To the Editor,
Complement deficiencies can be differentiated based on acquired or hereditary causes. Acquired complement deficiencies are seen more frequently, occurring in a variety of settings such as autoimmunity, reduced hepatic synthesis, renal loss, the presence of autoantibodies or in cases of transient activation/depletion such as sepsis or viremia [1]. Inherited complement deficiencies are rare, with an estimated prevalence of 0.03% in the general population [1]. Manifestations of inherited deficiencies typically include infectious, autoimmune or renal complications dependent on the deficient component [2]. C2 deficiency is the most common inherited human complement deficiency and is often associated with autoimmunity [1,2]. Inherited C3 deficiency is extremely rare with only 20–50 cases reported in the literature [2]. Inherited C3 deficiencies typically presents as severe, recurrent infections shortly after birth [1] with autoimmune disease and/or glomerulonephritis additionally occurring in approximately 20% of cases [2], highlighting the central role of C3 in the classical, lectin and alternative complement pathways [1,3].
We present the case of a 16-year-old female who presented with mild myalgia's lasting 6 months. Her symptoms never interfered with daily functioning and occurred in varied locations including bilateral upper and lower extremities. She had a mild viral illness at symptom onset but was otherwise well with an unremarkable review of systems. A rheumatologic evaluation was sought, notable for a C3 of 16 mg/dL (normal 90–180 mg/dL) and CH50 of 29 CAE units (normal 60–144 CAE units). Evaluation of C2, C4, CRP, ESR, quantitative immunoglobulins, complete blood count with differential, complete metabolic panel and urine analysis were within normal limits. She had an unremarkable medical history without history of infections, renal disease or symptoms concerning for a rheumatologic condition. The patient was from a non consanguineous background with no family history of autoimmunity of immunodeficiency. Her physical exam was unremarkable and without lipodystrophy.
The patient's C3 remained decreased over the following 3–4 months, despite a course of oral steroids. Upon referral to our institution, we found her C3 to be persistently decreased at 14 mg/dL (normal 90–180 mg/dL). Inherited causes of a C3 deficiency were considered, such as a deficiency in C3 itself or inherited deficiencies of Factors H or I [1], regulators of complement activation which lead to uncontrolled consumption and C3 depletion in a deficient state [3]. Similar to inherited cases of C3 deficiency, hereditary Factor H and Factor I deficiencies are exceedingly rare, with <50 cases reported in the literature [2]. Patients with inherited deficiencies of C3, Factor H or Factor I typically present with infections, immune complex disease or renal disease [2], which are notably absent in our patients. Thus, given the rarity of these conditions and the absence of suggestive clinical findings we felt an inherited complement deficiency was unlikely.
Regarding acquired causes of C3 deficiency, the previously discussed differential of autoimmunity, reduced hepatic synthesis, renal loss, the presence of autoantibodies and transient activation/depletion was considered. In our patient's case, the unremarkable laboratory and rheumatologic evaluations made reduced hepatic synthesis, renal loss or autoimmunity unlikely. Initially, we hypothesized our patient had a transient depletion in C3 due to a prior viral infection, however given its persistence this became less likely. Thus, testing for autoantibodies known to cause C3 depletion, namely C3 Nephritic Factor (C3NeF), was obtained.
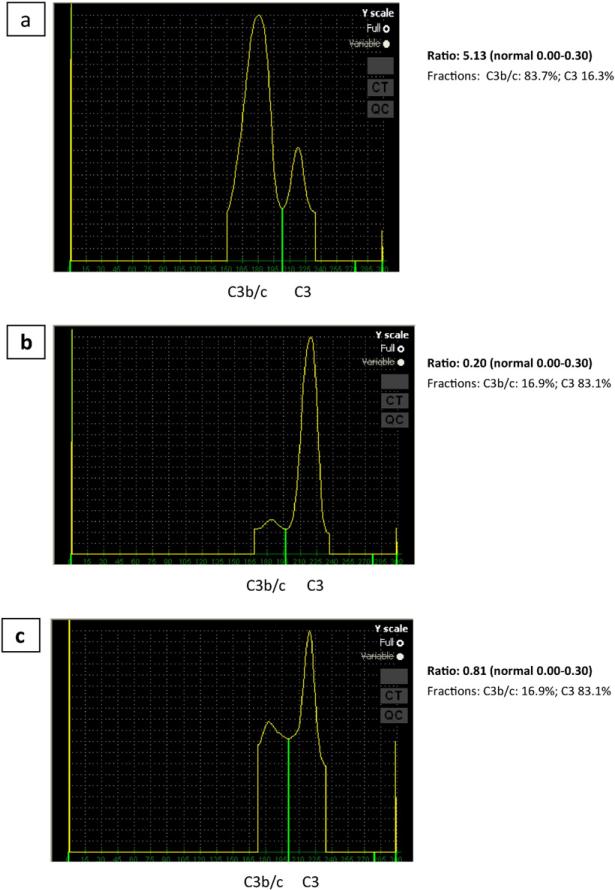
The patient was found to have a positive C3NeF in a ratio of 5.13 (normal 0.00–0.30; National Jewish Heath Complement Laboratory) as seen in Fig. 1. Fig.1a demonstrates increased conversion of C3 due to the presence of the patient's C3NeF revealed by mixing patient serum, normal serum and reagents allowing for alternative pathway activation. Fig. 1b includes the mixed serum but tested in the presence of reagents that do not allow for alternative pathway activation and Fig. 1c is a control of patient serum alone. C3NeF is an autoantibody specific for the alternative pathway C3 convertase, C3bBb, and stabilizes the convertase, blocking Factor-H mediated dissociation and leading to unregulated consumption of C3 [1]. Patients typically present with renal disease, infections or lipodystrophy [1]. The presence of C3NeF has been reported in up to 80% of patient cohorts with C3 glomerular disease [4]. However, despite the clear association between C3NeF and renal disease, it's role in pathogenesis is unclear at it has been previously reported in normal individuals [5]. Additionally, the prognosis in patients with C3NeF is unclear as evidenced by Schwertz et al. who followed 50 children with idiopathic membranoproliferative glomerulonephritis for 2–20 years and noted that while 30 patients had evidence of C3NeF at study onset, C3 levels remained normal in 6 patients and C3NeF became undetectable in 6 patients, demonstrating a marked heterogeneity in clinical outcomes [6].
Fig. 1.
Positive C3 Nephritic Factor analysis. A: 3 parts normal serum, 1 part patient's serum, Mg++ and EDTA (alternative pathway activation possible); B. 3 parts normal serum + 1 part patient's serum + EDTA (alternative pathway activation not possible); C. Contains 3 parts buffer + 1 part patient's serum (control for patient's serum by itself).
A prior case report describes the presence of C3NeF in a healthy individual who remained well during a 2-year follow up [5]. It is possible our patient represents an additional asymptomatic individual, as the prevalence of C3NeF in the general population is not known. As our patient was clinically well, we deferred pursuing treatment strategies used in patients with C3NeF, such as rituximab or mycophenolate mofetil, as they have been primarily used in the setting of C3 glomerulonephritis with mixed results [7]. However, as it remains unknown if she will develop complications associated with C3NeF we recommended her vaccinations be up to date, clinical evaluations with illnesses and routine screening for the development of membranoproliferative glomerulonephritis with urine protein analysis.
Contributor Information
Maureen Egan, Division of Allergy and Immunology, Department of Medicine, The Icahn School of Medicine at Mount Sinai, One Gustave L Levy Place, Box 1089, 10029 New York, NY, USA, MaureenSEgan@gmail.com.
Kathleen Sullivan, Division of Allergy Immunology, Department of Pediatrics, The Children's Hospital of Philadelphia, 3615 Civic Center Blvd., Philadelphia, PA 19104, USA, Sullivank@mail.med.upenn.edu.
Ashley Frazer-Abel, Division of Cell Biology, Department of Pediatrics, National Jewish Health Advanced Diagnostic Laboratories, 1400 Jackson St., Denver, CO 80206, USA, frazer-abelA@njhealth.org.
Charlotte Cunningham-Rundles, Division of Allergy and Immunology, Department of Medicine, The Icahn School of Medicine at Mount Sinai, One Gustave L Levy Place, Box 1089, 10029 New York, NY, USA.
References
- 1.Wen L, Atkinson JP, Giclas PC. Clinical and laboratory evaluation of complement deficiency. J. Allergy Clin. Immunol. 2004 Apr;113(4):585–593. doi: 10.1016/j.jaci.2004.02.003. quiz 594. [DOI] [PubMed] [Google Scholar]
- 2.Skattum L, van Deuren M, van der Poll T, Truedsson L. Complement deficiency states and associated infections. Mol. Immunol. 2011 Aug;48(14):1643–1655. doi: 10.1016/j.molimm.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. seventh ed. Saunders/Elsevier; Philadelphia: 2012. [Google Scholar]
- 4.Riedl M, Thorner P, Licht C. C3 glomerulopathy. Pediatr. Nephrol. 2016 Apr;7 doi: 10.1007/s00467-015-3310-4. [DOI] [PubMed] [Google Scholar]
- 5.Gewurz AT, Imherr SM, Strauss S, Gewurz H, Mold C. C3 nephritic factor and hypocomplementaemia in a clinically healthy individual. Clin. Exp. Immunol. 1983 Oct;54(1):253–258. [PMC free article] [PubMed] [Google Scholar]
- 6.Schwertz R, Rother U, Anders D, Gretz N, Scharer K, Kirschfink M. Complement analysis in children with idiopathic membranoproliferative glomerulonephritis: a long-term follow-up. Pediatr. Allergy Immunol. 2001 Jun;12(3):166–172. doi: 10.1034/j.1399-3038.2001.012003166.x. [DOI] [PubMed] [Google Scholar]
- 7.Pickering MC, D'Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, et al. C3 glomerulopathy: consensus report. Kidney Int. 2013 Dec;84(6):1079–1089. doi: 10.1038/ki.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]