult, resulting in loss of vitellogenin and damage to eggs, an effect readily apparent in in vitro assays where these tissues provided a readily utilizable nutrient source supporting fungal growth. In B. tabaci, the number of ovarioles per adult varies between 8 and 18 depending on the time (days) after eclosion, with an average of 13–15 within 12 days65. Examination of dissected ovaries revealed no significant differences in the total number of ovarioles between uninfected and I. fumosorosea treated insects, as well as no differences were seen across the B. tabaci generations (1st to 5th) whether or not they were infected by I. fumosorosea.
As cell-free culture supernatants of I. fumosorosea have been shown to be insecticidal54, we also investigated any effects these extracts may have on dissected B. tabaci fat body and ovaries. Our data showed that these cell-free extracts have toxigenic activity on immune and reproductive tissues, suggesting a means by which both lethal and sub-lethal effects can be exerted in biological control. A number of lethal and sub-lethal effects of cell-free culture supernatants derived from entomopathogenic fungi have been noted. Complex outcomes were reported in the application of cell-fee M. anisopliae culture supernatant extracts towards the Mediteranean fruit fly, Ceratitis capitata66,67. Aside from direct toxicity (death), female fly fecundity was reduced upon initial exposure, but little to no effects were seen with respect to the egg fertility or mortality of larvae, although pupae were affected. Here we show that the extracts can result in direct damage to tissues in the absence of the presence of the fungal agent itself. Damage to the fat body, ovaries, and degradation of vitellogenin was observed in vitro, indicating the presence of toxic compounds in the culture supernatant. Although further characterization of the extract is warranted, a number of potential candidate compounds exist. Dipicolinic acid (2,6-pyridine dicarboxylic acid, DPA) is known to be an inhibitor of the prophenoloxidase activation38 and I. fumosorosea produces abundant amounts of DPA68. A wide range of potential insecticidal toxic metabolites have been reported in M. anisopliae and B. bassiana69,70,71 however work in I. fumosorosea has lagged behind. Our data confirm the potential for I. fumosorosea as an agent for B. tabaci control as part of Integrated Pest Management practices and indicate that subsequent generations are likely to continue to be susceptible to the fungus.
Methods
Fungal strain and insect maintenance
Strain PF01-N10 of I. fumosorosea (CCTCC No. M207088) was originally isolated from a B. tabaci nymph27. For routine use, I. fumosorosea was grown on potato dextrose agar (PDA) and conidia were prepared as described24. Conidia of I. fumosorosea were counted in a Fuchs-Rosenthal hemocytometer using a compound microscope and adjusted to indicate spore suspensions (104–107 conidia/ml) in 0.05% Tween-80 water. Spore viability was examined by spreading 0.2 ml of the 1 × 104 conidia/ml suspension on PDA and counting the number of germinated cells after 24 h of incubation at room temperature. Cells were considered germinated/viable if the germ tube length was as long as the width of the conidia. Conidial viability was assessed for each batch of cells and only batches estimated to be >95% viable were considered used in experiments. B. tabaci was originally collected from plant of Brassica campestris L. in Guangzhou and then maintained on the same plant in a greenhouse. Identity of the insect was confirmed by PCR-restriction fragment-length polymorphism analysis and mtCOI sequencing as described72. The identified mtCOI sequence was identical to the GenBank sequence accession no. GQ332577. Second instar B. tabaci were reared and prepared as described24 for use in fungal virulence bioassays. Plants of B. campestris L. were grown in plastic pots and incubated in an artificial climate room at 26 ± 2 °C. Sufficient slow release fertilizer (N/P/K = 13:7:15) was added as required to maintain normal plant growth. Intact plants were maintained in greenhouse and used in this experiment at the six to eight leaf stages with 12 to 18 cm tall.
Preparation of fungal cell-free culture supernatant ethyl acetate extracts
Fungal conidia (10 ml, 1 × 107 conidia ml−1) of I. fumosorosea were inoculated into shake cultures in a 1 L flask containing 300 ml of Czapek-Dox broth supplemented with 1% peptone (CZP) and incubated with aeration (180 rpm) at 26 ± 1 °C for 3 d for the production of seed inoculum. The seed inoculum was added to fresh CZP at a 1:9 ratio (v/v, 3 L total volume) and the mixture was incubated with aeration (200 rpm) at 26 ± 1 °C for an additional 6 d after which fungal cells were removed by centrifugation (12000 × g, 15 min) and the cell-free culture supernatant was stored at 4 °C. Metabolites were extracted from the cell-free culture supernatant using ethyl acetate (EthOAc). The cell-free supernatant was mixed with an equal volume of ethyl acetate (1:1, 6 L total final volume) and mixed vigorously for 30 min. The organic phase was collected and concentrated by rotary evaporation (RE −52A, Shanghai Ya Rong Biochemical Instrument Factory, Shanghai, China) under reduced pressure and then stored at −20 °C for use.
Insect bioassays
Isaria fumosorosea insect bioassays were performed using standard methods as described24. Newly molted 2nd instars of B. tabaci were treated by dipping infested leaves (not excised leaves) into indicated concentrations of I. fumosorosea (0, 104 and 107 conidia/ml) for 10 seconds. Each treatment (each concentration) involved at least four leaves with >50 whitefly nymphs per leaf and the entire experiment repeated three times with new batches of insects and new conidial suspensions. Within experiment treatments were performed at the same time, using randomized groups of insects from a single batch. Plants with treated insects/leaves were placed in an air-conditioned room at 26 ± 2 °C, R.H.>85%, L:D = 14:10 h. Treatments were monitored daily until death or new adult emergence, with B. tabaci mortality recorded every 24 h after treatment. Dead insects were removed immediately upon detection and placed separately in a clear Petri dish to allow for fungal sporulation on the cadavers. If the sporulation of I. fumosorosea was observed, the insect was considered to have been killed as a result of infection by I. fumosorosea.
Selection of B. tabaci under I. fumosorosea pressure
Newly emerged individual adults (zero generation) were maintained for 1 d and allowed to mate. Mated adults were allowed to lay eggs on B. campestris plant leaves (not excised leaves). Eggs were allowed to hatch and the nymph was considered as the 1st generation after selective pressure (only second instar nymph treated with 104 and 107 conidia/ml of I. fumosorosea). Insects from the 1st generation were used in experiments re-treated as above to yield the second generation, and the experiment was continued for 5 generations. The nymph mortality of the F1, F3 and F5 generations to various concentrations of I. fumosorosea conidial suspensions was examined.
Measurement effect of I. fumosorosea on B. tabaci ovipositioning
B. tabaci newly emergence adults, derived from nymphs topically treated with indicated concentrations (0, 1 × 104, 1 × 107 conidia/ml) of I. fumosorosea conidia as described in the insect bioassays section, were placed in cages (60 × 60 × 60 cm) and allowed to mate for ~1 day. Mated B. tabaci pairs were placed separately in a plastic Petri dishes (Ø 9 cm diameter), containing a leaf disk (8 cm diameter) of B. campestris L. placed on sterile 2% water agar. Insects (cages and Petri dishes) were kept in an air-conditioned room at 26 ± 2 °C, R.H. >85%, L:D = 14:10 h. Egg production was recorded at 2 d intervals till death of the adult. Fresh leaf discs and bee honeydew were provided every 2 d to maximize ovipositioning and as a food supplement. Each treatment was repeated three times, for each repetition there were total 12–20 pairs of adult (selected at random from the mating adult pairs emergence at different day).
Microscopic visualization of the B. tabaci reproductive system
Newly emergence B. tabaci adults, derived from nymph treated as described above, i.e. topically with 0, 1 × 104, 1 × 107 conidia/ml I. fumosorosea, were placed in cages (60 × 60 × 60 cm), and allowed to mate. The cages were kept in an air-conditioned room at 26 ± 2 °C, R.H. >85%, L : D = 14 : 10 h. Mated insects were removed at 2, 5 and 8 d post-treatments and their ovaries dissected in PBS buffer solution. The number of ovarioles were observed under a microscope and recorded. Ovary morphology and vitellogenin were observed microscopically. Isolated ovaries were then washed 3 times with sterilize distilled water (ddH2O) and placed on Petri dishes containing PDA in order to observe any fungal outgrowth from infected tissues. Each treatment was repeated three times, and 10–15 ovaries were examined per replicate.
In separate experiments, the fat body and ovaries were dissected from un-infected B. tabaci adults and placed in a small Petri dish (Ø 3 cm) containing 15 ml of 5 × 104 conidia/ml of freshly harvested I. fumorososea conidia in sterilize 0.05% Tween-80 water. Ovaries placed in 15 ml sterilize 0.05% Tween-80 water were used as one control, and conidia (5.0 × 104 conidia/ml in sterile 0.05% Tween-80) placed in a Petri dish were used as another control. The morphology of the fat bodies, ovaries, and associated vitellogenin were observed every 6 h with a microscope. To observe growing hyphae and mycelia, select samples were stained with cotton blue dye (cotton blue 0.05 g, lactic acid 20 ml, phenol 20 g, glycerol 40 ml, ddH2O water 20 ml). Each treatment was repeated three times, for each repetition there were total 20 ovaries of adult selected at random from the different treatments to observe.
Fat bodies, ovaries, and associated vitellogenin dissected from un-infected B. tabaci were also treated with I. fumosorosea ethylacetate fractions (as isolated above). The evaporated I. fumosorosea EthOAc fraction was re-suspended in acetone at a concentration of 200 mg/mL and subsequently diluted to a working solution of 10 mg/mL sterilize 0.05% Tween-80. Dissected tissues (ovary), washed with ddH2O were placed in a small Petri dish (Ø 3 cm) containing 15 ml of the EthOAc extract working solution. Control samples included isolated tissues placed in 0.05% Tween-80:5% acetone. The morphology of the fat bodies, ovaries, and vitellogenin were observed every 6 h with a microscope.
Data analyses
Mortality and ovipositing data were analyzed by using one-way analysis of variance (ANOVA). Mean values were compared by Turkey’s student range test (Tukey’s HSD, a = 0.05)73.
Additional Information
How to cite this article: Gao, T. et al. Lack of resistance development in Bemisia tabaci to Isaria fumosorosea after multiple generations of selection. Sci. Rep. 7, 42727; doi: 10.1038/srep42727 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This research was funded by grants from the National Natural Science Foundation of China (31170391), Science and Technology Planning Project of Guangdong province (2016A050502049), the National Key Technology Support Program of China (201303019-02) and US-National Science Foundation grant IOS-1557704 to NOK.
Footnotes
The authors declare no competing financial interests.
Author Contributions Conceived and designed the experiments: Z.H. and N.O.K. Performed the experiments: T.G., Z.W. and Y.H. Analyzed the data: T.G. and Z.H. Wrote the paper: Z.H. and N.O.K.
References
- Boykin L. M. et al. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences. Mol. Phylogenet. Evol. 44, 1306–1319 (2007). [DOI] [PubMed] [Google Scholar]
- Dinsdale A., Cook L., Riginos C., Buckley Y. M. & De Barro P. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 103, 196–208 (2010). [Google Scholar]
- De Barro P. J., Liu S. S., Boykin L. M. & Dinsdale A. B. Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56, 1–19 (2011). [DOI] [PubMed] [Google Scholar]
- Jones D. R. Plant viruses transmitted by whiteflies. Eur. J. Plant. Pathol. 109, 195–219 (2003). [Google Scholar]
- Navas-Castillo J., Fiallo-Olive E. & Sanchez-Campos S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 49, 219–248 (2011). [DOI] [PubMed] [Google Scholar]
- Pan H. P. et al. Rapid spread of tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS ONE 7, e34817 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong T., Zhang B., Jiang Y. & Hu Q. Isolation and Classification of Fungal Whitefly Entomopathogens from Soils of Qinghai-Tibet Plateau and Gansu Corridor in China. PLoS ONE 11, e0156087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A. R. & Ishaaya I. Chemical control of Bemisia, management and application. Bemisia: 1995 Taxonomy, Biology, Damage, Control and Management (eds Gerling D. & Mayer R. T.), pp. 537–556 Intercept, Andover, UK (1996). [Google Scholar]
- Yuan L. Z. et al. Status of insecticide resistance and associated mutations in Q-biotype of whitefly, Bemisia tabaci, from eastern China. Crop Prot. 31, 67–71 (2012). [Google Scholar]
- Basit M., Saeed S., Saleem M. A., Denholm I. & Shah M. Detection of resistance, cross-resistance, and stability of resistance to new chemistry insecticides in Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 106, 1414–1422 (2013). [DOI] [PubMed] [Google Scholar]
- Horowitz A. R. & Ishaaya I. Dynamics of biotypes B and Q of the whitefly Bemisia tabaci and its impact on insecticide resistance. Pest Manag. Sci. 70, 1568–1572 (2014). [DOI] [PubMed] [Google Scholar]
- Wraight S. P., Carruthers R. J., Bradley C. A., Garza C. J. & Galani-Wraight S. Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol. Control. 17, 203–217 (2000). [Google Scholar]
- Faria M. & Wraight S. P. Biological control of Bemisia tabaci with fungi. Crop Prot. 20, 767–778 (2001). [Google Scholar]
- Quesada-Moraga E., Maranhao E. A. A., Valverde-García P. & Santiago-Aílvarez C. Selection of Beauveria bassiana isolates for control of the whiteflies Bemisia tabaci and Trialeurodes vaporariorum on the basis of their virulence, thermal requirements, and toxicogenic activity. Biol. Control. 36, 274–287 (2006). [Google Scholar]
- Cuthbertson A. G. S. et al. Bemisia tabaci: the current situation in the UK and the prospect of developing strategies for eradication using entomopathogens. Insect Sci. 18, 1–10 (2011). [Google Scholar]
- Lai T. & Su J. Assessment of resistance risk in Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) to chlorantraniliprole. Pest Manag. Sci. 67, 1468–1472 (2011). [DOI] [PubMed] [Google Scholar]
- Fan Y., Borovsky D., Hawkings C., Ortiz-Urquiza A. & Keyhani N. O. Exploiting host molecules to augment mycoinsecticide virulence. Nature Biotech. 30, 35–37 (2012). [DOI] [PubMed] [Google Scholar]
- Allen M. T. & Levy L. S. Parkinson’s disease and pesticide exposure - a new assessment. Crit. Rev. Toxicol. 43, 515–534 (2013). [DOI] [PubMed] [Google Scholar]
- Smalling K. L. et al. Environmental fate of fungicides and other current-use pesticides in a central California estuary. Mar. Pollut. Bull. 73, 144–153 (2013). [DOI] [PubMed] [Google Scholar]
- Cuthbertson A. G. S., Walters K. F. A. & Northing P. The susceptibility of immature stages of Bemisia tabaci to the entomopathogenic fungus Lecanicillium muscarium on tomato and verbena foliage. Mycopathologia 159, 23–29 (2005). [DOI] [PubMed] [Google Scholar]
- Scorsetti A. C., Humber R. A., Gregorio C. D. & López Lastra C. C. New records of entomopathogenic fungi infecting Bemisia tabaci and Trialeurodes vaporariorum, pest of horticultural crops, in Argentina. Biocontrol 53, 787–796 (2008). [Google Scholar]
- Xia J. et al. Analysis of whitefly transcriptional responses to Beauveria bassiana infection reveals new insights into insect–fungus interactions. PloS ONE 8, e68185 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarin G. M., Jackson M. A., Kobori N. N. & Delalibera I. Liquid culture fermentation for rapid production of desiccation tolerant blastospores of Beauveria bassiana and Isaria fumosorosea strains. J. Invertebr. Pathol. 120, 11–20 (2015). [DOI] [PubMed] [Google Scholar]
- Huang Z., Ali S., Ren S. & Wu J. Effect of Isaria fumosorosea on mortality and fecundity of Bemisia tabaci and Plutella xylostella. Insect Sci. 17, 140–148 (2010). [Google Scholar]
- Tian J., Hao C., Liang L. & Ma R. Y. Effects of temperature and relative humidity on conidial germination of Isaria fumosorosea (Hypocreales: Cordycipitaceae) IF-1106 and pathogenicity of the fungus against Bemisia tabaci (Homoptera: Aleyrodidae). Mycosystema 33, 668–679 (2014). [Google Scholar]
- Mascarin G. M., Kobori N. N., Quintela E. D. & Delalibera I. The virulence of entomopathogenic fungi against Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) and their conidial production using solid substrate fermentation. BioControl 66, 209–218 (2013). [Google Scholar]
- Luangsa-Ard J. J., Hywel-Jones N. L., Manoch L. & Samson R. A. On the relationships of Paecilomyces sect. Isarioidea species. Mycol. Res. 109, 581–589 (2005). [DOI] [PubMed] [Google Scholar]
- Huang Z. et al. The Ifchit1 chitinase gene acts as a critical virulence factor in the insect pathogenic fungus Isaria fumosorosea. Appl. Microbiol. Biotechnol. 100, 5491–5503 (2016). [DOI] [PubMed] [Google Scholar]
- Altre J. A. & Vandenberg J. D. Factors influencing the infectivity of isolates of Paecilomyces fumosoroseus against diamondback moth, Plutella xylostella. J. Invertebr. Pathol. 78, 31–36 (2001). [DOI] [PubMed] [Google Scholar]
- Gökce A. & Er M. K. Pathogenicity of Paecilomyces spp. to the glasshouse whitefly, Trialeurodes vaporariorum, with some observations on the fungal infection process. Turk. J. Agric. For. 29, 331–339 (2005). [Google Scholar]
- Avery P. B. et al. Effects of the fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci. Technol. 21, 1065–1078 (2011). [Google Scholar]
- Hunter W. B., Avery P. B., Pick D. & Powell C. A. Broad spectrum potential of Isaria fumosorosea against insect pests of citrus. Fla. Entomol. 94, 1051–1054 (2011). [Google Scholar]
- Pick D. A., Avery P. B., Hunter W. B., Powell C. A. & Arthurs S. P. Effect of Isaria fumosorosea (Hypocreales: Cordycipitaceae) and Lysiphlebus testaceipes, (Hymenoptera: Braconidae) on the brown citrus aphid: preliminary assessment of a compatibility study. Fla. Entomol. 95, 764–766 (2012). [Google Scholar]
- Ortiz-Urquiza A. & Keyhani N. O. Stress response signaling and virulence: insights from entomopathogenic fungi. Curr. Genet. 61, 239–249 (2015). [DOI] [PubMed] [Google Scholar]
- Ortiz-Urquiza A. & Keyhani N. O. Molecular genetics of Beauveria bassiana infection of insects, Adv. Genet. 94, 165–249 (2016). [DOI] [PubMed] [Google Scholar]
- Castellanos-Moguel J. et al. Virulence testing and extracellular subtilisin-like (Pr1) and trypsin-like (Pr2) activity during propagule production of Paecilomyces fumosoroseus isolates from whiteflies (Homoptera: Aleyrodidae). Rev. Iberoam. Micol. 24, 62–68 (2007). [DOI] [PubMed] [Google Scholar]
- Huang S. et al. Interplay between calcineurin and the Slt2 MAP-kinase in mediating cell wall integrity, conidiation, and virulence in the insect fungal pathogen Beauveria bassiana, Fung Genet. Biol. 83, 78–91 (2015). [DOI] [PubMed] [Google Scholar]
- Watanabe N. et al. Entomogenous fungi that produce 2,6-pyridine dicarboxylic acid (dipicolinic acid), J. Biosci. Bioeng. 102, 365–368 (2006). [DOI] [PubMed] [Google Scholar]
- Liu L. et al. Structure and biosynthesis of fumosorinone, a new protein tyrosine phosphatase 1B inhibitor firstly isolated from the entomogenous fungus Isaria fumosorosea. Fung. Genet. Biol. 81, 191–200 (2015). [DOI] [PubMed] [Google Scholar]
- Furlong M. J., Pell J. K. & Reddy G. V. Premortality effects of Zoophthora radicans infection in Plutella xylostella. J. Invertbr. Pathol. 70, 214–220 (1997). [DOI] [PubMed] [Google Scholar]
- Torrado-León E., Montoya-Lerma J. & Valencia-Pizo E. Sublethal effects of Beauveria bassiana (Balsamo) Vuillemin (Deuteromycotina: Hyphomycetes) on the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) under laboratory conditions. Mycopathologia 162, 411–419 (2006). [DOI] [PubMed] [Google Scholar]
- Arthurs S. & Thomas M. B. Effects of a mycoinsecticide on feeding and fecundity of the Brown locust Locustana pardalina. Biocontrol Sci. Technol. 10, 321–329 (2000). [Google Scholar]
- Blanford S. & Thomas M. B. Adult survival, maturation, and reproduction of the desert locust Schistocerca gregaria infected with the fungus Metarhizium anisopliae var acridum. J. Invertebr. Pathol. 78, 1–8 (2001). [DOI] [PubMed] [Google Scholar]
- Rauch N. & Nauen R. Identification of biochemical markers linked to neonicotinoid cross-resistance in Bemisia tabaci (Hemiptera: Aleyrodidae). Arch. Insect Biochem. Physiol. 54, 165–176 (2003). [DOI] [PubMed] [Google Scholar]
- Pedrini N. et al. Tenebrionid secretions and a fungal benzoquinone oxidoreductase form competing components of an arms race between a host and pathogen. Proc. Natl. Acad. Sci. USA 112, E3651–E3660 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Urquiza A. & Keyhani N. O. Action on the surface: entomopathogenic fungi versus the insect cuticle, Insects 4, 357–374 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Urquiza A., Luo Z. & Keyhani N. O. Improving mycoinsecticides for insect biological control, Appl. Microbiol. Biotechnol. 99, 1057–1068 (2015). [DOI] [PubMed] [Google Scholar]
- Dubovskiy I. M. et al. Can insects develop resistance to insect pathogenic fungi? PLoS ONE 8, e60248(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini N. et al. Control of Pyrethroid-Resistant Chagas Disease Vectors with Entomopathogenic Fungi. Plos Neglect. Trop. D. 3, e434 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A. F., N'Guessan R., Koenraadt C. J., Asidi A., Farenhourst M., Akogbeto M., Thomas M. B., Knols B. G. J. & Takken W. et al. The entomopathogenic fungus Beauveria bassiana reduces instantaneous blood feeding in wild multi-insecticide-resistant Culex quinquefasciatus mosquitoes in Benin. West Africa Parasites Vectors 3, 87 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambethgar V. Potential of entomopathgenic fungi in insecticide resistance management (IRM): a review. J. Biopesticides 2, 177–193 (2009). [Google Scholar]
- Howard A. F. V., Koenraadt C. J. M., Farenhorst M., Knols B. G. J. & Takken W. Pyrethroid resistance in anopheles gambiae leads to increased susceptibility to the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Malaria. J. 16, 168 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farenhorst M. et al. Fungal infection counters insecticide resistance in African malaria mosquitoes. PNAS 41, 17443–17447(2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. et al. Screening of Metarhizium anisopliae UV-induced mutants for faster growth yields a hyper-virulent isolate with greater UV and thermal tolerances, accepted, Appl. Microbiol. Biotechnol. 100, 9217–9228 (2016). [DOI] [PubMed] [Google Scholar]
- Liu H. & Bauer S. B. Microbial control of emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae) with Beauveria bassiana strain GHA: Greenhouse and field trials. Biol. Control 45, 124–132 (2008). [Google Scholar]
- Wu S. H., Youngman R. R., Kok L. T. & Laub C. A. Sublethal effect of Beauveria bassian and Metarhizium brunneum (Hypocreales: Clavicipitaceae) on Cyclocephala lurida (Coleoptera: Scarabaeidae). J. Entomol. Sci. 51, 43–53(2016). [Google Scholar]
- Dubois T., IIajek A. E., Jiafu I. I. & Li Z. Evaluating the efficiency of entomopathogenic fungi against the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae), using cages in the field. Environ. Entomol. 33, 62–74 (2004). [Google Scholar]
- Moore D., Reed M., Le Patourel G., Abraham Y. J. & Prior C. Reduction of feeding by the desert locust, Schistocerca gregaria, after infection with Metarhizium flavoviridae. J. Invert. Pathol. 60, 404–407 (1992). [Google Scholar]
- Noma T. & Strickler K. Effects of Beauveria bassiana on Lygus hesperus (Hemiptera: Miridae) feeding and oviposition. Environ. Entomol. 29, 394–402 (2000). [Google Scholar]
- de Crecy E., Jaronski S., Lyons B., Lyons T. J. & Keyhani N. O. Directed evolution of a filamentous fungus for thermotolerance. BMC Biotech. 9, 74 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada-Moraga E., Ruiz-Garcis A. & Stantiago-Alvarez C. Laboratory evaluation of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against puparia and adults of Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 99, 1955–1966 (2006). [DOI] [PubMed] [Google Scholar]
- Hornbostel V. L., Ostfeld R. S., Zhioua E. & Benjamin M. A. Sublethal effects of Metarhizium anisopliae (Deuteromycetes) on engorged larval, nymphal, and adult Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 41, 922–929 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang T., Reitz S. R., Wang H. & Lei Z. Sublethal effects of Beauveria bassiana (Ascomycota: Hypocreales) on life table parameters of Frankliniella occidentalis (Thysanoptera: Thripidae). J. Econ. Entomol. 108, 975–985 (2015). [DOI] [PubMed] [Google Scholar]
- Pelizza S. A., Scorsetti A. C. & Tranchida M. C. The sublethal effects of the entomopathic fungus Leptolegnia chapmanii on some biological parameters of the dengue vector Aedes aegypti. J. Insect. Sci. 13, 22 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammeel O. I. Some aspects of the mating and oviposition behavior of the cotton whitefly Bemisia tabaci (Germ.). Rev. Zool. Afr. 88, 784–788 (1974). [Google Scholar]
- Ortiz-Urquiza A., Keyhani N. O. & Quesada-Moraga E. Culture conditions affect virulence and production of insect toxic proteins in the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci. Technol. 23, 1199–1212 (2013). [Google Scholar]
- Ortiz-Urquiza A., Vergara-Oritiz A., Santiago-Alvare C. & Quesada-Morage E. Insecticidal and sublethal reproductive effects of Metarhizium anisopliae cultures supernatant protein extract in the Mediterranean fruit fly. J. Apl. Entomol. 134, 581–591 (2010). [Google Scholar]
- Asaff A., Cerda-Garcia-Rojas C. & de la Torre M. Isolation of dipicolinic acid as an insecticidal toxin from Paecilomyces fumosoroseus. Appl. Microbol. Biotech. 68, 542–547 (2005). [DOI] [PubMed] [Google Scholar]
- Quesada-Moraga E. & Vey A. Bassiacridin, a protein toxic for locusts secreted by the entomopathogenic fungus Beauveria bassiana. Mycol. Res. 108, 441–452 (2004). [DOI] [PubMed] [Google Scholar]
- Gibson D. M., Donzelli B. G. G., Krasnoff S. B. & Keyhani N. O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Natural Products Review 10, 1287–1305 (2014). [DOI] [PubMed] [Google Scholar]
- Molnar I., Gibson D. M. & Krasnoff S. B. Secondary metabolites from entomopathogenic Hypocrealean fungi. Nat. Prod. Reports 27, 1241–1275 (2010). [DOI] [PubMed] [Google Scholar]
- Qing L., Wang J., Bing X. L. & Liu S. S. Identification of nine cryptic species of Bemisia tabaci (Hemiptera: Aleyrodidae) from China by using the mtCOI PCR-RFLP technique. Acta Entomol. Sin. 56, 186–194 (in Chinese with English abstract 2013). [Google Scholar]
- SPSS. SPSS Statistics for Windows, Version 17.0, Chicago IL, SPSS Inc (2008).