Abstract
The dynamic addition and removal of covalent posttranslational modifications (PTMs) on histone proteins serves as a major mechanism regulating chromatin-templated biological processes in eukaryotic genomes. Histone PTMs and their combinations function by directly altering the physical structure of chromatin and as rheostats for effector protein interactions. In this chapter, we detail microarray-based methods for analyzing the substrate specificity of lysine methyltransferase and demethylase enzymes on immobilized synthetic histone peptides. Consistent with the “histone code” hypothesis, we reveal a strong influenceof adjacent and,surprisingly,distant histonePTMs onthe ability of histone-modifying enzymes to methylate or demethylate their substrates. This platform will greatly facilitate future investigations into histone substrate specificity and mechanisms of PTM signaling that regulate the catalytic properties of histone-modifying enzymes.
1. INTRODUCTION
Eukaryotic genomes are tightly packaged in cell nuclei by their ability to associate with histone proteins. These histone-DNA complexes are first organized into nucleosomes, which are further folded into higher-order chromatin fibers that are poorly understood (Khorasanizadeh, 2004; Kornberg & Lorch, 1999; Luger, Mader, Richmond, Sargent, & Richmond, 1997). A major focus of modern biomedical research has been to understand how genetic information is accessed in the context of chromatin to control DNA-templated processes like gene transcription, replication, and repair, and how deregulation of these processes contributes to the initiation and progression of human disease (Detrich, 1986; Maze, Noh, & Allis, 2013; Portela & Esteller, 2010).
Posttranslational modifications (PTMs) on histone proteins have emerged as key epigenetic regulators of genome accessibility and function (Kouzarides, 2007). Fundamental breakthroughs in our understanding of chromatin regulation have been made through the identification of protein machineries that add (write), remove (erase), and bind (read) these marks (Rothbart & Strahl, 2014). Fueled by technological advances in mass spectrometry-based proteomics, more than 20 unique histone PTMs have been identified at upward of 80 different histone residues, many of which cluster in the unstructured N- and C-terminal tail domains that protrude from the nucleosome core (Huang, Sabari, Garcia, Allis, & Zhao, 2014; Zhao & Garcia, 2015).
In 2000, the concept of a “histone code” emerged as a hypothesis to stimulate new thinking about how histone PTMs might function in a combinatorial manner to dynamically regulate chromatin interactions of histone reader proteins (Strahl & Allis, 2000). In addition, it was postulated that much like histone PTM readers, the enzymes that write and erase these marks would themselves be influenced by preexisting PTM patterns. While significant effort has been placed on identifying and characterizing enzymes and effector proteins responsible for writing, erasing, and reading histone marks (Fig. 1), deciphering regulatory mechanisms of combinatorial PTM patterning has proven challenging, due in part to the sheer complexity of the histone PTM landscape.
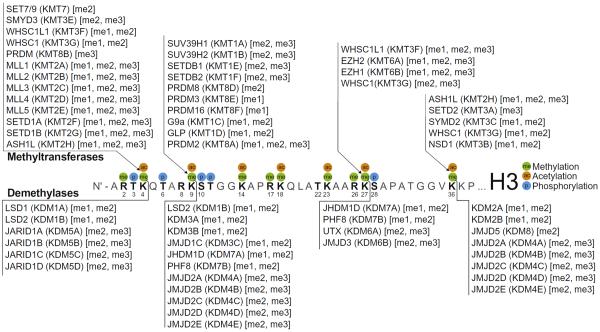
Fig. 1.
The dynamic regulation of lysine methylation on histone H3. Shown are major sites of methylation (me), acetylation (ac), and phosphorylation (p) on the N-terminal tail domain of histone H3. Known writers (methyltransferases; KMTs) and erasers (demethylases; KDMs) of lysine methylation are clustered by major histone substrate residue(s). Methylation products and substrates (mono-, me1; di-, me2; tri-, me3) of KMT and KDM reactions, respectively, are listed. Enzyme identification reflects both conventional and generic (Allis et al., 2007) nomenclature.
To address this issue, we developed a high-density histone peptide microarray platform to enable rapid and high-throughput biochemical characterization of histone PTM-specific antibodies and readers in the context of complex histone PTM patterns (Fuchs, Krajewski, Baker, Miller, & Strahl, 2011; Rothbart et al., 2015; Rothbart, Krajewski, Strahl, & Fuchs, 2012). Briefly, synthetic biotinylated peptides, posttranslationally modified with up to eight physiologically relevant combinations of lysine acetylation and methylation (mono-, di-, and trimethyl), arginine methylation (mono, symmetric dimethyl, and asymmetric dimethyl) and citrullination, and serine/threonine phosphorylation, are deposited on streptavidin-coated glass slides that can then be used to examine aspects of protein function like binding and enzymatic activity.
Methods for the synthesis of combinatorially modified biotinylated histone peptides, microarray fabrication using these peptides, and the characterization of histone readers and antibodies with histone peptide microarrays were previously described (Rothbart et al., 2012). In this chapter, we now detail the utility of this same peptide microarray platform, which is commercially available through Epicypher and Millipore, for high-throughput substrate specificity profiling of histone lysine methyltransferases (KMTs) and demethylases (KDMs). We further reveal the influence of neighboring, and surprisingly distant, PTMs on the catalytic properties of histone-modifying enzymes.
2. ASSAY OPTIMIZATION
2.1 Design of Custom Print Formats
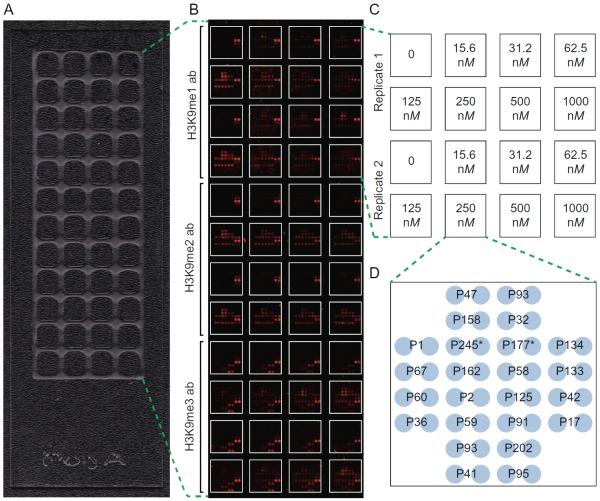
A number of variables should be considered when designing an enzyme assay for microarray screening. For instance, incubation times and enzyme concentrations that have been optimized for solution-based assays may not translate to a microarray format, particularly since substrate concentrations of an immobilized peptide or protein can be limiting by several orders of magnitude. To optimize assay conditions, including variables of time, buffer composition, enzyme concentration, and detection reagent, we used our recently developed open source software package, ArrayNinja (see chapter “ArrayNinja: An Open Source Platform for Unified Planning and Analysis of Microarray Experiments” by Dickson et al.). ArrayNinja unifies the planning and analysis of microarray experiments to facilitate streamlined microarray customization and data processing. Shown in Fig. 2A is a format we have found useful for enzyme assay optimization that partitions 48 subarrays on a single slide by hydrophobic wax. Detailed procedures for wax deposition using a stamping device are described elsewhere (Partyka, Wang, Zhao, Cao, & Haab, 2014). These wax boundaries produce a well around each subarray of 64 unique features (275 μm spot diameter and 100 μm between spots) and allows for hybridization of 6 μL reaction volumes. Representative slides for the optimization of a KMT assay with G9a are shown, where we were able to test seven enzyme concentrations and three detection antibodies (ie, antibodies that recognize the orders of H3K9 methylation that are generated by G9a), all in duplicate, on a single slide (Fig. 2B).
Fig. 2.
Optimization of a G9a KMT assay on microarrays. (A) A 48-well optimization array partitioned by hydrophobic wax. (B and C) Scanned image of an optimization array assay following a 2-h incubation with seven G9a concentrations, a no enzyme control (0), and three detection antibodies (H3K9me1, EpiCypher #13-0014; H3K9me2, Abcam #1220; H3K9me3, Active Motif #39161), all in duplicate. (D) Print layout of individual arrays partitioned on 48-well optimization slides. Each array consists of 24 unique histone peptides printed in duplicate. Peptide identifications numbers correspond to those used previously (Rothbart et al., 2015). P245 and P177 are H3(1–32) and H3(1–43), respectively. Biotin-4-Fluorescein (not shown) is printed in the corners of each array as a spotting control and a landmark to aid in alignment for data analysis.
2.2 Detection Reagent Considerations
A critical optimization step when designing an assay for histone-modifying enzyme activity on microarrays is determining how catalysis will be monitored. Detection strategies most successful in our hands have relied on radioisotope incorporation or histone PTM-specific antibodies. Advantages and disadvantages of both radioisotopic and antibody readout of enzyme function are discussed in Sections 2.2.1 and 2.2.2, and methods for performing these assays are described in Section 3.
2.2.1 Radioisotopes
The activity of enzymes that write histone PTMs, including methyltransferases, acetyltransferases, and kinases can be monitored on microarrays by radioisotope incorporation. The use of radioisotopes for profiling the substrate specificity of the enzymes responsible for erasing histone PTMs presents a significant technical challenge for microarray analysis, as this would require the tedious synthesis of large peptide libraries that contain these isotopes. Radioactivity assays are advantageous when substrate specificity is unknown, and with enough chemical diversity in a substrate library, can be informative in identifying the target residue. However, the degree or order of modification (eg, mono-, di-, or trimethylation on lysine residues) cannot be defined with this detection strategy. Use of radioisotopes also enables a clear assessment of the influence neighboring PTMs have on enzymatic activity, since these direct labeling strategies are not confounded by the influence of PTMs on antibody-epitope recognition (Rothbart et al., 2015).
A major drawback of radioactivity assays, specifically methyltransferase and acetyltransferase assays, is the time required to collect data from an experiment. Tritium and 14C are low-energy beta emitters, requiring upward of 1 month exposure time to X-ray film to visualize results. Autoradiography enhancing reagents do not appear to be compatible with this array format. While it may be possible to decrease exposure times with the aid of phosphor screens, this has not been empirically tested. Kinases are more amenable to radioisotope-based microarray assays, as they rely on the use of the high-energy beta emitter [γ-33P] ATP as a cofactor (Rothbart et al., 2012), which is detectable by autoradiography following a brief exposure.
2.2.2 Antibodies
Histone PTM-specific antibodies are widely used chromatin biochemistry tools, and more than 1000 commercial antibodies are available that recognize the diverse modification states on histone proteins. There are a number of advantages to using antibodies as detection reagents for histone-modifying enzymes on microarrays. Unlike radioisotopic readouts, antibody detection methods enable site-specific analysis of a particular substrate residue of interest and can discriminate between the various states of methylation found on histones. In addition, a number of pan PTM antibody reagents have been developed, many of which react well with the intended modifications on histones (data not shown). However, a number of these pan reagents show epitope specificity problems, albeit less strict than conventional antibodies, resulting in unequal discrimination among PTMs when presented in diverse sequence contexts. Antibody hybridization procedures are straightforward, require little material, do not generate radioactive waste, and can be detected within several hours.
A significant caveat to using antibodies as detection reagents is the influence, both positive and negative, of neighboring PTMs. Inclusion of control detection experiments in the absence of modifying enzyme or cofactor can help assess the behavior of the antibody being used. In addition, we recently characterized the specificity of over 100 commercially available histone PTM antibodies by microarray, and we created a publicfacing web portal, the Histone Antibody Specificity Database (www.histoneantibodies.com) (Rothbart et al., 2015). This online resource is a useful tool for aiding in the selection of antibodies suitable for enzyme profiling experiments.
3. ASSAY METHODOLOGY
Later we detail methods for assaying the methyltransferase activity of G9a and the demethylase activity of JMJD2A–both enzymes that write and erase H3K9 methylation, respectively–on our previously described peptide microarray platform (Rothbart et al., 2012). This print format benefits from the ability to assay >250 unique peptide substrates in a single assay. In addition, two identical subarrays are printed on each slide and can be separated by a silicon adhesive seal (eg, Epicypher #11-3001), by hydrophobic wax deposited with a slide imprinter (eg, Gel Company #WSP48-1), or by a PAP pen (eg, Sigma #Z377821). Subarray separation is useful for comparison of multiple conditions on the slide. For example, we commonly incubate the array in the absence or presence of enzyme on the same slide prior to antibody hybridization. We detail an antibody-based detection procedure, applicable to both classes of enzymes, and we also describe radioisotopic assays using the cofactor adenosyl-l-methionine, S-[methyl-3H] (3H-SAM) for histone methyltransferase assays on microarrays and in solution.
3.1 Microarray-Based Lysine Methyltransferase (KMT) Assays with G9a
Equipment and reagents
Tris–HCl, pH 8.8
MgCl2
Recombinant human G9a, amino acids 913-1210 (Epicypher #15-1008)
S-adenosyl methionine (SAM)
Dithiothreitol (DTT)
Cold PBS, pH 7.6
Powdered bovine serum albumin (BSA)
Tween-20
Nontreated four-well dish (such as Thermo Scientific #267061)
25×60 mm cover slips
Biotinylated histone peptide microarray, custom printed as previously described (Rothbart et al., 2012), or commercially available through Epicypher (#11-2001) or Millipore (#16-671).
Humidified microarray incubation chamber (such as VWR#97000-384).
Additional equipment and reagents specific to antibody detection
Histone PTM-specific primary antibody.
Fluorescent-labeled secondary antibody (such as Alexa Fluor® 647-conjugated anti-rabbit (Thermo #A-21244) or anti-mouse (Thermo #A-21235)).
Microarray scanner capable of scanning at ≤25 μm and equipped with lasers compatible with your secondary antibody conjugates.
Additional equipment and reagents specific to radioisotope detection
3H-SAM (Perkin Elmer NET155001MC)
X-ray film
Film developer
General procedure
Equilibrate a microarray slide in KMT reaction buffer (50 mM Tris–HCl, pH 8.8, 5 mM MgCl2, 4 mM DTT) for 10 min at room temperature.
Incubate the slide in a humidified microarray incubation chamber with 200 μL1 KMT reaction buffer containing 1 μM enzyme2 and 60 μM SAM3 under a coverslip4 at 30°C for 2 h.
Wash the slide 2×5 min with PBS, pH 7.6 in the cold.
Dry the slide. We prefer a mini-centrifuge for this purpose, such as Sigma #Z674672. Alternatively, slides can be dried over a stream of filtered compressed air or by centrifugation in a 50-mL conical tube in a swinging-bucket rotor at 800×g for 1 min.
Follow procedures below for detection with radioisotope or antibody.
Additional procedures and considerations for radioisotope detection
Step 3 above should be performed 5×5 min, and liquid waste should be discarded in accordance with your institution's radiation safety procedures.
For containment, the preferred drying method in step 4 above is centrifugation in a 50-mL conical tube.
Following step 4 above, expose the dry slide to X-ray film in an autoradiography cassette at −80°C for at least 1 month.
Bring the sealed autoradiography cassette to room temperature and process film through a developer.
Additional procedures and considerations for antibody detection
Prepare a dilution of primary antibody in hybridization buffer (PBS, pH 7.6, 5% BSA (w/v), 0.1% Tween-20). In general, begin with a dilution factor at the high end of the detection range by western blot.
Incubate the slide with 200 μL of diluted antibody under a coverslip for 1 h at 4°C in a humidified microarray incubation chamber.
Remove the coverslip and wash 3×5 min with cold PBS.
Prepare a dilution of fluorescent secondary antibody (1:5000–1:10,000 for Alexa Fluor® 647) in hybridization buffer and incubate the slide for 30 min in the dark with gentle rotation at 4°C.
Wash the slide 3×5 min with PBS in the dark at 4°C.
Dip the slide 5× in 0.1× PBS to remove excess salt.
Dry the slide by centrifugation or compressed air as described earlier.
Scan the slide using a microarray scanner at ≥25 μm with a laser appropriate for your fluorescent secondary antibody.
Slides can be stored at 4°C protected from light for several days without appreciable loss of fluorescent signal.
3.2 Solution-Based KMT Assays with G9a
Equipment and reagents
Tris–HCl, pH 8.8
MgCl2
Recombinant human G9a, amino acids 913-1210 (Epicypher #15-1008)
DTT
3H-SAM (Perkin Elmer #NET155001MC)
H3(1–20) peptide (Epicypher #12-0001)
Trifluoroacetic acid (TFA)
NaHCO3, pH 9.0
P81 phosphocellulose filter paper (Whatman)
General procedure
Dilute G9a to a final concentration of 25 nM in KMT reaction buffer (50 mM Tris–HCl, pH 8.8, 5 mM MgCl2, 4 mM DTT) containing 0.5 μCi 3H-SAM.
Start reactions by addition of substrate peptide at a final concentration of 10 μM.
Quench reactions by addition of TFA to a final concentration of 0.5% (w/v).
Spot equal amounts of quenched reactions on P81 filter paper and dry at room temperature for 10 min.
Wash filter papers in a single beaker 4×5 min with 50 mM NaHCO3, pH 9.0.
Dry filter papers at room temperature for 20 min.
Place filter papers in scintillation vials and add an appropriate amount of scintillation fluid to submerge the paper.
Count tritium with a scintillation counter.
3.3 Microarray-Based Lysine Demethylase (KDM) Assays with JMJD2A
Equipment and reagents
HEPES, pH 7.5
NaCl
Recombinant human JMJD2A, amino acids 1–350 (Krishnan, Collazo, Ortiz-Tello, & Trievel, 2012; see chapter “Purification, Biochemical Analysis, and Structure Determination of JmjC Lysine Demethylases” by Krishnan and Trievel)
(NH4)2Fe(SO4)2
l-Ascorbic acid
2-OG
Cold PBS, pH 7.6
Powdered BSA
Tween-20
Nontreated four-well dish (such as Thermo Scientific #267061)
Biotinylated histone peptide microarray, custom printed as previously described (Rothbart et al., 2012) or commercially available through Epicypher (#11-2001) or Millipore (#16-671)
Humidified microarray incubation chamber (such as VWR#97000-384)
Histone PTM-specific primary antibody
Fluorescent-labeled secondary antibody (such as Alexa Fluor® 647-conjugated anti-rabbit (Thermo #A-21244) or anti-mouse (Thermo #A-21235))
Microarray scanner capable of scanning at ≥25 μm and equipped with lasers compatible with your secondary antibody conjugates
General procedure
Equilibrate a microarray slide in KDM reaction buffer (50 mM HEPES, pH 7.5, 50 mM NaCl, 50 μM (NH4)2Fe(SO4)2, 1 mM l-ascorbic acid, 1 mM 2-OG) for 10 min at room temperature.
Incubate the slide in a humidified microarray incubation chamber with 500 μL KDM reaction buffer containing 0.313 μM enzyme5 at 25°C for 18 h.
Wash the slide 3×5 min with PBS, pH 7.6 in the cold.
Dry the slide. We prefer a mini-centrifuge for this purpose, such as Sigma #Z674672. Alternatively, slides can be dried by over a stream of filtered compressed air or by centrifugation in a 50-mL conical tube in a swinging-bucket rotor at 800×g for 1 min.
Prepare a dilution of primary antibody in hybridization buffer (PBS, pH 7.6, 5% BSA (w/v), 0.1% Tween-20). In general, begin with a dilution factor at the high end of the detection range by western blot (1:1000–1:5000).
Incubate the slide with 500 μL of diluted antibody 1 h at 4°C in a humidified microarray incubation chamber.
Wash the slide 3×5 min with cold PBS.
Prepare a dilution of fluorescent secondary antibody (1:5000–1:10,000 for Alexa Fluor® 647) in hybridization buffer and incubate the slide for 30 min in the dark with gentle rotation at 4°C.
Wash the slide 3×5 min with PBS in the dark at 4°C.
Dip the slide 5× in 0.1× PBS to remove excess salt.
Dry the slide by centrifugation or compressed air as described earlier.
Scan the slide using a microarray scanner at ≤25 μm with a laser appropriate for your fluorescent secondary antibody.
Slides can be stored at 4°C protected from light for several days without appreciable loss of fluorescent signal.
4. ENZYME SPECIFICITY PROFILING BY MICROARRAY
Histone peptide microarrays have been a robust biochemical tool for the in vitro characterization of histone antibodies and readers in the context of complex histone PTM patterns (Fuchs et al., 2011; Rothbart et al., 2015, 2012). We therefore sought to expand the utility of this competitive assay platform for the interrogation of histone-modifying enzymes that act on peptide substrates. Here, we detail results obtained from successful screening of histone methyltransferase and demethylase activities using the methods described in Section 3.
4.1 High-Throughput Profiling of G9a KMT Activity
Since the seminal discovery of the first histone lysine methyltransferase (Rea et al., 2000), and the link between site-specific histone methylation and gene transcription (Lee, Teyssier, Strahl, & Stallcup, 2005), much research has focused on the role of histone methylation signaling through chromatin in human health and disease (Greer & Shi, 2012). Notably, over 50 known and predicted lysine methyltransferase enzymes have been identified (Petrossian & Clarke, 2011), many of which have histone substrates (Fig. 1). The lysine methyltransferase G9a (EKMT2/KMT1C) is a SET (Su(var)3-9-Enhancer of zeste-Trithorax) domain-containing protein identified as the major enzyme responsible for catalysis of mono- and dimethylation on H3K9 (Collins et al., 2005; Tachibana, Sugimoto, Fukushima, & Shinkai, 2001). G9a activity through euchromatic H3K9 methylation has primarily been associated with gene silencing (Shankar et al., 2013). Several additional histone substrates of G9a have been identified, including H3K27 (Tachibana et al., 2001; Wu et al., 2011), H3K56 (Yu et al., 2012), and H1 (Tachibana et al., 2001; Trojer et al., 2009; Weiss et al., 2010). G9a also methylates a number of nonhistone proteins (Rathert et al., 2008; West et al., 2010).
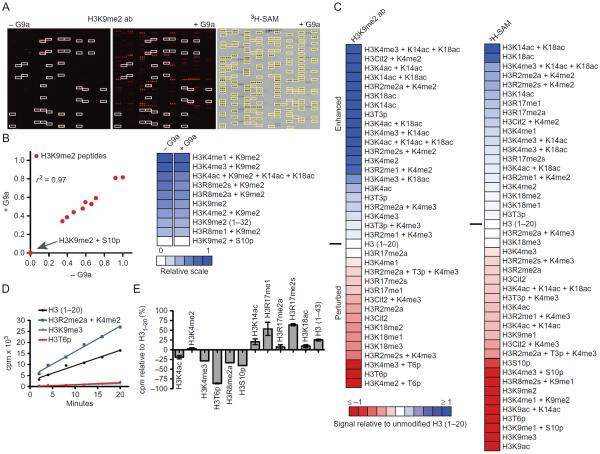
Using assay conditions optimized on 48-well microarrays (Fig. 2), we subjected G9a to methyltransferase assays on a large array format. This platform allows for simultaneous monitoring of enzymatic activity on over 250 unique substrates in a competitive assay format, yielding over 2000 specific catalytic measurements in a single experiment. Following incubation with G9a, we first detected new methylation of H3K9 with an H3K9me2-specific antibody (Fig. 3A). Antibody reactivity in the absence of G9a was screened as a control. Consistent with previous observations (Rothbart et al., 2015), reactivity of this antibody with H3K9me2-containing peptides was both positively and negatively impacted by neighboring modifications. As discussed earlier, this behavior of histone PTM-specific antibodies is a potential disadvantage of using these reagents to detect enzymatic activity (Fig. 3B). In particular, phosphorylation of H3S10 completely inhibited this antibody from recognizing H3K9me2. Based on this observation, H3S10p peptides were excluded from subsequent analysis following detection with this antibody to circumvent false-negative conclusions.
Fig. 3.
Analysis of G9a histone substrate specificity and the combinatorial PTM influence on KMT activity. G9a histone substrate specificity was profiled using the described antibody and radioisotopic detection strategies on large-format histone peptide micro-arrays. (A) Representative images of arrays detected with an H3K9me2 antibody (Abcam #1220) following hybridization in the absence or presence of 1 μM G9a for 2 h (left). White boxes demarcate peptides detected by H3K9me2 antibody in the absence of G9a. Image of autoradiography film exposed for 1.5 months following a 2-h array assay with 1 μM G9a and 5 μCi 3H-SAM (right). For comparison, yellow boxes demarcate peptides detected with H3K9me2 antibody in the presence of G9a. (B) Scatter plot (left) and heat map (right) of H3K9me2 peptides detected by the above-mentioned H3K9me2 antibody in the absence or presence of G9a. Correlation coefficient (r2) was calculated by linear regression analysis using GraphPad Prism v6. For heat maps, relative signal intensities are plotted using JavaTreeView (Saldanha, 2004) from 0 (white, no binding) to 1 (blue, strong binding). (C) Heat maps depicting the effects of combinatorial PTMs on the enzymatic activity of G9a from panel (A). Enhanced (1, blue) and occluded (−1, red) effects are depicted. Peptide signal intensities are presented relative to H3(1–20) (0, white) following detection with the above-mentioned H3K9me2 antibody (left) or autoradiography (right). (D) In-solution 3H-SAM filter-binding assays monitoring G9a activity as a function of time on the listed histone peptide substrates. Data points are presented as counts per minute (cpm), each from three independent measurements. (E) In-solution 3H-SAM filter-binding assays monitoring G9a activity following a 10-min incubation with the listed histone peptide substrates. Data points are presented as cpm relative to an H3(1–20) substrate. Error bars represent ±S.E.M. from three independent experiments.
Another caveat of detecting the products of enzyme reactions on arrays with site-specific antibodies is the potential for missing substrates (ie, residues being methylated other than H3K9 would not be detected by the antibody). We therefore developed a procedure to screen for G9a activity by monitoring radioisotope incorporation using 3H-SAM as a cofactor (see method above). The majority of peptides detected by the H3K9me2 antibody after G9a incubation also incorporated tritiated methyl groups (Fig. 3A), consistent with the conclusion that the preferential histone target of G9a is H3K9 (Collins et al., 2005; Tachibana et al., 2001). Notably, a number of distinct methylation substrates in the sequence context of H3(15–41) were detected by radioactivity, consistent with the observation that G9a can also methylate H3K27 in vitro (Tachibana et al., 2001; Wu et al., 2011). We were unable to detect H3K56 methylation by G9a on our array platform.
Analysis of quantified signal intensities from both antibody and radioisotope detection methods revealed new insights regarding the behavior of G9a catalytic activity toward H3K9 in the presence of adjacent and distant histone PTMs (Fig. 3C). Modifications that enhanced and perturbed G9a activity toward H3(1–20) peptides were largely consistent between detection strategies. The unbiased nature of radioisotope incorporation allowed us to scrutinize the contribution of H3S10p to G9a activity, which, consistent with a previous observation (Rathert, Dhayalan, et al., 2008), was largely inhibited by this neighboring mark. In general, single PTMs toward the N-terminus of H3(1–20) inhibited G9a activity on H3K9, and PTMs toward the C-terminus of H3(1–20) enhanced G9a activity. In addition, several combinations of H3R2 modification (methylation and citrullination) with H3K4 methylation enhanced G9a activity. These latter results were surprising, connecting distant PTMs and their combinations to G9a activity and function. Understanding how these long-range PTMs contribute to G9a activity will be an exciting area of future study.
To validate the dynamics of combinatorial PTMs to G9a activity observed on microarrays, we performed in-solution filter-binding assays with 3H-SAM (Fig. 3D). The rate of methyl incorporation using H3(1–20) peptide substrates was determined to be linear over the course of 20 min, and no activity was measured on H3K9me3 peptides. Consistent with single time point measurements on the arrays and a previous observation (Rathert, Dhayalan, et al., 2008), H3T6p completely inhibited G9a catalysis (Fig. 3D). In addition, we validated an enhancement of G9a activity on H3(1–20) peptides modified with asymmetric dimethylation at arginine 2 (H3R2me2a) in combination with H3K4me2. Furthermore, single in-solution filter-binding assay measurements along this linear scale for a number of other histone PTMs was consistent with the changes observed on the arrays (Fig. 3C and E). Collectively, these results demonstrate the utility of histone peptide microarrays in capturing the dynamics of histone methylation in the context of the “histone code,” and reveal combinatorial histone PTM patterns that may regulate the chromatin activity of G9a.
4.2 High-Throughput Profiling of JMJD2A KDM Activity
Methylation on lysine residues was long thought to be an irreversible modification until the discovery of the first lysine demethylase (KDM), LSD1 (lysine-specific demethylase 1), shown to remove mono- and dimethylation on histone H3K4 using its FAD-dependent amine oxidase activity (Shi et al., 2004). Since this landmark discovery, over 30 KDMs with activity specific for all of the major lysine methylation sites on histones have been identified (Fig. 1; Dimitrova, Turberfield, & Klose, 2015). This includes the discovery of an additional class of demethylase enzymes, the Jumonji demethylases, that coordinate iron and utilize α-ketoglutarate (2-OG) as a cofactor (Tsukada et al., 2006). JMJD2A (KMD4A), a Jumonji family KDM, was the first demethylase discovered to remove lysine trimethylation (Klose et al., 2006; Whetstine et al., 2006). JMJD2A is known to catalyze the removal of trimethylation at H3K9 and H3K36 and has been implicated in the regulation of gene expression, DNA damage signaling, DNA replication, and site-specific copy-number regulation (Black et al., 2013).
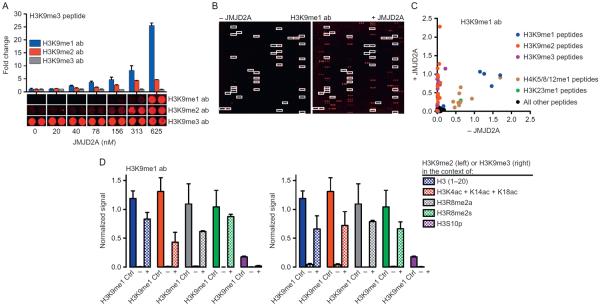
JMJD2A demethylase assays on histone peptide microarrays were first optimized using a 48-well array (Fig. 2). Using a single optimization slide, we simultaneously detected signal with three different antibodies to the states of H3K9 methylation following hybridization with six concentrations of JMJD2A, all in duplicate (Fig. 4A). Consistent with previous reports (Tsukada et al., 2006), we show that H3K9me3 peptides are reduced to lower order methyl states (K9me2 and K9me1) in the presence of JMJD2A. The H3K9me2 antibody used for these experiments cross reacts with H3K36me2 (Rothbart et al., 2015), and we were able to observe demethylation of H3K36me3-containing peptides at the higher JMJD2A concentrations used in these optimization experiments (data not shown). In general, detecting reaction products was a more robust readout for enzyme activity than monitoring the disappearance of substrate (Fig. 4A). This is perhaps due to the high sensitivity of primary antibodies and the amplification provided by secondary antibody conjugation.
Fig. 4.
Analysis of JMJD2A histone substrate specificity and the combinatorial PTM influence on KDM activity. (A) Optimization of JMJD2A demethylase activity on H3(1–20)K9me3 peptides in a 48-well microarray (see Fig. 2A). The indicated orders of H3K9 methylation were detected by antibody hybridization (H3K9me1, EpiCypher #13-0014; H3K9me2, Active Motif #39239; H3K9me3, Active Motif #39161) following 18 h incubation with the indicated concentrations of JMJD2A. Fold change is expressed relative to signal intensity in the absence of enzyme. Error bars represent ±S.E.M. (B) Representative images of arrays detected with an H3K9me1 antibody (EpiCypher #13-0014) following hybridization in the absence or presence of 313 nM JMJD2A for 18 h. White boxes demarcate peptides detected by H3K9me1 antibody in the absence of JMJD2A. (C) Scatter plot of all peptides on the large array detected with the abovementioned H3K9me1 antibody following hybridization in the absence or presence of JMJD2A. Signal intensities are normalized to IgG control spots. (D) Bar graphs depicting the effects of combinatorial PTMs on the enzymatic activity of JMJD2A from panel (B). Signal intensities from H3K9me1 antibody detection are normalized to IgG control spots. Shown are signals for the indicated peptides that also contain H3K9me2 (left) or H3K9me3 (right), both in the absence (−) and presence (+) of JMJD2A. To control for H3K9me1 antibody specificity in the context of these additional PTMs, normalized signal intensities from these peptides that also contain H3K9me1 (H3K9me1 Ctrl) are plotted as a reference.
Using assay conditions optimized on the 48-well microarray, we performed JMJD2A demethylase assays on a large-format array. Following incubation with JMJD2A, we monitored the appearance of H3K9me1 by antibody detection (Fig. 4B). Antibody reactivity in the absence of JMJD2A was tested as a control. Analysis of quantified signal intensities from both JMJD2A treated and untreated arrays revealed JMJD2A is able to demethylate the majority of H3K9me3- and H3K9me2-containing peptides, whereas the H3K9me1-containing peptides are not substrates (Fig. 4C). The H3K9me1 antibody used also cross-reacted with H3K23me1 and monomethylation of lysines 5, 8, and 12 on the H4 tail, and none of these PTMs were substrates of JMJD2A (Fig. 4C).
We next sought to determine how neighboring PTMs influenced the catalysis of JMJD2A toward H3K9me2 and H3K9me3. As discussed earlier, the behavior of histone PTM antibodies (positive and negative) when presented with combinatorially modified PTM epitopes is a factor that must be considered when using these reagents for enzyme assay detection. The arrays used for these experiments displayed five combinatorially modified H3(1–20) peptides that also harbored all three methylation states at H3K9 (Fig. 4D). Focusing on these peptides allowed us to properly control for the behavior of the H3K9me1 antibody toward the product of JMJD2A demethylation in the context of neighboring PTMs. We saw little effect of H3 poly-acetylation or H3R8 dimethylation on the activity of JMJD2A toward H3K9me2 or H3K9me3 substrates. However, we show that like G9A (above), and consistent with a previous observation (Ng et al., 2007), JMJD2A activity on H3K9me2 and H3K9me3 is blocked by neighboring H3S10 phosphorylation (Fig. 4D). These results demonstrate the utility of histone peptide microarrays for profiling the substrate specificity of KDMs and for deciphering the impact of the “histone code” on JMJD2A activity.
5. SUMMARY AND PERSPECTIVES
Peptide microarrays have been a valuable tool for studying the influence of histone PTMs on antibody and reader protein recognition (Rothbart et al., 2012). The methods presented in this chapter describe the expanded utility of this tool for investigating how PTMs influence the enzymatic activity of the writers and erasers of these marks. While others have previously used array formats for analyzing substrate specificity of histone methyltransferases, libraries for these assays were designed primarily for motif mining through amino acid substitutions (Dhayalan, Kudithipudi, Rathert, & Jeltsch, 2011; Kudithipudi, Dhayalan, Kebede, & Jeltsch, 2012; Kudithipudi, Kusevic, Weirich, & Jeltsch, 2014; Kudithipudi, Lungu, Rathert, Happel, & Jeltsch, 2014; Rathert, Dhayalan, et al., 2008; Rathert, Zhang, Freund, Cheng, & Jeltsch, 2008; Smith, Settles, Hallows, Craven, & Denu, 2011). Our studies describe a robust high-throughput assay format optimized for both substrate specificity profiling and analysis of the impact that PTMs have on both KMT and KDM activity. It is now possible to investigate one of the key tenets of the original “histone code” hypothesis, that neighboring PTMs on histones impact the function of the enzymes that write and erase these marks. Indeed, we found that PTMs adjacent to the target amino acid both enhance and abrogate the KMT and KDM activity of both G9a and JMJD2A, respectively. Furthermore, we identified several distant PTMs that strongly perturb and enhance G9a catalytic function.
While peptide microarrays are a powerful technology for studying the activity of histone-modifying enzymes, there are several limitations that should be noted. First, not all histone-modifying enzymes are active on peptide substrates, limiting the enzymes for which this platform can be employed. This is an outstanding issue for all peptide-based approaches, and an important challenge for the field, as substrate profiling of these enzymes remains challenging. Another important consideration when using antibodies as the detection method is ensuring the proper peptide diversity is represented in the displayed library. For example, the peptide that corresponds to the product of catalysis in the context of neighboring PTMs should be represented to accurately control for antibody specificity.
Characterization of the impact that PTMs have on the catalytic activity of histone-modifying enzymes will be crucial in understanding the dynamic histone PTM language. It is important to note that combined, nearly 80 KMTs and KDMs have been identified. Additionally, the methods described above can serve as guidelines for adapting this microarray approach for histone-modifying enzymes that catalyze the addition or removal of modifications other than lysine methylation. Thus, microarray-based enzyme assays and similar approaches are poised to be widely used tools to aid in unraveling how the histone PTM landscape is created, maintained, and erased.
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institutes of Health to S.B.R. (CA181343) and B.D.S. (GM110058). B.D.S. also acknowledges support from the W.M. Keck Foundation and is a cofounder of EpiCypher.
Footnotes
The volume requirement for a silicon adhesive seal is 350 and 500 μL for arrays separated by PAP pen.
Enzyme concentration was optimized on 48-well slides at a range from 0 to 1 μM (Fig. 2B); 1 μM G9a was used for large-format arrays (Fig. 3A).
For radioisotope assays, use 5 uCi 3H-SAM.
48-well optimization slides were incubated with 6 μL in each well without a coverslip.
REFERENCES
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131(4):633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Black JC, Manning AL, Van Rechem C, Kim J, Ladd B, Cho J, Whetstine JR. KDM4A lysine demethylase induces site-specific copy gain and rereplication of regions amplified in tumors. Cell. 2013;154(3):541–555. doi: 10.1016/j.cell.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RE, Tachibana M, Tamaru H, Smith KM, Jia D, Zhang X, Cheng X. In vitro and in vivo analyses of a Phe/Tyr switch controlling product specificity of histone lysine methyltransferases. The Journal of Biological Chemistry. 2005;280(7):5563–5570. doi: 10.1074/jbc.M410483200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrich HW., 3rd Isolation of sea urchin egg tubulin. Methods in Enzymology. 1986;134:128–138. doi: 10.1016/0076-6879(86)34081-3. [DOI] [PubMed] [Google Scholar]
- Dhayalan A, Kudithipudi S, Rathert P, Jeltsch A. Specificity analysis-based identification of new methylation targets of the SET7/9 protein lysine methyltransferase. Chemistry & Biology. 2011;18(1):111–120. doi: 10.1016/j.chembiol.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Dimitrova E, Turberfield AH, Klose RJ. Histone demethylases in chromatin biology and beyond. EMBO Reports. 2015;16(12):1620–1639. doi: 10.15252/embr.201541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs SM, Krajewski K, Baker RW, Miller VL, Strahl BD. Influence of combinatorial histone modifications on antibody and effector protein recognition. Current Biology. 2011;21(1):53–58. doi: 10.1016/j.cub.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Shi Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nature Reviews. Genetics. 2012;13(5):343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Sabari BR, Garcia BA, Allis CD, Zhao Y. SnapShot: Histone modifications. Cell. 2014;159(2):458–458.e451. doi: 10.1016/j.cell.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorasanizadeh S. The nucleosome: From genomic organization to genomic regulation. Cell. 2004;116(2):259–272. doi: 10.1016/s0092-8674(04)00044-3. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Yamane K, Bae Y, Zhang D, Erdjument-Bromage H, Tempst P, Zhang Y. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature. 2006;442(7100):312–316. doi: 10.1038/nature04853. [DOI] [PubMed] [Google Scholar]
- Kornberg RD, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98(3):285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Collazo E, Ortiz-Tello PA, Trievel RC. Purification and assay protocols for obtaining highly active Jumonji C demethylases. Analytical Biochemistry. 2012;420(1):48–53. doi: 10.1016/j.ab.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Kudithipudi S, Dhayalan A, Kebede AF, Jeltsch A. The SET8 H4K20 protein lysine methyltransferase has a long recognition sequence covering seven amino acid residues. Biochimie. 2012;94(11):2212–2218. doi: 10.1016/j.biochi.2012.04.024. [DOI] [PubMed] [Google Scholar]
- Kudithipudi S, Kusevic D, Weirich S, Jeltsch A. Specificity analysis of protein lysine methyltransferases using SPOT peptide arrays. Journal of Visualized Experiments. 2014;93:e52203. doi: 10.3791/52203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudithipudi S, Lungu C, Rathert P, Happel N, Jeltsch A. Substrate specificity analysis and novel substrates of the protein lysine methyltransferase NSD1. Chemistry & Biology. 2014;21(2):226–237. doi: 10.1016/j.chembiol.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Lee DY, Teyssier C, Strahl BD, Stallcup MR. Role of protein methylation in regulation of transcription. Endocrine Reviews. 2005;26(2):147–170. doi: 10.1210/er.2004-0008. [DOI] [PubMed] [Google Scholar]
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Maze I, Noh KM, Allis CD. Histone regulation in the CNS: Basic principles of epigenetic plasticity. Neuropsychopharmacology. 2013;38(1):3–22. doi: 10.1038/npp.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SS, Kavanagh KL, McDonough MA, Butler D, Pilka ES, Lienard BM, Oppermann U. Crystal structures of histone demethylase JMJD2A reveal basis for substrate specificity. Nature. 2007;448(7149):87–91. doi: 10.1038/nature05971. [DOI] [PubMed] [Google Scholar]
- Partyka K, Wang S, Zhao P, Cao B, Haab B. Array-based immunoassays with rolling-circle amplification detection. Methods in Molecular Biology. 2014;1105:3–15. doi: 10.1007/978-1-62703-739-6_1. [DOI] [PubMed] [Google Scholar]
- Petrossian TC, Clarke SG. Uncovering the human methyltransferasome. Molecular & Cellular Proteomics. 2011;10(1) doi: 10.1074/mcp.M110.000976. M110.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela A, Esteller M. Epigenetic modifications and human disease. Nature Bio-technology. 2010;28(10):1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- Rathert P, Dhayalan A, Murakami M, Zhang X, Tamas R, Jurkowska R, Jeltsch A. Protein lysine methyltransferase G9a acts on non-histone targets. Nature Chemical Biology. 2008;4(6):344–346. doi: 10.1038/nchembio.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathert P, Zhang X, Freund C, Cheng X, Jeltsch A. Analysis of the substrate specificity of the Dim-5 histone lysine methyltransferase using peptide arrays. Chemistry & Biology. 2008;15(1):5–11. doi: 10.1016/j.chembiol.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406(6796):593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- Rothbart SB, Dickson BM, Raab JR, Grzybowski AT, Krajewski K, Guo AH, Strahl BD. An interactive database for the assessment of histone antibody specificity. Molecular Cell. 2015;59(3):502–511. doi: 10.1016/j.molcel.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart SB, Krajewski K, Strahl BD, Fuchs SM. Peptide microarrays to interrogate the “histone code”. Methods in Enzymology. 2012;512:107–135. doi: 10.1016/B978-0-12-391940-3.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart SB, Strahl BD. Interpreting the language of histone and DNA modifications. Biochimica et Biophysica Acta. 2014;1839(8):627–643. doi: 10.1016/j.bbagrm.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha AJ. Java Treeview—Extensible visualization of microarray data. Bioinformatics. 2004;20(17):3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- Shankar SR, Bahirvani AG, Rao VK, Bharathy N, Ow JR, Taneja R. G9a, a multipotent regulator of gene expression. Epigenetics. 2013;8(1):16–22. doi: 10.4161/epi.23331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Smith BC, Settles B, Hallows WC, Craven MW, Denu JM. SIRT3 substrate specificity determined by peptide arrays and machine learning. ACS Chemical Biology. 2011;6(2):146–157. doi: 10.1021/cb100218d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. The Journal of Biological Chemistry. 2001;276(27):25309–25317. doi: 10.1074/jbc.M101914200. [DOI] [PubMed] [Google Scholar]
- Trojer P, Zhang J, Yonezawa M, Schmidt A, Zheng H, Jenuwein T, Reinberg D. Dynamic histone H1 isotype 4 methylation and demethylation by histone lysine methyltransferase G9a/KMT1C and the Jumonji domain-containing JMJD2/KDM4 proteins. The Journal of Biological Chemistry. 2009;284(13):8395–8405. doi: 10.1074/jbc.M807818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439(7078):811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- Weiss T, Hergeth S, Zeissler U, Izzo A, Tropberger P, Zee BM, Schneider R. Histone H1 variant-specific lysine methylation by G9a/KMT1C and Glp1/KMT1D. Epigenetics & Chromatin. 2010;3(1):7. doi: 10.1186/1756-8935-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West LE, Roy S, Lachmi-Weiner K, Hayashi R, Shi X, Appella E, Gozani O. The MBT repeats of L3MBTL1 link SET8-mediated p53 methylation at lysine 382 to target gene repression. The Journal of Biological Chemistry. 2010;285(48):37725–37732. doi: 10.1074/jbc.M110.139527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Shi Y. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125(3):467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Wu H, Chen X, Xiong J, Li Y, Li H, Ding X, Zhu B. Histone methyltransferase G9a contributes to H3K27 methylation in vivo. Cell Research. 2011;21(2):365–367. doi: 10.1038/cr.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Song C, Zhang Q, DiMaggio PA, Garcia BA, York A, Grunstein M. Histone H3 lysine 56 methylation regulates DNA replication through its interaction with PCNA. Molecular Cell. 2012;46(1):7–17. doi: 10.1016/j.molcel.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Garcia BA. Comprehensive catalog of currently documented histone modifications. Cold Spring Harbor Perspectives in Biology. 2015;7(9):a025064. doi: 10.1101/cshperspect.a025064. [DOI] [PMC free article] [PubMed] [Google Scholar]