Abstract
Phenotypic plasticity describes the ability of a given genotype to produce distinct phenotypes in different environments. We use the temperature sensitivity of abdominal pigmentation in Drosophila melanogaster females as a model to analyse the effect of the environment on development. We reported previously that thermal plasticity of abdominal pigmentation in females involves the pigmentation gene tan (t). However, the expression of the pigmentation gene yellow (y) was also modulated by temperature in the abdominal epidermis of pharate females. We investigate here the contribution of y to female abdominal pigmentation plasticity. First, we show that y is required for the production of black Dopamine-melanin. Then, using in situ hybridization, we show that the expression of y is strongly modulated by temperature in the abdominal epidermis of pharate females but not in bristles. Interestingly, these two expression patterns are known to be controlled by distinct enhancers. However, the activity of the y-wing-body epidermal enhancer only partially mediates the effect of temperature suggesting that additional regulatory sequences are involved. In addition, we show that y and t co-expression is needed to induce strong black pigmentation indicating that y contributes to female abdominal pigmentation plasticity.
Phenotypic plasticity is “the property of a given genotype to produce different phenotypes in response to distinct environmental conditions”1. This is frequently observed in the wild2,3, and has also major implications in medicine and agricultural sciences. Furthermore, it is thought to facilitate evolution4,5,6,7. Indeed, cryptic genetic variations can be revealed by environmental changes. These variations can subsequently be selected, thus contributing to evolution. Although phenotypic plasticity has been described in many animals and plants, its molecular bases are only beginning to be understood. Strikingly, environmental conditions can dramatically affect the transcriptome and several studies have identified modulated genes involved in the plasticity of particular morphological traits8,9,10,11.
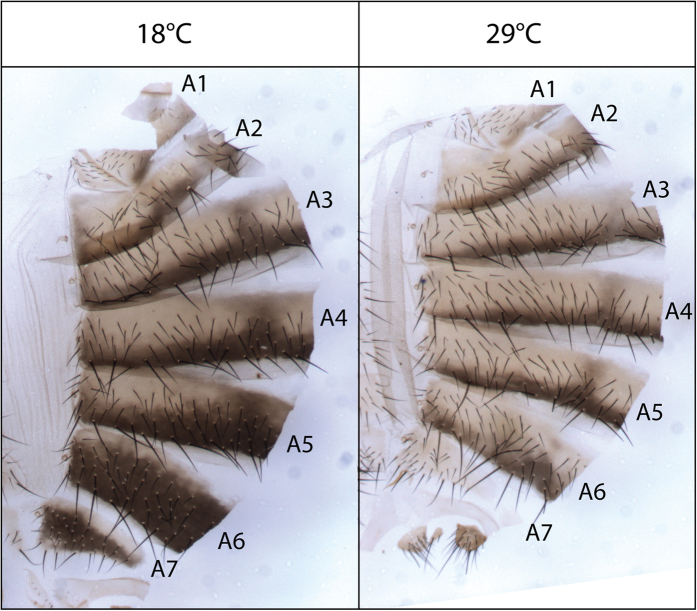
Abdominal pigmentation in Drosophilids represents an attractive model to dissect the molecular bases of phenotypic plasticity as it is sensitive to temperature in many species12. Abdominal pigmentation of Drosophila melanogaster females is darker when they develop at low temperature13. This is particularly pronounced in posterior abdominal segments (A5, A6 and A7) (Fig. 1). Plasticity of abdominal pigmentation is likely to have functional consequences as abdominal pigmentation has been linked to thermoregulation and resistance to UV, pathogens or parasites13,14,15,16. Abdominal pigmentation was also associated to resistance to desiccation17, but this was not confirmed in a recent study18. Abdominal pigmentation differs between males and females in several Drosophila species and has been used as a model to dissect the genetic bases of sexual dimorphism19,20. Furthermore, as abdominal pigmentation is highly evolvable21, it has been investigated to study the molecular bases of morphological variation within or between species22,23,24,25,26,27,28,29. The genes involved in Drosophila abdominal pigmentation are relatively well known, in particular those encoding the enzymes required for the synthesis of cuticle pigments30,31,32,33 (Fig. 2). We reported recently that the thermal plasticity of female abdominal pigmentation in Drosophila melanogaster involves transcriptional modulation of the pigmentation gene tan (t)34. This gene encodes a hydrolase implicated in the production of melanin35 (Fig. 2). t is seven times more expressed at 18 °C than at 29 °C in the posterior abdominal epidermis of young adult females. Furthermore, genetic experiments showed that t plays an essential role in the plasticity of female abdominal pigmentation. However, we also reported that the expression of the pigmentation genes yellow (y), ebony (e), Dopa-decarboxylase (Ddc) and black (b) was modulated in pharates (late pupae) but more moderately (less than two fold). This modulation of their expression by temperature is consistent with a darker pigmentation at low temperature and likely explains the residual pigmentation plasticity observed in t loss-of-function mutants.
Figure 1. Plasticity of abdominal cuticle pigmentation upon temperature in females.
Abdominal cuticles of w1118 females grown at 18 °C or 29 °C. Pigmentation is modulated by temperature in particular in the posterior abdomen (A5, A6 and A7) as previously shown34.
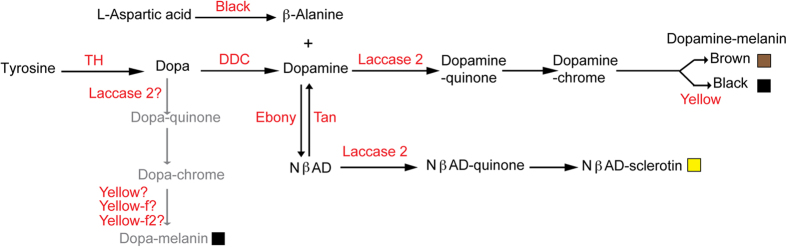
Figure 2. Synthesis pathway of cuticular pigments.
Enzymes are indicated in red. In this representation, black melanin is considered as Dopamine-melanin (see text for experimental justification) and the alternative pathway mentioned by several authors leading to Dopa-melanin is represented in grey. NβAD: N-β-Alanine-Dopamine.
We investigate here the contribution of y to female abdominal pigmentation plasticity. The y gene has been known for a hundred years as it was among the first described Drosophila melanogaster mutants36. It is required for the production of black melanin and in the absence of y, black melanin is replaced by brown melanin (see Fig. 2)37. In Drosophila melanogaster, y is sex-specifically regulated in the posterior abdomen in correlation with the sexual dimorphism of the melanic pattern observed in adults25. Furthermore, the evolution of wing or abdominal pigmentation patterns between Drosophila species correlates with modifications of y spatial expression27,37,38,39,40,41. We show here that temperature modulates the spatial expression of y in the abdominal epidermis of pharate females in correlation with the modulation of cuticle pigmentation observed in adults. By contrast, y expression associated with bristles is not modulated by temperature. y is known to be required but not sufficient for black melanin production37. Our data indicate that this black melanin is Dopamine-melanin and not Dopa-melanin. Furthermore, we show that combined over-expression of y and t at 29 °C is necessary and sufficient to reproduce the black phenotype observed at 18 °C. Thus, the stronger expression of y at 18 °C contributes to thermal plasticity of female abdominal pigmentation.
Results
yellow is required for the production of Dopamine-melanin
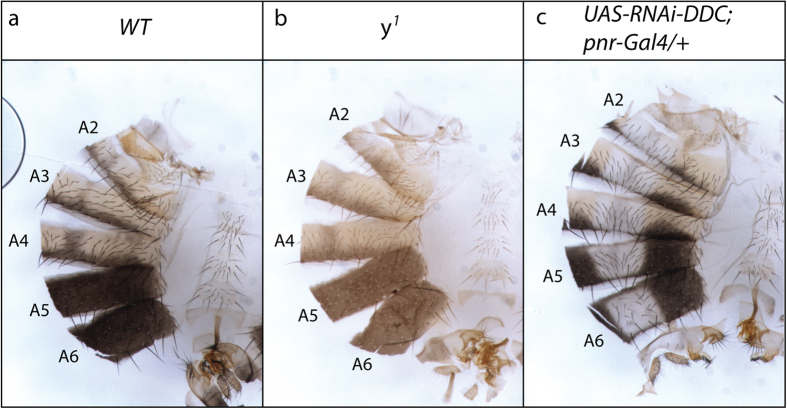
There is a relative uncertainty in the literature on the nature of the black pigment found in the abdominal cuticle of Drosophila when y is functional. Indeed, as Yellow is related to two other enzymes, Yellow-f and Yellow-f2, which can use as substrate Dopa-chrome with a higher efficiency than Dopamine-chrome33, some authors have proposed that the black pigment in abdominal cuticle was Dopa-melanin produced from Dopa21,22 (Fig. 2). However, incubation of abdominal cuticles or wings of unpigmented pharates with Dopamine is sufficient to produce black pigment, which suggests that this black pigment is produced from Dopamine and is therefore Dopamine-melanin30,32. We reasoned that if the black pigment of cuticle were Dopa-melanin, the inactivation of Ddc should leave it unaffected (Fig. 2). On the opposite, if black pigment were Dopamine-melanin, the inactivation of Ddc should lead to loss of black and brown pigments. We therefore took advantage of the Gal4/UAS system to down-regulate the expression of Ddc in the dorsal abdominal epidermis of males using a Ddc RNAi transgene42 and the pannier-Gal4 driver (pnr-Gal4) (Fig. 3). As pnr is expressed only in the dorso-central region of the body43, the lateral regions can be used as internal controls. We chose males because of their characteristic black pigmentation in abdominal segments A5 and A6 (Fig. 3a), which is well known to require y (Fig. 3b). We observed that Ddc down-regulation leads to a complete loss of black and brown pigments (Fig. 3c). We therefore concluded that the black pigment is Dopamine-melanin and not Dopa-melanin.
Figure 3. y is required for the production of Dopamine-melanin.
The posterior abdominal segments of Drosophila melanogaster males (A5 and A6) are black (a). In y1 mutant males, the black pigment is lost and only brown pigment is visible (b). Dorsal down-regulation of Ddc using pnr-Gal4 to drive an UAS-RNAi-Ddc transgene in males leads to loss of both black and brown pigments (c).
Temperature modulates the spatio-temporal expression of y in abdominal epidermis
We showed previously by RT-qPCR that y expression is modulated by temperature in the epidermis of abdominal segments A5, A6 and A7 in female pharates (1.97 fold more expressed at 18 °C than at 29 °C)34. In order to analyse the spatial expression of y, we performed in situ hybridization of female pharates grown at 18 °C or 29 °C. We could distinguish three stages of y expression (A, B and C) based on the degree of maturation of abdominal bristles (Fig. 4). These stages correspond approximately to a transition from stage P11(i) to stage P12(ii) as described by Bainbridge and Bownes with morphological markers at 25 °C44.
Figure 4. Temperature modulates the spatio-temporal expression of y in female pharates.
y in situ hybridization in the abdomen of w1118 female pharates (stage A,B and C) grown at 18 °C or 29 °C. Cuticles were cut next to the dorsal midline. When the cut was made more distantly, the dorsal midline is indicated by a dashed line in the preparations. Small frames in each picture show the staining associated with bristles at a higher magnification.
In stage A pharates, two cells at the base of bristles expressed y. This expression had a similar intensity when pharates were raised at 18 °C and at 29 °C. These two cells are likely to be the socket and the shaft, the only pigmented cells of the bristle organ. In addition, y was expressed in the posterior region of each tergite in segments A2 to A6. This expression was much broader and stronger in pharates grown at 18 °C compared to 29 °C. In A6, y was expressed in the whole tergite at 18 °C, and only in the posterior region of the tergite at 29 °C. In A7, at 18 °C, the whole tergite expressed y at a high level, whereas it was much weaker at 29 °C.
In stage B pharates, y expression was reduced in the socket and the shaft, while the bristle began to be pigmented. Furthermore, y was still more expressed in the abdominal epidermis of pharates grown at 18 °C than at 29 °C.
In stage C pharates, y was no longer expressed at the base of bristles and the bristles were almost fully pigmented. Furthermore, its overall expression in tergites was reduced compared to stage B and more similar between pharates grown at 18 °C and 29 °C.
Therefore, these data showed that temperature modulates the expression of y in stages A and B pharates, i.e. when its expression is the highest (corresponding approximately to stages P11(i) to P12(i)44).
The temperature sensitivity of y is only partially mediated by the y-wing-body enhancer
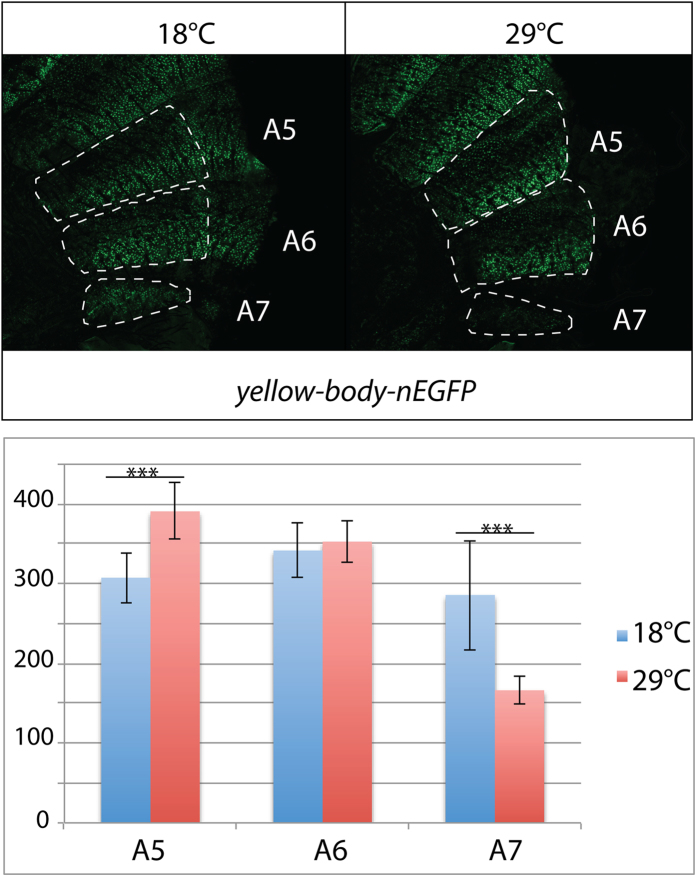
Expression of y in wings and abdominal epidermis was shown to be controlled by an enhancer called y-wing-body located within the 3 kb upstream of y transcription start site25. We used the transgenic line y-wing-body-nEGFP (nuclear Enhanced Green Fluorescent Protein) to test the effect of temperature on the activity of this enhancer (Fig. 5). Unexpectedly, nEGFP was more expressed at 29 °C than at 18 °C in A5. Furthermore, no difference was detected in A6. However, in A7, nEGFP was more expressed at 18 °C than at 29 °C (1.72 fold). In conclusion, nEGFP expression mimicked y expression only in A7.
Figure 5. Effect of temperature on the activity of y wing-body-enhancer.
Top: abdominal epidermis of y-wing-body–nEGFP transgenic females grown at 18 °C or 29 °C. The hemi-tergites A5, A6 and A7 used for nEGFP quantification have been circled with white dashed lines. Representative pictures are shown.
Bottom: quantification of nEGFP. ***p < 0.001 (n = 10 for each temperature). Exact p-values: A5 p = 2.73E-5; A6 p = 0.43; A7 p = 3.24E-4.
y and t co-overexpression at 29 °C is sufficient to reproduce the phenotype observed at 18 °C
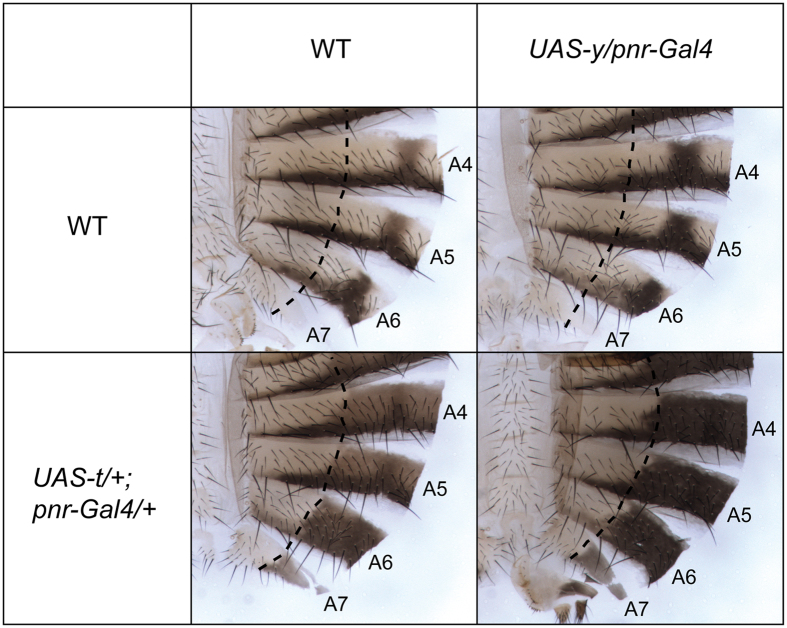
y is required but not sufficient for production of black pigment37. Indeed, y gain- of-function must be combined to e down-regulation or t up-regulation to induce a fully black pigmentation24,37. In order to test whether the strong expression of y an t is sufficient to explain the black pigmentation observed at 18 °C, we increased their expression in abdominal epidermis at 29 °C to mimic the effect of lower temperature. We compared the cuticles of wild-type females and females over-expressing either y (pnr-Gal4/UAS-y), t (UAS-t/+; pnr-Gal4/+) or both y and t (UAS-t/+; UAS-y/pnr-Gal4) at 29 °C (Fig. 6). As previously described, y over-expression did not change pigmentation whereas t over-expression induced dark pigmentation in the anterior region of the tergites22,37. However, careful examination revealed that this ectopic pigmentation was not as dark as the normal pigmentation in the posterior region of the tergites. This was more visible in A4 and A5 segments. By contrast, when both y and t were over-expressed in the dorsal region of the abdomen, the anterior region of the tergites was as black as the posterior border of the tergites. Thus, these data show that y and t combined over-expression at 29 °C is necessary and sufficient to reproduce the pigmentation phenotype observed at low temperature.
Figure 6. y and t combined over-expression at 29 °C is sufficient to mimic the pigmentation phenotype observed at 18 °C.
Abdominal cuticle phenotypes of wild-type females, females over-expressing y (UAS-y/pnr-Gal4), females over-expressing t (UAS-t/+; pnr-Gal4/+) and females over-expressing both y and t (UAS-t/+; UAS-y/pnr-Gal4) grown at 29 °C. The domain of pnr expression is located to the right of the dashed line. Over-expression of y is not sufficient to induce the production of black pigment. Over-expression of t induces dark pigmentation but pigmentation in the anterior part of the tergites (ectopic pigmentation induced by pnr) is lighter than in the posterior part of the tergites (orthotopic plus ectopic pigmentation). By contrast, combined over-expression of t and y leads to strong and uniform black pigmentation.
Discussion
In Drosophila, y is required for the production of black pigment that we demonstrate here to be Dopamine-melanin. This identification of Drosophila black pigment as Dopamine-melanin is in agreement with recent studies analysing the effect of RNAi against Ddc on melanin production in other insects45,46. The diversification of wing or abdomen pigmentation patterns during Drosophila evolution was shown to correlate with the modification of y expression through changes of either cis-regulatory sequences or trans-regulator(s)27,37,38,39,40,41. We provide here a detailed description of y expression in the abdomen of female pharates grown at 18 °C and 29 °C. We show that temperature modulates the spatio-temporal expression of y in the abdominal epidermis of pharates in correlation with the pigmentation pattern observed in adults. Interestingly, expression of y in bristle cells is not modulated by temperature. The expression of y in bristle cells and in abdominal epidermis is known to be regulated by distinct cis-regulatory sequences. In bristle cells, y is controlled by an enhancer located in the large intron, whereas in abdominal epidermis it is controlled by the y-wing-body enhancer25,47. The regulatory sequences that control y expression in bristle cells are likely to be very constrained as they are found in this intron in 6 Drosophila species, whereas those that control y expression in the abdominal epidermis are more flexible as they have changed location between these species47. Thus, the evolutionary flexibility of y regulatory sequences and the temperature sensitivity of y expression might be related.
Our results show that temperature sensitive expression of y in the epidermis of A7 is mediated at least partly by the y-wing-body enhancer, demonstrating that regulation of y by temperature occurs at the level of transcription. However, this enhancer does not confer temperature sensitivity to y expression in the other segments. The fact that we observed the plastic response in A7 (nEGFP signal 1.72 fold stronger at 18 °C than 29 °C, similarly as the 1.97 fold difference of expression of y previously measured by RT-qPCR) is not in favour of a lack of sensitivity of the method. We cannot exclude that genomic sequences flanking the insertion site of the transgene influence the activity of the enhancer and reduce the effect of temperature on its activity specifically in A5 and A6. However, this could also indicate that other regulatory sequences than the y-wing-body enhancer, located in the vicinity of the endogenous y locus, participate or condition the sensitivity of y transcription to temperature. Interestingly, y maps close to the telomere of the X chromosome, a region with particular properties such as reduced recombination rate and low genetic variation48. Furthermore, y is juxtaposed to a binding site for the insulator protein Su(Hw) which separates it from the achaete-scute complex49. The sensitivity of y transcription to temperature changes might therefore be influenced by telomeric chromatin and/or by this insulator. Deciphering these effects would require to manipulate the endogenous y locus. Alternatively, upstream regulators of y might be themselves temperature sensitive. It would be interesting to identify such factors and find out whether they also regulate other pigmentation genes notably t or e. Indeed, a recent study identified several transcription factors involved in the regulation (at least indirect) of both t and e50. Lastly, temperature sensitivity of an enhancer was shown to result from a particular arrangement of binding sites for transcriptional regulators51. Hence, another hypothesis would be that the architecture of the y-wing-body enhancer itself explains its temperature sensitive activity.
Down-regulation of e is required to see the effect of y over-expression indicating that y is not sufficient for black pigment production37. We have shown previously that e is slightly less expressed at 18 °C than at 29 °C in the anterior abdominal segments of young adult females34. Thus, the broader expression of y in combination with the lower expression of e might contribute to the weak but distinguishable enhancement of black pigment production at 18 °C in anterior tergites.
In posterior abdominal segments, dramatic modulation of t expression by temperature is essential for thermal plasticity of pigmentation in females34. However, as overexpression at 29 °C of both y and t, but not the one of t alone, is necessary and sufficient to reproduce the black pigmentation phenotype observed at 18 °C, we can conclude that modulation of y expression by temperature also contributes to thermal plasticity of pigmentation in the posterior abdominal segments of females.
Our results are remarkably similar to those obtained in the lepidopteran Junonia coenia, a species with contrasting seasonal morphs10. Spring and autumn morphs have markedly different wing pigmentation patterns. In this butterfly, environmental conditions dramatically modulate the expression of t in late pupal stage and y in earlier pupal stage. Phenotypic plasticity of pigmentation in insects appears therefore to be a complex process based on transcriptional modulation of multiple pigmentation genes at distinct developmental stages corresponding to their peak of expression. A few of them, such as t, play a paramount role whereas others, like y, have a more modest contribution.
Methods
Fly stocks
We used a w1118 inbred line as wild-type. The y allele used was y1. The UAS-t line was a gift of Dr Nicolas Gompel. The y-wing-body-nEGFP line was from the lab of Sean Carroll. The pnr-Gal4 (BL3039) and UAS-y (BL3043) lines were from the Bloomington stock centre. The UAS-RNAi-Ddc (GD3329) line was from the VDRC stock centre (Vienna, Austria).
Cuticle preparations
Adult flies between 3 and 5 days old were stored for 8 days in ethanol 75% before dissection. Abdominal cuticles were cut just beyond the dorsal midline. After dissection, cuticles were rehydrated in PBS-glycerol for 4 hrs. They were then mounted in Hoyer’s medium.
For nEGFP observations, abdomens were dissected in PBS, fixed 20 minutes in
3.7% paraformaldehyde in PBS, washed twice 10 minutes in PBS and mounted in Mowiol.
In situ hybridization
A 822 bp y fragment was amplified by PCR with primers 5′-TGACTTGACCACGGATACGC-3′ and 5′-GGTGGACCCATTGGCAAAAC-3′ from cDNAs previously prepared from pupal abdominal epidermis34. This PCR fragment was cloned by Topo-Cloning and LR-Recombination (Gateway) in pBlueScript vector (Invitrogen). We used two clones where the y fragments were inserted in opposite directions so that sense and antisense Digoxygenin-labeled probes could be synthesized with the same RNA polymerase (T7, Roche) after linearization of the plasmids. In situ hybridizations were performed according to the Carroll’s lab protocol (http://carroll.molbio.wisc.edu). Precisely, we followed the protocole “in situ hybridization on adult abdomen” modified from the Mark Rebeiz’s one by Héloise D. Dufour. Specificity of the antisense probe was assessed by comparison with signal from the sense probe (Figure S1). Morphological markers (wing color, degree of maturation of abdominal bristles, localization of meconium in the abdomen) were used to stage pharates and collect them at a similar developmental stage whether grown at 18 °C or 29 °C44. In situ hybridization experiments were performed twice, on individuals grown at 18 °C or 29 °C (between 10 and 20 individuals for each temperature). Staining was stopped after 90 minutes and 80 minutes for the first and the second experiment respectively. Similar results were obtained in each experiment. Representative pictures from the first in situ hybridization are shown in Fig. 4.
Image acquisition
Adult cuticles and abdominal in situ hybridizations were imaged with a binocular equipped with Leica DC480 digital camera using the Leica IM50 Image Manager software. An annular lamp was used to ensure homogeneous lighting. Care was taken to use identical settings in each set of experiments. The higher magnification pictures showing y expression associated with bristles as well as abdominal epidermes of y-body-nEGFP pharates were acquired using a micro-apotome (Zeiss). nEGFP was measured in hemi-tergites A5, A6 and A7 using maximum intensity projections of stacks of 14 to 16 pictures (39 to 45 μm thick) using the Zen software.
Statistical analysis
The effect of temperature on nEGFP expression in A5, A6 and A7 was tested by a t-test. Homogeneity of variances was previously checked using a Levene test and the appropriate option of the t-test was used (homo- or heteroscedasticity).
Additional Information
How to cite this article: Gibert, J.-M. et al. Modulation of yellow expression contributes to thermal plasticity of female abdominal pigmentation in Drosophila melanogaster. Sci. Rep. 7, 43370; doi: 10.1038/srep43370 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
Flybase provided information useful for this study. We thank the Bloomington Drosophila stock centre and the Vienna Drosophila Resource Centre for fly stocks. We thank Dr Héloise D. Dufour for the y-wing-body-nEGFP stock and Dr Nicolas Gompel for the UAS-tan stock. We thank other members of the lab for fruitful discussions.
Footnotes
The authors declare no competing financial interests.
Author Contributions J.M.G. designed the study and performed the experiments. J.M.G., E.M.V. and F.P. analysed the data and wrote the paper.
References
- Pigliucci M. Phenotypic Plasticity, Beyond Nature and Nurture. (2001).
- West-Eberhard M. J. Developmental plasticity and evolution. (Oxford University Press, 2003). [Google Scholar]
- Gilbert S. & Epel D. Ecological Developmental Biology: Integrating Epigenetics, Medecine, and Evolution. (Sinauer Associates, 2008). [Google Scholar]
- Espinosa-Soto C., Martin O. C. & Wagner A. Phenotypic plasticity can facilitate adaptive evolution in gene regulatory circuits. BMC Evol Biol 11, 5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierst J. L. A history of phenotypic plasticity accelerates adaptation to a new environment. J Evol Biol 24, 1992–2001 (2011). [DOI] [PubMed] [Google Scholar]
- Moczek A. P. et al. The role of developmental plasticity in evolutionary innovation. Proc Biol Sci, doi: 10.1098/rspb.2011.0971 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard M. J. Developmental plasticity and the origin of species differences. Proc Natl Acad Sci UA 102 Suppl 1, 6543–9 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Campbell T. G., Stone E. A., Mackay T. F. & Anholt R. R. Phenotypic plasticity of the Drosophila transcriptome. PLoS Genet 8, e1002593 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. F., Li Y., Meyer A. & Gunter H. M. Regulatory gene networks that shape the development of adaptive phenotypic plasticity in a cichlid fish. Mol. Ecol. 23, 4511–4526 (2014). [DOI] [PubMed] [Google Scholar]
- Daniels E. V., Murad R., Mortazavi A. & Reed R. D. Extensive transcriptional response associated with seasonal plasticity of butterfly wing patterns. Mol Ecol, doi: 10.1111/mec.12988 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runcie D. E. et al. Genetics of gene expression responses to temperature stress in a sea urchin gene network. Mol Ecol 21, 4547–62 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkash R., Ramniwas S., Lambhod C. & Kajla B. Adaptive changes in the plasticity of body melanisation in generalist, cold and warm adapted Drosophila species. Acta Entomol. Sin. 54, 1155–64 (2011). [Google Scholar]
- Gibert P., Moreteau B. & David J. R. Developmental constraints on an adaptive plasticity: reaction norms of pigmentation in adult segments of Drosophila melanogaster. Evol Dev 2, 249–60 (2000). [DOI] [PubMed] [Google Scholar]
- Bastide H., Yassin A., Johanning E. J. & Pool J. E. Pigmentation in Drosophila melanogaster reaches its maximum in Ethiopia and correlates most strongly with ultra-violet radiation in sub-Saharan Africa. BMC Evol Biol 14, 179 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck I. & Jaenike J. Ecological genetics of abdominal pigmentation in Drosophila falleni: a pleiotropic link to nematode parasitism. Evolution 58, 587–96 (2004). [PubMed] [Google Scholar]
- Kutch I. C., Sevgili H., Wittman T. & Fedorka K. M. Thermoregulatory strategy may shape immune investment in Drosophila melanogaster. J Exp Biol 217, 3664–9 (2014). [DOI] [PubMed] [Google Scholar]
- Parkash R., Rajpurohit S. & Ramniwas S. Impact of darker, intermediate and lighter phenotypes of body melanization on desiccation resistance in Drosophila melanogaster. J Insect Sci 9, 1–10 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpurohit S., Peterson L. M., Orr A. J., Marlon A. J. & Gibbs A. G. An Experimental Evolution Test of the Relationship between Melanism and Desiccation Survival in Insects. PloS One 11, e0163414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A., Duncan I., Godt D. & Carroll S. B. Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature 408, 553–9 (2000). [DOI] [PubMed] [Google Scholar]
- Williams T. M. et al. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 134, 610–23 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp P. J., Carroll S. B. & Kopp A. Evolution in black and white: genetic control of pigment patterns in Drosophila. Trends Genet 19, 495–504 (2003). [DOI] [PubMed] [Google Scholar]
- Wittkopp P. J. et al. Intraspecific polymorphism to interspecific divergence: genetics of pigmentation in Drosophila. Science 326, 540–4 (2009). [DOI] [PubMed] [Google Scholar]
- Rebeiz M., Pool J. E., Kassner V. A., Aquadro C. F. & Carroll S. B. Stepwise modification of a modular enhancer underlies adaptation in a Drosophila population. Science 326, 1663–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S. et al. The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell 132, 783–93 (2008). [DOI] [PubMed] [Google Scholar]
- Jeong S., Rokas A. & Carroll S. B. Regulation of body pigmentation by the Abdominal-B Hox protein and its gain and loss in Drosophila evolution. Cell 125, 1387–99 (2006). [DOI] [PubMed] [Google Scholar]
- Rogers W. A. et al. Recurrent modification of a conserved cis-regulatory element underlies fruit fly pigmentation diversity. PLoS Genet 9, e1003740 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camino E. M. et al. The evolutionary origination and diversification of a dimorphic gene regulatory network through parallel innovations in cis and trans. PLoS Genet. 11, e1005136 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastide H. et al. A Genome-Wide, Fine-Scale Map of Natural Pigmentation Variation in Drosophila melanogaster. PLoS Genet 9, e1003534 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembeck L. M. et al. Genetic Architecture of Abdominal Pigmentation in Drosophila melanogaster. PLoS Genet. 11, e1005163 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel F., Vorkel D. & Eaton S. Megalin-dependent yellow endocytosis restricts melanization in the Drosophila cuticle. Development 138, 149–58 (2011). [DOI] [PubMed] [Google Scholar]
- Massey J. H. & Wittkopp P. J. The Genetic Basis of Pigmentation Differences Within and Between Drosophila Species. Curr. Top. Dev. Biol. 119, 27–61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. F. et al. Catecholamine metabolism and in vitro induction of premature cuticle melanization in wild type and pigmentation mutants of Drosophila melanogaster. Arch Insect Biochem Physiol 31, 219–33 (1996). [DOI] [PubMed] [Google Scholar]
- Han Q. et al. Identification of Drosophila melanogaster yellow-f and yellow-f2 proteins as dopachrome-conversion enzymes. Biochem J 368, 333–40 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert J.-M., Mouchel-Vielh E., De Castro S. & Peronnet F. Phenotypic Plasticity through Transcriptional Regulation of the Evolutionary Hotspot Gene tan in Drosophila melanogaster. PLoS Genet. 12, e1006218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- True J. R. et al. Drosophila tan Encodes a Novel Hydrolase Required in Pigmentation and Vision. PLoS Genet 1, e63 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan T. J. & Bridges C. B. Sex-linked inheritance in Drosophila. Publs Carnegie Inst 1–88 (1916). [Google Scholar]
- Wittkopp P. J., True J. R. & Carroll S. B. Reciprocal functions of the Drosophila yellow and ebony proteins in the development and evolution of pigment patterns. Development 129, 1849–58 (2002). [DOI] [PubMed] [Google Scholar]
- Wittkopp P. J., Vaccaro K. & Carroll S. B. Evolution of yellow gene regulation and pigmentation in Drosophila. Curr Biol 12, 1547–56 (2002). [DOI] [PubMed] [Google Scholar]
- Gompel N., Prud’homme B., Wittkopp P. J., Kassner V. A. & Carroll S. B. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature 433, 481–7 (2005). [DOI] [PubMed] [Google Scholar]
- Ordway A. J., Hancuch K. N., Johnson W., Wiliams T. M. & Rebeiz M. The expansion of body coloration involves coordinated evolution in cis and trans within the pigmentation regulatory network of Drosophila prostipennis. Dev Biol 392, 431–40 (2014). [DOI] [PubMed] [Google Scholar]
- Arnoult L. et al. Emergence and diversification of fly pigmentation through evolution of a gene regulatory module. Science 339, 1423–6 (2013). [DOI] [PubMed] [Google Scholar]
- Dietzl G. et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–6 (2007). [DOI] [PubMed] [Google Scholar]
- Calleja M. et al. Generation of medial and lateral dorsal body domains by the pannier gene of Drosophila. Development 127, 3971–80 (2000). [DOI] [PubMed] [Google Scholar]
- Bainbridge S. P. & Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol 66, 57–80 (1981). [PubMed] [Google Scholar]
- Liu J., Lemonds T. R. & Popadić A. The genetic control of aposematic black pigmentation in hemimetabolous insects: insights from Oncopeltus fasciatus. Evol. Dev. 16, 270–277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonds T. R., Liu J. & Popadić A. The contribution of the melanin pathway to overall body pigmentation during ontogenesis of Periplaneta americana. Insect Sci, doi: 10.1111/1744-7917.12356 (2016). [DOI] [PubMed] [Google Scholar]
- Kalay G. & Wittkopp P. J. Nomadic Enhancers: Tissue-Specific cis-Regulatory Elements of yellow Have Divergent Genomic Positions among Drosophila Species. PLoS Genet. 6, e1001222 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munté A., Aguadé M. & Segarra C. Divergence of the yellow gene between Drosophila melanogaster and D. subobscura: recombination rate, codon bias and synonymous substitutions. Genetics 147, 165–175 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovnin A. et al. An endogenous Su(Hw) insulator separates the yellow gene from the Achaete-scute gene complex in Drosophila. Dev. Camb. Engl. 130, 3249–3258 (2003). [DOI] [PubMed] [Google Scholar]
- Rogers W. A. et al. A survey of the trans-regulatory landscape for Drosophila melanogaster abdominal pigmentation. Dev Biol, doi: 10.1016/j.ydbio.2013.11.013 (2013). [DOI] [PubMed] [Google Scholar]
- Crocker J. et al. Low affinity binding site clusters confer hox specificity and regulatory robustness. Cell 160, 191–203 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.