Abstract
Background
Youth-onset type 2 diabetes (T2D) is a disease that is newly emerging and behavioral strategies for its prevention are limited. Interventions that target the lifestyle behaviors of adolescents, to improve poor dietary quality and reduce excessive sedentariness, promise to reduce the risk of developing T2D. Health coaching is effective for promoting healthy behaviors in patients who have chronic disease, but few experimental studies are in adolescents. This randomized controlled trial, in adolescents with prediabetes, will determine the effectiveness of a health coaching intervention to facilitate adoption of healthy diet and activity behaviors that delay or prevent development of T2D.
Methods/design
The Dietary Intervention for Glucose Tolerance In Teens (DIG IT) trial will involve an evaluation of a health coaching intervention in adolescents with prediabetes. Eligible participants will be randomized to receive 6 months of health coaching or a single dietary consultation that is standard-of-care. The primary outcome will be 2-hour oral glucose tolerance test concentration. Secondary outcomes will include measures of glycemia and insulin action as well as dietary, physical activity and sedentary behaviors measured using an electronic food record, and by inclinometer. Data will be collected before and after the intervention (at 6 months) and at 12 months (to assess sustainability).
Discussion
This trial will determine whether a health coaching intervention, a personalized and low-cost approach to modify dietary and activity behaviors, is effective and sustainable for prevention of youth-onset T2D, relative to standard-of-care. Health coaching has the potential to be widely implemented in clinical or community settings.
1. Background
Youth-onset type 2 diabetes (T2D) is an emerging condition linked with pediatric obesity. The prevalence has increased approximately 30% in the United States (U.S.) between 2001 and 2009 [1]. Prediabetes is the precursor of T2D and recent studies have estimated its prevalence in adolescents to be 16.1% [2]. Once an adolescent has developed T2D, the prognosis is worrisome because of poor glycemic control, even with the use of conventional medications, leading to early progression of chronic complications and lower life expectancy [3–5]. The problem of youth-onset T2D is especially challenging since obesity is difficult to treat in children [6].
The Diabetes Prevention Program (DPP) was a landmark behavior modification intervention which was conducted in adults with prediabetes [7]. The DPP and other major clinical trials demonstrated the superiority of lifestyle intervention, which promoted weight loss, over medication, for preventing T2D [7–9]. Following the success of the DPP, efforts were underway to translate the program from academic to clinical and community settings [10]. The Bright Bodies study, conducted in youth with prediabetes, demonstrated that behavioral modification was effective in reducing glycemia [11]. However, the study was conducted in an academic setting, required on-site participation, and presumably, was labor intensive. Given the high prevalence of prediabetes in youth [2], who are therefore at risk for developing T2D, there is a critical need for a low-cost, home-based prevention program that can be individualized and easily integrated into families.
Health coaching is a personalized approach that is being widely used to improve health behaviors in patients with chronic conditions including prediabetes [12,13]. In adolescents, health coaching is a promising strategy that promotes healthy behaviors using validated techniques like motivational interviewing [14,15]. Although data from randomized clinical trials in adolescents are limited [16–18], a health coaching behavior modification program may be an effective, low-cost, personalized and flexible program that can be implemented through clinical or community settings.
A growing body of literature, from longitudinal studies, suggests that diet [19–29], physical activity [30,31], and sedentary behavior [32] may modify the risk of development of T2D, independent of effects on adiposity. Adolescence is associated with low consumption of fruits, vegetables and whole grains, excessive consumption of refined grains, saturated fats and added sugars, and high amounts of time spent in sedentary behavior [33–35]. In adolescents, we [36] and others [37–39] have shown that high dietary intakes of fat as well as sugary foods and beverages are negatively associated with elevated glycemia and insulin resistance. Also, high sedentariness negatively influenced insulin secretion and sensitivity in youth [40]. It is reasonable therefore that a diabetes prevention program that targets specific diet and activity behaviors which are associated with T2D may be effective for management of glycemia.
We have proposed herein an intervention to be conducted in adolescents with prediabetes that aims to improve glycemia through modification of specific diet and activity behaviors, which reflect guidelines from the American Diabetes Association, American Academy of Pediatrics, American Heart Association and U.S. Department of Health and Human Services and Department of Agriculture [41–44]. The objective of this trial is to compare the effectiveness of a 6-month, home-based, individualized health coaching program, which focuses on modification of diet, physical activity, and sedentary behavior, versus a single dietary consultation, in preventing T2D in adolescents who are overweight or obese and have prediabetes. Health coaches will facilitate behavior change regarding consumption of specific foods and time spent in physical activity/inactivity using techniques such as motivational interviewing and goal setting that have been shown to increase self-efficacy [14,15] (see conceptual framework Fig. 1). We hypothesize that compared to receiving a single dietary consult, which is the usual care [45], patients who receive a one-on-one health coaching program will exhibit reduced glycemia as measured by 2 h oral glucose tolerance test (OGTT) at the end of the 6-month intervention, and that this impact will be sustained for an additional 6 months. We expect that increased consumption of specific nutrients and foods (dietary fiber, proteins, whole grains and fruits and vegetables), and decreased consumption of foods and beverages that are high in saturated fat and in added sugars, will modulate improvements which are observed in insulin sensitivity and beta-cell function. The research will also explore the contribution of biological, social, and behavioral factors as predictors or covariates of gluco-regulation including anthropometric measures of total and regional adiposity, measures of sleep behavior, depression, and food insecurity, as well as readiness and self-efficacy for behavior change. Perceptions of the health and behavior of the adolescent participants, by their parents/guardians, will also be obtained. This manuscript describes the study design and methodology for recruitment, data collection and data analysis that will test the stated hypothesis.
Fig. 1.
Conceptual framework of study.
2. Methods/design
2.1. Recruitment
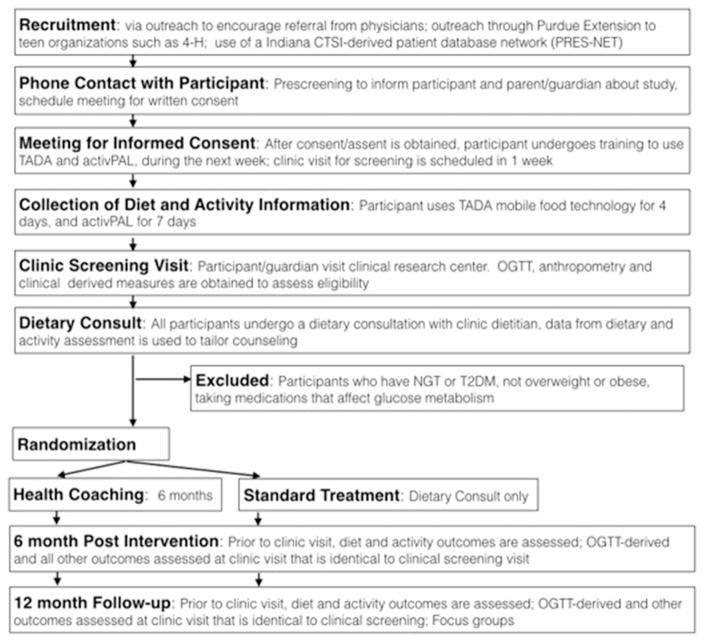
The main activities of the study are presented in a flow diagram (Fig. 2). Boys and girls who are between the ages of 10–21 years and are overweight or obese (body mass index > 85th percentile for age and sex) will be recruited into the study. In Indiana, the most recent estimates suggest 15.9% of adolescents are overweight and 12.8% are obese, which together make up one-third of the adolescent population [46]. Various outreach strategies will be used to inform participants/families and health care providers as well as the general community about the study. Participants who are recruited through primary care clinics will be considered to be recruited by clinic referral. Physicians at pediatric primary care practices in the Central Indiana region have been made aware of the study through in-service presentations by the study investigators at Indiana University School of Medicine and at Purdue University. In addition, we will recruit subjects from Riley Youth Diabetes Prevention Clinic (YDPC), Adolescent Medicine Diabetes Clinic, and HealthNet Pediatric and Adolescent Care Center with recruitment assistance by the Indiana Pediatric Research Network (PRES-NET, https://www.indianactsi.org/programs/researchnetworks/presnet). Participants who are recruited from community-outreach efforts will be considered to be recruited via self-referral. Community outreach strategies include sending mass emails and flyers to targeted audiences, either through Purdue Extension, or to employees of Purdue University. In addition, information about the study can be found at ClinicalTrials. gov, registration number NCT02535169.
Fig. 2.
2.2. Informed consent procedure
Potential participants and their parents/guardians will meet the study team at a public venue to obtain informed consent/assent. During this time, participants and families will be provided with a detailed description of the study including responsibilities, as well as risks, and benefits of participation. Procedures for obtaining consent have been reviewed and approved by the Indiana University and Purdue University Institutional Review Boards (IRB Study #: 1403986016). After reviewing the materials, and signing the consent forms, adolescents will undertake further screening for eligibility to participate in the study. During the screening process, participants will undergo a detailed assessment of diet and activity behavior to be followed by a clinical assessment of glycemic status and insulin action. Diet and activity behavior will be assessed with electronic technology using validated methods that are enabled for use in free living individuals.
2.3. Dietary assessment
Dietary information will be collected for four days, including at least one weekend day, using the Technology Assisted Dietary Assessment (TADA) mobile food record [47]. The TADA application enables individuals to create a mobile food record by capturing food and beverage images “before” and “after” eating occasions using the camera on an iPod® [47]. The TADA application has been validated in the adolescent population [47,48]. Graduate assistants, who are trained in techniques of dietary assessment at the Dietary Assessment Center at Purdue, will be involved in the data collection for dietary assessment. Their responsibilities will be to 1) review all images with study participants to verify quantities and to obtain missing information; and to 2) to create a food record by evaluating each before and after image, and determining the food types and portion sizes of each food item, using a fiducial marker as a reference. If the fiducial marker is absent, other reference markers such as standard plates or utensils will be used. The quantities consumed of foods and beverages which are identified from the images will be evaluated using the Nutrition Data System for Research (NDSR, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). Reported daily intake is calculated as a mean of the four days. Levels of intake of nutrients will be compared to those recommended for adolescent girls and boys, in the Dietary Guidelines for Americans reports [49,50]. Completeness of dietary records will be evaluated by determining energy requirements and calculating the percentage of calories reported versus estimated energy requirements. Energy requirements were estimated using equations for overweight children and adolescents who are in a weight maintenance situation [51]. Underreporting of energy intake will be indicated if energy intake is less than 1 standard deviation below predicted energy requirements [50,52].
2.4. Assessment of physical activity and inactivity
Time spent in seated, standing, and ambulatory physical activity will be assessed over 7 days using an activPAL™monitor (PAL Technologies, Glasgow, United Kingdom), a device which utilizes accelerometer and inclinometer functions [53]. activPAL™ is considered the reference method for estimating sedentary time in free-living individuals [54]. The inclinometer function is used to detect posture and thus can distinguish between sitting and standing. The device is highly objective and more accurate than traditional methods such as self-report and survey, and has been validated in the adolescent population [53,55]. The instrument also has the capability to measure various tempos of physical activity by generating stepping cadence. Data from the activPAL™ will be categorized into six variables including four activity intensity levels (light physical activity [LPA], moderate physical activity [MPA], vigorous physical activity [VPA] and moderate-to-vigorous physical activity [MVPA], plus total steps taken and total stepping time. Children will be classified as sedentary if they obtain less than 30 min of MPA; low active if they obtain 30 to 60 min of MPA; and active if they obtain greater than 60 min of MPA [51].
2.5. Clinic screening visit
Immediately following the week of collection of dietary and activity information, the study volunteer and their parent/guardian will attend a clinical research center (Clinical Research Centers at Indiana University Hospital or Riley Hospital for Children) for assessment of clinical and anthropometrical measures, which are used to determine their eligibility for the study. The participant will attend the screening visit in the morning after an 8-hour overnight fast. The clinical and anthropometric measures will include blood pressure, and indices of general and abdominal obesity (height, weight, body mass index [BMI], waist circumference [WC], hip circumference, and sagittal abdominal diameter [SAD]) [56]. BMI percentiles and Z-scores will be calculated from age-and sex-specific reference values as defined by the Centers for Disease Control and Prevention (CDC) growth charts [57]. Blood pressure will be measured after 5 min of rest, on the right arm in the supine position, using a sphygmomanometer with an appropriately sized cuff [58]. An intravenous line will be placed and blood is drawn for biochemistries, including concentrations of blood glucose, hemoglobin A1c, liver enzymes (alanine amino transferase and aspartate amino transferase), as well as a comprehensive lipid profile, and a metabolic panel. Concentrations of plasma lipids (total cholesterol, triglycerides [TG], high-density lipoprotein [HDL]), will be measured in the Indiana University Core Laboratory, using Alere Cholestech LDX System (Alere, Orlando, FL). Low-density lipoprotein (LDL) cholesterol will be calculated using the Friedewald equation [59].
Glycemic status (categorized as having normal glucose tolerance [NGT], prediabetes or T2D based on American Diabetes Association criteria) [60] will be assessed using an OGTT. Blood samples will be obtained at −15, 0, 15, 30, 60, 90, 120 min relative to ingestion of a glucose drink in a dose of 1.75 g/kg body weight (maximum 75 g glucose). Concentrations of blood glucose and HbA1c will be measured using the i-STAT System (Abbott Point of Care, Princeton, NJ) and DCA Vantage Analyzer (Siemens Medical Solutions USA, Inc., Malvern, PA) instrumentation respectively, which enable point-of-care testing. For measures of insulin action, serum fractions from the OGTT will be frozen at −80 °C until analysis. Concentrations of glucose will be determined using an automated chemistry analyzer (COBAS Integra 800, Roche Diagnostics, Indianapolis, IN); insulin, and c-peptide will be assessed using an Elecsys Systems immunoassay analyzer (Roche Diagnostics). The homeostasis model assessment of insulin resistance (HOMA-IR) will be calculated from glucose and insulin concentrations in the fasting state [61]. Whole-body insulin sensitivity index (WBISI), the insulinogenic index (IGI), and the oral disposition index (DI) will be calculated as described [62,63].
2.6. Eligibility criteria
Participants who meet eligibility criteria, by having a diagnosis of prediabetes, will be enrolled into the study intervention and randomly assigned into the single dietary consult group or the health coaching intervention. Prediabetes will be defined by having one or more of the criteria recommended by the American Diabetes Association, namely: 1) impaired fasting plasma glucose (IFG) concentration of 5.6 to 6.9 mmol/L; 2) impaired glucose tolerance (IGT) a 2 hour OGTT glucose concentration of 7.8 to 11.0 mmol/L, or 3) an elevated hemoglobin A1c concentration of 5.7 to 6.4% [60]. Exclusion criteria include pregnancy, use of medications that affect glucose metabolism (such as glucocorticoid-containing medications or atypical antipsychotics), and syndromic obesity (such as Prader Willi, hypothalamic obesity, or Laurence-Moon-Biedl). Participants who are characterized as NGT or T2D will not be eligible to enroll in the study and complete the study at this point. At the end of the screening clinic visit, all participants, regardless of glycemic status, will undergo a consultation with a clinic dietitian regarding diet and lifestyle behaviors.
2.7. Randomization
Randomization will be carried out at subject level, by using the random number generator built in the Research Electronic Data Capture software (REDCap, Vanderbilt University, Nashville, TN). Specifically, participants will be randomly assigned to the Health Coaching or the Single Dietary Consult groups. A randomization table that pre-defines how each subject will be randomized as they are added to the study will be generated by a statistician and uploaded to the REDCap database. Neither the participants and their families nor the study team will be blinded to treatment allocation.
2.8. Description of health coaching intervention and single dietary consult groups
2.8.1. Single dietary consult
For participants who are enrolled in the single dietary consult group, a dietary consultation at the end of the screening visit serves as the standard of care intervention. The clinic dietitian will provide to the participant and their parental guardian, evidence-based, individualized nutrition and physical activity information regarding expert guidelines for prevention of obesity and diabetes (U.S. Dietary Guidelines, American Heart Association, American Diabetes Association, and American Academy of Pediatrics) [41–43,49]. Evidence-based nutrition messages will include: reducing consumption of sugary sweetened beverages and foods, increasing fruit and vegetable consumption, practicing portion control, reducing the frequency of eating out, and choosing healthy snacks. Evidence-based physical activity messages will include: being physically active for 60 min daily including cardiovascular, weight bearing, and flexibility exercises, and reducing screen time to 120 min or less per day. Other than the single dietary consult, during the intervention, participants in this group will not receive any additional consultation from the study dietitian; for further guidance they will be advised about provider services in their region.
2.8.2. Health coaching intervention
In addition to receiving a single dietary consultation, participants who are randomized to the health coaching intervention will receive assistance from a health coach for 6 months to modify diet and activity behavior. Health coaches will have undergraduate training from nutrition and kinesiology programs and will be highly knowledgeable about clinical practice guidelines regarding diet and physical activity strategies for obesity and diabetes management. The protocol for the health coaching intervention builds on the DPP framework [64], but will be adapted to be delivered via one-on-one counseling with a health coach and tailored to fit each participant’s needs. Thus the health coaching program is designed to be a patient-centered approach, focused on assisting participants to adopt healthy nutrition and physical activity behaviors, through motivational-interviewing and other engagement techniques. Health coaches will also tailor the delivery of information and the setting of goals to the participant’s readiness for change of specific diet and activity behaviors.
The program consists of weekly phone calls and monthly in person or “face” contacts over the course of six months. Participants will meet weekly by phone with the health coach at scheduled appointment times. The health coach will offer availability during the evening to best accommodate appointment times that are convenient for participants. Communication may be delivered by email if preferred.
2.9. Follow-up clinic visits at 6 and 12 months
At 6 months following randomization to the groups, participants will undergo a follow-up assessment. As before, during the week prior to a clinic visit, dietary and activity behaviors will be assessed under free-living conditions, using the TADA and activPAL™ tools. Participants will then return to the clinic for assessment of all other outcomes including status of glycemia. To determine the durability of the study intervention, all enrolled participants will undergo a similar assessment of diet, activity and glycemic measures at 12 months following the random assignment to groups. After this assessment, participants will have completed the study. At this time, focus groups will be conducted with participants and their parents/guardians to obtain feedback about their experience, and the discussions will also include quantitative and qualitative measures of patient satisfaction [65].
2.10. Outcomes
Outcomes will to be obtained at baseline and at 6 and 12 months following random assignment into the health coaching or dietary consult groups. A listing of all study outcomes and the timing of collection is provided in Table 1.
Table 1.
Description of outcome measures.
| Measures | Detail of measures obtained | Instrument |
|---|---|---|
| To be collected at screening/baseline, end of intervention (6 month) and follow-up (12 month) | ||
| Primary outcomes | ||
| Glycemia | Glucose at 2 h OGTT | COBAS Integra 800 |
| Secondary outcomes | ||
| Glycemia | Fasting glucose, HbA1c | Elecsys Systems |
| Insulin action | WBISI, IGI, DI HOMA |
Elecsys Systems |
| Dietary intake | Nutrients (saturated fat, fiber); foods and food groups | TADA |
| Activity behavior | LPA, MPA, VPA, MVPA, total steps, total stepping time | activPAL™ |
| Sedentary behavior | Time spent sitting/lying | activPAL™ |
| Anthropometry | Height, weight, BMI, waist and hip circumference, SAD | Per NHANES protocols [56] |
| Metabolic risk profile | Plasma lipids (TC, TG, HDL, LDL), blood pressure (SBP, DBP) | Indiana University Core Laboratory and [58] |
| Sleep quality | Sleep duration, sleep debt, sleep disorders | Cleveland Adolescent Sleepiness Questionnaire [69]; Sleep Disturbances Scale [70] |
| Quality of life | Functioning (physical, emotional, social and school) | PedsQL – Child Self Report [71] |
| Depression | 9-Items of depressive signs and symptoms | Patient Health Questionnaire [72] |
| Disordered eating | Screening for anorexia or bulimia nervosa | SCOFF Questionnaire [73] |
| Stage of change for health behavior | Use of behavior strategies, self-efficacy | Behavioral Strategies Questionnaire [74] [75] |
| Perception of food insecurity | Food security status | Child Food Security Survey Module [76] |
| Measures obtained from parent/guardian | ||
| Parental perceptions of behavior | Perceived problems with weight-related behavior and self-efficacy | The parental perceptions of participant’s behavior: the lifestyle behavior checklist [77] |
| Stage of change for health behavior | Parent perception of use of behavioral strategies | Behavioral Strategies Questionnaire – for parents [74] [75] |
| Quality of life | Parent’s perception of child’s functioning (physical, emotional, social, and school) | PedsQL parent proxy-report [78] |
| Food security | Prevalence of food insecurity | U.S. Household Food Security Survey Module [76] |
| To be collected at 12 month follow-up | ||
| Focus groups | Participant/guardian satisfaction with the study | Quantitative and qualitative questions about satisfaction with study [65] |
Abbreviations: HbA1c, hemoglobin A1c; OGTT, oral glucose tolerance test; WBISI, whole body insulin sensitivity index; IGT, insulinogenic index; DI, disposition index; HOMA, homeostatic model assessment; TADA, Technology Assisted Dietary Assessment; LPA, light physical activity; MPA, moderate physical activity; VPA, vigorous physical activity; MVPA, moderate to vigorous physical activity; NHANES, National Health and Nutrition Examination Survey; TC, total cholesterol; TG, triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure.
2.11. Data analysis and sample size estimates
Data will be analyzed using linear mixed-effect models and controlled for a subject level effect. Adjusted means will be reported for the different treatments at the different time points (baseline, 6 month post intervention, and 12 month follow up). Confounding factors such as sex. Race/ethnicity and age, as well as dietary intakes of nutrients and physical activity known to influence glycaemia and insulin action dynamics, will be controlled for in the linear mixed models. Since other outcomes including sleep [66] and mood disturbances [67] may affect study outcomes, we may include these in the model. Continuous data will be tested for normality and if the data is skewed, log transformation may be used to normalize data or non-parametric statistics will be used. Differences between groups will be analyzed using Chi-squared for categorical data and independent t-tests for continuous data. Data will be expressed as mean ± standard error, and P values less than or equal to 0.05 will be considered to indicate significant differences.
A sample size of 35 per group will be needed to determine an effect size of 0.67 standard deviations as statistically significant at a two sided an alpha level of 0.05 and power of 0.8. This sample size will detect a difference of 0.17 mg/dL 0.94 mM in concentration of glucose at 2 h following the OGTT as statistically significant assuming a standard deviation of approximately 25 mg/dL (1.4 mM) [11]. To account for an attrition of 30%, this sample size will be increased to 46 per group.
3. Discussion
The DIG IT intervention aims to determine if health coaching can be used to prevent type 2 diabetes by improving self-efficacy of adolescents (and their families) regarding incorporation of healthy diet and activity behaviors. The prevalence of prediabetes in adolescents is high [2], fueled by the rising rates of obesity in this population [68]. In the context of the emerging epidemic of youth-onset T2D, an emphasis on strategies for prevention is warranted, especially since effective strategies for treatment are lacking. Lifestyle behaviors of adolescents are characteristically low in amount of physical activity, and excessively sedentary, high in dietary intake of refined grains, saturated fats and added sugars, and low in intake of fruits and vegetables. Independent of obesity, these lifestyle factors may promote the risk of developing T2D [36–40]. An intensive behavior modification program, demonstrated the efficacy of lifestyle intervention to improve nutrition and physical activity for reducing the risk of type 2 diabetes in adolescents, but it was conducted in the academic setting [11]. Such studies demonstrate efficacy but may be limited in ability to reach broad populations. Therefore there is a critical need for randomized controlled trials to translate diabetes prevention programs to the community or clinical setting.
Health coaches use a personalized approach that involves goal setting and motivational interviewing to assist individuals with behavior change. This approach may be successful in promoting the self-efficacy of adolescents, and their families, to adopt a healthy lifestyle. Potential advantages of using health coaches are 1) they are highly knowledgeable about evidence-based practices for lifestyle modification; 2) they can tailor recommendations to meet individual challenges and barriers; and 3) interactions between health coaches and participants can be convenient and inexpensive since they will be conducted through phone calls and email. The DIG IT protocol described will determine whether health coaching can be used to promote behavior change in adolescents in the free-living environment.
The impact of health coaching on diet and physical activity behaviors of the participants will be evaluated using objective measures that are non-invasive and well suited to the free-living environment. The TADA and activPAL instruments have been validated in adolescents to obtain highly-detailed information on diet and activity/inactivity. The measures will be used to assess participant’s adherence to the messages received from the intervention in terms of health coaching or single dietary consult. Thus we will be able to evaluate the fidelity of the treatment by directly assessing target behaviors. In addition, diabetes risk will be assessed using oral glucose tolerance testing which yields dynamic measures of insulin resistance and beta-cell function. We believe that the detail of information on diet, physical activity/inactivity as well as glucose and insulin action is a novel aspect of this project. Limitations of the protocol are that the neither the participants nor the study team will be blinded to treatment allocation, thus findings will be subjected to expectation bias. The data obtained may inform about direct influence of specific diet, activity as well as sedentary factors on markers of diabetes risk. This information will provide useful information about specific modifiable targets, besides weight loss, for preventing diabetes in adolescents who are at risk. Weight loss is particularly challenging to achieve in adolescents [6], therefore a low-cost, health coaching approach which enables adolescents to incorporate lifestyle behaviors that are anti-diabetogenic may have a positive and widespread impact on this emerging major public health issue.
Acknowledgments
We are grateful to the participants of the study. This publication was made possible, in part, with support from the Indiana Clinical and Translational Sciences Institute funded, in part by Project Development Teams (PDT) pilot grants (Grant #UL1TR001108) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Other support included funding from Riley Hospital for Children Indiana University Health, and the Center for Pediatric Obesity and Diabetes Prevention Research.
Footnotes
Competing interests
None of the authors of the manuscript have any competing interests.
References
- 1.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, Bell R, Badaru A, Talton JW, Crume T, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care. 2009;32(2):342–347. doi: 10.2337/dc08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci. 2015;1353(1):113–137. doi: 10.1111/nyas.12939. [DOI] [PubMed] [Google Scholar]
- 4.Today Study Group. Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, Cuttler L, Nathan DM, Tollefsen S, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with type 2 diabetes mellitus. Diabet Med. 2012;29(4):453–463. doi: 10.1111/j.1464-5491.2011.03542.x. [DOI] [PubMed] [Google Scholar]
- 6.Peirson L, Fitzpatrick-Lewis D, Morrison K, Warren R, Usman Ali M, Raina P. Treatment of overweight and obesity in children and youth: a systematic review and meta-analysis. CMAJ Open. 2015;3(1):E35–E46. doi: 10.9778/cmajo.20140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The-Diabetes-Prevention-Program-Research-Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 9.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 10.Ackermann RT, Liss DT, Finch EA, Schmidt KK, Hays LM, Marrero DG, Saha C. A randomized comparative effectiveness trial for preventing type 2 diabetes. Am J Public Health. 2015;105(11):2328–2334. doi: 10.2105/AJPH.2015.302641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savoye M, Caprio S, Dziura J, Camp A, Germain G, Summers C, Li F, Shaw M, Nowicka P, Kursawe R, et al. Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care. 2014;37(2):317–324. doi: 10.2337/dc13-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill B, Richardson B, Skouteris H. Do we know how to design effective health coaching interventions: a systematic review of the state of the literature. Am J Health Promot. 2015;29(5):e158–e168. doi: 10.4278/ajhp.130510-LIT-238. [DOI] [PubMed] [Google Scholar]
- 13.Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG, Stafford RS. Translating the diabetes prevention program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113–121. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Resnicow K, Davis R, Rollnick S. Motivational interviewing for pediatric obesity: conceptual issues and evidence review. J Am Diet Assoc. 2006;106(12):2024–2033. doi: 10.1016/j.jada.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Alm M, Soroudi N, Wylie-Rosett J, Isasi CR, Suchday S, Rieder J, Khan U. A qualitative assessment of barriers and facilitators to achieving behavior goals among obese inner-city adolescents in a weight management program. Diabetes Educ. 2008;34(2):277–284. doi: 10.1177/0145721708314182. [DOI] [PubMed] [Google Scholar]
- 16.Bean MK, Powell P, Quinoy A, Ingersoll K, Wickham EP, 3rd, Mazzeo SE. Motivational interviewing targeting diet and physical activity improves adherence to paediatric obesity treatment: results from the MI values randomized controlled trial. Pediatr Obes. 2015;10(2):118–125. doi: 10.1111/j.2047-6310.2014.226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saelens BE, Sallis JF, Wilfley DE, Patrick K, Cella JA, Buchta R. Behavioral weight control for overweight adolescents initiated in primary care. Obes Res. 2002;10(1):22–32. doi: 10.1038/oby.2002.4. [DOI] [PubMed] [Google Scholar]
- 18.Jefferson V, Jaser SS, Lindemann E, Galasso P, Beale A, Holl MG, Grey M. Coping skills training in a telephone health coaching program for youth at risk for type 2 diabetes. J Pediatr Health Care. 2011;25(3):153–161. doi: 10.1016/j.pedhc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig DS, Pereira MA, Kroenke CH, Hilner JE, Van Horn L, Slattery ML, Jacobs DR., Jr Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA. 1999;282(16):1539–1546. doi: 10.1001/jama.282.16.1539. [DOI] [PubMed] [Google Scholar]
- 20.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 21.Salmeron J, Hu FB, Manson JE, Stampfer MJ, Colditz GA, Rimm EB, Willett WC. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr. 2001;73(6):1019–1026. doi: 10.1093/ajcn/73.6.1019. [DOI] [PubMed] [Google Scholar]
- 22.Meyer KA, Kushi LH, Jacobs DR, Jr, Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care. 2001;24(9):1528–1535. doi: 10.2337/diacare.24.9.1528. [DOI] [PubMed] [Google Scholar]
- 23.Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;100(1):218–232. doi: 10.3945/ajcn.113.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livesey G, Taylor R, Livesey H, Liu S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am J Clin Nutr. 2013;97(3):584–596. doi: 10.3945/ajcn.112.041467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drehmer M, Pereira MA, Schmidt MI, Del Carmen BMM, Alvim S, Lotufo PA, Duncan BB. Associations of dairy intake with glycemia and insulinemia, independent of obesity, in Brazilian adults: the Brazilian longitudinal study of adult health (ELSA-Brasil) Am J Clin Nutr. 2015;101(4):775–782. doi: 10.3945/ajcn.114.102152. [DOI] [PubMed] [Google Scholar]
- 26.Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ. 2010;341:c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Fan Y, Zhang X, Hou W, Tang Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: meta-analysis of prospective cohort studies. BMJ Open. 2014;4(11):e005497. doi: 10.1136/bmjopen-2014-005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nettleton JA, Hivert MF, Lemaitre RN, McKeown NM, Mozaffarian D, Tanaka T, Wojczynski MK, Hruby A, Djousse L, Ngwa JS, et al. Meta-analysis investigating associations between healthy diet and fasting glucose and insulin levels and modification by loci associated with glucose homeostasis in data from 15 cohorts. Am J Epidemiol. 2013;177(2):103–115. doi: 10.1093/aje/kws297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominguez LJ, Bes-Rastrollo M, Basterra-Gortari FJ, Gea A, Barbagallo M, Martinez-Gonzalez MA. Association of a Dietary Score with incident type 2 diabetes: the dietary-based diabetes-risk score (DDS) PLoS One. 2015;10(11):e0141760. doi: 10.1371/journal.pone.0141760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015;30(7):529–542. doi: 10.1007/s10654-015-0056-z. [DOI] [PubMed] [Google Scholar]
- 31.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30(3):744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 32.Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123–132. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 33.Slining MM, Mathias KC, Popkin BM. Trends in food and beverage sources among US children and adolescents: 1989–20 10. J Acad Nutr Diet. 2013;113(12):1683–1694. doi: 10.1016/j.jand.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Increase the proportion of adolescents who meet current federal physical activity guidelines for aerobic physical activity and for muscle-strengthening activity. U.S.-Department-of-Health-and-Human-Services; Washington, DC: 2014. Healthy People 2020. PA-3. [Google Scholar]
- 35.Dietary Guidelines Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. United States Department of Agriculture, Government Printing Office; 2015. [Google Scholar]
- 36.Wagner KA, Armah SM, Smith LG, Pike J, Tu W, Campbell WW, Boushey CJ, Hannon TS, Gletsu-Miller N. Associations between diet behaviors and measures of glycemia, in clinical setting, in obese adolescents. Child Obes. 2016;12(5):341–347. doi: 10.1089/chi.2015.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prince RL, Kuk JL, Ambler KA, Dhaliwal J, Ball GD. Predictors of Metabolically Healthy Obesity in Children. Diabetes Care. 2014;37(5):1462–1468. doi: 10.2337/dc13-1697. [DOI] [PubMed] [Google Scholar]
- 38.Mollard RC, Senechal M, MacIntosh AC, Hay J, Wicklow BA, Wittmeier KD, Sellers EA, Dean HJ, Ryner L, Berard L, et al. Dietary determinants of hepatic steatosis and visceral adiposity in overweight and obese youth at risk of type 2 diabetes. Am J Clin Nutr. 2014;99(4):804–812. doi: 10.3945/ajcn.113.079277. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Light K, Henderson M, O’Loughlin J, Mathieu ME, Paradis G, Gray-Donald K. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J Nutr. 2014;144(1):81–86. doi: 10.3945/jn.113.182519. [DOI] [PubMed] [Google Scholar]
- 40.Sardinha LB, Andersen LB, Anderssen SA, Quiterio AL, Ornelas R, Froberg K, Riddoch CJ, Ekelund U. Objectively measured time spent sedentary is associated with insulin resistance independent of overall and central body fat in 9- to 10-year-old Portuguese children. Diabetes Care. 2008;31(3):569–575. doi: 10.2337/dc07-1286. [DOI] [PubMed] [Google Scholar]
- 41.Bantle JP, Wylie-Rosett J. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 42.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 43.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 44.U.S.-Department-of-Health-and-Human-Services-and-U.S.-Department-of-Agriculture; USDA/HHS, editor. Dietary guidelines for Americans, 2010. Washington, DC: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeitler P, Fu J, Tandon N, Nadeau K, Urakami T, Barrett T, Maahs D. ISPAD Clinical Practice Consensus Guidelines, Type 2 diabetes in the child and adolescent. Pediatr Diabetes. 2014;2014(15 Suppl 20):26–46. doi: 10.1111/pedi.12179. [DOI] [PubMed] [Google Scholar]
- 46.Centers For Disease Control and Prevention, Division of Nutrition, Physical Activity and Obesity; Department of Health and Human Services, U.S. Government Printing Office, editor. Pediatric Nutrition Surveillance System. 2010. [Google Scholar]
- 47.Six BL, Schap TE, Zhu FM, Mariappan A, Bosch M, Delp EJ, Ebert DS, Kerr DA, Boushey CJ. Evidence-based development of a mobile telephone food record. J Am Diet Assoc. 2010;110(1):74–79. doi: 10.1016/j.jada.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boushey CJ, Harray AJ, Kerr DA, Schap TE, Paterson S, Aflague T, Bosch Ruiz M, Ahmad Z, Delp EJ. How willing are adolescents to record their dietary intake? The mobile food record. JMIR Mhealth Uhealth. 2015;3(2):e47. doi: 10.2196/mhealth.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dietary Guidelines for Americans 2015–2020. 8. Department-of-Health-and-Human-Services-and-Department-of-Agriculture; Washington, DC: 2016. [Google Scholar]
- 50.US-Department-of-Health-and-Human-Services-and-US-Department-of-Agriculture. Dietary guidelines for Americans. 6. US Government Printing Office; Washington, DC: 2005. [Google Scholar]
- 51.Food and Nutrition Board Dietary Reference Intakes for Energy; Institute of Medicine, editor. Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, DC: 2005. [DOI] [PubMed] [Google Scholar]
- 52.Huang TT, Roberts SB, Howarth NC, McCrory MA. Effect of screening out implausible energy intake reports on relationships between diet and BMI. Obes Res. 2005;13(7):1205–1217. doi: 10.1038/oby.2005.143. [DOI] [PubMed] [Google Scholar]
- 53.Dowd KP, Harrington DM, Bourke AK, Nelson J, Donnelly AE. The measurement of sedentary patterns and behaviors using the activPAL professional physical activity monitor. Physiol Meas. 2012;33(11):1887–1899. doi: 10.1088/0967-3334/33/11/1887. [DOI] [PubMed] [Google Scholar]
- 54.Judice PB, Santos DA, Hamilton MT, Sardinha LB, Silva AM. Validity of GT3X and Actiheart to estimate sedentary time and breaks using ActivPAL as the reference in free-living conditions. Gait Posture. 2015;41(4):917–922. doi: 10.1016/j.gaitpost.2015.03.326. [DOI] [PubMed] [Google Scholar]
- 55.Dowd KP, Harrington DM, Hannigan A, Donnelly AE. Light intensity physical activity is associated with adiposity in adolescent females. Med Sci Sports Exerc. 2014 doi: 10.1249/MSS.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 56.National Health and Nutrition Examination Survery (NHANES) Anthropometry Procedures Manual. www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf.
- 57.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 58.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 59.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 60.Anonymous: (2) Classification and diagnosis of diabetes. Diabetes Care. 2014;38(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 61.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 62.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 63.Hannon TS, Rofey DL, Lee S, Arslanian SA. Depressive symptoms and metabolic markers of risk for type 2 diabetes in obese adolescents. Pediatr Diabetes. 2013;14(7):497–503. doi: 10.1111/pedi.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ackermann RT, Marrero DG. Adapting the diabetes prevention program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ. 2007;33:69–78. doi: 10.1177/0145721706297743. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen B, Shrewsbury VA, O’Connor J, Lau C, Steinbeck KS, Hill AJ, Baur LA. A process evaluation of an adolescent weight management intervention: findings and recommendations. Health Promot Int. 2015;30(2):201–212. doi: 10.1093/heapro/dau110. [DOI] [PubMed] [Google Scholar]
- 66.Rangaraj VR, Knutson KL. Association between sleep deficiency and cardiometabolic disease: implications for health disparities. Sleep Med. 2016;18:19–35. doi: 10.1016/j.sleep.2015.02.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Groot M, Crick KA, Long M, Saha C, Shubrook JH. Lifetime duration of depressive disorders in patients with T2D. Diabetes Care. 2016 doi: 10.2337/dc16-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spilsbury JC, Drotar D, Rosen CL, Redline S. The Cleveland adolescent sleepiness questionnaire: a new measure to assess excessive daytime sleepiness in adolescents. J Clin Sleep Med. 2007;3(6):603–612. [PMC free article] [PubMed] [Google Scholar]
- 70.Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, Giannotti F The Sleep Disturbance Scale for Children (SDSC) Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5(4):251–261. doi: 10.1111/j.1365-2869.1996.00251.x. [DOI] [PubMed] [Google Scholar]
- 71.Varni JW, Limbers CA, Burwinkle TM. How young can children reliably and validly self-report their health-related quality of life?: an analysis of 8,591 children across age subgroups with the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:1. doi: 10.1186/1477-7525-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kocalevent RD, Hinz A, Brahler E. Standardization of the depression screener patient health questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry. 2013;35(5):551–555. doi: 10.1016/j.genhosppsych.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 73.Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467–1468. doi: 10.1136/bmj.319.7223.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nothwehr F. Self-efficacy and its association with use of diet-related behavioral strategies and reported dietary intake. Health Educ Behav. 2008;35(5):698–706. doi: 10.1177/1090198106296771. [DOI] [PubMed] [Google Scholar]
- 75.Berg CA, Butner JE, Butler JM, King PS, Hughes AE, Wiebe DJ. Parental persuasive strategies in the face of daily problems in adolescent type 1 diabetes management. Health Psychol. 2013;32(7):719–728. doi: 10.1037/a0029427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.U.S.-Department-of-Agriculture. US household food security module. U.S.-Department-of-Agriculture; 2012. [Google Scholar]
- 77.West F, Sanders MR. The lifestyle behaviour checklist: a measure of weight-related problem behaviour in obese children. Int J Pediatr Obes. 2009;4(4):266–273. doi: 10.3109/17477160902811199. [DOI] [PubMed] [Google Scholar]
- 78.Cremeens J, Eiser C, Blades M. Factors influencing agreement between child self-report and parent proxy-reports on the Pediatric Quality of Life Inventory 4.0 (PedsQL) generic core scales. Health Qual Life Outcomes. 2006;4:58. doi: 10.1186/1477-7525-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]