Abstract
Background
Promoting quality of life (QoL) is a key priority in cancer care. We investigated the hypothesis that, in comparison to usual care, exercise post-neoadjuvant chemoradiation therapy/prior to surgical resection will reduce pain, fatigue, and insomnia, and will improve physical and mental health perceptions in patients with locally advanced stage rectal cancer.
Methods
In this non-randomized controlled pilot trial, patients in the supervised exercise group (EG; M age = 64 years; 64% male) and in the control group (CG; M age = 72 years; 69% male) completed the European Organization for Research and Treatment of Cancer core Quality of Life questionnaire and the RAND 36-Item Health Survey three times: pre-neoadjuvant chemoradiation therapy (Time 1; n EC = 24; n CG = 11), post-neoadjuvant chemoradiation therapy/pre-exercise intervention (Time 2; n EC = 23; n CG = 10), and post-exercise intervention (Time 3; n EC = 22; n CG = 10). The 6-week exercise intervention was delivered in hospital and comprised of interval aerobic training. Patients trained in pairs three times per week for 30 to 40 min. Data were analyzed by Mann–Whitney tests and by Wilcoxon matched-pairs signed-rank tests.
Results
No significant between-group differences in changes were found for any of the outcomes. In both groups, fatigue levels decreased and physical health perceptions increased from pre- to post-exercise intervention. Pain levels also decreased from pre- to post-exercise intervention, albeit not significantly.
Conclusions
The findings from this study can be used to guide a more definitive trial as they provide preliminary evidence regarding the potential effects of pre-operative exercise on self-reported pain, fatigue, insomnia, and health perceptions in patients with locally advanced rectal cancer. Trial registration: This study has been registered with clinicaltrials.gov (NCT01325909; March 29, 2011).
Keywords: Rectal cancer, Advanced stage, Exercise, Experimental study design, Patient-reported outcomes, Quality of life
Background
Approximately 813,613 men and 663,689 women were diagnosed with rectal cancer worldwide in 2012 [1]. Of these, 50–65% were diagnosed with locally advanced rectal cancer. Treatment for locally advanced rectal cancer often involves neoadjuvant chemoradiation therapy followed by surgical resection with the aim of improving resectability and disease control [2]. Although these standard treatments can prolong survival, they can result in adverse physical side effects, including pain, fatigue, constipation or diarrhea, upset stomach, nausea, sexual problems, infertility, acute toxicity, and decreased physical fitness [3, 4]. They can also result in adverse psychological side effects, including anxiety and distress [5]. As a result of these treatment-related side effects, patients’ quality of life (QoL) is often impaired [6]. Considering that QoL is a significant prognostic factor for cancer recurrence and all-cause mortality in patients with advanced colorectal cancer [7], identifying therapies to reduce treatment-related side effects and enhance QoL is a priority in the care of patients with advanced rectal cancer.
Exercise is one type of therapy that may improve outcomes for patients with advanced cancer at different stages of the disease trajectory. For example, researchers have reported that post-operative exercise can prolong survival after cancer diagnosis [8, 9], as well as enhance QoL by helping patients with advanced stage cancer manage physical and psychological side effects [10]. In addition, researchers have reported that pre-operative exercise is beneficial for patients with colorectal [11], colon [12, 13], and rectal cancer [14]. Specifically, they have shown that it can improve cardiorespiratory fitness [14], muscle strength [14], peak power output [13], heart rate [13], oxygen uptake [13], and respiratory muscle endurance [12]. This provides evidence that pre-operative exercise can elicit favourable changes in physiological outcomes in patients with advanced stage cancer [15, 16]. However, limited data are currently available to determine the effects of exercise post-neoadjuvant chemoradiation therapy and prior to surgical resection on key patient-reported outcomes (e.g., pain, fatigue, insomnia, health perceptions) in patients with advanced rectal cancer. Considering that advanced rectal cancer and neoadjuvant chemoradiation therapy can adversely affect patients’ general physical and mental health perceptions and increase fatigue, pain, and insomnia [17, 18], which can negatively affect recovery [5], it is important to examine whether participating in pre-operative exercise can help prevent or reduce these adverse consequences reported by patients.
The present study
We delivered a 6-week exercise intervention to patients diagnosed with locally advanced rectal cancer immediately post-neoadjuvant chemoradiation therapy and prior to surgical resection in order to examine the benefits of exercise at this particular stage of the disease trajectory. We examined changes in various patient-reported outcomes resulting from the exercise intervention using quantitative and qualitative methods. The aim of our qualitative inquiry was to capture in-depth accounts of changes in QoL associated with the exercise intervention from patients’ perspectives [19]. We had several aims in mind for our quantitative inquiry. Herein, we focus on the two aims related to changes in QoL. The first aim was to assess the effects of the exercise intervention on indicators of QoL in comparison to usual care (i.e., assess differences in changes between groups). The second aim was to quantify the extent to which the exercise intervention had a positive effect on indicators of QoL (i.e., assess within-group changes). We focused on pain, fatigue, insomnia, and physical and mental health perceptions as indicators of QoL because (i) patients with rectal cancer report these as main concerns [17], (ii) these symptoms appear in the National Institute of Health call for more efforts toward symptom management in cancer [20], and (iii) they represent different dimensions of health relevant to patients with cancer [21].
Methods
Data analyzed for this study were collected as part of a single-site, non-randomized controlled pilot trial. We have published analyses using this sample elsewhere [22, 23]. Additional details of the methods that are not relevant to this study can be found in those publications. The protocol was approved by the North West – Liverpool East Committee for Research Ethics (11/H1002/12) and it was registered with clinicaltrials.gov (NCT01325909; March 29, 2011). Patients provided informed consent to participate in this study prior to us conducting any study-related procedures.
Participants and procedures
From March 2011 to February 2013, patients referred to the colorectal multidisciplinary team were recruited for this study. Inclusion criteria were: (i) ≥ 18 years of age, (ii) confirmed diagnosis of magnetic resonance imaging defined locally advanced circumferential margin threatened resectable rectal cancer (i.e., ≥ stage T2/N+ with no distant metastasis), (iii) scheduled for standardized neoadjuvant chemoradiation therapy, and (iv) performance status score of ≤ 2 on the Eastern Co-operative Oncology Group (ECOG)/World Health Organization (WHO) system [24]. Patients were not eligible if they: (i) were unable to give informed consent, (ii) had been diagnosed with non-resectable cancer, (iii) were unable to perform a cardiopulmonary exercise test (CPET) or exercise, (iv) had declined surgery or neoadjuvant chemoradiation therapy, and/or (v) had received non-standard neoadjuvant chemoradiation therapy.
All patients in this study underwent 5 weeks of standardized neoadjuvant chemoradiation therapy. Standardized radiotherapy consisted of 45 Gray (Gy) in 25 fractions on weekdays using a three-dimensional conformal technique with computerized tomography guidance. A booster dose was given (5.4 Gy in 3 fractions) to the primary tumour only. Oral capecitabine at a dose of 825 mg.m−2 was given twice daily on radiotherapy days. No patient received brachytherapy.
After completing neoadjuvant chemoradiation therapy, all patients were assigned to the exercise group by default (i.e., there was no allocation concealment) by the colorectal multidisciplinary team unless they were unable to commit to the exercise schedule or lived > 15 miles from the hospital. These latter patients were asked to act as contemporaneously recruited controls. A total of 39 patients were recruited into the study, though four dropped out immediately. Thirty-five patients completed QoL assessments prior to receiving neoadjuvant chemoradiation therapy (Time 1 data analyzed) and went on to receive neoadjuvant chemoradiation therapy. Thereafter, 24 were allocated to the exercise group and 11 to the control group, though 1 patient switched immediately to the control group. At this time, 23 patients in the exercise group and 10 patients in the control group completed QoL assessments prior to the 6-week exercise intervention (Time 2 data analyzed). After the exercise intervention, 22 patients remained in the exercise group and completed QoL assessments along with 10 patients in the control group (Time 3 data analyzed). Figure 1 displays the flow of patients through each stage of this study from enrolment to analysis. We note that the sample size for analysis herein is slightly different from previous publications [22, 23] due to the completeness of relevant data (i.e., the previous publications used CPET data and the current study used QoL data).
Fig. 1.
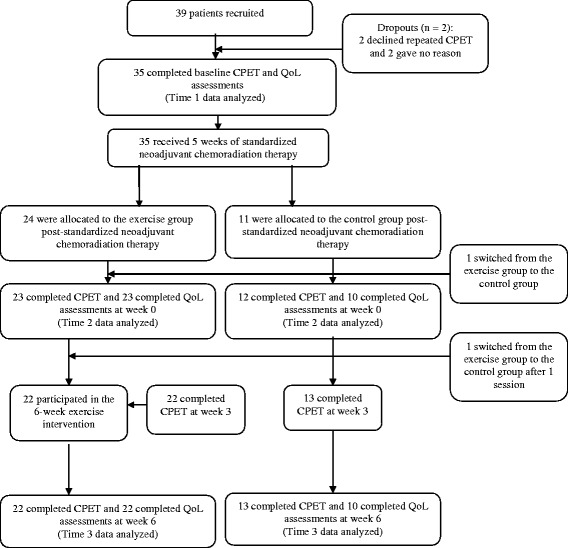
Flow chart of recruitment and participation in this study
Study procedures
Assessments
Patients completed questionnaires prior to neoadjuvant chemoradiation therapy (Time 1), before starting the exercise intervention (i.e., immediately post-neoadjuvant chemoradiation therapy; Time 2), and immediately post-exercise intervention (Time 3). They also underwent a standardized CPET to assess their cardiovascular, respiratory, and skeletal muscle systems (see [25] for protocol details) at these three time points1; however, an additional CPET was performed mid-way through the exercise intervention so as to modify the exercise prescription according to patients’ changing fitness levels. Prior to receiving the exercise intervention, patients received usual care from their oncology care team.
Exercise intervention
The exercise protocol was progressive and lasted 6 weeks. Patients exercised in pairs three times per week under the supervision of a trained exercise specialist in a hospital. Initially, exercise intensities were tailored for each patient based on his/her standardized CPET results post-chemoradiation therapy and modified thereafter according to his/her results mid-way through the exercise intervention. Each patient was instructed to engage in interval training on an electromagnetically braked cycle ergometer (Optibike Ergoline GmbH, Germany). A chip-and-pin card with patients’ pre-loaded target interval intensities was used to ensure they engaged in 3 min of moderate-intensity intervals (i.e., work rate of 80% of oxygen uptake at lactate threshold) interspersed with 2 min of vigorous-intensity intervals (i.e., work rate of 50% of the difference in work rates between peak oxygen uptake and oxygen uptake at lactate threshold). For the first three sessions, training consisted of a total time of 30 min, which was then increased to 40 min for the rest of the training sessions. All sessions included 5 min of warm-up and 5 min of cool-down.
Outcome measures
At each of the three time points, we used the European Organization for Research and Treatment of Cancer 30-item core Quality of Life questionnaire (EORTC QOL-C30) version 3 [26] to assess patients’ levels of pain, fatigue, and insomnia, and used the RAND 36-Item Health Survey [27] to assess their general physical and mental health perceptions.
The EORTC QLQ-C30 is a self-report questionnaire developed to assess cancer patients’ QoL. It comprises five multi-item functional subscales (i.e., role, physical, cognitive, emotional, and social functioning), three multi-item symptom scales (i.e., fatigue, pain, and nausea), five single items assessing common symptoms experienced (i.e., dyspnea, insomnia, appetite loss, constipation, and diarrhea), and two questions assessing global health status/QoL. Each item has four response options: (1) not at all, (2) a little, (3) quite a bit, and (4) very much, except for the two questions assessing global health status/QoL [response options range from (1) very poor to (7) excellent]. Higher scores on the functional subscales and global health status/QoL scale represent a better level of functioning and global health status/QoL, whereas higher scores on symptom subscales represent higher levels of symptomatology. Given that cancer and neoadjuvant chemoradiation therapy can increase fatigue, pain, and insomnia [17, 18], which can negatively affect recovery [5], these scales were the focus of the current analyses.
The RAND 36-Item Health Survey is a self-report questionnaire that consists of eight subscales assessing the health domains of physical functioning, social functioning, role limitations due to physical health problems, role limitations due to emotional health problems, vitality/energy, bodily pain, general health perceptions, and mental health perceptions. It includes the same items as those in the 36-item Short-Form (SF-36) Health Survey [28]; however, each item is scored on a scale ranging from 0 to 100. Scores on the physical functioning, role limitations due to physical health problems, bodily pain, and general health perceptions subscales were averaged into a physical component summary score. Scores on the social functioning, role limitations due to emotional health problems, vitality/energy, and mental health subscales were averaged into a mental component summary score. Higher scores represent better physical and mental health perceptions.
Statistical analysis
All statistical analyses were performed using SPSS version 23 and included all data available at any given point. Missing values were not imputed for analysis. Descriptive data were used to describe differences on the QoL measures across time points, and are expressed as medians and inter-quartile ranges at each time point. As the distribution of the variables was significantly different from normal based on Kolmogorov-Smirnov tests for three variables (i.e., pain, insomnia, and mental health perceptions), non-parametric tests were used. Specifically, Mann–Whitney tests were used to assess whether changes in fatigue, pain, insomnia, and health perceptions across time points differed between the exercise group and the control group (i.e., Aim 1; assess differences in changes between the two groups). Wilcoxon matched-pairs signed-rank tests were used to identify any changes in fatigue, pain, insomnia, and health perceptions across time points within-groups (i.e., Aim 2; assess within-group changes). Of note, testing using parametric tests (i.e., t-tests) for variables with normal distributions yielded results similar to those obtained with the non-parametric tests (data not shown). To correct for multiple comparisons, we used the Simes procedure [29] – a modification of the Bonferroni correction method. Accordingly, level of statistical significance was set to p < .017.
Results
Patients in the exercise group had a mean age of 64 years (range = 45 – 82), 64% were male, and they had a mean body mass index of 27.4 kg/m2 (SD = 5.1). Forty-five percent were currently smoking, and 46% had a past medical history of diabetes, health failure, or ischemic heart disease. Most (82%) scored ‘0’ on the ECOG/WHO system meaning that they were asymptomatic (i.e., fully active and able to carry on all pre-disease activities without restriction). The rest (18%) scored ‘1’ meaning they were symptomatic but completely ambulatory (i.e., restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature). No patient scored ‘2’ meaning none were symptomatic (i.e., <50% in bed during the day, ambulatory and capable of all self care but unable to carry out any work activities, and up and about > 50% of waking hours). Overall, patients adhered well to the exercise protocol, as the mean (SD) attendance for the patients who took part in the exercise intervention was 96% (5.0). There were no adverse events reported.
Patients in the control group had a mean age of 72 years (range = 62 – 84), 69% were male, and they had a mean body mass index of 24.9 kg/m2 (SD = 3.9). Thirty-one percent were currently smoking, and 54% had a past medical history of diabetes, health failure, or ischemic heart disease. Most (62%) scored ‘0’ on the ECOG/WHO, 23% scored ‘1’, and 15% scored ‘2’.
Aim 1: Examining differences in changes between groups
There was no evidence that changes in pain (p = .67), fatigue (p = .10), insomnia (p = .89), physical health perceptions (p = .34), and mental health perceptions (p = .90) observed from pre- to post-exercise intervention differed significantly between the exercise group and the control group.
Aim 2: Examining within-group changes
Prior to neoadjuvant chemoradiation therapy, median scores of pain, fatigue, and insomnia were 17.0, 22.0, and 33.0 for the total sample, respectively, which are comparable to published norms [30]. Median scores were 52.8 and 56.8 for physical and mental health perceptions, respectively, which also fall close to normative values [31]. Descriptive statistics for all outcomes for the exercise group and the control group by time point are presented in Table 1.
Table 1.
Summary of scores for each group by time point expressed as medians and inter-quartile ranges
| Pain | Fatigue | Insomnia | Physical health | Mental health | |
|---|---|---|---|---|---|
| Baseline | |||||
| Control (n = 11) | 0 (0,33.0) | 11.0 (11.1,44.0) | 0 (0,33.0) | 52.8 (32.8,64.4) | 59.0 (53.3,63.4) |
| Intervention (n = 24) | 16.7 (0,33.3) | 27.5 (11.0,50.3) | 33.3 (0,67.0) | 53.1 (33.5,63.1) | 56.0 (51.3,63.4) |
| Total (n = 35) | 17.0 (0,33.0) | 22.0 (11.0,44.0) | 33.0 (0,67.0) | 52.8 (33.0,63.4) | 56.8 (51.8,63.4) |
| Pre-exercise intervention | |||||
| Control (n = 10) | 33.0 (29.0,62.5) | 33.0 (19.3,49.8) | 33.0 (0,42.5) | 29.6 (24.4,34.7) | 52.0 (43.3,61.3) |
| Intervention (n = 23) | 33.0 (17.0,50.0) | 33.0 (22.0,67.0) | 33.0 (33.0,67.0) | 39.2 (26.6,55.2) | 57.0 (51.3,62.4) |
| Total (n = 33) | 33.0 (17.0,50.0) | 33.0 (22.0,67.0) | 33.0 (16.5,67.0) | 36.4 (26.4,53.3) | 55.0 (50.3,61.7) |
| Post-exercise intervention | |||||
| Control (n = 10) | 8.5 (0,41.5) | 22.0 (0,35.8) | 0 (0,49.8) | 56.8 (30.7,64.1) | 55.1 (51.2,58.6) |
| Intervention (n = 22) | 8.5 (0,37.3) | 22.0 (11.0,33.0) | 33.0 (0,67.0) | 57.3 (37.3,63.1) | 56.1 (53.5,60.7) |
| Total (n = 32) | 8.5 (0,33.0) | 22.0 (2.3,33.0) | 16.5 (0,67.0) | 57.3 (37.1,63.3) | 55.5 (53.0,59.5) |
Pain
There were changes in levels of pain from pre- to post-neoadjuvant chemoradiation therapy (ps < .03), wherein patients in both groups reported more pain immediately post-neoadjuvant chemoradiation therapy compared to pre-neoadjuvant chemoradiation therapy. Whereas patients in both groups reported less pain post-exercise intervention, these were not statistically different from those pre-exercise intervention (ps > .14).
Fatigue
There were changes in levels of fatigue from pre- to post-neoadjuvant chemoradiation therapy (ps < .001) and from pre- to post-exercise intervention (ps < .01). Specifically, patients in both groups reported more fatigue immediately post-neoadjuvant chemoradiation therapy compared to pre-neoadjuvant chemoradiation therapy, and reported less fatigue post-exercise intervention compared to pre-exercise intervention.
Insomnia
There were changes in levels of insomnia for patients in the control group from pre- to post-neoadjuvant chemoradiation therapy (p = .05) and from pre- to post-exercise intervention (p = .04), albeit not significantly based on the corrected critical p-value. These patients reported more insomnia immediately post-neoadjuvant chemoradiation therapy compared to pre-neoadjuvant chemoradiation therapy, and reported less insomnia post-exercise intervention compared to pre-exercise intervention. There were no significant differences in levels of insomnia across time points (ps ≥ .26) for patients in the exercise group.
Physical health
There were changes in physical health perceptions from pre- to post-neoadjuvant chemoradiation therapy (ps < .007) and from pre- to post-exercise intervention (ps < .004). Patients in both groups reported poorer physical health perceptions immediately post-neoadjuvant chemoradiation therapy compared to pre-neoadjuvant chemoradiation therapy, and better physical health perceptions post-exercise intervention compared to pre-exercise intervention.
Mental health
There were no changes in mental health perceptions across time points for either of the groups (ps ≥ .43).
Discussion
The wait period between the completion of neoadjuvant chemoradiation therapy and prior to surgery can be challenging for patients with advanced rectal cancer. Debilitating side effects can impair recovery and reduce QoL in this population [5]. Yet, relatively few studies have been conducted to examine whether pre-operative exercise is an effective approach to help patients manage treatment-related side effects and promote QoL during this time. In this study, we explored the effects of a 6-week exercise intervention on pain, fatigue, insomnia, and health perceptions in patients with locally advanced cancer who had recently completed neoadjuvant chemoradiation therapy.
We found no evidence that an exercise intervention delivered in hospital and that comprised of interval aerobic training resulted in greater effects for any of the outcomes in comparison to usual care, and thus failed to support the notion that this type of exercise intervention is more effective than usual care for reducing treatment-related side effects and improving QoL. However, it is important to note that our study procedures may explain these findings. In the current study, all patients were assigned to the exercise group by default, unless they were unable to commit to the exercise schedule or lived > 15 miles from the hospital. In retrospect, presenting patients in the control group with the exercise intervention could have prompted them to reflect on their current behaviour, made them recognize that there is a need to change their behaviour, and in some cases, led them to make changes to it. Indeed, patients in both groups increased their average number of steps from pre- to post-exercise intervention (see [22], Figure 4). Thus, this may have led to an under-estimation of the effects of the exercise intervention in comparison to usual care. With this in mind, we believe that there are potentially some patients that may not need this type of pre-operative intervention to manage their treatment-related side effects and improve their QoL as they may be active on their own. Observed improvements for the control group may also be explained by other factors. For example, those in the control group may have sought other types of treatments (e.g., pharmaceuticals, psychological therapy, group therapy), which could have had positive effects on the outcomes we assessed. To control for this, we recommend conducting a randomized controlled trial in which participation in various therapies and exercise is measured and controlled for. We are currently conducting a randomized controlled trial (NCT01914068) in order to mitigate these study design limitations.
Whilst our findings do not support the notion that this type of exercise intervention is more effective than usual care in reducing treatment-related side effects and improving QoL, they demonstrate the likely value of exercise post-neoadjuvant chemoradiation therapy/prior to surgery for patients with advanced rectal cancer. This is because we observed a significant improvement in physical health perceptions and a decrease in levels of fatigue post-exercise intervention for patients in the exercise group. Moreover, we noted decreases in levels of pain post-exercise intervention for these patients, though these did not reach statistical significance.
Previous observational and experimental studies have demonstrated that post-operative exercise reduces fatigue in adults with cancer [10, 32]. Our findings extend these observations, demonstrating that a pre-operative exercise intervention can decrease fatigue – which happens to be the most frequent symptom cited [17] – in a group of patients who had completed neoadjuvant chemoradiation therapy for advanced stage rectal cancer. This finding is important when considering that patients’ levels of fatigue significantly increased after neoadjuvant chemoradiation therapy, and that fatigue can negatively affect QoL more than any other symptom such as vomiting, nausea, pain, and depression [33, 34]. While the exact process through which exercise reduced patients’ levels of fatigue remains to be determined, it could be that it helped to restore their physical capacity and fitness [35]. Indeed, for patients in the exercise group, their oxygen uptake at lactate threshold significantly improved post-exercise intervention (data reported elsewhere; [22]). Thus, future research attempting to determine which aspects of pre-operative exercise helps to reduce fatigue would be beneficial to optimize pre-operative exercise interventions aimed at reducing fatigue in this population.
Although we did not observe a statistically significant difference in change between groups, we observed that exercise post-neoadjuvant chemoradiation therapy significantly improved patients’ physical health perceptions. This finding is consistent with previous studies in which patients receiving treatment for either a primary, recurrent incurable cancer or advanced cancer showed improvements in health perceptions post-exercise [36–38]. These findings are significant because decreases in physical health are common during the post-neoadjuvant chemoradiation therapy period [3, 33, 34] and lead to more adverse surgical outcomes (e.g., prolonged hospital stay; [39]). Moreover, this may have clinical significance because self-rated health is a significant predictor of survival in adults with advanced cancer [40].
Though our results suggest that our exercise intervention did not have a statistically significant effect on pain, these should be interpreted cautiously. The non-significant trend for patients to report less pain post-exercise intervention as compared to pre-exercise intervention may have been the result of insufficient power. Hence, it is necessary to keep in mind that patients’ levels of pain decreased post-exercise intervention, and that they were lower than their pre-neoadjuvant chemoradiation therapy levels. Further, compared to reference data published for patients with rectal cancer [30], patients in this study reported lower levels of pain post-exercise intervention. Thus, it is recommended that studies with larger samples sizes be conducted to assess the extent to which exercise may have an impact on pain during this time in this population.
In contrast to previous research that suggests exercise can reduce anxiety, depression, and sleep disturbances during and post-treatment in adults with cancer [38], we did not find statistically significant improvements in insomnia or mental health perceptions. Neither insomnia nor mental health perceptions worsened during neoadjuvant chemoradiation therapy, and levels were comparable to normative levels [30]. This may have left less room for improvement than if patients had high levels of insomnia and poor mental health perceptions after undergoing neoadjuvant chemoradiation therapy. Alternatively, the non-significant effects of exercise on these outcomes might be due to the short duration of our intervention (i.e., 6 weeks). Based on previous reports [41], longer interventions might be necessary to change mental health perceptions and insomnia. Patients could have also been taking pharmaceuticals or have received psychological therapy (data not collected) to manage their insomnia and/or mental health issues [42], which may have confounded the effects of exercise on these outcomes. Last, the measures used, though valid and reliable, might not have been sensitive enough to capture changes in these two patient-reported outcomes. For instance, insomnia was only measured using one item, which may fail to capture insomnia symptoms along several dimensions (i.e., severity, duration, and impact). Assessing insomnia using questionnaires that capture the nature, severity, and impact of insomnia may be more effective for determining if exercise has an impact on insomnia. As well, previous studies have shown that adults with cancer are likely to experience unanticipated fear, anxiety, and psychological stress about major surgery [5]. The mental health summary score derived from the RAND 36-Item Health Survey might not be sensitive to measuring these specific cancer-related mental health issues (e.g., pre-operative anxiety) that might have been affected by exercise. These possible explanations should be investigated in future research.
Limitations
Perhaps the most significant limitation of this study is the small sample size of the control group that could have introduced Type II error when testing for differences between the exercise group and the control group. Indeed, power calculations were only made to determine the sample size required to detect a minimum difference in oxygen uptake at lactate threshold of 1.5 ml kg−1 min−1 and a SD of 1.1 ml kg−1 min−1 [22], not QoL. Relatedly, because the sample size was small and the data were not normally distributed for three variables, non-parametric statistical tests that do not require the assumptions of normality be met were used. However, it should be noted that non-parametric tests are more conservative and are appropriate for hypothesis testing when the sample size is small. Other limitations include the reliance on a convenience sample, our inability to report the rate of recruitment because the number of patients eligible was not recorded, and the non-randomization. The latter increases the likelihood of there being differences between the exercise group and the control group in factors (known and unknown) that could affect the outcomes we assessed. Also, this study has the potential for ascertainment bias due to the fact that patients were given a choice to participate in the exercise intervention. Consequently, the effects observed may be biased upwards. A final limitation is the lack of follow-up data to determine if the observed improvements were maintained over time and whether pre-operative exercise reduced the incidence of post-operative complications. Thus, a larger, adequately powered randomized controlled trial with long-term follow-ups is needed to compare the effects of exercise post-neoadjuvant chemoradiation therapy/prior to surgical resection on pain, fatigue, insomnia, and physical and mental health perceptions, in comparison to usual care, in patients with locally advanced stage rectal cancer.
Conclusions
Pain, fatigue, and insomnia are prevalent and disturbing side effects of treatment for advanced rectal cancer. Furthermore, treatment for advanced rectal can result in diminished health perceptions and QoL. The notion that exercise has a greater effect on self-reported pain, fatigue, insomnia, and health perceptions than usual care was not confirmed in this study. Nevertheless, we did observe an increase in physical health perceptions and a decrease in levels of fatigue post-exercise intervention for patients in the exercise group. We also found small, but not statistically significant, decreases in levels of pain post-exercise intervention for these patients. In light of the limitations associated with this study, it is important that a larger randomized controlled trial be conducted to assess the effectiveness of exercise in comparison to usual care, and to provide precise estimates of the effects of exercise on key patient-reported outcomes. Such a study would provide valuable insight into the extent to which pre-operative exercise is effective in treating patients’ side effects and promoting improvements in the quality of their lives above and beyond usual care.
Acknowledgements
The authors would like to thank all the participants who took part in the study and Lisa Loughney for her help with collecting data and supervising the exercise sessions. They would also like to thank Rebecca Asher and Eftychia Psarelli for their assistance with the data analysis.
Funding
This work was funded by the Royal College of Anaesthetists BOC Fellowship awarded by the National Institute of Academic Anaesthesia and the National Institute of Health Research for the Fit-4-Surgery program of research. This manuscript was prepared while the first author was supported by a Canadian Cancer Society Career Development Award in Prevention.
Availability of data and materials
The dataset used and analyzed for this study is available from the corresponding author on reasonable request.
Authors’ contributions
JB, MAW, SJ, and MPWG made substantial contributions to the study conception and design. MAW and SJ made substantial contributions to the acquisition of data. JB, SB, and MAW were involved in drafting the manuscript. JB, SB, MPWG, MAW, and SJ were involved in revising it critically for important intellectual content, and gave final approval of the version to be published.
Authors’ information
JB is an Assistant Professor in the School of Human Kinetics at the University of Ottawa and holds appointments as an Affiliate Investigator at the Ottawa Hospital Research Institute and as a Research Member at the Montfort Hospital Research Institute. She is also the recipient of the Canadian Association for Psychosocial Oncology New Investigator Award and the John Charles Polanyi Prize in Physiology and Medicine. She is working to develop and evaluate evidence-based interventions aimed at increasing physical activity levels among individuals reporting particularly low levels of physical activity, such as cancer patients/survivors, women, and youth. She also works collaboratively with many health care providers and researchers on different research projects which are centred on physical activity. Her research interests are primarily focused on understanding the psychological and social influences on, and consequences of, physical activity participation.
SB is a lecturer in exercise and health psychology in the Faculty of Biological Sciences at the University of Leeds. She is also the program leader for sport and exercise sciences. Her research focuses on the role of physical activity in promoting psychological health and well-being. She is particularly interested in physical activity as a complimentary therapy to manage the adverse side effects of cancer and improve quality of life across the disease continuum. She is also interested in the advancement of qualitative research methods within clinical and health services research.
MPWG is a Professor of Anaesthesia and Critical Care Medicine at the University of Southampton (UoS) where he leads the Centre for Human Integrative Physiology. He is also a consultant in Critical Care Medicine at University Hospital Southampton NHS Foundation Trust (UHS) where he leads the critical care research area of the UHS-UoS NIHR Respiratory Biomedical Research Unit. He is the NIHR CRN Specialty National Lead for Anaesthesia, Perioperative Medicine and Pain Management and also leads the Xtreme-Everest Oxygen Research Consortium and the Fit-4-Surgery Group. He is Director of the NIAA Health Services Research Centre and chairs the National Emergency Laparotomy Audit. He is also Joint Editor-in-Chief of the BioMedCentral journal Extreme Physiology and Medicine. His research interests include human responses to hypoxia, measuring and improving outcome following surgery, acute lung injury, and fluid therapy.
MAW was the Clinical Lead for Perioperative Cardio Pulmonary Exercise testing service at Aintree University Hospitals NHS Foundation Trust, Liverpool, UK. He was a NIHR funded Clinical Research Fellow at the University of Liverpool supported by two National Institute for Health Research, Research for Patient Benefit grants. MW was research lead for the Colorectal Research Group in Aintree, which is part of the Fit-4-Surgery research collaboration. He has taken time out of his surgical training to pursue a PhD in exercise physiology, perioperative surgical risk stratification and mitochondrial energetics in rectal cancer patients. He has recently been awarded a prestigious NIHR Surgical Academic Clinical Fellowship at the University of Southampton. His research interests include surgical risk stratification, cancer therapies and their effect on physical fitness, outcome and morbidity following cancer surgery.
SJ was Director of the Clinical Diagnostic and Pre-operative Assessment Exercise service at Aintree University Hospitals NHS Foundation Trust. She was an investigator on the recent Xtreme Everest 2 expedition where she led on hypoxic ventilator control tests. She is currently a Consultant Clinician Scientist in the Anaesthesia and Critical Care Research Unit at University Hospital Southampton NHS Foundation Trust, Southampton and NIHR Southampton Respiratory Biomedical Research Unit and Integrated Physiology and Critical Illness Group, Clinical and Experimental Sciences, Faculty of Medicine, University of Southampton. She is also currently an Associate Professor at the University of Liverpool, University of Southampton and University College London. Her research interests are primarily exercise physiology in health and disease with a special interest in the ventilatory control responses in patients with idiopathic hyperventilation. More recently her research interests have been on the use of exercise testing in pre-operative assessment and perioperative management including pre-habilitation in cancer patients undergoing major surgery.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Approval for this study was obtained from the North West – Liverpool East Committee for Research Ethics (11/H1002/12). All participants provided informed consent.
Abbreviations
- CG
Control group
- CPET
Cardiopulmonary exercise test
- ECOG
Eastern Co-operative Oncology Group
- EG
Exercise group
- EORTC QOL-C30
European Organization for Research and Treatment of Cancer 30-item core Quality of Life questionnaire
- Gy
Gray
- QoL
Quality of life
- SF-36
36-item Short-Form Health Survey
- WHO
World Health Organization
Footnotes
Contributor Information
Jennifer Brunet, Email: jennifer.brunet@uottawa.ca.
Shaunna Burke, Email: s.burke@leeds.ac.uk.
Michael P.W. Grocott, Email: mike.grocott@soton.ac.uk
Malcolm A. West, Email: m.west@soton.ac.uk
Sandy Jack, Email: s.jack@soton.ac.uk.
References
- 1.GLOBOCAN 2012 v 1.0. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. http://globocan.iarc.fr. Accessed 28 Sept 2015.
- 2.Foster JD, Jones EL, Falk S, Cooper EJ, Francis NK. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum. 2013;56:921–30. doi: 10.1097/DCR.0b013e31828aedcb. [DOI] [PubMed] [Google Scholar]
- 3.Pucciarelli S, Del Bianco P, Efficace F, Serpentini S, Capirci C, De Paoli A, Amato A, Cuicchi D, Nitti D. Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study. Ann Surg. 2011;253:71–7. doi: 10.1097/SLA.0b013e3181fcb856. [DOI] [PubMed] [Google Scholar]
- 4.West M, Lythgoe D, Barben C, Noble L, Kemp G, Jack S, Grocott M. Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: a prospective blinded observational study. Br J Anaesth. 2014;112:665–71. doi: 10.1093/bja/aet408. [DOI] [PubMed] [Google Scholar]
- 5.Simunovic M, Gagliardi A, McCready D, Coates A, Levine M, DePetrillo D. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. Can Med Assoc J. 2001;165:421–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver KE, Forsythe LP, Reeve BB, Alfano CM, Rodriguez JL, Sabatino SA, Hawkins NA, Rowland JH. Mental and physical health–related quality of life among US cancer survivors: population estimates from the 2010 national health interview survey. Cancer Epidemiol Biomarkers Prev. 2012;21:2108–17. doi: 10.1158/1055-9965.EPI-12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong CK, Law W-L, Wan Y-F, Poon JT-C, Lam CL-K. Health-related quality of life and risk of colorectal cancer recurrence and all-cause death among advanced stages of colorectal cancer 1-year after diagnosis. BMC Cancer. 2014;14:337. doi: 10.1186/1471-2407-14-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–34. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 9.Davies N, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer. 2011;105:S52–S73. doi: 10.1038/bjc.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrecht TA, Taylor AG. Physical activity in patients with advanced-stage cancer: a systematic review of the literature. Clin J Oncol Nurs. 2012;16:293–300. doi: 10.1188/12.CJON.293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim D, Scott S, Mayo N. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. 2010;97:1187–97. doi: 10.1002/bjs.7102. [DOI] [PubMed] [Google Scholar]
- 12.Dronkers J, Lamberts H, Reutelingsperger I, Naber R, Dronkers-Landman C, Veldman A, van Meeteren N. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clin Rehabil. 2010;24:614–22. doi: 10.1177/0269215509358941. [DOI] [PubMed] [Google Scholar]
- 13.Kim DJ, Mayo NE, Carli F, Montgomery DL, Zavorsky GS. Responsive measures to prehabilitation in patients undergoing bowel resection surgery. Tohoku J Exp Med. 2009;217:109–15. doi: 10.1620/tjem.217.109. [DOI] [PubMed] [Google Scholar]
- 14.Timmerman H, de Groot J, Hulzebos H, de Knikker R, Kerkkamp H, Van Meeteren N. Feasibility and preliminary effectiveness of preoperative therapeutic exercise in patients with cancer: a pragmatic study. Physiother Theory Pract. 2011;27:117–24. doi: 10.3109/09593981003761509. [DOI] [PubMed] [Google Scholar]
- 15.Singh F, Newton RU, Galvão DA, Spry N, Baker MK. A systematic review of pre-surgical exercise intervention studies with cancer patients. Surg Oncol. 2013;22:92–104. doi: 10.1016/j.suronc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 16.O’Doherty A, West M, Jack S, Grocott M. Preoperative aerobic exercise training in elective intra-cavity surgery: a systematic review. Br J Anaesth. 2013;110:679–89. doi: 10.1093/bja/aes514. [DOI] [PubMed] [Google Scholar]
- 17.Butt Z, Rosenbloom SK, Abernethy AP, Beaumont JL, Paul D, Hampton D, Jacobsen PB, Syrjala KL, Von Roenn JH, Cella D. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw. 2008;6:448–55. doi: 10.6004/jnccn.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osoba D, Hsu M-A, Copley-Merriman C, Coombs J, Johnson FR, Hauber B, Manjunath R, Pyles A. Stated preferences of patients with cancer for health-related quality-of-life (HRQOL) domains during treatment. Qual Life Res. 2006;15:273–83. doi: 10.1007/s11136-005-0580-5. [DOI] [PubMed] [Google Scholar]
- 19.Burke SM, West MA, Grocott MP, Brunet J, Jack S. Exploring the experience of adhering to a prescribed pre-surgical exercise program for patients with advanced rectal cancer: a phenomenological study. Psychol Sport Exerc. 2015;16:88–95. doi: 10.1016/j.psychsport.2014.09.005. [DOI] [Google Scholar]
- 20.National Health Institute Symptom management in cancer: pain, depression and fatigue: state-of-the-Science Conference Statement. J Pain Palliat Care Pharmacother. 2003;17:77–97. doi: 10.1080/j354v17n01_12. [DOI] [PubMed] [Google Scholar]
- 21.Deshpande PR, Rajan S, Sudeepthi BL, Nazir CA. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2:137–44. doi: 10.4103/2229-3485.86879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West M, Loughney L, Lythgoe D, Barben C, Sripadam R, Kemp G, Grocott M, Jack S. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth. 2014;114:244–51. doi: 10.1093/bja/aeu318. [DOI] [PubMed] [Google Scholar]
- 23.West MA, Loughney L, Barben CP, Sripadam R, Kemp GJ, Grocott MPW, Jack S. The effects of neoadjuvant chemoradiotherapy on physical fitness and morbidity in rectal cancer surgery patients. Eur J Surg Oncol. 2014;40:1421–8. doi: 10.1016/j.ejso.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–55. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 25.West M, Parry M, Lythgoe D, Barben C, Kemp G, Grocott M, Jack S. Cardiopulmonary exercise testing for the prediction of morbidity risk after rectal cancer surgery. Br J Surg. 2014;101:1166–72. doi: 10.1002/bjs.9551. [DOI] [PubMed] [Google Scholar]
- 26.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The european organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 27.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 28.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): conceptual framework and item selection. Med Care. 1992;30:473–83. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Simes RJ. An improved bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–4. doi: 10.1093/biomet/73.3.751. [DOI] [Google Scholar]
- 30.Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, Groenvold M, Gundy C, Koller M, Petersen MA, Sprangers MAG. EORTC QLQ-C30 reference values. Brussels: Quality of Life Department; 2008. [Google Scholar]
- 31.Ware JE, Kosinski M, Dewey JE, Gandek B. SF-36 health survey: manual and interpretation guide. Lincoln: Quality Metric Inc.; 2000.
- 32.Windsor PM, Nicol KF, Potter J. A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer. 2004;101:550–7. doi: 10.1002/cncr.20378. [DOI] [PubMed] [Google Scholar]
- 33.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK. Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. Oncologist. 2000;5:353–60. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 34.Hawthorn M. Fatigue in patients with advanced cancer. Int J Palliat Nurs. 2010;16:536–41. doi: 10.12968/ijpn.2010.16.11.80023. [DOI] [PubMed] [Google Scholar]
- 35.Dimeo FC. Effects of exercise on cancer-related fatigue. Cancer. 2001;92:1689–93. doi: 10.1002/1097-0142(20010915)92:6+<1689::AID-CNCR1498>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 36.Adamsen L, Quist M, Andersen C, Møller T, Herrstedt J, Kronborg D, Baadsgaard MT, Vistisen K, Midtgaard J, Christiansen B. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McClellan R. Exercise programs for patients with cancer improve physical functioning and quality of life. J Physiother. 2013;59:57. doi: 10.1016/S1836-9553(13)70150-4. [DOI] [PubMed] [Google Scholar]
- 38.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Libr. 2012;8:CD008465. [DOI] [PMC free article] [PubMed]
- 39.Valkenet K, van de Port IG, Dronkers JJ, de Vries WR, Lindeman E, Backx FJ. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clin Rehabil. 2011;25:99–111. doi: 10.1177/0269215510380830. [DOI] [PubMed] [Google Scholar]
- 40.Shadbolt B, Barresi J, Craft P. Self-rated health as a predictor of survival among patients with advanced cancer. J Clin Oncol. 2002;20:2514–9. doi: 10.1200/JCO.2002.08.060. [DOI] [PubMed] [Google Scholar]
- 41.Ferrer RA, Huedo-Medina TB, Johnson BT, Ryan S, Pescatello LS. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41:32–47. doi: 10.1007/s12160-010-9225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traeger L, Greer JA, Fernandez-Robles C, Temel JS, Pirl WF. Evidence-based treatment of anxiety in patients with cancer. J Clin Oncol. 2012;30:1197–205. doi: 10.1200/JCO.2011.39.5632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and analyzed for this study is available from the corresponding author on reasonable request.


