Abstract
Importance
Voice prosthesis (VP) device life is a limiting factor of tracheoesophageal (TE) voice restoration that drives patient satisfaction, health care costs, and overall burden. Historic data suggest that TE VPs have an average device life of generally 3 to 6 months, but these data are typically derived from small samples using only 1 or 2 devices.
Objective
To reexamine current device life in a large, contemporary cancer hospital in the United States that uses a wide assortment of VPs.
Design, Setting, and Participants
This retrospective observational study included 390 laryngectomized patients with a tracheoesophageal puncture (TEP) who had VP management at MD Anderson Cancer Center between July 1, 2003, and December 31, 2013.
Main Outcomes and Measures
Tracheoesophageal voice–related outcomes were: (1) device life duration to VP removal, and (2) treatment-related and prosthetic-related factors influencing device failure. Primary independent variables included treatment history (extent of surgery and radiation history), VP type (indwelling vs nonindwelling, size, specialty features), and reason for removal (leakage, complication, other). Duration was examined using Kaplan-Meier analysis. Disease, treatment, and patient-specific factors were analyzed as predictors of duration.
Results
Overall, 3648 VPs were placed in the 390 patients (median [range] age, 62 [34-92] years). Indwelling prostheses accounted for more than half (56%) of the devices placed (55%, 20-Fr diameter; 33%, 8-mm length). More than two-thirds (69%) of prostheses were removed because of leakage, while the rest were removed for other reasons. Median device life was 61 days for all prostheses. Indwelling and nonindwelling VPs had median device lives of 70 and 38 days, respectively. There was no significant difference between specialty prostheses compared with standard devices (median duration, 61 vs 70 days, respectively). The Provox ActiValve (Atos Medical) had the longest life. Neither radiation therapy nor extent of surgery had a meaningful impact on device life.
Conclusions and Relevance
Our data suggest that VP duration demonstrates a lower durability than historically reported. This may reflect the intensification of treatment regimens that complicate TEP management in an era of organ preservation; however, further investigation is needed.
Keywords: voice, tracheoesophageal, speech, alaryngeal, laryngectomy
Introduction
Tracheoesophageal (TE) speech is regarded as the gold standard for rehabilitation following total laryngectomy1 and is considered a superior method to esophageal speech and the use of an electrolarynx in properly selected patients.2Tracheoesophageal speech utilizes a voice prosthesis (VP) that is inserted through a puncture in the common wall separating the trachea from the esophagus. As the speaker occludes the stoma, air from the lungs is forced through the prosthesis via a one-way valve, causing the pharyngoesophageal segment to vibrate. This vibration egresses into the oral cavity, and the articulators move to create speech. The VP allows air exchange into the pharyngoesophagus for speech production while preventing aspiration of food and liquid into the lungs.
Over the years, manufacturers have produced an assortment of TE VPs that vary in length, diameter, valve strength, material, and insertion method. Voice prostheses are commonly distinguished using 2 categories: nonindwelling and indwelling devices. Properly trained patients or others may insert nonindwelling devices to allow for greater independence and minimize the number of return clinic visits. Speech language pathologists, physicians, or other trained clinicians must place indwelling devices.3 Indwelling devices are considered for patients who experience frequent leakage through nonindwelling VPs or for those who are unable or do not wish to exchange their own devices.4 The most commonly cited cause of device failure is leakage of liquids through the prosthesis, a problem often attributed to the formation of biofilm on the valve. Although the current literature is limited, other factors may influence device life, including cancer type and extent of treatment.5
Recent manufacturing advances in TE VPs have focused on the enhancement of device function and prolongation of device life or time to device failure. Such advances include the use of magnets in the prosthesis valve to increase valve resistance6and the incorporation of specialty biomaterials such as silver oxide to reduce biofilm formation.7 The duration of VPs can have substantial effects on patient burden including cost, frequency of clinic visits, and overall quality of life,6 making new and improved specialty VPs an attractive option to improve the health and overall satisfaction of TE speakers.
Historical data suggest an average lifetime of 4 to 6 months for most indwelling VPs, but these data have not been revisited in a contemporary practice in which total laryngectomy is often performed as a salvage procedure after radiation failure. Limited data have been published comparing various VPs, especially with regard to their relative device lifetimes, and the existing data show conflicting results.8,9 The purpose of this 10-year retrospective analysis was to (1) estimate device life in a contemporary cohort of TE voice prosthesis users and (2) examine the treatment and prosthetic-related factors that influence device failure.
Methods
Study Methods and Eligibility
An institutional review board–approved retrospective study was completed using records of 390 patients who underwent a total laryngectomy with a TE puncture (TEP) and who had VP management at MD Anderson Cancer Center (MDACC) between July 1, 2003, and December 31, 2013. A waiver of informed consent was obtained. Data were collected from the MDACC TEP tracking database and by review of electronic medical records. Eligibility criteria included a record of VP placement in the MDACC TEP tracking database of patients who underwent total laryngectomy, total laryngectomy with partial pharyngectomy, or total laryngopharyngectomy and whose prosthesis management took place at MDACC during the study period. Records of dummy prostheses, records with incomplete durational data, records of prostheses that were placed within 90 days after TEP whose exchange was likely influenced by change in tract length during early postsurgical healing rather than device failure due to leakage, records postdating recurrent disease after TEP, records of patients presenting to the clinic with dislodged VPs for which duration was unclear, and records of patients who required temporary prosthesis removal prior to procedures (ie, dilation and TE injection) were excluded from analysis. Additionally, records with incomplete durational data based on patient self-replacement of their own prostheses and records with non-MDACC replacement data were also excluded.
Institutional TEP Management
Both primary and secondary TEP are performed at MDACC. A variety of indwelling and nonindwelling TE VPs are used within the institution (InHealth Technologies; Atos Medical). Nonindwelling devices were defined as those cleared by the US Food and Drug Administration (FDA) for insertion by the patient at home. Indwelling devices were defined as those FDA-cleared for insertion by a clinician. Specialty devices were defined as those designed and manufactured to address the need for a stronger, more durable device with increased resistance to airflow. Types of prostheses used in this study are listed and categorized in eTable 1 in the Supplement.
Statistical Analysis
Voice prothesis duration was summarized using descriptive methods. Variables analyzed in relationship to VP duration included patient sex and age, tumor location, stage of disease, extent of treatment, type of VP, and reason for VP removal. Device life comparisons were plotted according to the Kaplan-Meier method with log-rank tests for statistical comparison of device lifetimes. A sensitivity analysis examining these potential predictors of VP duration was subsequently conducted by restricting device life comparisons to the 2530 prostheses replaced for leakage. For all analyses, statistical significance was considered as P less than .05. STATA data analysis software, version 14, was used for statistical analyses (StataCorp LP).
Results
Patient Population
The study population included 390 patients with a history of total laryngectomy and TEP who had VPs placed at MDACC between July 1, 2003, and December 31, 2013. Table 1 outlines patient characteristics. The median (range) age of patients at time of TEP was 62 (34-92) years. Eighty-one percent of the cohort was male. Extended total laryngectomy procedures were performed in the minority of patients, including partial (n = 62 [16%]) or total (n = 32 [8%]) pharyngectomy. The majority of patients had a history of radiation therapy (n = 388 [87%]); 248 patients (64%) underwent a primary TEP, while the remainder of patients had a secondary TEP.
Table 1.
Patient Characteristics
| Variable | No. (%) | |
|---|---|---|
| Voice Prostheses (n = 3648) | Unique Patients (n = 390) | |
| Age at TEP, median (range) [mean] | 61 (34-92) [61] | 62 (34-92) [62] |
| Sex | ||
| Male | 2929 (80.3) | 317 (81.3) |
| Female | 719 (19.7) | 73 (18.7) |
| Tumor site | ||
| Glottic | 1929 (52.9) | 214 (54.9) |
| Hypopharynx | 310 (8.5) | 33 (8.5) |
| Oropharynx | 77 (2.1) | 7 (1.8) |
| Subglottic | 201 (5.5) | 24 (6.1) |
| Supraglottic | 1004 (27.5) | 96 (24.6) |
| Thyroid | 87 (2.4) | 10 (2.6) |
| Other | 40 (1.1) | 6 (1.5) |
| T stage | ||
| T0-T2 | 158 (4.3) | 17 (4.4) |
| T3 | 559 (15.3) | 45 (11.5) |
| T4 | 1024 (28.1) | 124 (31.8) |
| Recurrent | 1713 (47.0) | 179 (45.9) |
| Unknown | 194 (5.3) | 25 (6.4) |
| N stage | ||
| N0 | 923 (25.3) | 91 (23.3) |
| N+ | 815 (22.3) | 94 (24.1) |
| Recurrent | 1713 (47.0) | 179 (45.9) |
| Unknown | 197 (5.4) | 26 (6.7) |
| Surgery | ||
| TL | 2833 (77.7) | 296 (75.9) |
| TL + PP | 580 (15.9) | 62 (15.9) |
| TLP | 235 (6.4) | 32 (8.2) |
| Reconstruction | ||
| Circumferential | 235 (6.4) | 32 (8.2) |
| Patch | 704 (19.3) | 81 (20.8) |
| Tongue | 3 (0.1) | 1 (0.2) |
| None | 2706 (74.2) | 276 (70.8) |
| Timing of TEP | ||
| Primary | 2422 (66.4) | 248 (63.6) |
| Secondary | 1226 (33.6) | 142 (36.4) |
| Radiation therapy | ||
| Preoperative | 1723 (47.2) | 182 (46.7) |
| Postoperative | 1347 (37.0) | 148 (38.0) |
| Both | 41 (1.1) | 8 (2.0) |
| None | 537 (14.7) | 52 (13.3) |
Abbreviations: TEP, tracheoesophageal puncture; TL, total laryngectomy; TLP, total laryngopharyngectomy; PP, partial pharyngectomy.
Voice Prosthesis Characteristics
During the study period, 3648 prostheses were placed in the 390 patients. Indwelling prostheses accounted for over half of all devices placed (76%). The most common prosthesis placed was the Blom-Singer Indwelling (InHealth Technologies) (38%). Over half of the devices (55%) placed were 20 Fr in diameter, and the 2 most common lengths of prostheses were 8 mm (33%) and 10 mm (27%). Sixty-nine percent of prostheses were removed due to leakage, while the remainder were removed because of other etiologies unrelated to leakage. Nineteen percent of removals that included the prostheses of patients who requested replacement at the time of their physician return visit while at the clinic for regular surveillance as a matter of convenience regardless of problems were categorized as routinely related. Twelve percent were removed for nonleakage complications including extrusion, common wall swelling, gastric filling, or leakage around the prosthesis. Table 2 and the eTable 2 in the Supplement summarize device characteristics for all VPs.
Table 2.
Characteristics of VPs
| Characteristic | No. (%) |
|---|---|
| Total No. | 3648 |
| Duration, median (range) [mean], d | 61 (1-816) [86] |
| VP Category | |
| Nonindwelling | 564 (15.5) |
| Indwelling | 2027 (55.6) |
| Specialty indwelling | 733 (20.0) |
| Custom | 324 (8.9) |
| VP Manufacturer and Type | |
| Smiths MedicaI | |
| Bivona Duckbill | 10 (0.3) |
| Bivona Ultra Low | 20 (0.6) |
| InHealth Technologies | |
| Blom-Singer Duckbill | 4 (0.1) |
| Blom-Singer Low Pressure | 255 (7.0) |
| Blom-Singer Indwelling | 1383 (37.9) |
| Blom-Singer Indwelling Standard Enlarged Flange | 205 (5.6) |
| Blom-Singer Advantage | 251 (6.9) |
| Atos Medical | |
| Provox NiD | 340 (9.3) |
| Provox Vega | 44 (1.2) |
| Provox 2 | 1096 (30.0) |
| Provox ActiValve | 40 (1.1) |
| Reason for removal | |
| Leakage | 2530 (69.4) |
| Complication | 423 (11.6) |
| Other | 695 (19.0) |
Abbreviation: VP, voice prosthesis.
Device Life Based on Patient Characteristics
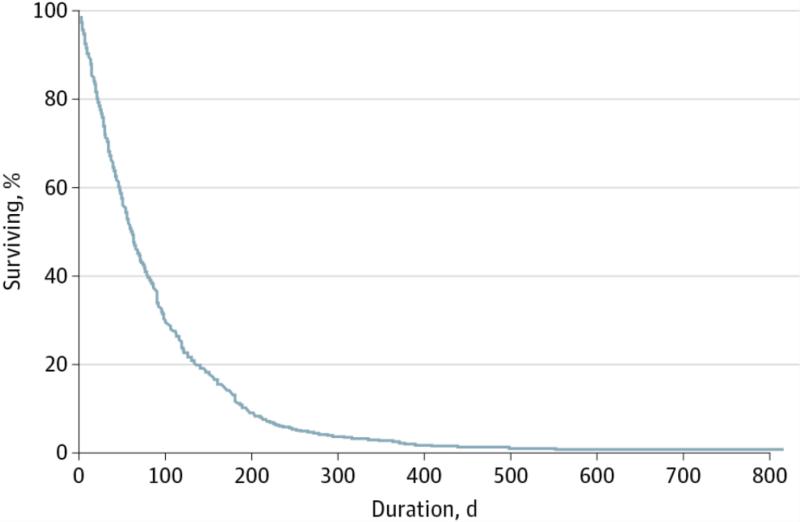
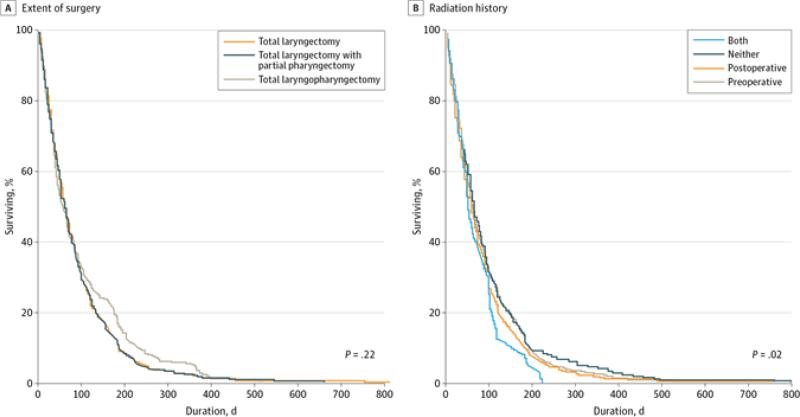
Median (range) device life was 61 (1-816) days for all prostheses, and mean (SD) device life was 86 (87) days, as summarized in Figure 1 and Table 2. Extent of surgery did not significantly affect device life; patients treated with total laryngectomy, total laryngectomy with partial pharyngectomy, and total laryngopharyngectomy had median lifetimes of 62, 57, and 56 days, respectively (P = .22) (Figure 2A). There was a significant difference in prosthetic life in patients who had a history of radiation therapy (median, 59 days) compared with those who did not (median, 66 days), but this was on average only 1 week longer in duration than in the nonirradiated laryngectomy group (Figure 2B). Twenty five patients had subglottic disease or cervical esophageal extension necessitating lower surgical fields and possibly more distal location of TEP; VP duration was not significantly different among patients with subglottic disease relative to other sites (median: subglottic, 60 days vs other, 66 days; P = .19). There was a significant difference in prosthetic life based on timing of TEP (median: primary, 63 days vs secondary, 54 days; P = .003), but this represented less than 2 weeks in durational difference. Reason for VP removal significantly affected device life (P < .001), as shown in the eFigure in the Supplement. Patients whose prosthesis was removed for reasons other than leakage through the valve experienced poorer device life (median, 28 days) compared with prostheses that were removed for leakage through the valve (median, 64 days) or for prostheses regularly exchanged as part of routine clinic return visits (median, 61 days).
Figure 1.
Device Life. The median device life of 3648 voice prostheses was 61 days.
Figure 2.
Device Life Stratified by Treatment History. A, Median device life for total laryngectomy was 62 days; total laryngectomy with partial pharyngectomy, 57 days; total laryngopharyngectomy, 56 days. B, Median device life for patients who underwent both preoperative and postoperative radiation therapy (RT) was 49 days; neither preoperative nor postoperative RT, 66 days; postoperative RT only, 57 days; preoperative RT only, 61 days. This analysis included 3648 voice prostheses.
Device Life Based on Prosthesis Type
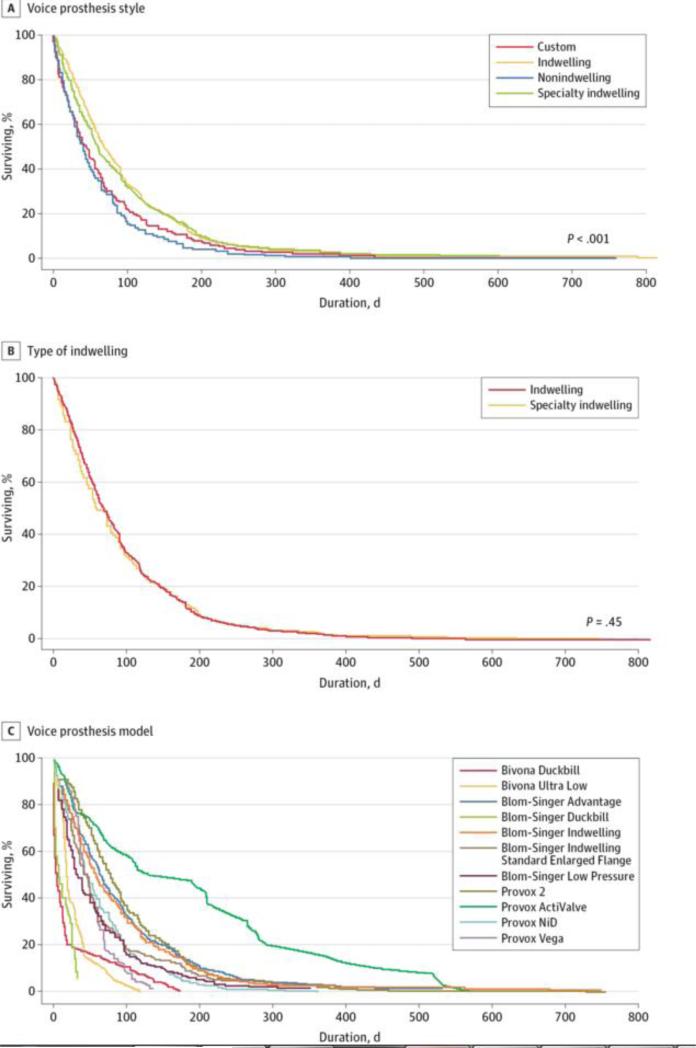
Indwelling VPs had significantly longer device life than nonindwelling types (Figure 3A). Device life did not differ significantly between specialty and standard VPs (Figure 3B). The VP with the longest life was the Provox ActiValve (Atos Medical) with a median of 161 days. eTable 3 in the Supplement shows a stratified summary of VP duration, and the survival curve in Figure 3C shows a comparison of all types of prostheses included in the study.
Figure 3.
Device Life Stratified by Prosthetic Characteristics for 3648 Voice Prostheses. A and B, Median device life for custom protheses was 44 days; indwelling prostheses, 70 days; nonindwelling prostheses, 28 days; specialty indwelling prostheses, 61 days. C, Median device life for the Bivona Duckbill (Smiths Medical) was 7 days; Bivona Ultra-Low (Smiths Medical), 20 days; Blom-Singer Advantage (InHealth Technologies), 67 days; Blom-Singer Duckbill (InHealth Technologies), 18 days; Blom-Singer Indwelling (InHealth Technologies), 59 days; Blom-Singer Indwelling Standard Enlarged Flange (InHealth Technologies), 42 days; Blom-Singer Low Pressure (InHealth Technologies), 33 days; Provox 2 (Atos Medical), 77 days; Provox ActiValve (Atos Medical), 161 days; Provox NiD (Atos Medical), 47 days; Provox Vega (Atos Medical), 45 days. This analysis included 3648 voice prostheses.
Sensitivity Analysis
A sensitivity analysis restricted to only the 2530 VPs that were removed for leakage demonstrated that inclusion of all prostheses in our comparative analyses of device life (including those changed in routine follow-up) did not alter our results. Comparing results obtained in the 2530 VPs removed for leakage to all 3648 VPs, device life estimates among subgroups based on surgical extent, reconstruction, radiotherapy, TEP timing, and tumor site differed by only 1 to 7 days and statistical significance was unchanged for all comparisons.
Discussion
Voice prosthesis device life is a limiting factor of TE voice restoration. Device life drives patient satisfaction, health care costs, and overall rehabilitative burden. In this large, contemporary laryngectomy cohort of TE prosthesis users, the average device life was roughly 2 months, with minimal effects of treatment history and device type observed. Our device life results are lower than historic reports that are generally derived from small samples using only 1 or 2 devices, indicating typical device life between 3 and 6 months. Our results are comparable to those reported in a more recent 3-year European cohort of TE users that found a 3-month average duration (median, 74 days) of indwelling voice prostheses in a group of 102 laryngectomy survivors.6 Both studies suggest a lower than previously estimated average of 4 to 6 months duration of VPs in contemporary users prior to TE prosthesis failure.4,10,11
Accurate estimations of device life are critical to set realistic expectations for postlaryngectomy voice rehabilitation for both health care providers and patients. Preoperative counseling is considered best practice for the patient considering total laryngectomy with or without a TEP procedure. In these preoperative visits, it is customary to examine candidacy for TEP and to counsel the patient regarding expected outcomes and postoperative care. Collectively, ours and other recent device life studies suggest that, on average, the typical TE prosthesis user should anticipate roughly 4 to 6 prosthesis replacements within the calendar year.6,10,11 This is a critical consideration for patients with limited access to expert providers trained in TE voice restoration. In addition, in countries in which health care is not universally provided, such as the United States, the cost associated with the replacement of TE voice prostheses may be an unexpected obstacle to successful TE voice restoration. Thus, the frequently held belief that everyone is a candidate for TE voice restoration may be misleading. In our clinic setting, the translation of these data to our preoperative assessment algorithm is that it is now routine to include a frank discussion regarding the patient's ability and willingness to commit to regular return visits and potential costs associated with likely 4 to 6 new prostheses per year.
Indwelling VPs were associated with significantly longer device life, but this was on average only a 1 month durational advantage. Despite the fact that indwelling prostheses are associated with longer device life and less burden for the patient in terms of self-management, there still may be advantages to the use of non-indwelling devices in some patients. Nonindwelling devices offer a more affordable option because the product is less expensive and does not require a clinic return that generates a hospital procedure charge, a cost advantage that is beneficial, regardless of the payer. These factors suggest that for select patients, the relative advantage of self-insertion of the nonindwelling device in lieu of the indwelling prosthesis may represent a worthwhile departure from current practice in which placement of the indwelling prosthesis has, in many cases, become the standard approach.
In most practices, factors related to radiation treatment and extent of surgery are commonly proposed as major drivers of device life; however, our data suggest otherwise. Although, our findings showed that radiotherapy was significantly associated with shorter device life, this outcome may simply represent a statistical but not a clinically meaningful difference since the median duration differed by only 7 days among radiated and nonirradiated TE speakers. Likewise, the extent of surgery was not statistically predictive of device life. We agree that radiotherapy and extent of surgery are important factors that influence the success of TE voice restoration and postlaryngectomy functioning and have been described robustly in areas related to TE voice quality, swallowing, and postlaryngectomy complication.12-14 However, neither radiation nor extent of surgery showed any meaningful difference in device life in this study.
Finally, a likely assumption that specialty designs of VPs, such as those that are manufactured with increased resistance to airflow or use antimicrobial materials among other substances, will result in better performance and therefore, facilitate longer time to device failure, may in fact be misleading. When the data from all indwelling devices was aggregated, our findings suggested that specialty indwelling prostheses did not outperform standard indwelling devices in terms of long-lasting device life. Rather they provided a similar duration comparable to the duration of standard devices used by normal, uncomplicated TE speakers. In other words, specialty prostheses are typically placed in patients who experience suboptimal device life using standard prostheses because of unique problems or characteristics related to physiology, anatomy, airflow, etc.15,16 Our results showed that specialty prostheses as a consortium, offered users an average duration comparable to standard devices, but they did not extend device life beyond the group level. That is, both the median device life for standard and specialty devices approximated 2 months. However, when data were examined based on individual performance, the ActiValve as an individual device offered the longest longevity relative to other VPs, representing a roughly 3-month longer duration beyond standard device life. This outcome is not unexpected but rather seems likely given the advanced design of a magnet-driven valve coupled with biofilm-resistant biomaterials. Unfortunately, despite the known benefit, the cost of the ActiValve makes it less accessible to patients in many health care settings. A better understanding of the relative benefit of specialty devices over their standard counterparts requires future controlled analysis comparing products using a within subject design to account for these selection biases.
Conclusions
Herein, we report to our knowledge the largest series to date examining VP device life in an unselected, contemporary cohort of TE speakers. Our data suggest that overall VP device life demonstrates a lower durability than historically has been reported, potentially representing the difficulties associated with comparison of studies in which methodologies and clinical practices differ and thus, conclusions are disparate. Perhaps a more plausible explanation is that TE VPs are wearing out sooner in modern TE speakers because of the challenges associated with TE voice restoration in a medically and socially complex population as a natural consequence of the effects of cancer treatment intensification in an era of organ preservation. Despite the limitations inherent to retrospective design and clinician biases, our data provide important information that reinforce the need for better design and manufacture of VPs that are readily accessible to and meet the challenges of contemporary TE speakers. Finally, the results of this study highlight the importance of proper counseling so that TEP candidate selection and patient expectations are optimized to facilitate successful TE voice restoration and postoperative quality of life.
Supplementary Material
Key Points.
Question What is the duration of device life and how is this influenced by tracheoesophageal (TE) voice prostheses?
Findings In this retrospective study of 390 laryngectomized patients with TE puncture (3648 total prosthesis replacements), median device life was 61 days for all prostheses. Most prostheses (69%) were replaced because of leakage. Neither radiation nor extent of surgery had a meaningful effect on device life.
Meaning Voice prothesis duration demonstrates lower durability than historically reported, highlighting the need for better voice prothesis design and proper patient counseling to ensure appropriate TE puncture candidate selection and accurate patient expectations for successful TE speech outcomes.
Acknowledgments
Funding/Support: Drs Lewin and Hutcheson have received research funds from the National Institutes of Health Center Core Grant (grant No. 5P30CA016672). Dr Hutcheson receives grant support from the MD Anderson Institutional Research Grant Program and the National Cancer Institute (grant No. R03 CA188162). Dr Hutcheson also receives grant support from the National Institute of Dental and Craniofacial Research (grant No.1R56DE025248-01).
Role of the Funder/Sponsor: The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Presented in part at the Multidisciplinary Head and Neck Cancer Symposium, February 18, 2016, Scottsdale, AZ.
Additional Contributions: We are grateful for the editorial assistance of Janet Hampton and project administration and support by Denise A. Barringer, MS, CCC-SLP; they were not compensated for their contributions. We also acknowledge the Section of Speech Pathology & Audiology at MD Anderson Cancer Center for data collection efforts in the TEP Clinic.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No disclosures were reported.
Previous Presentation: A portion of this research was presented at the Multidisciplinary Head and Neck Cancer Symposium; February 18, 2016; Scottsdale, Arizona.
References
- 1.Pawar PV, Sayed SI, Kazi R, Jagade MV. Current status and future prospects in prosthetic voice rehabilitation following laryngectomy. J Cancer Res Ther. 2008;4(4):186–191. doi: 10.4103/0973-1482.44289. [DOI] [PubMed] [Google Scholar]
- 2.Schwandt LQ, van Weissenbruch R, van der Mei HC, Busscher HJ, Albers FW. Effect of dairy products on the lifetime of Provox2 voice prostheses in vitro and in vivo. Head Neck. 2005;27(6):471–477. doi: 10.1002/hed.20180. [DOI] [PubMed] [Google Scholar]
- 3.Lewin JS, Portwood MA, Wang Y, Hutcheson KA. Clinical application of the Provox NiD voice prosthesis: a longitudinal study. Laryngoscope. 2014;124(7):1585–1591. doi: 10.1002/lary.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hancock KL, Lawson NR, Ward EC. Device life of the Provox Vega voice prosthesis. Eur Arch Otorhinolaryngol. 2013;270(4):1447–1453. doi: 10.1007/s00405-012-2154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graville DJ, Palmer AD, Andersen PE, Cohen JI. Determining the efficacy and cost-effectiveness of the ActiValve: results of a long-term prospective trial. Laryngoscope. 2011;121(4):769–776. doi: 10.1002/lary.21380. [DOI] [PubMed] [Google Scholar]
- 6.Kress P, Schäfer P, Schwerdtfeger FP, Rösler S. Are modern voice prostheses better? A lifetime comparison of 749 voice prostheses. Eur Arch Otorhinolaryngol. 2014;271(1):133–140. doi: 10.1007/s00405-013-2611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilgers FJ, Ackerstaff AH, Jacobi I, Balm AJ, Tan IB, van den Brekel MW. Prospective clinical phase II study of two new indwelling voice prostheses (Provox Vega 22.5 and 20 Fr) and a novel anterograde insertion device (Provox Smart Inserter). Laryngoscope. 2010;120(6):1135–1143. doi: 10.1002/lary.20925. [DOI] [PubMed] [Google Scholar]
- 8.Delsupehe K, Zink I, Lejaegere M, Delaere P. Prospective randomized comparative study of tracheoesophageal voice prosthesis: Blom-Singer versus Provox. Laryngoscope. 1998;108(10):1561–1565. doi: 10.1097/00005537-199810000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Ramalingam W, Chikara D, Rajagopal G, Mehta AR, Sarkar S. Tracheo-esophageal puncture (TEP) for voice rehabilitation in laryngectomised patients Blom-Singer® vs Provox® Prosthesis: our experience. Med J Armed Forces India. 2007;63(1):15–18. doi: 10.1016/S0377-1237(07)80098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackerstaff AH, Hilgers FJ, Meeuwis CA, et al. Multi-institutional assessment of the Provox 2 voice prosthesis. Arch Otolaryngol Head Neck Surg. 1999;125(2):167–173. doi: 10.1001/archotol.125.2.167. [DOI] [PubMed] [Google Scholar]
- 11.Lequeux T, Badreldin A, Saussez S, Thill MP, Oujjan L, Chantrain G. A comparison of survival lifetime of the Provox and the Provox2 voice prosthesis. J Laryngol Otol. 2003;117(11):875–878. doi: 10.1258/002221503322542881. [DOI] [PubMed] [Google Scholar]
- 12.Sweeny L, Golden JB, White HN, Magnuson JS, Carroll WR, Rosenthal EL. Incidence and outcomes of stricture formation postlaryngectomy. Otolaryngol Head Neck Surg. 2012;146(3):395–402. doi: 10.1177/0194599811430911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutcheson KA, Sturgis EM, Lewin JS. Early risk factors for enlargement of the tracheoesophageal puncture after total laryngectomy: nodal metastasis and extent of surgery. Arch Otolaryngol Head Neck Surg. 2012;138(9):833–839. doi: 10.1001/archoto.2012.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewin JS, Barringer DA, May AH, et al. Functional outcomes after laryngopharyngectomy with anterolateral thigh flap reconstruction. Head Neck. 2006;28(2):142–149. doi: 10.1002/hed.20308. [DOI] [PubMed] [Google Scholar]
- 15.Kress P, Schäfer P, Schwerdtfeger FP. [Clinical use of a voice prosthesis with a flap valve containing silver oxide (Blom-Singer Advantage), biofilm formation, in-situ lifetime and indication]. Laryngorhinootologie. 2006;85(12):893–896. doi: 10.1055/s-2006-925292. [DOI] [PubMed] [Google Scholar]
- 16.Soolsma J, van den Brekel MW, Ackerstaff AH, Balm AJ, Tan B, Hilgers FJ. Long-term results of Provox ActiValve, solving the problem of frequent candida- and “underpressure”-related voice prosthesis replacements. Laryngoscope. 2008;118(2):252–257. doi: 10.1097/MLG.0b013e318159ebde. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.