Abstract
Objective:
This study was to evaluate the effect of systemic injection of an anti-tumor necrosis factor alpha (TNF-α) monoclonal antibody (mAb) on endotoxin-induced uveitis (EIU).
Materials and Methods:
Fifty-six male Wistar rats (6–8 weeks old) were randomly divided into three groups: EIU, anti-TNF-α mAb + EIU, and control. EIU was induced by injecting Escherichia coli O55:B5 lipopolysaccharide (LPS) into the hind footpad of the rats (150 μg/rat). The anti-TNF-α mAb (1 μg/kg) was administrated 30 min before LPS injection through one-time intravenous injection. The onset time and peak time of EIU were recorded. The serum and aqueous humor (AH) TNF-α, interleukin (IL)-6, and IL-10 levels were measured by ELISA at 4, 24, and 72 h post-LPS injection. Clinical manifestations of EIU and eye histopathology were scored.
Results:
Compared with the EIU rats, anti-TNF-α mAb + EIU rats showed significantly delayed onset of uveitis (t = 7.41, P < 0.001), lower clinical scores and histopathological grades (t = 3.18/2.22, P < 0.001), reduced levels of TNF-α (F = 15.06/59.43, P < 0.001) and IL-6 (F = 99.63/14.92, P < 0.001), and increased levels of IL-10 (F = 24.94/8.99, P < 0.001) in the serum and AH. AH TNF-α, serum IL-6, and AH IL-6 levels are positively correlated, whereas serum IL-10 levels were negatively correlated with EIU activity.
Conclusion:
Antagonizing TNF-α by system injection of the anti-TNF-α mAb protects against EIU in rats. Blocking TNF-α signaling could be a useful strategy for managing uveitis.
Keywords: Endotoxin-induced uveitis, interlekuin-6, interlekuin-10, lipopolysaccharide, tumor necrosis factor alpha
Uveitis is a group of diseases characterized by intraocular inflammation. Predominantly affecting young people, uveitis leads to a high percentage of blindness in Europe, America, and China.[1,2,3,4] Uveitis often exhibits a long disease course and high recurrence, with no ideal prevention and treatment methods established to date. While many cases are idiopathic, evidence suggests that uveitis involves both the adaptive immune system (e.g., activation of T cells) and the innate immune elements (e.g., production of inflammatory cytokines).[5]
Many cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-10 have been implicated in the development of uveitis.[6] TNF-α is a pro-inflammatory cytokine released mainly from macrophages and T cells during inflammatory responses and functions to mediate leukocyte activation and infiltration and T helper 1 lymphocyte responses within tissues.[7] In the case of uveitis, high levels of TNF-α promote disruption of the blood-retina barrier and increase vascular permeability, leading to edema.[8] IL-6 represents another pleiotropic pro-inflammatory cytokine often abundantly present in the intraocular fluids of patients with uveitis.[9] It is also produced by a variety of cell types (e.g., macrophages, monocytes, and leukocytes), and can be induced by TNF-α. IL-6 plays an important role in T helper 17 cell development and is a major regulator of the acute phase response.[10] IL-10, on the contrary, is a key anti-inflammatory and anti-angiogenic cytokine in most of the ocular inflammations.[11] IL-10 suppresses the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and interferon gamma (IFN-γ) and regulates differentiation and proliferation of multiple immune cells such as T and B cells, antigen-presenting cells, and granulocytes. Elevated intraocular IL-10 levels are often associated with the activity of uveitis,[9] likely representing an attempt to control the overt inflammation.
Given their crucial roles in uveitis pathogenesis, biological therapies that intend to modulate cytokines and cytokine-mediated inflammatory/immune responses have been proposed for the disease. Among them, TNF-α blockers are the most commonly used class of biologics for managing uveitis.[12,13] However, the efficacy and safety of anti-TNF-α agents for the treatment of uveitis need further demonstration.[14]
The main aim of this study was to evaluate the effects of systemic administration of an anti-TNF-α monoclonal antibody (mAb) on endotoxin-induced uveitis (EIU), a prototypical experimental model for acute anterior uveitis,[15] and on pro-inflammatory IL-6 and anti-inflammatory IL-10 levels. In this model, low doses of lipopolysaccharide (LPS), a component of the Gram-negative bacterial cell wall, are given to rats through hind footpad injections to activate TLR4-MyD88 signaling pathway, resulting in cytokine production, leukocyte infiltration, and inflammatory eye symptoms that resemble those seen clinically of uveitis.[16] Our hypothesis was that the anti-TNF-α mAb treatment would prevent EIU development and ameliorate EIU-associated pro-inflammatory cytokine production and clinical manifestations.
Materials and Methods
Ethics statement
Rats were provided by the Institute of Radiation Medicine Chinese Academy of Medical Sciences. All experiments were conducted according to the Association for Research in Vision and Ophthalmology statement on the use of animals. Ethics approval for this study was obtained from the Animal Ethics Committee of Institute of Radiation Medicine Chinese Academy of Medical Sciences.
Reagents
LPS extracted from Escherichia coli O55:B5 was purchased from Sigma-Aldrich, USA. The anti-rat TNF-α mAb (Cat. MAB510) was purchased from R and D, Minneapolis, Minnesota, USA. The ELISA kits for rat TNF-α and IL-10 were obtained from Wuhan Boshide Biotechnology Co., Ltd., (Wuhan, Hubei, China). The rat IL-6 ELISA kit was from R and D, USA.
Animal cohorts
Fifty-six male Wistar rats (6–8 weeks) were randomly assigned to three groups: The EIU group (n = 24), the anti-TNF-α mAb + EIU group (n = 24), and the control group (n = 8). Rats in the EIU group and the anti-TNF-α mAb + EIU group were further divided into three groups (8 per group) for examination at 4, 24, 72 h post-LPS injection. EIU was induced by injecting LPS (150 µg/rat) into the hind footpad of rats. The anti-TNF-α mAb was administrated 30 min before LPS injection through one-time intravenous injection at 1 µg/kg body weight. Comparable doses were used previously in a rodent EIU model.[17]
Endotoxin-induced uveitis observation and scoring
The clinical presentation of EIU post-LPS injection was monitored using slit-lamp biomicroscope (Kowa, Japan) and ophthalmoscope (Suzhou Liuliu Optics, China) hourly in the first 12 h post and then every 2 h. The EIU onset time and peak time were recorded. EIU clinical manifestations were scored following the method described previously.[18] After rats had been sacrificed at indicated time points, the left eye was dissected, and histopathology was observed using the OLYMPUS BX50 microscope and graded as described previously.[19]
Determination of tumor necrosis factor alpha, interlekuin-6, and interlekuin-10
Blood (2 ml) was extracted from the angular vein, and aqueous humor (AH) was extracted from the right eye from each rat at indicated time points. Blood samples were centrifuged at 4000 r/min for 10 min, and then took 400 µL of clear serum for analysis. Cytokine production was assessed by ELISA, performed following the manufacturers' instructions. Briefly, a standard curve was first established for each of the cytokines using reagents provided in ELISA kits. And then, TNF-α, IL-6, and IL-10 levels in serum and AH were determined by referring to the standard curve.
Statistics
Data were presented as mean ± standard deviation. Statistical analyses, performed in SPSS 11.5, (SPSS company, Chicago, USA) including t-test for histology score and grade comparison, Pearson correlation coefficient test for correlation between TNF-α, IL-6 and disease severity, and one-way ANOVA test followed by least significant difference test for cytokine level comparison. Significance was determined at P < 0.05 for a given parameter.
Results
Systemic administration of the anti-tumor necrosis factor alpha monoclonal antibody delayed and attenuated endotoxin-induced uveitis
While the untreated controls remained normal, both the EIU and anti-TNF-α mAb + EIU rats showed signs of uveitis, such as vascular engorgement, fibrinous exudate, and neutrophil infiltration in the anterior chamber, retinal vasculitis, and pupil miosis [Fig. 1]. However, the average onset time of EIU was determined to be 3.88 ± 0.62 h for the EIU rats and 5.75 ± 1.06 h for the anti-TNF-α mAb + EIU rats. The anti-TNF-α mAb pretreatment significantly delayed EIU development (t = 6.09, P < 0.001).
Figure 1.
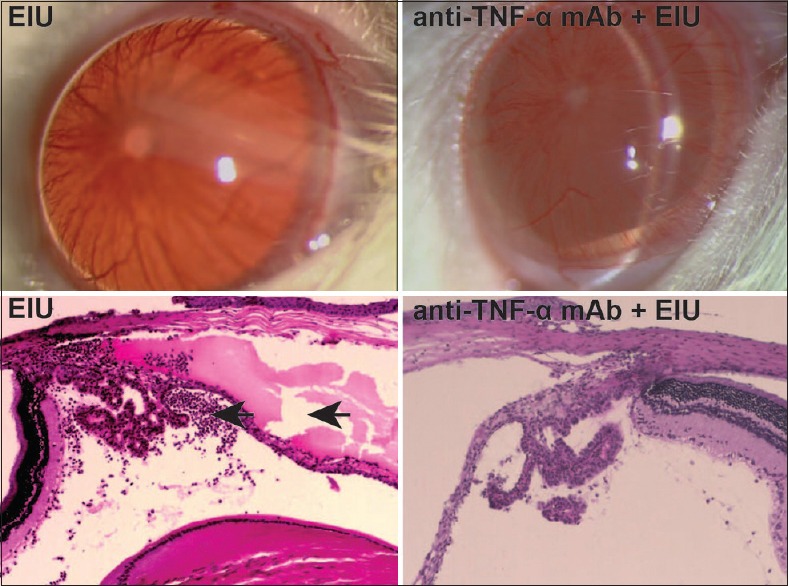
Anti-tumor necrosis factor alpha monoclonal antibody administration attenuated endotoxin-induced uveitis. The top left figure is endotoxin-induced uveitis rat and the top right figure is the anti-tumor necrosis factor alpha monoclonal antibody + endotoxin-induced uveitis rat 24 h postlipopolysaccharide injection. The endotoxin-induced uveitis rat showed iris vascular engorgement, fibrinous exudate at pupillary margin, and pupil miosis. The bottom left figure is the eye histology picture from an endotoxin-induced uveitis rat, and the bottom right figure is from an anti-tumor necrosis factor alpha monoclonal antibody + endotoxin-induced uveitis rat 24 h postlipopolysaccharide injection. The endotoxin-induced uveitis rat's eye had large exudate (right arrow) and massive neutrophil infiltration (left arrow) in the anterior chamber, which was attenuated in the anti-tumor necrosis factor alpha monoclonal antibody + endotoxin-induced uveitis rat
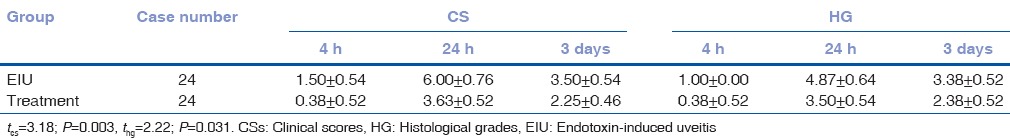
We then scored EIU manifestations clinically and histologically. The EIU rats had an average clinical score (CS) of 3.67 ± 1.97 and histopathological grading of 3.08 ± 1.69, whereas the anti-TNF-α mAb + EIU rats had a score of 2.08 ± 1.44 and grading of 2.08 ± 1.41. EIU CSs (t = 3.18, P = 0.003) as well as histopathological grades (t = 2.22, P = 0.031) were significantly higher in the EIU groups, suggesting that the anti-TNF-α mAb pretreatment attenuated EIU [Table 1].
Table 1.
Comparison of clinical scores and histological grades (x̄±s)
Systemic administration of the anti-tumor necrosis factor alpha monoclonal antibody reduced tumor necrosis factor alpha, interlekuin-6 and increased interlekuin-10 production
To further characterize the effect of systemic administration of the anti-TNF-α mAb, we measured EIU-associated production of cytokines, in particular, the pro-inflammatory TNF-α and IL-6 and the anti-inflammatory IL-10.
The serum and AH TNF-α levels of the control rats were determined to be 51.5 ± 7.90 ng/L and 26.8 ± 11.98 ng/L, respectively. The EIU rats had significantly higher serum (F = 15.06, P < 0.001) and AH (F = 59.43, P < 0.001) TNF-α levels than the control and anti-TNF-α mAb + EIU rats [Table 2]. Both the EIU and anti-TNF-α mAb + EIU rats showed higher levels of TNF-α in AH than that in serum (t = 8.49, P < 0.001).
Table 2.
Concentrations of tumor necrosis factor-alpha in the serum and aqueous humor (x̄±s, ng/L)

The serum and AH IL-6 levels of the control rats were determined to be 3.2 ± 1.14 ng/L and 2.5 ± 0.62 ng/L, respectively. Similar to TNF-α, IL-6 levels were significantly higher in the EIU rats than in the control and anti-TNF-α mAb + EIU rats, in both serum (F = 99.62, P < 0.001) and AH (F = 14.92, P < 0.001) [Table 3]. In contrast to TNF-α though, IL-6 was more abundant in serum than AH (t = 9.87, P < 0.001) and was higher in the EIU than anti-TNF-α treated rats.
Table 3.
Concentrations of interleukin-6 in the serum and aqueous humor (x̄±s, ng/L)

The control rats had 136.4 ± 97.91 ng/L of IL-10 in the serum and 172.5 ± 210.79 ng/L in the AH [Table 4]. Interestingly, both the control and EIU rats had significantly lower IL-10 levels than anti-TNF-α mAb + EIU rats, in serum (F = 24.94, P < 0.001) and AH (F = 8.99, P < 0.001). Serum IL-10 levels were higher than those in AH in anti-TNF-α mAb + EIU rats (t = 4.64, P = 0.008). On the contrary, AH contained more IL-10 than serum in EIU rats (t = 2.90, P = 0.008).
Table 4.
Concentrations of interleukin-10 in the serum and aqueous humor (x̄±s, ng/L)

Together, our data suggest that systemic administration of the anti-TNF-α mAb inhibited production of the pro-inflammatory cytokines TNF-α and IL-6 and elevated levels of the anti-inflammatory IL-10 in EIU rats.
Tumor necrosis factor alpha and interlekuin-6 were positively whereas interlekuin-10 was negatively correlated with endotoxin-induced uveitis severity
To investigate the kinetics of these cytokines during EIU pathogenesis, we further assessed the correlation between their levels and disease activities in EIU rats. We found that AH TNF-α, AH IL-6, and serum IL-6 levels were all positively correlated with EIU CSs (r = 0.48, P < 0.02; r = 0.49, P < 0.005; and r = 0.42, P < 0.005, respectively) and histopathological grades (r = 0.34, P < 0.02; r = 0.77, P < 0.005; and r = 0.71, P < 0.005, respectively). Serum IL-10 levels were inversely related to EIU CSs (r = −0.57, P < 0.001) and histopathological grades (r = −0.55, P < 0.001).
Interestingly, we found that serum TNF-α, AH TNF-α, serum IL-6, and AH IL-6 levels are all negatively correlated with the EIU onset time (r = −0.45/−0.69/−0.60/−0.63, P < 0.001). Serum IL-10 levels are inversely related to EIU onset time (r = 0.62, P < 0.001).
Discussion
In this study, we investigated the effects of systemic administration of an anti-TNFα mAb on EIU in rats. We found that the anti-TNF-α mAb pretreatment prevented EIU development, attenuated disease manifestations, inhibited production of pro-inflammatory cytokines TNF-α and IL-6, and elevated levels of the anti-inflammatory IL-10. We conclude that blocking TNF-α signaling is protective against EIU and can be a useful strategy for managing uveitis. In addition, we found that serum IL-6 levels were positively whereas serum IL-10 levels were negatively correlated with EIU activity in rats, providing further evidence for the involvement of these cytokines in uveitis pathogenesis and possible markers for monitoring uveitis progression.
We found that TNF-α and IL-6 levels, in serum and AH, were significantly upregulated shortly after LPS injection (as early as 4 h postinjection), suggesting that the two inflammatory cytokines are among the early response factors to LPS stimuli. Interestingly, after EIU induction, TNF-α levels in the AH were much higher than those in the serum, a phenomenon not observed for IL-6. Moreover, we found that AH TNF-α levels were positively correlated with EIU activity, supporting the note that TNF-α rather than IL-6 is the driving force behind EIU pathogenesis. Our findings are consistent with the previous reports using LPS to induce uveitis in animal models.[20,21,22] And indeed, TNF-α is crucial for uveitis development as Mo et al. showed that direct injection of TNF-α into the vitreous body exacerbated leukocyte infiltration into the anterior chamber and protein leakage.[20]
We demonstrated that systemic administration of the anti-TNF-α mAb delayed EIU onset, attenuated EIU CSs and histological grades, and downregulated production of pro-inflammatory cytokines TNF-α and IL-6. This observation was in line with other studies done with experimental animal uveitis models. For example, Mo et al. showed that intravitreal injection of an anti-TNF-α mAb to rabbits dramatically alleviates LPS-induced uveitis, reducing neutrophil infiltration by 50%, monocyte infiltration by 59%, and protein leakage by 42%.[20] Johnsen-Soriano et al. demonstrated that the anti-TNF-α drugs, adalimumab and infliximab, restored the histopathological changes of EIU although only infliximab partially prevented EIU development.[23] Others have reported that subcutaneous injection of recombinant TNF receptor P75 (etanercept) before EIU induction prevented leukocyte adhesion and activation, reduced vascular permeability, inhibited retinal cell apoptosis, and significantly improved clinical uveitis scores.[24,25] Together, these studies demonstrated the efficacy of blocking TNF-α for managing uveitis. However, others have reported that systemically blocking TNF-α during EIU appeared to exacerbate the disease.[26,27] The paradoxical effects might depend on the models, reagents, and administration methods used in these studies and be explained by the redundant and pleiotropic nature of the cytokine network.
Importantly, as demonstrated by our and others' studies, blocking TNF-α alone failed to abrogate uveitis, suggesting that other cytokines are also responsible for the disease pathogenesis and that a combined approach antagonizing TNF-α, as well as other pro-inflammatory cytokines, might be more effective. Supporting the note, Mo et al. showed that combining an anti-TNF-α mAb and recombinant rabbit IL-1 receptor antagonist gave more pronounced results than the anti-TNF-α mAb alone in an LPS-induced uveitis rat model.[20]
In our model, IL-10 was significantly upregulated following EIU induction. This likely represents a host response in an effort to control the over immune activation. Anti-TNF-α mAb pretreatment significantly elevated IL-10 levels, suggesting that TNF-α inhibits IL-10 production during EIU development. Given the anti-inflammatory and anti-angiogenic role of IL-10, enhancing IL-10 production/signaling might be beneficial for uveitis management. Indeed, Rosenbaum and Angell reported that IL-10 effectively reduced EIU in rats and mice.[28] Rizzo et al. showed that IL-10 treatment after induction of experimental autoimmune uveoretinitis ameliorated subsequent CSs and downregulated antigen-specific production of TNF-α and IFN-γ.[29] Using a different model, experimental autoimmune anterior uveitis, Fang et al. demonstrated that systemic adenovirus-mediated IL-10 gene therapy significantly improved immune-mediated ocular inflammation.[30]
Conclusion
Using the EIU rat model, we have demonstrated that blocking TNF-α signaling using an anti-TNF-α mAb delayed EIU development and attenuated disease manifestations. Based on our observation, we propose that a combined biological therapy targeting TNF-α as well as other cytokines (e.g., IL-6, IL-10) would be a valuable approach to uveitis. Future studies are needed to comprehensively characterize the kinetics of other important cytokines involved in uveitis pathogenesis and their changes on anti-TNF-α therapy, which will provide a better understanding of the complex cytokine network responses in the disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Yang P, Ren Y, Li B, Fang W, Meng Q, Kijlstra A. Clinical characteristics of Vogt-Koyanagi-Harada syndrome in Chinese patients. Ophthalmology. 2007;114:606–14. doi: 10.1016/j.ophtha.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Wakefield D, Chang JH. Epidemiology of uveitis. Int Ophthalmol Clin. 2005;45:1–13. doi: 10.1097/01.iio.0000155938.83083.94. [DOI] [PubMed] [Google Scholar]
- 4.Yang P, Fang W, Meng Q, Ren Y, Xing L, Kijlstra A. Clinical features of chinese patients with Behcet's disease. Ophthalmology. 2008;115:312–8.e4. doi: 10.1016/j.ophtha.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 5.Perez VL, Caspi RR. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015;36:354–63. doi: 10.1016/j.it.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ooi KG, Galatowicz G, Calder VL, Lightman SL. Cytokines and chemokines in uveitis: Is there a correlation with clinical phenotype? Clin Med Res. 2006;4:294–309. doi: 10.3121/cmr.4.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jap A, Chee SP. Immunosuppressive therapy for ocular diseases. Curr Opin Ophthalmol. 2008;19:535–40. doi: 10.1097/ICU.0b013e3283126d20. [DOI] [PubMed] [Google Scholar]
- 8.Yang PZ, Vos AF, Broersma L, Kijlstra A. Studies on tissue wholemounts and tissue sections of endotoxin induced uveitis in lewis rats. Chin J Ocul Fundus Dis. 1996;12:33–6. [Google Scholar]
- 9.Valentincic NV, de Groot-Mijnes JD, Kraut A, Korosec P, Hawlina M, Rothova A. Intraocular and serum cytokine profiles in patients with intermediate uveitis. Mol Vis. 2011;17:2003–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimura T, Sonoda KH, Ohguro N, Ohsugi Y, Ishibashi T, Cua DJ, et al. Involvement of Th17 cells and the effect of anti-IL-6 therapy in autoimmune uveitis. Rheumatology (Oxford) 2009;48:347–54. doi: 10.1093/rheumatology/ken489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghasemi H, Ghazanfari T, Yaraee R, Owlia P, Hassan ZM, Faghihzadeh S. Roles of IL-10 in ocular inflammations: A review. Ocul Immunol Inflamm. 2012;20:406–18. doi: 10.3109/09273948.2012.723109. [DOI] [PubMed] [Google Scholar]
- 12.Uchiyama E, Papaliodis GN, Lobo AM, Sobrin L. Side-effects of anti-inflammatory therapy in uveitis. Semin Ophthalmol. 2014;29:456–67. doi: 10.3109/08820538.2014.959203. [DOI] [PubMed] [Google Scholar]
- 13.Barry RJ, Nguyen QD, Lee RW, Murray PI, Denniston AK. Pharmacotherapy for uveitis: Current management and emerging therapy. Clin Ophthalmol. 2014;8:1891–911. doi: 10.2147/OPTH.S47778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cordero-Coma M, Yilmaz T, Onal S. Systematic review of anti-tumor necrosis factor-alpha therapy for treatment of immune-mediated uveitis. Ocul Immunol Inflamm. 2013;21:19–27. doi: 10.3109/09273948.2012.723107. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–3. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- 16.Chang JH, McCluskey PJ, Wakefield D. Recent advances in Toll-like receptors and anterior uveitis. Clin Exp Ophthalmol. 2012;40:821–8. doi: 10.1111/j.1442-9071.2012.02797.x. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum JT, Woods A, Kezic J, Planck SR, Rosenzweig HL. Contrasting ocular effects of local versus systemic endotoxin. Invest Ophthalmol Vis Sci. 2011;52:6472–7. doi: 10.1167/iovs.11-7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoekzema R, Murray PI, van Haren MA, Helle M, Kijlstra A. Analysis of interleukin-6 in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1991;32:88–95. [PubMed] [Google Scholar]
- 19.Dick AD, Cheng YF, McKinnon A, Liversidge J, Forrester JV. Nasal administration of retinal antigens suppresses the inflammatory response in experimental allergic uveoretinitis. A preliminary report of intranasal induction of tolerance with retinal antigens. Br J Ophthalmol. 1993;77:171–5. doi: 10.1136/bjo.77.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mo JS, Matsukawa A, Ohkawara S, Yoshinaga M. Involvement of TNF alpha, IL-1 beta and IL-1 receptor antagonist in LPS-induced rabbit uveitis. Exp Eye Res. 1998;66:547–57. doi: 10.1006/exer.1997.0451. [DOI] [PubMed] [Google Scholar]
- 21.de Vos AF, van Haren MA, Verhagen C, Hoekzema R, Kijlstra A. Kinetics of intraocular tumor necrosis factor and interleukin-6 in endotoxin-induced uveitis in the rat. Invest Ophthalmol Vis Sci. 1994;35:1100–6. [PubMed] [Google Scholar]
- 22.Planck SR, Huang XN, Robertson JE, Rosenbaum JT. Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Invest Ophthalmol Vis Sci. 1994;35:924–30. [PubMed] [Google Scholar]
- 23.Johnsen-Soriano S, Sancho-Tello M, Arnal E, Díaz-Llopis M, Navea A, Miranda M, et al. Comparison of the acute effects of anti-TNF-alpha drugs on a uveitis experimental model. Ocul Immunol Inflamm. 2010;18:208–15. doi: 10.3109/09273940903521964. [DOI] [PubMed] [Google Scholar]
- 24.Koizumi K, Poulaki V, Doehmen S, Welsandt G, Radetzky S, Lappas A, et al. Contribution of TNF-alpha to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003;44:2184–91. doi: 10.1167/iovs.02-0589. [DOI] [PubMed] [Google Scholar]
- 25.Avunduk MC, Avunduk AM, Oztekin E, Baltaci AK, Ozyazgan Y, Mogolkoc R. Etanercept treatment in the endotoxin-induced uveitis of rats. Exp Eye Res. 2004;79:357–65. doi: 10.1016/j.exer.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Kasner L, Chan CC, Whitcup SM, Gery I. The paradoxical effect of tumor necrosis factor alpha (TNF-alpha) in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1993;34:2911–7. [PubMed] [Google Scholar]
- 27.De Vos AF, Van Haren MA, Verhagen C, Hoekzema R, Kijlstra A. Systemic anti-tumor necrosis factor antibody treatment exacerbates endotoxin-induced uveitis in the rat. Exp Eye Res. 1995;61:667–75. doi: 10.1016/s0014-4835(05)80017-x. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbaum JT, Angell E. Paradoxical effects of IL-10 in endotoxin-induced uveitis. J Immunol. 1995;155:4090–4. [PubMed] [Google Scholar]
- 29.Rizzo LV, Xu H, Chan CC, Wiggert B, Caspi RR. IL-10 has a protective role in experimental autoimmune uveoretinitis. Int Immunol. 1998;10:807–14. doi: 10.1093/intimm/10.6.807. [DOI] [PubMed] [Google Scholar]
- 30.Fang IM, Lin CP, Yang CH, Chiang BL, Yang CM, Chau LY, et al. Inhibition of experimental autoimmune anterior uveitis by adenovirus-mediated transfer of the interleukin-10 gene. J Ocul Pharmacol Ther. 2005;21:420–8. doi: 10.1089/jop.2005.21.420. [DOI] [PubMed] [Google Scholar]


