Abstract
Background
Age-related changes of the immune system, termed immunosenescence, may underlie the increased risk of infections and morbidity in the elderly. Little is known about the effects of acute exercise on peripheral immune parameters in octogenarians. Therefore, we investigated acute exercise-induced changes in phenotype and function of the immune system in octogenarians participating in the 2013 edition of the Nijmegen Four Days Marches. Blood sampling was performed at baseline and immediately after 4 days of the walking exercise (30 km/day). A comprehensive set of adaptive and innate immune traits were enumerated and analyzed by flow-cytometry. Peripheral blood mononuclear cells, isolated before and after walking were stimulated with LPS and supernatants were analysed for IL-1β, IL-6, IL-8 and TNF-α concentrations by ELISA. CMV serostatus was determined by ELISA.
Results
The walking exercise induced a clear leucocytosis with numerical increases of granulocytes, monocytes and lymphocytes. These exercise-induced changes were most profound in CMV seropositive subjects. Within lymphocytes, numerical increases of particularly CD4+ T cells were noted. Further T cell differentiation analysis revealed profound increases of naïve CD4+ T cells, including naïve Treg. Significant increases were also noted for CD4+ memory T cell subsets. In contrast, only slight increases in naïve and memory CD8+ T cell subsets were detected. Exercise did not affect markers of immune exhaustion in memory T cell subsets. NK cells demonstrated a numerical decline and a change in cellular composition with a selective decrease of the mature CD56dim NK cells. The latter was seen in CMV seronegative subjects only. Also, a higher IL-6 and IL-8 production capacity of LPS-stimulated PBMC was seen after walking.
Conclusion
In this exceptional cohort of octogenarian walkers, acute exercise induced changes in immune cell numbers and functions. A clear response of CD4+ T cells, rather than CD8+ T cells or NK cells was noted. Remarkably, the response to exercise within the CD4+ T cell compartment was dominated by naïve CD4+ subsets.
Electronic supplementary material
The online version of this article (doi:10.1186/s12979-017-0087-2) contains supplementary material, which is available to authorized users.
Keywords: T cells, Recent thymic emigrants, NK cells, Monocytes, Ageing, Immune System
Background
Age-related changes of the immune system may contribute to increased vulnerability for infectious disease, impaired responses to vaccination and the development of late-onset chronic inflammatory diseases [1–3]. This process, termed immunosenescence, is caused by changes in both the adaptive and innate immune system. The causes underlying immunosenescence may be largely environmental as a recent systems level analysis in healthy twins revealed that non-heritable (environmental) factors rather than heritable factors shape the immune system over time [4]. In particular, the broad impact of human Cytomegalovirus (CMV) infection, a non-heritable factor, on the phenotype of the immune system was demonstrated, thereby confirming previous findings [5, 6]. The effects of exercise as another non-heritable (behavioural) factor on the phenotype of the ageing immune system has been less well studied.
The development of immunosenescence includes the decline of naïve T cells due to thymus involution, increases in late-stage effector memory T cells, decreased CD4/CD8 ratio’s and the development of immune exhaustion [7, 8]. These changes result in inadequate T cell help to B cells, thereby affecting the development of productive immune responses. CMV infection is known to accelerate immune ageing through oligoclonal expansion of CMV- specific CD8 effector memory T cells [5, 6]. In addition, several studies report on increases in T regulatory cells (Treg) leading to increased Treg/Teffector ratio’s in healthy elderly which may further add to the development of immunosenescence [9–11].
Whilst adaptive immune responses decline with age, the activity of the innate immune system appears to increase with age. This is evidenced by numerical increases in natural killer (NK) cells and monocytes and by increased serum levels of acute phase proteins and inflammatory cytokines such as interleukin-1 β (IL-1β), Interleukin-6 (IL-6), interleukin-8 (IL-8) and Tumor Necrosis Factor-α (TNFα) [12, 13]. The molecular mechanisms underlying this chronic, low grade inflammation (coined inflamm-ageing) are currently unknown but may be associated with an altered innate response to an altered gut microbiota [12, 14].
NK cells are key in the protection against infection and cancer. Ageing-associated alterations have shown an increase in the more mature CD56dim subset and a decline of the immature, CD56bright NK subset, irrespective of CMV infection [15]. CD56dim NK cells are the most abundant subset in the blood, and demonstrate a higher cytotoxicity, whereas the CD56bright NK cells demonstrate higher cytokine production. CMV chronic infection is associated with an expansion of a “memory-like” (CD56dim) NK cell subset characterized by NKG2C expression and lack of NKG2A [15].
Physical activity and exercise have profound effects on the immune system and contribute to health, well-being and longevity [16, 17]. Single bouts of exercise induce a prominent leukocytosis followed by a redistribution of immune effector cells to the tissue compartments [18]. This biphasic response to exercise may enhance the immune response against pathogens in the lymph nodes and in peripheral tissues (e.g. skin, mucosa, lungs). In adult individuals, exercise-induced lymphocytosis is largely attributed to NK cells and CD8 effector memory T cells [19, 20]. Interestingly, these subsets share functional characteristics such as cytotoxicity and tissue migration, which are important in immunosurveillance. Notably, CMV serostatus was found to influence the magnitude and the kinetics of the NK and CD8+ memory T cell responses to exercise [21, 22]. To our knowledge, the effects of acute exercise on the phenotype and function of the immune system in octogenarians have not yet been documented.
In the current study we thus investigated the effects of acute exercise on a comprehensive set of adaptive and innate immune traits in a small cohort of 20 elderly octogenarian walkers participating in the 2013 Nijmegen Four Days Marches. All participants walked a total 120 km in four consecutive days (4 x 30 km) at a self-selected pace. Blood samples were drawn at baseline and immediately after completion of the march at day 4. A post hoc analysis was performed on the contribution of CMV serostatus to exercise-induced immune changes.
Methods
Participants
Twenty elderly male and female participants (mean age 81.3 ± 1.9 years) of the 2013 Nijmegen Four Days Marches volunteered to participate in our study. The Nijmegen Four Days Marches represents the largest mass participation walking event in the world with approximately 45,000 participants annually. Based on sex and age, individuals walk 30, 40 or 50 km per day for 4 consecutive days. Blood samples were drawn before and after (within 10 min after exercise cessation) the 4 day walking event.
Experimental design
Blood sampling logistics were performed as described previously [23]. In brief, all participants reported twice to our laboratory, which was located at the start/finish area of the event. Baseline measurements were performed 12–36 h preceding the start (Table 1). Thereafter, the participants walked 30 km a day, for four consecutive days at a self selected pace. Exercise was performed under temperate ambient conditions with daily maximum wet bulb globe temperatures ranging between 24–27 °C. The recovery phase (fluid intake, food intake, sleep) between walking stages was uncontrolled and not monitored. Walking duration was recorded every day, while speed was calculated accordingly. Immediately (within 10 min) after finishing on the fourth day, all baseline measurements were repeated. Heart rate, as part of exercise intensity, was measured during day 1 as described previously [24]. Mean heart rate during exercise was presented in absolute values (beats per minute, (bpm)) and as a percentage of the predicted maximal heart rate. Predicted maximal heart rate (HRmax) was calculated using the Tanaka’s formula: HRmax = 208 - 0.7*age [25]. During the experiment, dry bulb, wet bulb and globe temperatures were measured every 30 min using a portable climate monitoring device (Davis instruments Inc., Hayward, USA), which was positioned at the start/finish area. The wet bulb globe temperature index (WBGT) was calculated using the formula: WBGT = 0.1 (Tdry bulb) + 0.7 (Twet bulb) + 0.2 (Tglobe).
Table 1.
Demographics, health status and exercise characteristics of volunteers
| Men (n = 11) | Women (n = 9) | |
|---|---|---|
| Demographic characteristics | ||
| Age (yr) | 81.0 ± 1.2 | 81.6 ± 2.7 |
| Height (cm) | 174 ± 5.2 | 159 ± 6.7 |
| Weight (kg) | 75.8 ± 6.6 | 55.5 ± 6.6 |
| Body-mass index (kg/m2) | 25.0 ± 1.6 | 21.8 ± 1.9 |
| Lean body mass (kg) | 57.2 ± 4.7 | 37.8 ± 5.2 |
| Health status | ||
| Physical activity (hrs/week) | 8.5 ± 8.0 | 5.8 ± 5.9 |
| ≥ 5 times/week ≥30 min exercise (%) | 73 | 56 |
| Blood pressure | ||
| Systolic (mmHg) | 140 ± 18 | 146 ± 17 |
| Diastolic (mmHg) | 81 ± 11 | 82 ± 10 |
| Resting heart rate (bpm) | 64 ± 20 | 63 ± 14 |
| Gait speed (km/h) | 4.8 ± 0.7 | 4.5 ± 0.7 |
| Grip strength (kg) | 41 ± 8.0 | 25 ± 4.5 |
| CMV seropositive | 6 (55%) | 7 (78%) |
| Use of prescribed medicine | ||
| Anti-hypertensive drugs | 2 (18%) | 3 (33%) |
| Statins | 2 (18%) | 1 (11%) |
| Analgesics | 3 (27%) | 0 (0%) |
| Anti-diabetics | 0 (0%) | 0 (0%) |
| Othera | 2 (18%) | 1 (11%) |
| Pathology | ||
| Hypertension | 2 (18%) | 3 (33%) |
| Cardiovascular disease | 2 (18%) | 0 (0%) |
| Hypercholestorolemiab | 2 (18%) | 1 (11%) |
| Diabetes | 0 (0%) | 0 (0%) |
| Cancer (not further differentiated) | 2 (18%) | 1 (11%) |
| Othera | 1 (9%) | 2 (22%) |
| Exercise characteristics | ||
| Exercise duration per day (hh:mm) | 7:28 ± 1:10 | 8:07 ± 0:52 |
| Speed (km/h) | 4.2 ± 0.1 | 3.7 ± 0.1 |
| Average heart rate day 1 (bpm) | 99.5 ± 11.9 | 113.6 ± 14.3 |
| Peak heart rate day 1 (bpm) | 112.5 ± 13.1 | 125.0 ± 14.1 |
| Exercise intensity day 1 (% of age-adjusted max. heart rate) | 65.7 ± 7.7 | 75.4 ± 9.6 |
| Fluid balance | ||
| Fluid intake (L)c | 1.88 ± 0.8 | 1.98 ± 0.8 |
| Change in body mass (absolute kg)c | -0.80 ± 0.9 | -0.19 ± 0.6 |
| Change in body mass (relative %)c | -1.04 ± 1.1 | -0.38 ± 1.0 |
| Change in plasma volume (relative %)d | +3.1 ± 3.2 | +4.5 ± 3.5 |
aVolunteers who were diagnosed and treated for cancer, rheumatoid arthritis, allergy and glaucoma. bHypercholesterolemia is defined as total cholesterol levels of >6.5 mmol, as previously diagnosed by a physician. cDaily fluid intake and changes in body mass during walking. dChanges in plasma volume during walking estimated according to Dill and Costill [27]. Data are presented as mean ± standard deviation
Subject characteristics
Body mass (Seca 888 scale, Hamburg, Germany) and body height were measured and body mass index (BMI) was calculated. A four-point skin fold thickness measurement (biceps, triceps, sub-scapular, supra-iliac) was obtained in order to calculate the lean body mass [26]. Resting heart rate and blood pressure were measured twice using an automated sphygmomanometer (M5-1 intellisense, Omron Healthcare, Hoofddorp, the Netherlands) after 5 min supine rest. Finally, all subjects completed a questionnaire about their physical activity and health status.
Blood analyses
White blood cell differential counts, hemoglobin and hematocrit levels were directly analyzed on a Sysmex XE-5000 system (Sysmex Corporation, Kobe, Japan). Relative changes in plasma volume were calculated from blood hematocrit and hemoglobin concentrations using Dill and Costill’s equation [27]. C-reactive protein (CRP) and creatinine measurements were measured in batches of frozen sera. Sera aliquots were stored at -20 °C directly after collection and thawed directly before analysis. All analyses were coded and anonymized. Serum CRP and creatinine were both measured on the c16000 Architect (Abbott Diagnostics, Abbott Park, IL).
Detection of CMV-specific IgG
Serum levels of CMV-specific IgG was essentially done as previously described [7]. In brief, 96-well ELISA plates (Greiner) were coated with lysates of CMV-infected fibroblasts overnight. Lysates of non-infected fibroblasts were used as negative controls. Following coating, dilutions of serum samples were incubated for 1 h. Goat-anti-human IgG was added and incubated for 1 h. Samples were incubated with phosphatase for 15 min, and the reaction was stopped with NaOH. The plates were scanned on a Versamax reader (Molecular Devices). A pool of sera from 3 seropositive individuals with known titers of CMV-specific IgG was used to quantify CMV IgG titers in the test samples.
Flowcytometry
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation with Lymphoprep (Axis-Shield) and stored in -180 °C until staining. PBMCs (106 cells) were stained simultaneously employing 4 different staining panels to asses markers of T cell differentiation, Treg and proliferation, NK cell inhibitory receptors and NK cell activating receptors (Table 2). All cell subsets are expressed as cell counts per liter unless indicated otherwise. Cell counts of these subsets were based on data from the full leucocyte differential in combination with the flowcytometry data. As an example, we used the absolute lymphocyte count from the full leucocyte differential and the percentage of CD3 T cells in the lymphocyte gate as assessed by flowcytometry for calculation of the T cell count. To perform intracellular staining with monoclonal antibodies to Cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), forkhead box P3 (FoxP3) and the proliferation marker ki-67, the cells were first fixed and permeabilized with a FoxP3 staining buffer set (eBioscience). Samples were measured on a LSR-II (BD) and data were analyzed with Kaluza software (Beckman Coulter).
Table 2.
Overview of staining panels and reagents for flowcytometry
| Panel | Mab reagent | Clone | Provider |
|---|---|---|---|
| T cell differentiation | CD3-Efluor 605 | okt-03 | Ebioscience, San Diego, CA, USA |
| CD8-APC-H7 | RPA-T8 | Ebioscience, San Diego, CA, USA | |
| CD45RO-FITC | UCHL-1 | BD bioscience, San Jose, CA, USA | |
| CCR7-PE-Cy7 | 3D12 | BD bioscience, San Jose, CA, USA | |
| CD31-AF647 | WM-59 | BD bioscience, San Jose, CA, USA | |
| CD28-AF700 | 28.2 | Biolegend, San Diego, CA, USA | |
| PD1-PE | EH12.2H7 | Biolegend, San Diego, CA, USA | |
| CD4-PcP | SK3 | BD bioscience, San Jose, CA, USA | |
| CTLA-4-BV421 | BNI3 | BD bioscience, San Jose, CA, USA | |
| Treg/Proliferation | CD8-PE-Cy7 | RPA-T8 | BD bioscience, San Jose, CA, USA |
| CD25-APC | BC96 | Ebioscience, San Diego, CA, USA | |
| CD45RA-Efluor605 | HI100 | Ebioscience, San Diego, CA, USA | |
| CD19-FITC | HD37 | Dako, Santa Clara, CA, USA | |
| CD4-APC-H7 | RPA-T4 | BD bioscience, San Jose, CA, USA | |
| FOXP3-PE | PCH101 | BD bioscience, San Jose, CA, USA | |
| Ki-67-PcP-Cy5.5 | B56 | BD bioscience, San Jose, CA, USA | |
| NK cell | CD16-FITC | 3G8 | Beckman Coulter, Brea, CA, USA |
| Inhibitory receptors | CD56-ECD | N901 | Beckman Coulter, Brea, CA, USA |
| CD3-APC-AF750 | UCHT1 | Beckman Coulter, Brea, CA, USA | |
| CD45-KO | J.33 | Beckman Coulter, Brea, CA, USA | |
| CD159c (NKG2C)-PE | 134591 | R&D Systems, Minneapolis, MN, USA | |
| CD158b (KIR2DL2/3)-PE-Cy7 | GL183 | Beckman Coulter, Brea, CA, USA | |
| CD158e1 (KIR3DL1)-APC | Z27.3.7 | Beckman Coulter, Brea, CA, USA | |
| CD158a (KIR2DL1)-APC-AF700 | EB6B | Beckman Coulter, Brea, CA, USA | |
| CD159a (NKG2A)-PB | Z199 | Beckman Coulter, Brea, CA, USA | |
| NK cell | CD16-FITC | 3G8 | Beckman Coulter, Brea, CA, USA |
| Activating receptors | CD56-ECD | N901 | Beckman Coulter, Brea, CA, USA |
| CD3-APC-AF750 | UCHT1 | Beckman Coulter, Brea, CA, USA | |
| CD45-KO | J.33 | Beckman Coulter, Brea, CA, USA | |
| CD336 (NKp44)-PE | Z231 | Beckman Coulter, Brea, CA, USA | |
| CD337 (NKp30)-PE-Cy5.5 | Z25 | Beckman Coulter, Brea, CA, USA | |
| CD335 (NKp46)-PE-Cy7 | BAB281 | Beckman Coulter, Brea, CA, USA | |
| CD314 (NKG2D)-APC | ON72 | Beckman Coulter, Brea, CA, USA | |
| CD244 (2B4) -APC-AF700 | C1.7.1 | Beckman Coulter, Brea, CA, USA | |
| CD161-PB | 191B8 | Beckman Coulter, Brea, CA, USA |
Lipopolysacharide (LPS)-stimulated PBMC cytokine production
PBMCs at 1 x 106 cells/mL were stimulated with 1 μg/mL LPS (Sigma-Aldrich, St. Louis, MO, USA) or left unstimulated. Cells were cultured in polypropylene tubes (BD bioscience) in RPMI with 10% FCS for 24 h. After 24 h, supernatants were collected and stored at -20 °C. Culture supernatants were analyzed for production of the cytokines IL-1β, IL-6, IL-8 and TNF-α by enzyme-linked immunosorbent assay (ELISA, Duoset, R&D system, Minneapolis, MN, USA) according to the manufacturer’s instructions, and read with a Versamax reader (Molecular Devices, Sunnyvale, CA, USA). The assay sensitivity was 8 pg/mL for IL-1β, 31 pg/mL for IL-6 and TNF-α, and 312 pg/mL for IL-8. The net cytokine production was calculated as cytokine production of the stimulated sample minus the cytokine production of the non-stimulated sample.
Statistical analyses
Statistical analysis of data was carried out using IBM SPSS Statistics 20 (IBM, Chicago, IL, USA) and Graphpad Prism 5 (Graph Pad Software, San Diego, CA, USA). Wilcoxon Signed Rank test, unless indicated otherwise, was used to compare the same volunteers before and after walking. Two-tailed p-values of less than 0.05 were considered statistically significant.
Results
Subject characteristics
All study participants (n = 20) successfully completed the Four Days Marches at a self selected pace (4.0 ± 0.7 km/h). On average the participants walked 7 h and 47 min daily and had an average heart rate during the first day of 106 ± 15 bpm, representing an average exercise intensity of 70 ± 10%. Thus, the walking exercise in these elderly is qualified as a daily bout of moderate intensity exercise for 4 consecutive days. An overview of all subject characteristics is presented in Table 1. An exercise-induced plasma volume expansion of 3.7 ± 3.3% was observed over the 4 days, which coincided with a small, but significant decrease of hematocrit from 0.40 L/L at baseline to 0.38 L/L directly after 4 days of exercise (p < 0.0001).
Effects of acute exercise on the peripheral blood cellular composition
When examining the composition of the peripheral blood compartment, our data show that the walking exercise resulted in a clear leucocytosis with numerical increases of granulocytes, monocytes and lymphocytes (Table 3). When analyzing the lymphocyte compartment, clear numerical increases were noted for T cells and to a lesser extent B cells. In contrast, a decline in the number of NK cells was detected. The numerical increase in T cells was largely due to an increase in CD4+ T cells (Table 3). Although CD8+ T cell numbers showed a statistical significant increase after exercise, the absolute increase was very limited. The mean CD4/CD8 ratio, an age-appropriate value of 3 [9], was not significantly increased by the walking exercise (data not shown).
Table 3.
General and Immune parameters before and after walking
| Pre-Walking | Post-Walking | P-value | |
|---|---|---|---|
| Hemoglobin (mmol/L) | 8.7 (7.7–9.4) | 8.2 (7.3–9.1) | 0.0009 |
| Thrombocytes (109/L) | 233 (157–318) | 234 (160–349) | ns |
| CRP (mg/L) | 1 (1–9) | 1 (1–47) | 0.0078 |
| Creatinin (μmol/L) | 86 (47–147) | 100 (54–207) | 0.0008 |
| ASAT (U/L) | 28 (14–39) | (-) | (-) |
| ALAT (U/L) | 28 (21–46) | (-) | (-) |
| Leukocytes (109/L) | 6.6 (4.6–11.0) | 7.7 (5.7–14.3) | 0.0002 |
| Neutrophils (109/L) | 4.1 (2.4–8.3) | 5.1 (2.9–10.4) | 0.0008 |
| Eosinophils (109/L) | 0.15 (0.03–0.68) | 0.18 (0.06–0.78) | 0.0166 |
| Basophils (109/L) | 0.04 (0.01–0.07) | 0.03 (0.02–0.05) | ns |
| Monocytes (109/L) | 0.49 (0.29–0.90) | 0.67 (0.40–1.21) | 0.0005 |
| Lymphocytes (109/L) | 1.57 (1.00–2.21) | 1.82 (1.07–2.97) | 0.0045 |
| CD3+ T cells (109/L) | 0.75 (0.20–1.36) | 1.11 (0.48–1.98) | 0.0005 |
| CD4+ T cells (109/L) | 0.46 (0.06–0.99) | 0.59 (0.37–1.62) | 0.0007 |
| CD8+ T cells (109/L) | 0.15 (0.03–0.45) | 0.15 (0.05–0.71) | 0.0061 |
| CD19+ B cells (109/L) | 0.23 (0.05–0.40) | 0.30 (0.09–0.51) | 0.0023 |
| CD16 + CD56+ NK cells (109/L) | 0.40 (0.21–0.87) | 0.31 (0.13–0.70) | 0.0178 |
Medians + range are indicated (n = 20). The minimal increase in exercise-induced plasma volume (3.7%) did not influence any of the significant differences found
As carriage of CMV has pronounced effects on the immune system, we compared the effects of the walking exercise between CMV seropositive (n = 13) and CMV seronegative subjects (n = 7). Although an exercise induced leucocytosis was seen in both CMV seropositive and seronegative individuals, increases in granulocytes, monocytes and lymphocytes were statistically significant in CMV seropositive subjects but did not reach significance in CMV seronegative subjects (Table 4). Lymphocytosis in CMV seropositive subjects was largely caused by T cells, rather CD4 than CD8, and to a lesser extent B cells, whereas NK cell numbers remained unchanged. B cell numbers increased irrespective of CMV serostatus. Interestingly, a decrease of NK cells was seen only in CMV seronegative subjects. Thus, acute exercise induced responses were more clear in CMV seropositive octogenarians.
Table 4.
Immune parameters before and after walking in CMV seropositive and seronegative participants
| CMV+ | CMV- | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| Hemoglobina (mmol/L) | 8.4 (7.7–9.4) | 7.9 (7.3–8.7)** | 8.9 (7.9–9.0) | 8.7 (7.5–9.1) |
| CRP (mg/L) | 1 (1–9) | 1 (1–10) | 1 (1–6) | 9 (1–47) |
| Leukocytes (109/L) | 6.6 (4.6–11.0) | 7.9 (5.7–14.3)** | 6.6 (4.8–9.1) | 7.2 (6.3–12.3)* |
| Neutrophils (109/L) | 3.8 (2.4–8.3) | 5.0 (2.9–10.4)* | 4.2 (3.2–6.6) | 5.1 (4.2–9.2)* |
| Eosinophils (109/L) | 0.12 (0.03–0.68) | 0.19 (0.06–0.78)** | 0.17 (0.08–0.28) | 0.15 (0.08–0.33) |
| Basophils (109/L) | 0.03 (0.01–0.06) | 0.03 (0.02–0.05) | 0.05 (0.02–0.07) | 0.04 (0.03–0.05) |
| Monocytes (109/L) | 0.48 (0.29–0.69) | 0.68 (0.40–1.05)** | 0.57 (0.32–0.90) | 0.66 (0.42–1.21) |
| Lymphocytes (109/L) | 1.65 (1.45–2.21) | 2.19 (1.33–2.97)** | 1.37 (1.00–1.92) | 1.40 (1.07–2.08) |
| CD3+ T cells (109/L) | 0.77 (0.49–1.36) | 1.19 (0.73–1.98)** | 0.63 (0.20–1.00) | 0.76 (0.48–1.45) |
| CD4+ T cells (109/L) | 0.52 (0.17–0.99) | 0.73 (0.40–1.62)** | 0.40 (–0.06–0.69) | 0.57 (0.37–0.87) |
| CD8+ T cells (109/L) | 0.21 (0.04–0.45) | 0.21 (0.06–0.72)* | 0.08 (0.04–0.17) | 0.10 (0.05–0.19) |
| CD19+ B cells (109/L) | 0.26 (0.09–0.40) | 0.35 (0.11–0.51)* | 0.10 (0.05–0.40) | 0.16 (0.09–0.45)* |
| CD16 + CD56+ NK cells (109/L) | 0.32 (0.21–0.87) | 0.31 (0.13–0.70) | 0.43 (0.38–0.52) | 0.29 (0.22–0.43)* |
CMV+ subjects (n = 13) and CMV- subjects (n = 7). Medians and range are shown. Paired analysis was peformed separately for CMV+ and CMV- subjects before and after excersise. Statistical significance by Wilcoxon signed rank test is indicated as *p < 0.05 or **p < 0.01
aChanges in plasma volumes estimated according to Dill and Costill were not significantly different between CMV+ and CMV- subjects
Effect of acute exercise on T cells
We next examined the proliferative properties of circulating immune cells as measured by Ki-67 expression. Post walking, both CD4+ and CD8+ T cells demonstrated reduced percentages of proliferating cells (Fig. 1a). Similar observations were seen in CMV seropositive and seronegative subjects (Additional file 1: Figure S1a). Thus, the data suggest an exercise-induced increase of T cells with low proliferative capacity [20].
Fig. 1.
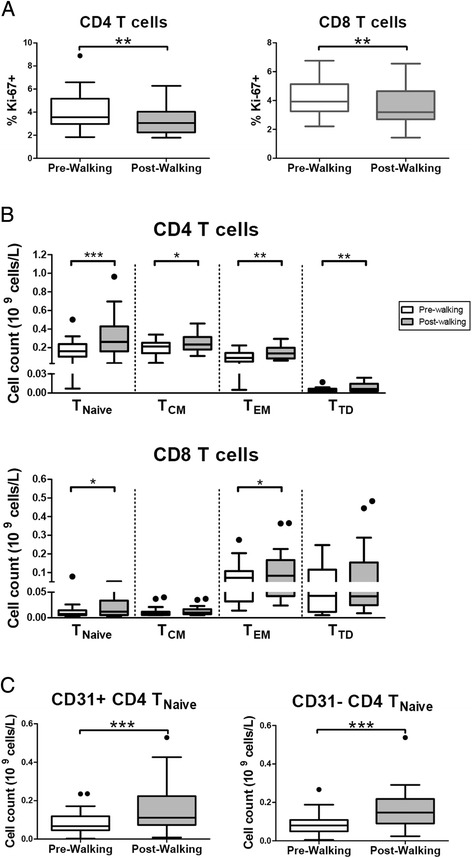
Exercise reduces rates of T cell proliferation and leads to redistribution of T cell subsets. a Rates of CD4 (left panel) and CD8 (right panel) proliferation before (Pre-Walking) and after exercise (Post-Walking) assessed by Ki-67 expression using flow-cytometry. Percentages of Ki-67 expressing cells within the CD4 and CD8 populations are shown. b Enumeration of CD4 (upper panel) and CD8 (lower panel) T cell differentiation subsets based on CD45RO and CCR7 expression Pre- and Post-Walking. Mean (+/- SEM) numbers (109 cells/L) of naïve T cells (TNaïve), central memory T cells (TCM), effector memory T cells (TEM) and terminally differentiated T cells (TTD) are shown. c Mean (+/- SEM) numbers (109 cells/L) of recent thymic emigrants defined as CD31 + CD4 + TNaïve and the central naïve CD31-CD4+ TNaïve subsets. Statistical significance by Wilcoxon signed rank test is indicated as *p < 0.05, **p < 0.01, ***p < 0.001
To determine if certain T cell populations respond differently, we next investigated exercise-induced changes in CD4+ and CD8+ differentiation subsets. A flow-cytometric analysis employing CD45RO and CCR7 was applied to identify naïve T cells (TNaïve), central memory T cells (TCM), effector memory T cells (TEM) and terminally differentiated T cells (TTD) [28]. Numerical changes of differentiation subsets were most profound in the CD4+ compartment (Fig. 1b) and appeared to be linked to CMV carriage (Additional file 1: Figure S1b). Although significant increases were noted for all four differentiation subsets, the most profound increase was noted in the CD4+ TNaïve subset (Fig 1b). Interestingly, we also noted a significant increase of CD4+ TNaïve in CMV seronegative subjects (Additional file 1: Figure S1b). When analysed for the contribution of recent thymic emigrants (RTE), defined as CD31pos CD4 + TNaïve and the central naïve CD31neg CD4 + TNaïve subsets, we found both subsets significantly increased [29] (Fig. 1c). In CMV seronegative subjects the rise in CD4+ TNaïve appeared due to the CD31neg CD4 + TNaïve subset (Additional file 1: Figure S1c). Moreover, the naïve CD4 + CD25dim subset, recently described to develop in secondary lymphoid organs upon TCR priming, was found significantly increased ([30], data not shown). The combined data suggest the exercise-induced response of CD4 + TNaïve and CD4+ memory subsets (TEM >TTD > TCM).
In the CD8+ compartment, slight but significant numerical increases were noted for both the CD8 TEM and the CD8 + TNaïve subsets, whereas the TCM and the TTD subsets were not changed (Fig. 1b). These changes appeared associated with CMV carriage (Additional file 1: Figure S1b). Taken together, our findings show an increase of peripheral naive CD4+ T cells particularly in response to exercise. Carriage of CMV is largely associated with enhanced numbers of both CD4 and CD8 subsets.
No effect of acute exercise on markers of T cell exhaustion
As we noted numerical increases in particularly the CD4+ TEM and the TTD subsets and to a lesser extent the CD8 TEM, but not the CD8 TTD subset, we examined these subsets for expression of the exhaustion markers CTLA-4 and PD-1 [8]. Frequencies of CTLA-4 and PD-1 in the CD4+ TEM and the TTD subsets were largely comparable before and after the walking exercise; although a slight decrease of PD-1 expressing cells in the TEM subset was detected (Additional file 2: Figure S2). In the CD8+ TEM and the TTD subsets, where only modest or no numerical increases were noted, respectively, only slight increases in the frequencies of CTLA4-, but not PD1-expressing cells were observed. Expression of these markers was generally lacking on TNaïve and TCM (data not shown). Thus, the walking exercise does not affect markers of immune exhaustion of either CD4 or CD8 TEM and TTD subsets.
Acute exercise-induced changes in nTreg but not memory Treg subsets
Based on CD45RA and FoxP3 expression [31], CD45RA + FoxP3low naïve (resting) Treg cells (nTreg) and CD45RA-FoxP3high- memory (activated) Treg cells (memTreg) were identified in the peripheral blood of elderly walkers (Fig. 2a). Post exercise, a clear numerical increase of nTreg was observed whereas the numbers of memTreg remained stable (Fig. 2b). The increase in nTreg was seen irrespective of CMV serostatus, although the increase was more clear in CMV seropositive subjects (Additional File 3, Figure S3). Thus, as seen with the conventional CD4+ TNaïve cells, the peripheral numbers of nTregs in elderly walkers also increased in response to acute exercise. In contrast, whereas exercise led to increases in conventional CD4+ memory T cells (Fig. 1b), exercise did not induce numerical increases of memTreg (Fig 2b).
Fig. 2.
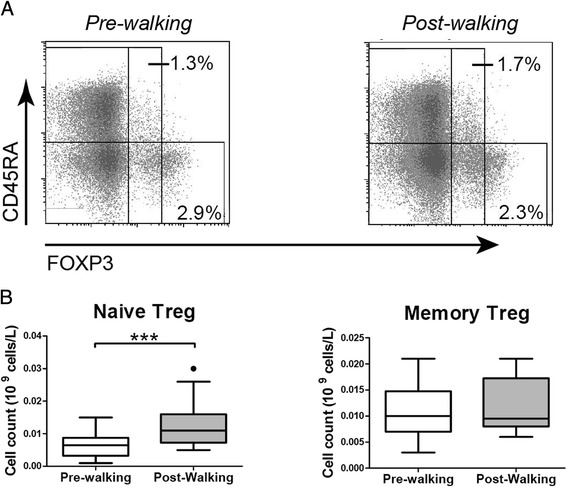
Exercise-induced changes of naïve Treg, but not memory Treg. a Representative CD45RA and FoxP3 staining in CD4 T cells before and after exercise (Pre-walking and Post-walking). Naïve Treg cells are identified by CD45RA + Foxp3dim and memory Treg cells by CD45RA-FoxP3high [31]. b Mean (+/- SEM) numbers (109 cells/L) of Naïve Treg and Memory Treg Pre-walking and Post-walking. Statistical significance by Wilcoxon signed rank test is indicated as ***p < 0.001
Effects of acute exercise on NK cells
Peripheral NK cell numbers were found reduced after exercise which was caused by a numerical decline of CD56dim but not CD56bright NK cells [15] (Table 3 and Fig. 3a). Notably, the decrease in CD56dim NK cells was seen in CMV seronegative donors only (Fig 3a). As CD56dim NK cells are most frequent in the blood (90% of total NK cells), we next investigated exercise-induced changes in the expression of inhibitory and activating NK receptors by this subset. In CMV seronegative subjects, we found comparable frequencies of cells positive for inhibitory receptors (KIR2DL1, KIR2DL2/3, KIR3DL1, NKG2A and NKG2C) and activating receptors (NKp30, NKp44, NKp46, 2B4 and NKG2D) within CD56dim NK cells before and after walking (Fig. 3bc). However, in CMV seropositive subjects, exercise did modulate frequencies of NK cells with inhibitory and activating receptors. More specifically, KIR2DL1-, KIR2DL2/3- and KIR3DL1-positive cells decreased with exercise, but frequencies of NKG2C-positive cells were increased (Fig. 3b). Also, frequencies of activating receptor NKG2D+ cells were found increased (Fig. 3c). Thus, the walking exercise led to a numerical decline of CD56dim NK cells in CMV seronegative subjects. In CMV seropositive subjects the walking exercise did not seem to affect the numbers of NK cells but down-modulated expression of most inhibitory receptors whereas the expression of activating receptors was largely unchanged, suggesting a less inhibited phenotype as a net result.
Fig. 3.
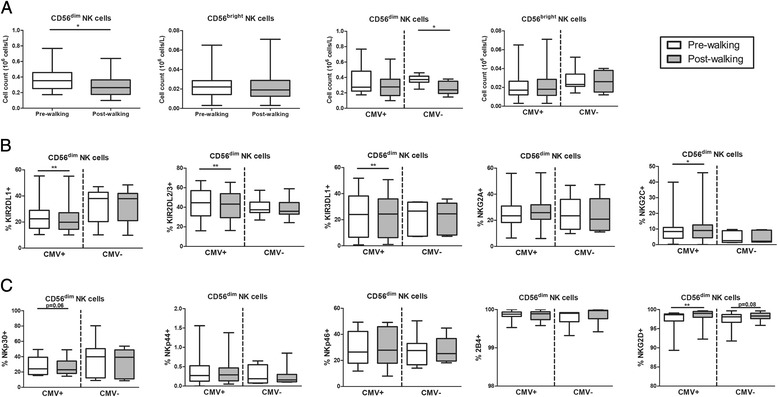
Exercise-induced changes in NK cells. a Mean (+/- SEM) numbers (109 cells/L) of CD56dim and CD56bright NK subsets Pre-walking and Post-walking in the study population (n = 20, left panels) and when stratified for CMV serostatus (CMV+; n = 13, CMV-; n = 7, right panels). b Percentages of KIR2DL1+, KIR2DL2/3+, KIR3DL1+, NKG2A+ and NKG2C+ NK cells within CD56dim NK cells Pre-walking and Post-walking in CMV+ and CMV- subjects. c Percentages of NKp30+, NKp44, NKp46+,2B4+, NKG2D+ within CD56dim NK cells Pre-walking and Post-walking in CMV+ and CMV- subjects. Statistical significance by Wilcoxon signed rank test is indicated as *p < 0.05, **p < 0.01
Exercise leads to higher LPS-stimulated cytokine production by PBMC
We next investigated the effects of exercise on PBMC function. Hereto, we analysed the LPS stimulated production of IL-1β, TNFα, IL-6 and IL-8 by PBMC before and after walking. The data show increases in production of IL-6 and IL-8, but not IL-1β and TNFα (Fig. 4). Similar data were obtained in CMV seropositive and seronegative subjects (data not shown).
Fig. 4.
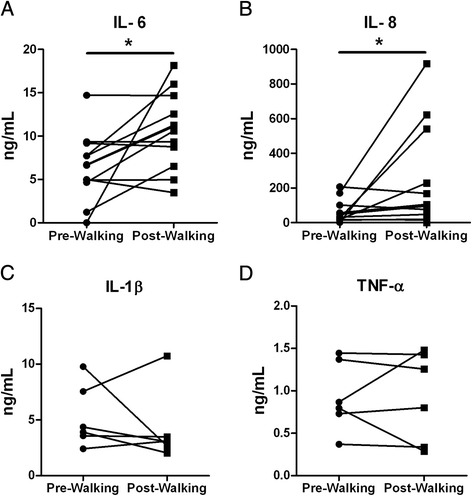
LPS-stimulated PBMC cytokine production before and after walking. Cytokines (a) IL-6, (b) IL-8, (c) IL-1β and (d) TNFα in the 24 h supernatant of LPS-stimulated PBMC isolated pre and post walking. Individual pre- and post- samples are connected by lines. Cytokines in supernatants were assessed with ELISA and expressed as ng/mL. The assay sensitivity was 8 pg/mL for IL-1β, 31 pg/mL for IL-6 and TNF-α, and 312 pg/mL for IL-8. The net cytokine production was calculated as cytokine production of the stimulated sample minus the cytokine production of the non-stimulated sample. IL-6 and IL-8 were assessed in 12 walkers (8 males and 4 females). IL-1β and TNF-α were assessed in 6 walkers (4 males and 2 females). Statistical significance by Wilcoxon signed rank test is indicated as *p < 0.05
Discussion
Our main finding is that acute exercise induced changes in immune cell numbers and functions in an exceptional cohort of octogenarian walkers. A clear response of CD4+ T cells, rather than CD8+ T cells or NK cells to exercise was noted. Moreover, the response was dominated by numerical increases of naïve CD4+ subsets.
Effects of exercise were evaluated in a paired sample design study enumerating a comprehensive set of peripheral cellular traits, before and directly after the walking event. As it is known that most exercise-induced changes in immune cell counts return to pre-exercise levels within a few hours, we emphasise that our data likely reflect the effect of the final day of exercise. We report on clear exercise-induced numerical increases of granulocytes, monocytes and lymphocytes. A retrospective analysis showed that these changes were associated with CMV carriage, thereby confirming the notion that infection history not only impacts the composition of the peripheral blood compartment but also the response to exercise, as recently suggested [21, 22].
Previous studies in adult subjects show that CD8 effector memory T cells and NK cells are the most exercise responsive lymphocytes [19, 20]. Preferential mobilization of these cells from the marginal pool is caused by increases in haemodynamic shear forces and by the relatively high expression of β-adrenergic receptors on these cells, leading to detachment of lymphocytes from endothelial cells upon catecholamine stimulation [22]. Following exercise cessation, both NK cells and CD8+ memory T cells quickly reallocate to the tissues. Only recently, it was documented that CMV latency enhances the exercise-induced mobilization of CD8 effector memory T cells in adult and middle aged (50–64 years old) subjects [21, 22, 32]. Interestingly, mobilization of NK cells was less pronounced in both adult and middle aged CMV carriers, suggesting that CMV infection may impair their mobilization [22, 33]. Moreover, CMV carriage delayed the egress of both CD8 TEM cells and NK cells to the tissues. The latter may be due to impaired β-adrenergic receptor signalling in CMV carriers [34].
Our study revealed a very modest increase of CD8 TEM cells in CMV carriers and a decrease of CD56dim NK cells in CMV non-carriers. This may be explained by the timing of the blood sampling, which was done within 10 min after exercise cessation, in the early recovery phase. The selective decrease of CD56dim NK cells may indeed suggest a rapid distribution of these cells to the tissues. In contrast, we found proportions of NKG2C+ CD56dim NK cells increased after exercise in CMV seropositive subjects, which would be in line with their delayed egress due to catecholamine insensitivity [22, 34]. Thus, also in octogenarians, CMV carriage may delay the egress of CD8 TEM cells and ‘memory-like’ NK cells to the tissues.
Latent CMV infection may also increase the mobilization of CD4+ effector memory T cells, albeit to a lesser extent than CD8 T cells [21, 32]. Our octogenarian walkers showed limited increases in CD4+ effector memory and in terminally differentiated effector memory T cells in response to exercise. This may be explained by the notion that most memory T cells are retained in peripheral tissues as tissue resident memory T cells [35]. In line with this, markers of immune exhaustion, such as PD-1 and CTLA-4, on memory subsets were unchanged.
Previously, the exercise induced mobilization of CD8+ naïve/early differentiated cells was found to be impaired in middle aged adults, irrespective of CMV serostatus [32]. Data on naïve CD4+ T cell mobilization in middle aged and elderly individuals are scarce. We here report on a robust response of naïve CD4+ T cells in our octogenarian cohort of habitual walkers. Numerical increases of naïve CD4+ T cells were seen irrespective of CMV status. In contrast, the response of naïve CD8 T cells was very limited and is in line with the higher turn-over rate of naïve CD8 T cells as a consequence of ageing and or frequent environmental challenges [7, 36–39]. Conversely, human CD4 naïve T cells are better maintained with age due to peripheral homeostatic proliferation mechanisms involving IL-2, a phenomenon not seen with naïve CD8 T cells [30, 40, 41]. The exercise-induced increases of CD4+ naïve T cells suggests that these cells are retained in the marginal pool and/or lymphoid tissues on to high age.
Few studies have investigated effects of exercise on diverse Treg subsets in elderly cohorts. We here report on exercise-induced changes of naïve/resting regulatory T cells but not activated/memory Treg. The increase in naïve Treg suggests, similar to the RTE and central naïve CD4+ T cells, the maintenance of these subsets on to high age in niches of the primary and secondary lymphoid organs. In contrast, memory Treg, even more so than conventional memory T cells, appear to be retained in the tissues [42].
We found serum CRP levels elevated in response to exercise. This is likely the result of higher levels of systemic IL-6. Indeed, a higher IL-6 and IL-8 production capacity upon LPS-stimulation of PBMC was seen after walking. Yet, production levels of IL-1β and TNFα were unaltered. The higher production levels of IL-6 and IL-8 may reflect the higher number of monocytes in the PBMC fraction after exercise but do not explain why the production capacity of IL-1β and TNFα were not elevated. An age-associated reduced inflammasome activation may underlie the reduced IL-1β and TNFα production capacity of elderly monocytes; a notion that would merit further investigation.
Our study cohort was obviously biased for physical fitness, as the participants were able to complete 4 consecutive days of moderate intensity exercise. The participants, however, were not selected as healthy individuals and 14 out of the 20 participants had at least one disease for which they were treated. Our cohort thus represents a selection of habitual elderly walkers who exercise in spite of having a disease. Yet, baseline values of both innate and adaptive immune cells compare well to values obtained in a healthy elderly cohort who were selected for their health ([9], data not shown).
Importantly, although our study was not designed and powered to definitely conclude on the effects of CMV carriage on the response to exercise, a retrospective analysis already revealed clear effects of CMV carriage on amplitude and/or kinetics of peripheral immune markers in this small cohort of octogenarians. Clearly, further studies in independent elderly cohorts are required to assess the effects of CMV carriage on acute exercise induced immune cell changes.
Conclusion
Acute exercise induced changes in immune cell numbers and functions in a group of octogenarian walkers. The massive exercise-induced response of naïve CD4+ T cells was most remarkable and adds to the notion of naïve CD4+ maintenance on to high age. The functional consequences of these changes for mobilization of immune responses to novel (and previously encountered) antigens remain to be established.
Acknowledgements
We are grateful to Bram van Cranenbroek (Dept Lab. Med. at Radboud university medical centre) for optimising the NK cell flow-cytometry panels.
We also recognize the excellent help of the organization of the Nijmegen Four Days Marches. Above all we would like to thank our volunteers (‘The Golden Oldies’) for their endurance, wonderful spirits and their participation in this study.
Funding
This work was supported by the Netherlands Organization for Scientific Research (Veni Grant 016.136.101 for JFMJ and Rubicon Grant 825.12.016 for TMHE). The funding agencies had no role in design of the study, collection, analysis and interpretation of the data nor the writing of the manuscript.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
KSMvdG, TMHE, HJPK, IJ, EB, MTEH, JFMJ and AMHB designed and organised the study. KSMvdG, QW, TMHE and JFMJ performed the study. KSMvdG, QW, TMHE, JFMJ and AMHB analyzed the data. KSMvdG and AMHB wrote the first draft of the paper. KSMvdG, TMHE, JFMJ and AMHB are responsible for the final content. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was reviewed and approved by the Medical Ethical Committee of the Radboud university medical centre, Nijmegen, The Netherlands. All participants gave written informed consent prior to the study (NL18245.091.07). This study was conducted in line with the Declaration of Helsinki.
Abbreviations
- BMI
Body mass index
- bpm
Beats per minute
- CCR7
C-C chemokine receptor type 7
- CRP
C-reactive protein
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- ELISA
Enzyme-linked immunosorbent assay
- Foxp3
Forkhead box P3
- HRmax
Maximal heart rate
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- LPS
Lipopolysacharide
- NK
Natural killer
- PBMC
Peripheral blood mononuclear cells
- PD-1
Programmed cell death protein 1
- RTE
Recent thymic emigrants
- TCM
Central memory T cells
- TCR
T cell receptor
- TEM
Effector memory T cells
- TNaïve
Naïve T cells
- TNF-α
Tumor necrosis factor-α
- Treg
Regulatory T cell
- TTD
Terminally differentiated T cells
- WBGT
Wet bulb globe temperature
Additional files
Exercise-induced T cell proliferation rates and T cell subset redistribution in CMV+ and CMV- subjects. (a) Rates of CD4 (left panel) and CD8 (right panel) proliferation before (Pre-Walking) and after exercise (Post-Walking) assessed by Ki-67 expression using flow-cytometry. Percentages of Ki-67 expressing cells within the CD4 and CD8 populations are shown. (b) Enumeration of CD4 and CD8 T cell differentiation subsets based on CD45RO and CCR7 expression Pre- and Post-Walking in CMV seropositive subjects (upper panel, n = 13) and CMV seronegative subjects (lower panel, n = 7). Mean (+/- SEM) numbers (109 cells/L) of naïve T cells (TNaïve), central memory T cells (TCM), effector memory T cells (TEM) and terminally differentiated T cells (TTD) are shown. (c) Mean (+/- SEM) numbers (109 cells/L) of recent thymic emigrants defined as CD31 + CD4 + TNaïve and the central naïve CD31-CD4+ TNaïve subsets in CMV+ and CMV- subjects. Statistical significance by Wilcoxon signed rank test is indicated as *p < 0.05, **p < 0.01. (DOCX 995 kb)
Exercise does not induce PD-1 and CTLA-4 on CD4 and CD8 memory T cells. (a) Percentages of PD-1 expressing cells in CD4 (left panel) and CD8 (right panel) effector memory T cells (TEM) and terminally differentiated T cells (TTD) pre- (white) and post-walking (grey). (b) Percentages of CTLA-4-expressing cells in CD4 (left panel) and CD8 (right panel) effector memory T cells (TEM) and terminally differentiated T cells (TTD) pre- (white) and post-walking (grey). (DOCX 775 kb)
Exercise-induced changes of naïve Treg, but not memory Treg. (a) Mean (+/- SEM) numbers (109 cells/L) of Naïve Treg and Memory Treg Pre-walking and Post-walking in CMV+ (n = 13) and CMV- (n = 7) subjects. Statistical significance by Wilcoxon signed rank test is indicated as *p < 0.05 and **p < 0.01. (DOCX 739 kb)
Contributor Information
Kornelis S. M. van der Geest, Email: k.s.m.van.der.geest@umcg.nl
Qi Wang, Email: q.wang01@umcg.nl.
Thijs M. H. Eijsvogels, Email: Thijs.Eijsvogels@radboudumc.nl
Hans J. P. Koenen, Email: Hans.Koenen@radboudumc.nl
Irma Joosten, Email: Irma.Joosten@radboudumc.nl.
Elisabeth Brouwer, Email: e.brouwer@umcg.nl.
Maria T. E. Hopman, Email: Maria.Hopman@radboudumc.nl
Joannes F. M. Jacobs, Email: H.Jacobs@radboudumc.nl
Annemieke M. H. Boots, Email: m.boots@umcg.nl
References
- 1.Fulop T, Kotb R, Fortin CF, Pawelec G, de Angelis F, Larbi A. Potential role of immunosenescence in cancer development. Ann N Y Acad Sci. 2010;1197(1):158. doi: 10.1111/j.1749-6632.2009.05370.x. [DOI] [PubMed] [Google Scholar]
- 2.Poland GA, Ovsyannikova IG, Kennedy RB, Lambert ND, Kirkland JL. A systems biology approach to the effect of aging, immunosenescence and vaccine response. Curr Opin Immunol. 2014;29:62. doi: 10.1016/j.coi.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000;30(6):931. doi: 10.1086/313792. [DOI] [PubMed] [Google Scholar]
- 4.Brodin P, Jojic V, Gao T, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160(1-2):37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fülöp T, Larbi A, Pawelec G. Human T cell aging and the impact of persistent viral infections. Front Immunol. 2013;4:271. doi: 10.3389/fimmu.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weltevrede M, Eilers R, de Melker HE, van Baarle D. Cytomegalovirus persistence and T-cell immunosenescence in people aged fifty and older: A systematic review. Exp Gerontol. 2016;77:87–95. doi: 10.1016/j.exger.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 7.van der Geest KS, Abdulahad WH, Horst G, et al. Quantifying Distribution of Flow Cytometric TCR-Vbeta Usage with Economic Statistics. PloS One. 2015a;10(4):e0125373. [DOI] [PMC free article] [PubMed]
- 8.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11(4):289. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 9.van der Geest KSM, Abdulahad WH, Tete SM, et al. Ageing disturbs the balance between effector and regulatory CD4+ T cells. Exp Gerontol. 2014;60:190. doi: 10.1016/j.exger.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Booth NJ, McQuaid AJ, Sobande T, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 11.Fessler J, Ficjan A, Duftner C, Dejaco C. The impact of aging on regulatory T-cells. Front Immunol. 2013;4:231. doi: 10.3389/fimmu.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Westra J, van der Geest KSM, et al. Reduced levels of cytosolic DNA sensor AIM2 are associated with impaired cytokine responses in healthy elderly. Exp Gerontol. 2016;2(78):39. doi: 10.1016/j.exger.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Biagi E, Nylund L, Candela M, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5):e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solana R, Campos C, Pera A, Tarazona R. Shaping of NK cell subsets by aging. Curr Opin Immunol. 2014;29:56. doi: 10.1016/j.coi.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Walsh NP, Gleeson M, Shephard RJ, et al. Position Statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 17.Eijsvogels TM, Thompson PD. Exercise Is Medicine: At Any Dose? JAMA. 2015;314(18):1915. doi: 10.1001/jama.2015.10858. [DOI] [PubMed] [Google Scholar]
- 18.Simpson RJ, Bosch JA. Special issue on exercise immunology: current perspectives on aging, health and extreme performance. Brain Behav Immun. 2014;39:1. doi: 10.1016/j.bbi.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Bigley AB, Rezvani K, Chew C, et al. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav Immun. 2014;39:160. doi: 10.1016/j.bbi.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Campbell JP, Riddell NE, Burns VE, et al. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun. 2009;6:767. doi: 10.1016/j.bbi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Turner JE, Aldred S, Witard OC. Latent Cytomegalovirus infection amplifies CD8 T-lymphocyte mobilisation and egress in response to exercise. Brain Behav Immun. 2010;24:1362. doi: 10.1016/j.bbi.2010.07.239. [DOI] [PubMed] [Google Scholar]
- 22.Simpson RJ, Bigley AB, Spielmann G, et al. Human cytomegalovirus infection and the immune response to exercise. Exerc Immunol Rev. 2016;22:8. [PubMed] [Google Scholar]
- 23.Jacobs JF, Eijsvogels TM, van der Geest KS, et al. The impact of exercise on the variation of serum free light chains. Clin Chem Lab Med. 2014;52(11):e239. doi: 10.1515/cclm-2014-0352. [DOI] [PubMed] [Google Scholar]
- 24.Eijsvogels TM, Veltmeijer MT, George K, Hopman MT, Thijssen DH. The impact of obesity on cardiac troponin levels after prolonged exercise in humans. Eur J Appl Physiol. 2012;112(5):1725. doi: 10.1007/s00421-011-2145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153. doi: 10.1016/S0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 26.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32(1):77. doi: 10.1079/BJN19740060. [DOI] [PubMed] [Google Scholar]
- 27.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2):247. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 29.Kimmig S, Przybylski GK, Schmidt CA, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Geest KS, Abdulahad WH, Teteloshvili N, et al. Low-affinity TCR engagement drives IL-2-dependent post-thymic maintenance of naive CD4+ T cells in aged humans. Aging Cell. 2015;14(5):744. doi: 10.1111/acel.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Spielmann G, Bollard CM, Bigley AB, et al. The effects of age and latent cytomegalovirus infection on the redeployment of CD8+ T cell subsets in response to acute exercise in humans. Brain Behav Immun. 2014;39:142. doi: 10.1016/j.bbi.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Bigley AB, Spielmann G, Agha N, Simpson RJ. The effects of age and latent Cytomegalovirus infection on NK-cell phenotype and exercise responsiveness in man. Oxid Med Cell Longev. 2015;2015:979645. doi: 10.1155/2015/979645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bigley AB, Rezvani K, Pistillo M, et al. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Part II: impact of latent cytomegalovirus infection and catecholamine sensitivity. Brain Behav Immun. 2015;49:59. doi: 10.1016/j.bbi.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 35.Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 2015;36(7):428. doi: 10.1016/j.it.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4(2):123. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 37.Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42:400. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackman MA, Woodland DL. The narrowing of the CD8 T cell repertoire in old age. Curr Opin Immunol. 2011;23:537. doi: 10.1016/j.coi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wertheimer AM, Bennett MS, Park B, et al. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014;192:2143. doi: 10.4049/jimmunol.1301721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.den Braber I, Mugwagwa T, Vrisekoop N, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Pekalski ML, Ferreira RC, Coulson RM, et al. Postthymic Expansion in Human CD4 Naive T Cells Defined by Expression of Functional High-Affinity IL-2 Receptors. J Immunol. 2013;190:2554. doi: 10.4049/jimmunol.1202914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thome JJ, Bickham KL, Ohmura Y, et al. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med. 2016;22(1):72. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


