TO THE EDITOR
Eosinophilic esophagitis (EoE) is a chronic inflammatory disease characterized by esophageal dysfunction with histological evidence of ≥15 eosinophils per high-powered field (HPF) that does not resolve with proton pump inhibitor therapy.1 Although EoE is often considered an allergic disorder, its specific immune mechanisms remain unclear.1,2 There is evidence in support of both immunoglobulin (Ig)-dependent (IgE and/or IgG)1-3 and Ig-independent mechanisms.2 This begs the question of whether both Ig-dependent and Ig-independent mechanisms are required to develop EoE.
Common variable immunodeficiency (CVID) is a primary immunodeficiency defined by markedly reduced serum Ig, impaired antigen-specific antibody responses, and susceptibility to sinopulmonary bacterial infections. Characteristic features also include impaired development of plasma cells and isotype-switched memory B cells.4 In CVID, plasma cells and immunoglobulins are typically markedly decreased or entirely absent from the gastrointestinal (GI) tract.5 Thus, CVID represents a unique model to study the pathogenesis of GI disorders in a setting of antibody and plasma cell deficiency. Here, we present what we believe to be the first reported case of EoE diagnosed in a patient with CVID.
The patient recalled a history of frequent sinopulmonary infections, particularly bronchitis, in childhood and typically received antibiotics at least twice a year. She was also diagnosed with pneumonia twice during her high school years and twice while in college. At 28 years of age, the patient was hospitalized twice in the same year for pneumonia. The following year, after suffering pneumonia once again, she was referred to clinical immunology for immunodeficiency evaluation.
Peripheral lymphocyte count was within the reference range (2.3 × 103/μL). Total B cells were in the normal range (15.9% of lymphocytes) with isotype-switched memory B cells reduced (1.87% of CD19 + B cells). Serum quantitative immunoglobulin levels were found to be markedly decreased with IgG of 334 mg/ dL (normal 700-1600 mg/dL), IgA < 6 mg/dL (normal 70-400 mg/dL), and IgM of 16 mg/dL (normal 40-230 mg/dL). She was also found to have nonprotective antibody responses for Haemophilus influenzae, measles, mumps, rubella, and varicella, and was diagnosed with CVID at the age of 29. Intravenous Ig therapy was initiated at 400 mg/kg per month, which was later transitioned to subcutaneous Ig replacement due to patient preference. On this regimen, she has not had any further episodes of pneumonia and has only required antibiotic treatment on 2 occasions for sinusitis.
At the age of 34, the patient was referred to gastroenterology for symptoms of dysphagia, recurrent mild esophageal food impactions triggered by alcohol and hard-textured foods, and a history of proximal esophageal stricture previously treated by balloon dilation. She reported that these symptoms progressively worsened over the prior 5-6 years. A trial of lansoprazole 30 mg twice daily was initiated. After 4 months on lansoprazole, the patient did not have any relief of symptoms and underwent esophagogastroduodenoscopy (EGD). Grossly, endoscopy showed no stricture, but it did reveal mild furrows, scattered white plaques, and a decreased vascular pattern. Esophageal biopsies demonstrated significant intraepithelial eosinophilia (up to 60-100 eosinophils per HPF in the proximal and distal esophagus as well as the gastroesophageal junction), eosinophilic microabscesses and degranulation, and lamina propria fibrosis, all consistent with the diagnosis of EoE. Gastric and duodenal biopsies revealed normal mucosae. Of note, the patient’s serum IgE was <2 kU/L, and the patient had no history of asthma, atopic dermatitis, allergic rhinitis, IgE-mediated food allergies, or any other atopic disorders.
On diagnosis of EoE, empiric dietary restriction was initiated with elimination of milk, egg, wheat, soy, peanuts and tree nuts, fish and shellfish, as well as beef and lamb from her diet. While adhering to this diet for 3 months, the patient reported symptomatic improvement, with decreased dysphagia and no further food impactions. However, repeat EGD with biopsies revealed similar endoscopic features (Figure 1, A) and persistent esophageal intraepithelial eosinophilia (up to 60-75 eosinophils per HPF in the proximal and distal esophagus) with eosinophilic microabscesses. Because of continued histopathologic and endoscopic evidence of EoE, the patient was started on swallowed fluticasone 220 μg/puff, 2 puffs twice a day, and dietary avoidance was discontinued. After 4 months of fluticasone therapy, repeat EGD demonstrated full resolution of plaques and furrows grossly (Figure 1, B) and esophageal eosinophilia on biopsy (Figure 2, A). Annual surveillance EGDs have demonstrated continued remission of EoE on fluticasone therapy after 2 years of follow-up. Additionally, a review of duodenal biopsies revealed markedly diminished levels of CD138+ plasma cells both before and after treatment (Figure 2, B), consistent with GI manifestation of CVID.6
FIGURE 1.
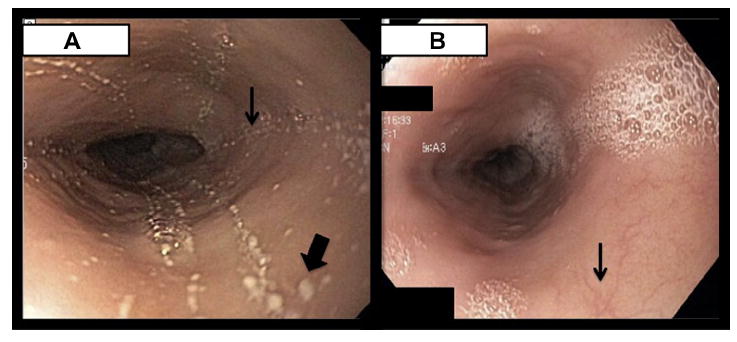
A, Before fluticasone therapy (but after treatment with lansoprazole and empiric dietary restriction), endoscopy grossly revealed findings consistent with eosinophilic esophagitis (EoE), including furrows (example marked with a thinner arrow), scattered white plaques (example marked with a thicker arrow), and a decreased vascular pattern. B, After 4 months of swallowed fluticasone therapy, repeat endoscopy demonstrated full resolution of plaques and furrows, along with return toward a normal vascular pattern (example marked with an arrow).
FIGURE 2.
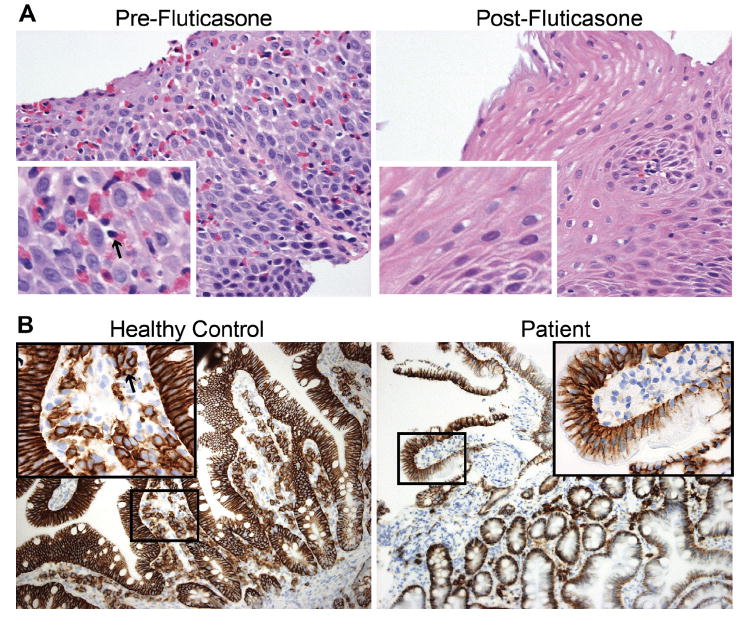
A, Esophageal biopsy showing eosinophils (example marked with an arrow in the “Pre-Fluticasone” inset) in mucosa before but not after fluticasone therapy. Large images at 400×, insets at 1600×. B, Duodenal biopsy from non—common variable immunodeficiency (CVID) control (left) and patient (right) with immunohistochemistry staining of CD138+ cells (example marked with an arrow in the “Healthy Control” inset) demonstrating the absence of plasma cells in the patient’s gastrointestinal (GI) tract. Large images at 200×. Zoomed-in areas are 400× and correspond to the box in the low magnification image.
Although the exact mechanisms underlying EoE are unclear, antigens from food, and possibly aeroallergens, are postulated to trigger an inflammatory response characterized by esophageal eosinophilia.1,2 Multiple studies suggest that Ig-dependent mechanisms may drive EoE, as significant increases in B cells and IgE-bound mast cells with evidence of local class switching have been found in the esophageal mucosa of patients with EoE.7 Additionally, the majority of patients with EoE demonstrate abnormal skin prick tests and increased food-specific IgE levels.2 However, the efficacy of elimination diets based on food-specific IgE allergy skin testing has been limited.8 Ig isotypes other than IgE may drive EoE, as one study reported omalizumab to be an ineffective treatment but found evidence for a possible IgG4-dependent mechanism.3
EoE occurred in our patient despite profoundly reduced serum Ig levels, impaired specific antibody responses, absent serum IgE, and markedly diminished GI plasma cells characteristic of CVID. Notably, EoE occurs in primary immunodeficiency disorders with variably impaired B-cell function, such as signal transducer and activator of transcription-3 and dedicator of cytokinesis-8 deficiency. As T cells, rather than B cells, are indispensable for the development of EoE in murine models, our case supports increasing evidence that EoE develops largely independent of IgE and perhaps Ig entirely.9 In addition, allergic rhinosinusitis and atopic asthma have been found to occur in some patients with CVID, illustrating that allergic diseases may not be IgE dependent in all instances. Further research is needed to determine whether Ig-independent endotypes exist in EoE as have been suggested for asthma and rhinosinusitis.
Clinical Implications.
Eosinophilic esophagitis can occur in the setting of common variable immunodeficiency. Further studies are warranted to determine whether antibody-independent mechanisms alone can underlie eosinophilic esophagitis.
Acknowledgments
No funding was received for this work.
D. A. Andreae is employed by New York Allergy and Sinus Center; and receives royalties from UpToDate. C. Cunningham-Rundles has received research support from the National Institutes of Health; and has received consultancy fees from Kedrion. M. Chehade has received consultancy fees from Receptos and Actelion Pharmaceuticals; and has received research support from American Gastrointestinal Association Research Foundation, Guthy-Jackson Foundation, Nutricia, Regeneron, and Patient-Centered Outcomes Research Institute.
Footnotes
Conflicts of interest: The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Lin SK, Sabharwal G, Ghaffari G. A review of the evidence linking eosinophilic esophagitis and food allergy. Allergy Asthma Proc. 2015;36:26–33. doi: 10.2500/aap.2015.36.3804. [DOI] [PubMed] [Google Scholar]
- 2.Wechsler JB, Bryce PJ. Allergic mechanisms in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:281–96. doi: 10.1016/j.gtc.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson L, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602–9. doi: 10.1053/j.gastro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 4.Ochtrop ML, Goldacker S, May AM, Rizzi M, Draeger R, Stehfest C, et al. T and B lymphocyte abnormalities in bone marrow biopsies of common variable immunodeficiency T and B lymphocyte abnormalities in bone marrow biopsies of common variable immunodeficiency. Blood. 2011;118:309–18. doi: 10.1182/blood-2010-11-321695. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal S, Smereka P, Harpaz N, Cunningham-Rundles C, Mayer L. Characterization of immunologic defects in patients with common variable immunodeficiency (CVID) with intestinal disease. Inflamm Bowel Dis. 2011;17:251–9. doi: 10.1002/ibd.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels JA, Lederman HM, Maitra A, Montgomery EA. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol. 2007;31:1800–12. doi: 10.1097/PAS.0b013e3180cab60c. [DOI] [PubMed] [Google Scholar]
- 7.Vicario M, Blanchard C, Stringer KF, Collins MH, Mingler MK, Ahrens A, et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias A, González-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014;146:1639–48. doi: 10.1053/j.gastro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Simon D, Cianferoni A, Spergel JM, Aceves S, Holbreich M, Venter C, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy. 2016;71:611–20. doi: 10.1111/all.12846. [DOI] [PubMed] [Google Scholar]


