Abstract
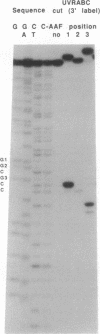
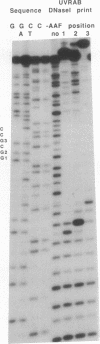
DNA fragments were constructed in which acetylaminofluorene adducts were introduced specifically at each one of the three different guanines of the 6-base-pair sequence -GGCGCC-. Incubation of the DNA with the UvrABC excision nuclease in vitro revealed major differences in the efficiency of adduct excision depending on the site of modification. Oligonucleotide excision of adducts bound to the second guanine was only 15% as efficient as excision of adducts at the first guanine, whereas the excision efficiency for adducts bound to the third guanine was intermediary. However, recognition of DNA damage appeared to occur with nearly 100% efficiency at all three adduct positions, as judged from DNase I footprint analysis of the DNA/protein binding complexes. Hence, it appears that the structural elements for DNA damage recognition by the UvrABC enzyme are different from the signals for excision. Furthermore, the repair pattern observed is not inversely correlated with the potential of these adducts to induce mutations since mutation analysis of single-adduct DNA has shown that only adducts at the third guanine are strongly premutagenic. We conclude that the effectiveness of excision repair depends on the context of the DNA sequence and that ineffectively repaired adduct sites are not necessarily mutational hot spots.
Full text
PDF
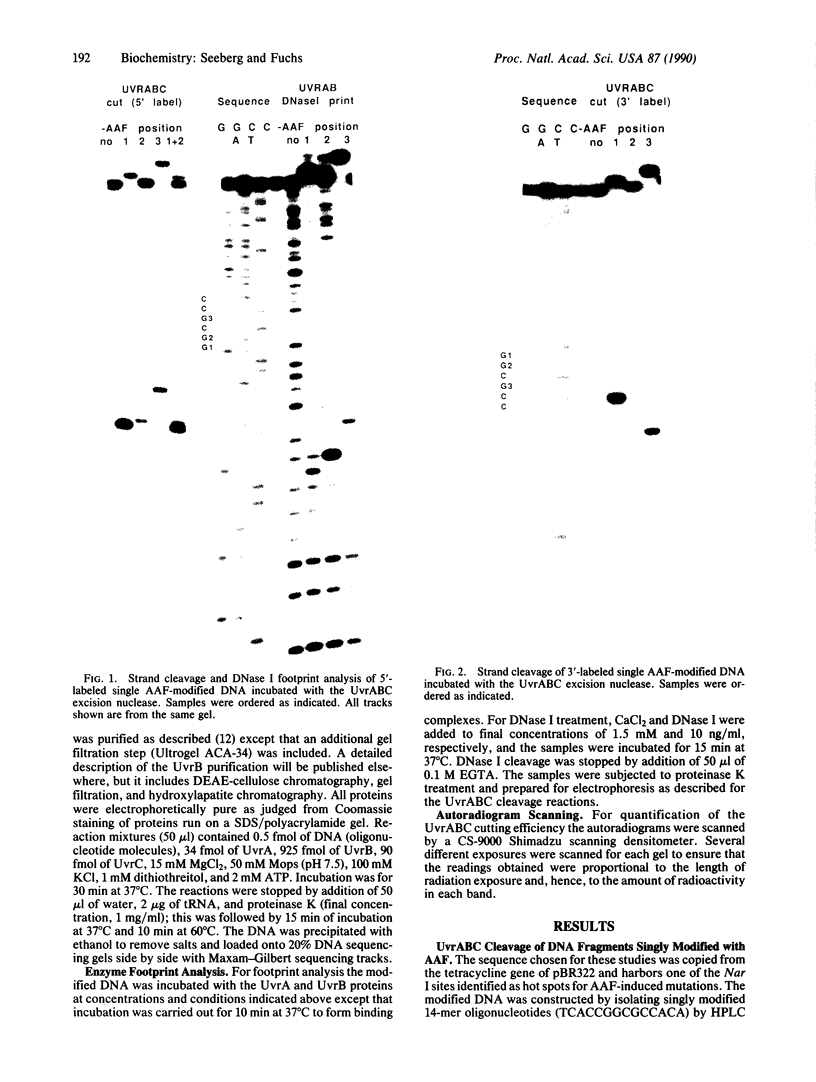
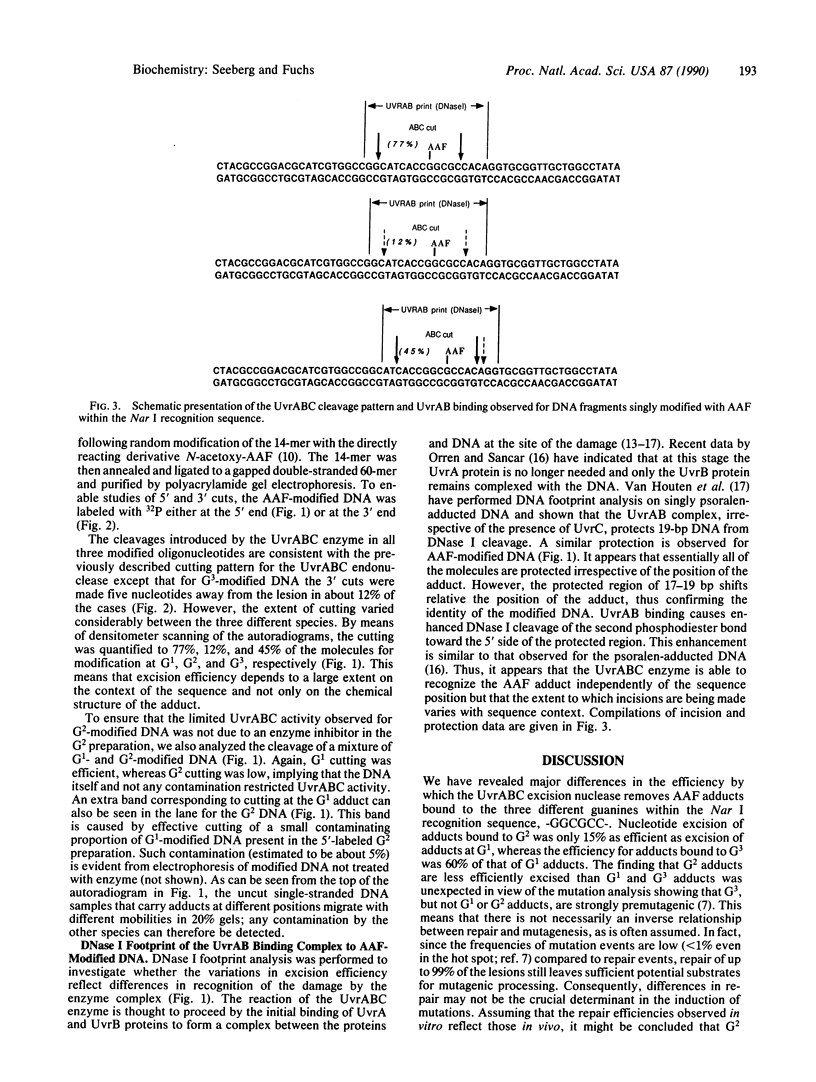

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnouf D., Koehl P., Fuchs R. P. Single adduct mutagenesis: strong effect of the position of a single acetylaminofluorene adduct within a mutation hot spot. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4147–4151. doi: 10.1073/pnas.86.11.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin W. A., Haseltine W. A. Removal of UV light-induced pyrimidine-pyrimidone(6-4) products from Escherichia coli DNA requires the uvrA, uvrB, and urvC gene products. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3821–3824. doi: 10.1073/pnas.81.12.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. P. DNA binding spectrum of the carcinogen N-acetoxy-N-2-acetylaminofluorene significantly differs from the mutation spectrum. J Mol Biol. 1984 Jul 25;177(1):173–180. doi: 10.1016/0022-2836(84)90063-9. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Seeberg E. pBR322 plasmid DNA modified with 2-acetylaminofluorene derivatives: transforming activity and in vitro strand cleavage by the Escherichia coli uvrABC endonuclease. EMBO J. 1984 Apr;3(4):757–760. doi: 10.1002/j.1460-2075.1984.tb01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. K., Yeung A. T. Repair of 4,5',8-trimethylpsoralen monoadducts and cross-links by the Escherichia coli UvrABC endonuclease. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8410–8414. doi: 10.1073/pnas.85.22.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacinski B. M., Rupp W. D. E. coli uvrB protein binds to DNA in the presence of uvrA protein. Nature. 1981 Dec 3;294(5840):480–481. doi: 10.1038/294480a0. [DOI] [PubMed] [Google Scholar]
- Koehl P., Burnouf D., Fuchs R. P. Construction of plasmids containing a unique acetylaminofluorene adduct located within a mutation hot spot. A new probe for frameshift mutagenesis. J Mol Biol. 1989 May 20;207(2):355–364. doi: 10.1016/0022-2836(89)90259-3. [DOI] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Verdier J. M., Bichara M., Freund A. M., Daune M. P., Fuchs R. P. Carcinogen-induced mutation spectrum in wild-type, uvrA and umuC strains of Escherichia coli. Strain specificity and mutation-prone sequences. J Mol Biol. 1984 Jul 25;177(1):33–51. doi: 10.1016/0022-2836(84)90056-1. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. A. Carcinogenesis by chemicals: an overview--G. H. A. Clowes memorial lecture. Cancer Res. 1970 Mar;30(3):559–576. [PubMed] [Google Scholar]
- Orren D. K., Sancar A. The (A)BC excinuclease of Escherichia coli has only the UvrB and UvrC subunits in the incision complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5237–5241. doi: 10.1073/pnas.86.14.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Franklin K. A., Sancar G., Tang M. S. Repair of psoralen and acetylaminofluorene DNA adducts by ABC excinuclease. J Mol Biol. 1985 Aug 20;184(4):725–734. doi: 10.1016/0022-2836(85)90316-x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Seeberg E. Reconstitution of an Escherichia coli repair endonuclease activity from the separated uvrA+ and uvrB+/uvrC+ gene products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2569–2573. doi: 10.1073/pnas.75.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L., Blingsmo O. R. Two separable protein species which both restore uvrABC endonuclease activity in extracts from uvrC mutated cells. Biochimie. 1982 Aug-Sep;64(8-9):825–828. doi: 10.1016/s0300-9084(82)80137-5. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L., Nordenskjöld M., Söderhäll S., Jernström B. Strand-break formation in DNA modified by benzo[alpha]pyrene diolepoxide. Quantitative cleavage by Escherichia coli uvrABC endonuclease. Mutat Res. 1983 Jun;112(3):139–145. doi: 10.1016/0167-8817(83)90036-6. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L. Purification and properties of the uvrA protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):988–992. doi: 10.1073/pnas.79.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E. Strand cleavage at psoralen adducts and pyrimidine dimers in DNA caused by interaction between semi-purified uvr+ gene products from Escherichia coli. Mutat Res. 1981 Jun;82(1):11–22. doi: 10.1016/0027-5107(81)90133-0. [DOI] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Sancar A., Hearst J. E. DNase I footprint of ABC excinuclease. J Biol Chem. 1987 Sep 25;262(27):13180–13187. [PubMed] [Google Scholar]
- Voigt J. M., Van Houten B., Sancar A., Topal M. D. Repair of O6-methylguanine by ABC excinuclease of Escherichia coli in vitro. J Biol Chem. 1989 Mar 25;264(9):5172–5176. [PubMed] [Google Scholar]
- Westhof E., Altschuh D., Moras D., Bloomer A. C., Mondragon A., Klug A., Van Regenmortel M. H. Correlation between segmental mobility and the location of antigenic determinants in proteins. Nature. 1984 Sep 13;311(5982):123–126. doi: 10.1038/311123a0. [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Grossman L. Protein complexes formed during the incision reaction catalyzed by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1986 Mar 25;14(6):2567–2582. doi: 10.1093/nar/14.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Grossman L. Enzymatic properties of purified Escherichia coli uvrABC proteins. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6157–6161. doi: 10.1073/pnas.80.20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]