Abstract
Two prominent features of tumors that contribute to oncogenic survival signaling are redox disruption, or oxidative stress phenotype, and high autophagy signaling, making both phenomena ideal therapeutic targets. However, the relationship between redox disruption and autophagy signaling is not well characterized and the clinical impact of reactive oxygen species (ROS)-generating chemotherapeutics on autophagy merits immediate attention as autophagy largely contributes to chemotherapeutic resistance. In this commentary we focus on melanoma, using it as an example to provide clarity to current literature regarding the roles of autophagy and redox signaling which can be applicable to initiation and maintenance of most tumor types. Further, we address the crosstalk between ROS and autophagy signaling during pharmacological intervention and cell fate decisions. We attempt to elucidate the role of autophagy in regulating cell fate following treatment with ROS-generating agents in preclinical and clinical settings and discuss the emerging role of autophagy in cell fate decisions and as a cell death mechanism. We also address technical aspects of redox and autophagy evaluation in experimental design and data interpretation. Lastly, we present a provocative view of the clinical relevance, emerging challenges in dual targeting of redox and autophagy pathways for therapy, and the future directions to be addressed in order to advance both basic and translational aspects of this field.
Keywords: cancer, autophagy, cell fate, reactive oxygen species, therapy
Graphical abstract
1. Introduction
Melanoma arises from the neoplastic transformation of normal, pigment-producing melanocytes, which reside in the basement layer of the epidermis, middle layer of the eye (uvea), and mucous membranes such as the gastrointestinal tract or oral cavity [1]. Recent studies in melanoma biology highlight the role of two critical cellular mechanisms as contributors to disease initiation and tumor maintenance; redox state and autophagy. In melanocytes, reactive oxygen species (ROS) serve as critical signaling mediators while autophagy serves as a cellular ‘quality control’, recycling mechanism. However, in melanoma cells, there exists a redox imbalance, or oxidative stress phenotype, where the accumulation of ROS is attributable to mitochondrial uncoupling, oncogenic mutations (i.e., BRAFV600E) and switch to glycolytic phenotype [2]. Compared to melanocytes, melanomas also display increased autophagic activity, which aids in tumor cell survival through sustained proliferative signaling, apoptosis evasion, and chemotherapeutic resistance [3-5]. Both redox signaling and autophagy have thus become attractive therapeutic targets to shut down pro-survival signaling features selectively in melanoma. However, there is a dearth of studies that examine the biological significance of the mechanistic relationship between ROS and autophagy in melanoma, feedback mechanisms, and the potential for pharmacological targeting of both pro-survival pathways as a therapeutic avenue. The purpose of this commentary is to address the known mechanisms that regulate the crosstalk between ROS and autophagy in melanoma cell fate outcomes and to present relevant research studies that contribute to our understaning of this signaling relationship. We provide clarification to current classifications of autophagy with regard to cell fate, particularly during pharmacological induction of oxidative damage, the technical challenges of measuring ROS and autophagy experimentally as well as the clinical relevance of dual targeting of redox and autophagy pathways for therapy. Finally, the preclinical and clinical challenges, and future directions in the field of redox-regulated autophagy as a therapeutic target are presented.
2. Role of ROS in melanoma
2.1 Pro-oxidant state potentiates melanomagenesis
Melanocytes are dendritic cells of neural crest origin, and synthesize melanin in specialized organelles called melanosomes. Biologically, melanin functions in the epidermal layer to absorb light as a UV-protective mechanism, which results in a high pro-oxidant state in melanocytes [6]. Melanocytes have an intrinsic antioxidant defense network in order to maintain redox homeostasis [7]. However, prolonged disruption of redox balance can result in rapid hydrogen peroxide (H2O2) generation and impaired antioxidant activity (catalase, heme oxygenase 1), culminating in an oxidative stress state, due to an imbalance of ROS and antioxidant capacity [8]. In addition to the pro-oxidant state of melanocytes, genetic polymorphisms in genes encoding antioxidant repair enzymes, specifically GPX1, have been associated with a significantly increased risk of breast cancer and melanoma [9, 10]. As such, elevated ROS and impaired antioxidant activity results in oxidative stress and melanomagenesis. Appropriately, melanoma has been termed a “ROS-driven tumor” [8]. Many preclinical studies have demonstrated that melanoma cells are particularly susceptible to increases in ROS compared with melanocytes [11-14]. This observed redox sensitivity is likely attributable to i) elevated antioxidant capacity in melanocytes, able to efficiently counteract oxidative insult compared with melanoma cells (which may have antioxidant gene mutations [15-17]) and ii) the metabolic requirement for and sensitivity to chronically elevated ROS levels for maintenance of oncogenic signaling [18]. A proposed model for the basis of this selective sensitivity of melanoma cells to ROS-generating therapeutic agents is summarized in Figure 1.
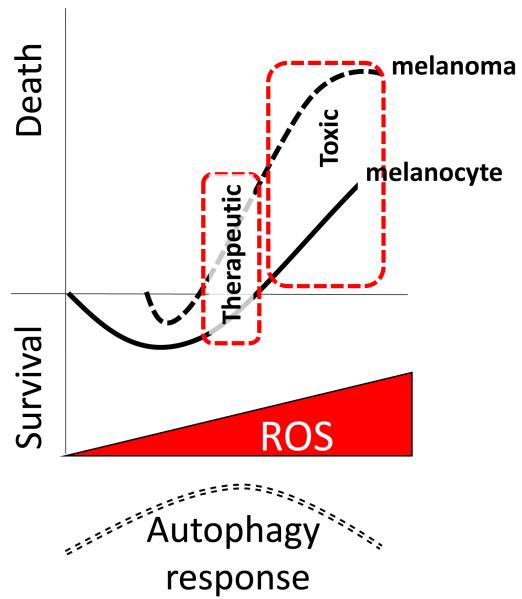
Figure 1. Hormetic effect of ROS increase on melanoma versus melanocyte cell fate: harnessing for therapeutic potential.
The proposed model is based on the threshold premise of reactive oxygen species having differential effects on cell response. The hormetic principle proposed here speculates that different levels of ROS are stimulatory for melanoma and melanocyte survival and provoke survival responses such as autophagy in attempt to rid the cell of oxidized/damaged contents. However, as ROS increases beyond the cell’s coping threshold, cell fate switches to death as apoptotic mechanisms ensue. The autophagy response (dashed curve, bottom) is induction as ROS increases, but eventual shut down/decline of the pro-survival autophagic mechanisms. We propose that the hormetic curve is altered in melanoma cells (dashed line) so that at the same amount of exogenous ROS generation, the melanocyte’s response (solid line) will be survival and the melanoma cell response will be death. This represents a therapeutic window for ROS-generating anti-cancer agents, and past a certain threshold, high enough ROS will lead to toxicity in both cell types. Contributing factors to this proposed hormetic alteration is based on literature showing decreased antioxidant capacity in melanoma cells owed to mutations in SOD1/2, and the existing basal oxidative stress state in melanoma cells that render them particularly susceptible to further ROS escalation.
2.2 ROS modulation as a therapeutic strategy
A variety of ROS molecules cause irreversible macromolecular damage. Chronically high levels of ROS in cancer cells promotes genomic instability and uncontrolled proliferation. The ‘threshold concept’ of ROS as a therapeutic target has emerged as a viable anti-cancer strategy that takes advantage of the selective vulnerability of cancer cells to disruptions in redox homeostasis [19]. We define ROS threshold as a window of redox cycling which a cell can sustain, without affecting or triggering cell death mechanisms. We have recently demonstrated that human melanoma cells have an elevated oxidative stress phenotype compared with melanocytes, and that disrupting the melanoma cell oxidative stress ‘threshold’ perturbs PI3K/AKT/mTOR oncogenic signaling required for survival, and culminates in cell death [20]. Clinical efforts that seek to modulate redox disruption in melanoma patients are reinforced by multiple studies showing increased serum levels of oxidative stress markers (malondialdehyde; MDA, oxidized protein products, SOD activity etc.) in metastatic melanoma patients compared with either healthy volunteers or non-metastatic patients [21, 22]. Figure 1 depicts the nature of selective ROS generation resulting in different cell fates between normal melanocytes and melanoma cells.
In the way of pro-oxidant therapeutics, we present the small molecule STA-4783 (Elesclomol) as a case study to highlight the utility of a pro-oxidant therapy, in which elevation of intracellular ROS results selectively in melanoma cell apoptosis. Elesclomol showed exquisite selectively in tumor reduction in vivo, that resulted in a significant doubling of mean progression free survival (PFS) when combined with paclitaxel for patients with metastatic melanoma [23-25]. Elesclomol complexes with (Cu)II to disrupt mitochondrial electron transport chain, rapidly depolarize mitochondrial membranes, and induce apoptosis [23]. The success of Elesclomol against aggressive, late stage melanoma in the clinic highlights the significance of developing ROS-inducing drugs as anti-melanoma agents. Further, Elesclomol treatment resulted in apoptosis in Vemurafenib-resistant melanoma cells, the result of a chronically elevated mitochondrial respiration and oxidative stress [14]. Elesclomol has also been evaluated in clinical trials for efficacy against solid tumors, lung, breast, and prostate cancers, and most recently acute myeloid leukemia [26-28]. At the time of submission of this commentary, Elesclomol is under evaluation for combined efficacy with Paclitaxel in a Phase II clinical trial for ovarian, fallopian tube, and primary peritoneal cancers, thus laying the foundation for the clinical relevance of pro-oxidant therapy. In addition to Elesclomol, there is overwhelming support for pharmacological redox modulation as a viable therapeutic target across cancer subtypes. Specifically, we highlight recent studies in which pharmacological redox modulation results in melanoma cell apoptosis, summarized in Table 1 [20, 29-33].
Table 1.
Effect of pharmacological modulators of ROS on melanoma cell fate.
| 20Modulator | Model | ROS effect | Techniques | Cell fate | Overall conclusion | Ref |
|---|---|---|---|---|---|---|
| Nexrutine | Melanoma cells & melanocytes |
Induction | H2DCFDA, mitoSOX; Oxidative stress markers |
Apoptosis | ROS, PI3K/mTOR inhibition & apoptosis induction |
[20] |
| Benzofuroxan N-Br & N-I derivatives |
B16F10-Nex2 cells; syngeneic mouse model |
Induction | Dihydroethidium; fluorescent microscopy; |
Apoptosis | Mitochondrial ROS & reduced tumor burden |
[29] |
| Luteolin | Melanoma cells | Induction | H2DCFDA | Apoptosis | ER stress/ROS with apoptosis | [30] |
| Isoliquiritigenin | B16F10 cells | Induction | H2DCFDA | Apoptosis | Mitochondrial ROS & apoptosis | [31] |
| DDSD | B16F10 cells & syngeneic mouse model |
Induction | H2DCFDA | Apoptosis | Mitochondrial apoptosis & reduced tumor burden |
[32] |
| 4-DACL | Melanoma cells, spheroids, melanocytes |
Induction | CellROX Green & Deep Red Reagent |
Apoptosis | Mitochondrial ROS, inhibition of proliferation and cell cycle |
[33] |
4-DACL: (±)-4-deoxyaustrocortilutein; DDSD: (5(R), 19-diacetoxy-15,18(R and S), dihydro spata-13, 16(E)-diene
2.3 Antioxidant modulation as a therapeutic strategy
In contrast to a pro-oxidant approach, disrupting the redox balance through modulation of the antioxidant defense system has proved to be much less efficacious as an anti-melanoma strategy [34]. We discuss two approaches and their cell death efficacy: 1) increasing cellular antioxidants and 2) depleting cellular antioxidants.
Historically, antioxidant supplementation has been proposed as a valid anticancer strategy, more often than depletion of cellular antioxidants. Preclinical studies have demonstrated that antioxidant supplementation leads to selective melanoma cell death through increased ROS-scavenging activity mediated by elevated SOD and catalase activity [35-37]. By contrast, dietary polyphenol antioxidants such as like resveratrol, tocopherol, and quercetin exert direct ROS-scavenging activity and have produced highly variable preclinical and clinical outcomes [38]. Clinical efforts have consistently failed to demonstrate antioxidant supplementation as an efficacious anti-cancer strategy, offering either negative or conflicting outcomes. A clinical trial showed that the incidence of melanoma increased in women receiving a combination of antioxidant supplementation [39]. Additionally, a pilot trial showed that alpha-tocopherol supplementation failed to provide protection against chemotherapy-induced DNA damage in melanoma patients [40]. The lack of clinical translation of early preclinical data had been perplexing, although newer preclinical data is pointing towards a lack of benefit of this modality. The utility of increasing the cellular antioxidant pool likely holds the greatest anti-cancer potential in prevention. The potential of antioxidnats to protect tumor cells when used as a therapeutic, may explanation the observed lack of clinical efficacy. As an alternative approach to antioxidant supplementation, the employment of antioxidant-depleting strategies has resulted in considerable higher efficacy in melanoma through excessive ROS generation and cell death [41, 42]. For example, downregulation of the NRF2 antioxidant defense pathway has shown marked anti-melanoma activity [43]. Additionally, depletion of the glutathione system in melanoma appears to have marked success in potentiating a pro-apoptotic response, as the accumulation of ROS leads to melanoma inhibition both in vitro and in vivo [44, 45]. Inhibition of SOD activity also shows selective anti-melanoma activity and represents an attractive therapeutic target [45, 46].
One consideration for antioxidant-modulating strategies is the finding that many antioxidant enzymes are found to be mutated in cancer, including SOD2, a mitochondrial matrix enzyme responsible for superoxide (O2.−) detoxification [41]. However, emerging evidence points to molecular redundancies in which SOD1 is overexpressed in cancer cells to cope with the genetic or functional loss of SOD2 [41]. Further, literature suggests that a concomitant antioxidant capacity is required to cope with increased oxidative stress and metabolic demands of cancer cells, implicating antioxidant modulation in tumorigenesis [47]. Therefore, future studies that evaluate the role of antioxidant gene mutations and loss of function will be necessary before the clinical implementation of an antioxidant-modulating therapy, which may promote melanoma development, as was found in prostate cancer prevention trial with selenium and vitamin E (SELECT) and the Nutritional Prevention of Cancer Trial (NPCT) [48].
2.4 Considerations for ROS evaluation
Experimental set up and data analyses to evaluate ROS can be quite complex. An exhaustive review of such methods is beyond the scope of this commentary, but a few basic guidelines are suggested. The fluctuating O2 tension in cell culture environment, and supplements contained in culture media can be sources of artifacts regarding measures of ROS and antioxidants, and must be taken into consideration [49]. In tissue culture assessments of ROS, there are many useful tools for generation and detection of total and specific ROS molecules. Further, the use of Electron Spin Resonance (ESR) spectroscopy is a robust methodology for qualitative and quantitative assessment of specific ROS molecules. The use of fluorescent-scanning microplate readers and fluorescence microscopy should be utilized judiciously, with careful considerations to minimize photobleaching and well-to-well scatter with use of black, clear-bottom culture plates. Chemiluminescent and chromogenic reagents are practical alternatives to validate ROS. The dynamics of ROS is an important criterion, as intracellular ROS are rapidly produced and detoxified. Therefore, kinetic assessment of ROS with controls (inducers and quenchers) is recommended. In animal models or patients, the modulation and detection of ROS is more complex than cell culture models, and as such, the detection of oxidative stress biomarkers from serum can provide clues to the overall basal redox status and after pharmacological modulation. Various biomarkers of oxidative stress including serum MDA, lipid peroxidation, reduced glutathione, catalase, glutathione peroxidase, and immunostaining for 8-hydroxy-2′-deoxyguanosine may be used. Therapy-naïve melanoma patients had an elevated oxidative stress profile, as assessed by increased plasma levels of Malondialdehyde (MDA) and decreased erythrocyte levels of SOD, compared with healthy control volunteers [22]. Interestingly, after complete surgical removal of melanoma tissues, patients showed decreased oxidative stress (serum MDA), which was elevated again after chemotherapy intervention with 5-(3,3-dimethyltriazene-1-yl)-imidazole-4-carboxamide (DTIC) and nitrosourea 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) [22]. Clinical evaluation of melanoma patient serum for thiols, catalase and SOD activity levels, and protein oxidation products has also been demonstrated as valid means to conclude overall level of oxidative stress [21]. For detailed information regarding the measurement of redox status, the reader is encouraged to read an excellent review by Liu-Smith et al [50].
3. Role of autophagy
3.1 Autophagy in melanoma development
Autophagy is an evolutionarily conserved catabolic process by which damaged proteins and organelles are encapsulated in the double-membraned autophagosome and degraded after delivery to the lysosome in a highly orchestrated series of molecular events. Although there are 3 subtypes of autophagy, the scope of this commentary focuses on macroautophagy, which will be referred to hereafter as “autophagy”. Under basal cellular conditions, autophagy serves to maintain homeostasis by recycling of cytosolic components, but is induced in response to a multitude of stimuli including starvation, viral invasion, hypoxia, increased intracellular ROS, and toxin/drug exposure [51]. Stress signaling kinases like JNK1 stimulate autophagy by phosphorylation of BCL2, which promotes the interaction of Beclin-1 and Vps34. Low ATP levels and hypoxia also stimulate autophagy by impairing mTOR kinase activity through reduced RHEB GTPase activity. Autophagy is generally recognized as a pro-survival mechanism, although classifications exist where autophagy is considered a type of programmed type-II cell death [52, 53]. The role of autophagy in melanoma, like other cancers, although complex, can be simplified as follows: protective against tumor initiation (pro-survival for normal melanocytes), and protective against chemotherapeutic agents, immune surveillance, and apoptotic signals (pro-survival for melanoma cells). So, while the published literature can appear overwhelmingly complex with regard to the function of autophagy in cell fate decisions, we present the viewpoint that the role of autophagy is exquisitely simple: cell survival at all costs.
A key autophagy-related protein 5 (ATG5; required for autophagosome formation), functions to induce senescence in melanocytes to prevent transformation [54, 55]. Melanocytes can undergo senescence due to increased polyploidy and subsequent autophagy activation during treatment with chemotherapeutic agents [56]. In contrast, loss of ATG7 results in melanocyte senescence and prevents melanoma development in BRAFV600E/PTEN-null mice, supporting a pro-tumorigenic role for autophagy [57]. Further, loss of ATG7 resulted in decreased melanocyte proliferation owed to increased p16Ink4a activity that triggered senescence. Perhaps in the background of BRAF and PTEN mutation, autophagy is required for continued tumor growth, but in non-transformed melanocytes it contributes to senescence. However, since autophagy is executed independently of ATG7, perhaps it is dispensable contextually, and evaluation of autophagy proteins like tumor suppressor Beclin-1 might provide a more complete picture of the significance of autophagy during melanocyte transformation. Mechanistically, Beclin-1 is a negative regulator of Myeloid Cell Leukemia 1 (Mcl-1) and promotes proteasomal degradation of Mcl-1 to restrain melanocyte transformation [58]. The autophagic protein, Beclin-1 constitutively suppressed melanocyte transformation, and loss of the protein in patients contributed to increased Mcl-1 and melanoma progression [58]. Further, autophagy is linked to melanogenesis and in melanocytes, LC3 has been shown to drive MITF expression and increase melanin content [59]. As such, autophagy-related proteins like LC3 would be detrimental when lost in normal melanocytes, as it is critical for melanogenesis and senescence activation.
In contrast to the anti-transformative role of autophagy in melanocytes, the role of autophagy in melanoma tumor development and progression is complex [5, 60, 61]. Specifically, we found several studies that conclude conflicting roles for autophagy in melanoma development and as prognostic markers. Mirraco et al found that Beclin-1 and LC3B messenger RNA levels had significant inverse correlation with melanoma progression, with the lowest expression seen in metastatic lesions, suggesting that loss of autophagy drives melanoma progression [62]. In addition, Liu et al demonstrated that ATG5 and LC3B protein levels decreased in metastatic melanoma patient samples compared with benign nevi, indicating that a loss of autophagy may promote tumor formation [63]. However, these two patient-based studies have limitations in that they cannot address the dynamic process of protein turnover during autophagy, as lower protein level could represent a high turnover and thus high autophagic activity. In contrast, several studies report high levels of autophagy proteins as predictors of melanoma, supporting the pro-survival role of autophagy in melanoma development and progression. Specifically, elevated LC3B expression, p62 and high autophagosome number in melanoma tumors are associated with aggressive disease and poor patient survival [5, 60, 61]. One possible explanation for these divergent conclusions of the role of autophagy may be owed to genetic mutations which modulate the autophagy process. Specifically, PIK3CA and PTEN mutations in the PI3K/AKT/mTOR pathway have been shown to drive high autophagic activity [64-67]. The clinical relevance of AKT-driven autophagic activity in melanoma is underscored by the clinical response of melanoma patients after combined inhibition of PI3K/AKT/mTOR activity and autophagy [4, 68]. A summary of studies that examine the effect of pharmacological modulation of autophagy on melanoma cell fate is included in Table 2 [69-74].
Table 2.
Effect of pharmacological modulators of autophagy on melanoma cell fate.
| Modulator | Model | Autophagy effect |
Measures of autophagy | Cell fate | Overall conclusion | Ref |
|---|---|---|---|---|---|---|
| CQ | Melanoma cells |
Inhibition | LC3B-I/II, ATG5, ATG7; autophagic flux |
Cell death | Sensitivity to autophagy is independent of BRAF |
[69] |
| α-Mangostin, Sorafenib |
Melanoma cells |
Inhibition | LC3B-I/II and ATG5 | Apoptosis | Inhibition of AKT/ERK, ER stress, autophagy inhibition |
[70] |
| AJ-5 | Melanoma cell lines |
Induction | TEM; GFP-LC3 puncta; autophagic flux by LC3B-I/II and Beclin-1 proteins |
Autophagic cell death; apoptosis |
ATM-CHK2 activation, apoptosis & autophagic cell death |
[71] |
| Pentoxifylline | Melanoma cells |
Induction | LC3B-I/II, p62, ATG5, Beclin-1; TEM; p62 co-localization with LC3B |
Apoptosis | Ca2+ overload, ER stress, mTOR inhibition & autophagy activation through ATG5 |
[72] |
| Mibefradil, Pimozide |
Melanoma cell lines |
Inhibition | LC3B-I/II, Beclin-1, ATG5-12, p62 |
Apoptosis | ER stress, autophagy inhibition, apoptosis induction |
[73] |
| Ursolic acid Resveratrol |
Melanoma cells |
Inhibition | Acridine orange; Beclin-1, LC3B-I/II & p62 protein |
Decreased viability | Reduced cell viability through autophagy inhibition |
[74] |
AJ-5: binuclear cyclometalated Pd(II) complex; CQ: chloroquine
3.2 Autophagy as a programmed death mechanism
With regard to autophagic cell death, there have been very few melanoma studies that show an exclusive role for autophagy as a cell death mechanism. Opinion in the field is that features of autophagy occur during cell death induction, and what really exists is cell death ‘with’ autophagy, not cell death ‘by’ autophagy [75]. The published literature shows that some studies conclude that apoptosis is the consequence of autophagy induction, while others argue that the two are independent cell death mechanisms. These differences can be corrected when examining the nature of external stimulus applied as was done in some studies [76-78].
Another consideration for evaluation of autophagic cell death is the contribution of the signaling pathways. For example, many natural compounds used in chemoprevention, have demonstrated anti-inflammatory, ant-oxidant and pro-apoptotic activities. Therefore, caution is warranted in the interpretation of studies with regard to autophagic cell death, due to the multiple signaling cascades being affected simultaneously. We must clarify that some components of the autophagic machinery have been shown to be involved in the “switch” from either pro-survival autophagy to pro-apoptotic, or from necrosis to apoptosis, which are mediated through the multifunctional, scaffolding protein SQSTM1/p62. These mechanisms can include p62 serving as a scaffold for caspase 8 oligomerization and subsequent processing (switch from autophagy to apoptosis) and failure of recruitment of Receptor-interacting serine/threonine-protein kinase 1 (RIPK1) to p62 (resulting in switch from apoptosis to necrosis) [79-82]. The contribution of autophagy proteins to cell death, either apoptotic or necrotic has been demonstrated, but the exclusive, upstream initiation of autophagy by ATG/Beclin-1-dependent mechanisms that lead to autophagosome formation and subsequent programmed cell death are lacking, particularly in melanocytes and melanoma biology [83]. We acknowledge that autophagic cell death, and autophagy induction can exist concurrently with the induction of apoptosis. Additionally, the emergence of ferroptosis- which is iron and ROS-dependent programmed cell death- certainly warrants further investigation, as a recent study shows that ferroptosis is an autophagic cell death process [84]. Future studies will be required to determine whether inducing autophagy actually results in autophagy-regulated programmed cell death, and the clinical relevance of inducing autophagy for melanoma therapy. Overall, preclinical and clinical studies highlight the therapeutic significance of pharmacological inhibition of autophagy with either chloroquine or hydroxychloroquine (CQ; HCQ) with melanoma regression and increased overall patient survival [5, 68, 85].
3.3. Challenges in autophagy measurement
Although in vivo measurements of autophagy can be more reliable than in vitro culture models, it is important to be aware of the limitations and challenges when interpreting data. While the ease of modulating autophagy dynamics, throughput nature and ability to use multiple cell lines and assays makes cell culture a good model system; it is essential to overcome the challenges of data interpretation. Caution is warranted in the measurement of mTOR-mediated autophagy, as both autophagic stimulation and inhibition can occur contextually. For example, under serum-starved conditions, mTOR-induced autophagy will be significantly higher than in serum-replete conditions, and surrogate markers of mTOR inhibition (phosphorylation of P70S6K targets phospho-rpS6, phospho-4EBP1), which might suggest autophagy induction, can actually conflict with other readouts of autophagy inhibition. Additionally, one must be cautious in interpretation of data after pharmacological inhibition of mTOR (i.e., rapamycin, rapalogs) compared with starvation-induced autophagy, as the result will often be different in magnitude. This difference can be attributed to measuring the “basal autophagic flux” during pharmacological inhibition, versus measuring the “maximal autophagic capacity” during serum-starved conditions. A further complication of mTOR-mediated autophagy is the use of autophagy inhibitors such as 3-methyladenine (3-MA), which block autophagosome formation by inhibition of class III PI3Ks. The caution of using 3-MA or wortmannin (similar mechanism of PI3K inhibition) as autophagy inhibitors comes from multiple reports that use of 3-MA (in combination with autophagy modulator of interest) actually stimulates autophagy due to the temporal-specific inhibition of class III PI3Ks (transient), and prolonged inhibition of class I PI3Ks, which in turn activates autophagic flux [86].
One particularly challenging aspect of measuring autophagy markers in clinical samples is the inability to capture the dynamic process of “autophagic flux”. In contrast to in vitro studies, obtaining end point measures of either LC3B-II, ATGs, Beclin-1, or selective autophagy substrates such as p62, in human samples are not conclusive measures of autophagy. There are many conflicting reports of autophagy protein levels being predictive of overall patient survival, or protein (IHC) staining correlating with disease state or prognosis [60-62, 87-89]. Presumably, these protein outputs can be increased in patients with high autophagy tumors, or increased in patients with low autophagy tumors, reflected by a lack of protein degradation. In addition to measuring autophagy protein levels by staining intensity, the evaluation of autophagosome formation by transmission electron microscopy (TEM) is considered the “gold standard” for autophagosome measurement. However, technical challenges of TEM are rampant in that clinical samples are often not preserved for subsequent TEM evaluation and pathology expertise is crucial for proper interpretation of TEM images, as melanosomes can be mistaken for double-membraned autophagosomes [90]. Further, melanosomes are degraded by autophagy and are present in the cell as macromelanosomes and autophagic giant melanosome complexes, which can add a significant complication to evaluation of autophagosome formation not owed to melanosome degradation [91]. Other barriers arise in TEM from the melanosome content in some but not all melanomas, and thus, one has to be cautious regarding the interpretation of autophagy by autophagosome number if melanosomes are highly enriched in a sample. However, the combination of proper experimental controls, sample processing, and pathology expertise can be implemented to take advantage of TEM as an excellent tool to evaluate autophagosome formation in experimental conditions.
Given the divergent conclusions of published studies regarding autophagy protein markers and autophagosome formation, the utility of autophagy inhibitors (lysosomotropic agents) such as chloroquine (CQ) or hydroxychloroquine (HCQ) in clinical trials for melanoma (either alone or as a combination therapy) supports conclusions in the literature that autophagy is a pro-survival feature of melanomas, and so inhibiting the process pharmacologically is not only of clinical relevance, but a priority [5, 68, 85]. A recent study by Kraya et al demonstrates the utility of assessing secreted proteins in serum from patients with metastatic melanoma as a way to evaluate the intracellular dynamics of autophagy in patients, and to stratify patients as having low versus high autophagy melanoma tumors, which could point to therapeutic response [92]. This study signifies the transition to validation and acceptance of more relevant approaches to evaluating patient autophagy levels as a prognostic factor or as a guide to a specific therapeutic approach. Overall, the clinical implementation of autophagy inhibitors seems to be most relevant in conjunction with current melanoma chemotherapies, presumably due to the role of autophagy in drug resistance and as a pro-survival mechanism in melanoma tumor cells. Specifically, autophagy inhibition has been shown to potentiate the anti-melanoma effect of multiple preclinical and clinical drugs, including mTOR inhibition by temsirolimus, BRAF inhibition with Vemurafenib, and DNA alkylation by temozolomide [85, 93].
4. ROS-autophagy crosstalk and therapeutic targeting
Reactive oxygen species are generated in response to chemotherapeutic agents [22]. Excessive ROS production results in disruption of the electron transport chain and production of O2.−, leading to mitochondrial membrane depolarization and initiation of mitochondria-induced apoptosis. It is generally accepted that ROS generation precedes downstream cellular cascades, including those that determine cell fate either survival (autophagy) or death (apoptosis, necrosis). However, ROS generation has also been shown to occur following apoptotic stimulation (TRAIL-induced), or autophagy inhibition, which places ROS downstream of cell fate cascades [94, 95]. As expected, ROS and autophagy can regulate each other depending on the stimulus, and so there exists much complexity in dissecting the interplay between ROS and autophagy in cell fate. Based on our review of the literature, ROS regulates autophagy and subsequent pro-survival versus pro-death cell fate contextually. Cell fate outcomes are largely dependent on the amount of ROS generated and the cell’s antioxidant response. A summary of studies that examine the effect of dual modulation of ROS and autophagy on melanoma preclinical and clinical outcomes is presented in Table 3 [43, 76, 85, 96-100].
Table 3.
Effect of pharmacological induction of ROS and subsequent effect on autophagy on melanoma models
| Modulator | Model system | Effect on ROS |
Effect on autophagy |
Outcome | Conclusion of ROS-autophagy relationship |
Ref |
|---|---|---|---|---|---|---|
| PFT-μ + NVP-AUY922 | Melanoma cells & xenograft model |
Induction | Inhibition | Apoptosis; decreased tumor growth |
ER stress, ROS induction and autophagy inhibition |
[43] |
| Graveoline | Melanoma cells | Induction | Induction | Apoptosis | ROS generation upstream of autophagy |
[76] |
| Hydroxychloroquine (HCQ) + temozolomide (TMZ) |
Metastatic melanoma patients |
TMZ induce ROS |
Inhibition | Stabilized disease and response |
Therapeutic potential of combination compared to TMZ alone |
[85] |
|
Polygonatum cyrtonema lectin (PCL) |
Melanocytes Melanoma cells |
Induction | Induction | Apoptosis | Glutathione depletion, mitochondrial ROS and induction of autophagy |
[96] |
| Cisplatin | Human melanoma cells | Induction | Inhibition | Apoptosis | ROS generation and inhibition of Beclin1/LC3B-mediated autophagic response |
[97] |
| Physalin A | Melanoma cells | Induction | Induction | Apoptosis | ROS generation, autophagy induction |
[98] |
| Fisetin | Melanoma cells | Induction | Transient induction |
Apoptosis | ER stress and ROS generation, autophagy induction |
[99] |
| Usnea barbata | B16 mouse melanoma | Induction | Induction | Apoptosis | Oxidative stress induces autophagosomes and apoptosis |
[100] |
During starvation, H2O2 is produced as a result of class III PI3K activation that stimulates autophagy through oxidation of ATG4. This inhibits the de-lipidation potential of ATG8, ultimately increasing the formation of lipidated LC3-rich autophagosomes [101]. Both O2.− and H2O2 can also induce autophagy through AMPK activation and subsequent mTOR inhibition, and by transcriptional regulation of autophagy genes such as SQSTM1 (p62) and BECN1 [102-104]. ROS may also activate KEAP1/NRF2 in which case, transcriptional upregulation of selective autophagy substrate SQSTM1 (p62) occurs [105]. Several studies have shown a similar trend where exogenously applied ROS leads to autophagy induction, and in a majority of studies the cell fate is apoptosis. A summary of the role of ROS and autophagy and resulting cell fate in melanocytes and melanoma can be found in Tables 1-3. A more critical evaluation of the published literature, specifically with reference to those studies that induce ROS with pharmacological agent and measure autophagy and apoptosis, reveals a lack of thorough evaluation of autophagy with proper experimental controls (see also Challenges in autophagy measurement). In melanoma, the lack of studies that evaluate a dose-response effect of ROS on autophagy (stimulation or inhibition) in a mechanistic fashion is very concerning from a therapeutic standpoint. Perhaps the role of autophagy is dispensable if the ultimate output is cell death? In the aforementioned published literature in which autophagy outputs are properly assessed and still found to be stimulated during redox disruption, the role of autophagy as a survival feature seems to be validated, even though ROS can stimulate autophagic and apoptotic signaling pathways concurrently. In our view, the cell’s ability to induce protective autophagy can co-exist with apoptosis, but at reaching a certain ‘threshold’, pro-survival autophagy cannot rescue the induction of a cell death cascade. Studies that cite induction of autophagy and apoptosis are essentially providing the viewpoint of what we might deem as cells that “die trying”. It must also be noted that the experimental endpoints of apoptosis and autophagic cell death in response to external stimulus cannot capture the potential cell-to-cell intracellular signaling variability (i.e. some cells induce apoptosis while others induce pro-survival autophagy). However, bonafide mechanistic measures of autophagy actually promoting apoptosis, as opposed to parallel intracellular assessments, might provide a more accurate account of a cell fate “switch” during exogenous ROS generation. Further, it cannot be overstated that conflicting measures of autophagy inhibition and induction can occur due to the pharmacological approach utilized. In multiple other cancers, the inhibition of autophagy during high ROS is better characterized, and the molecular mechanisms specifically, caspase activity point to a “switch” in the cellular signaling that blunts pro-survival autophagy and instead induces a pro-death apoptotic cascade [106]. In particular, cleavage of Beclin-1 by caspase-8 during high oxidative damage leads to failure of Beclin-1-regulated autophagy and subsequent autophagy inhibition [107]. Further, there is evidence of oxidative damage of autophagy gene promoter regions, including p62, which is significant enough to inhibit autophagy altogether [108]. There is also evidence that ROS induction inhibits autophagy through decreased expression of autophagy initiator ULK1 [109]. Additionally, autophagy proteins may be subject to redox-specific post-translational modifications at thiol residues, which may determine their role as pro-survival versus pro-death [110]. Specifically, phosphorylation of p62 at Ser. 349 during oxidative stress leads to increased NRF2 activity by competitive binding to KEAP1 [111].
Clearly, there is a contextual role of ROS on inhibition versus stimulation of autophagy and the resultant cell fate. We present a graphical illustration of the divergent cell fate outcomes with relation to ROS generation in Figure 2. Preclinical evaluation of chemotherapy-induced ROS by the anthracycline, Mitoxantrone further demonstrates that functional autophagy is a requisite for efficacious melanoma therapy, highlighting the clinical significance of ROS-autophagy considerations in translational approaches [112]. Future mechanistic studies of these autophagy inhibition mechanisms during ROS generation will ultimately unveil the potential impact of pro-oxidants in melanoma. Moreover, pro-oxidant chemotherapeutics that stimulate pro-survival autophagy may have enhanced efficacy when combined with autophagy inhibitors.
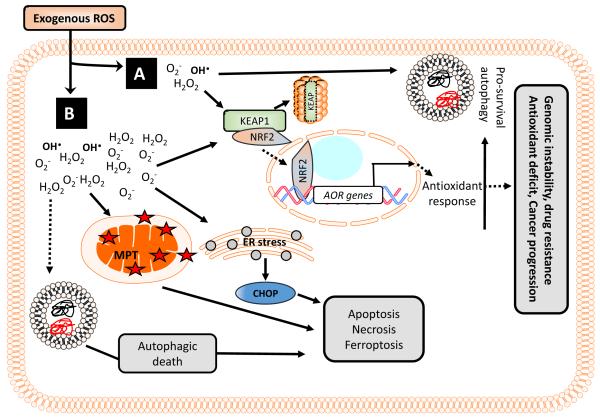
Figure 2. Role of ROS-mediated autophagy during melanoma cell fate determination.
(A) Cancer cells respond to low or transient exogenous ROS generation (chemotherapy, radiation) by KEAP1/NRF2-mediated oxidative stress response, and ER-mediated unfolded protein response to rid the cell of damaged lipids and proteins. Autophagy is initiated and sustained, leading to cell survival. The result is a cell with increased baseline autophagic activity, and continued exogenous ROS-induced stress leads to increased redox stress, genomic instability and therapeutic resistance over time. (B) Cancer cells respond to high or sustained exogenous ROS generation by an attempted KEAP1/NRF2-mediated oxidative stress response and initial autophagy activation. However, sustained ER stress, mitochondrial membrane depolarization, and activation of the caspase cascade results in the a pro-death response, by which time the autophagy response has been inhibited because the cell has committed to die. The molecular switch by which autophagy can no longer serve as a survival mechanism occurs when autophagy proteins become dysfunctional, participate in pro-apoptotic signaling, are post-translationally modified or the cell’s pro-apoptotic, necrotic, or ferroptotic signals have hierarchy above the proautophagic response. In some instances, a sustained autophagic response may result in autophagic cell death in the absence of pro-apoptotic signaling.
5. Future directions
There are several key issues that hamper the current understanding of the interplay between ROS and autophagy dynamics. Firstly, there is a lack of studies that evaluate the dynamic interplay between autophagy and redox signaling. In vivo studies that evaluate the relationship between ROS production and autophagy in melanoma are limited, and clinical studies are absent which evaluate autophagy measurements after traditional ROS-inducing chemotherapies. Evidence supporting ROS and autophagy, as individual targets are promising, but we urge the push for mechanistic evaluation of ROS-autophagy relationship, using proper pharmacological and genetic approaches, in panels of cell lines or human tissues, and use of multiple methodological assessments of autophagy. Another key issue is regarding the understanding of the role of autophagy as a programmed cell death mechanism. In future studies, there is a need to clarify the role of autophagy as a cell death mechanism using preclinical and clinical models. Molecular cross talk and genetic overlap between autophagy, apoptosis and necrosis mechanisms does exist but bonafide autophagic cell death mechanisms and molecular markers are needed.
One particular challenge to understanding ROS-autophagy dynamics is first in recognizing the significance of targeting two pro-survival mechanisms simultaneously. In many published studies we found that pro-oxidant treatment (inclding test compounds, chemotherapeutics) resulted in ROS-induced apoptosis and autophagy stimulation. Overall, the literature suggests that autophagy is induced as a cell survival mechanism, which can be concurrent with activation of an apoptotic response. It is essential to evaluate the utility of prooxidant and autophagy inhibitors in cases where autophagy induction is observed. As such, the efficacy of compounds may improve, as the pro-survival autophagy response is diminished. One challenge moving forward would be to judiciously evaluate published studies while determining the relevance of combination therapies. In our opinion, determining the role of autophagy in cell death during use of chemotherapeutic agents is an important challenge. While we suggest that autophagy is primarily a pro-survival mechanism in response to redox disruption, there may be cross-talk with cell death programming that remains to be elucidated. Evaluation of autophagy dynamics in relationship to treatment outcomes, survival curves, tumor burden, and progression-free survival in preclinical and clinical studies is needed. Specifically, determining if autophagy is a valid target for drug-resistant tumors, holds much promise. Furthermore, it is crucial to determine whether current chemotherapy regimens induce ROS, but also stimulate pro-survival tumor cell autophagy and thus contribute to drug-resistant populations.
In conjunction with the molecular interplay, determinants of oxidative stress and autophagy markers in patient serum can be used to evaluate the ROS-autophagy interplay during chemotherapy regimens. Such markers could also be an useful avenue for stratification to drug response, as was demonstrated with Elesclomol [113]. A future challenge and consideration is the reliability of autophagy proteins as actual autophagy determinants when evaluating therapy-naïve versus treated patients, as therapeutic modalities may modulate the overall tumor microenvironment and autophagy status [114, 115]. Retrospective studies, which evaluate tumor autophagy markers and correlate with a ROS-induced chemotherapy agent, may provide useful evidence about the ROS-autophagy interplay in patient outcomes. Assessment of immunotherapy-ineligible, or chemotherapy-resistant patients’ response to dual ROS-autophagy modulating agents would open a therapeutic route for these patients. These challenges present opportunities for future research in the emerging field of redox-autophagy dynamics.
Acknowledgements
This work was supported in part by R01 CA149516 (RG) and by the CTRC at UT Health Science Center San Antonio (UTHSCSA) through NCI support grant #2P30 CA 054174-17. Heather Hambright is supported by the Craniofacial Oral-Biology Student Training in Academic Research (COSTAR) Training Grant (NIDCR T32 DE14318) and ACRCF funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Parichy DM, Spiewak JE. Origins of adult pigmentation: diversity in pigment stem cell lineages and implications for pattern evolution. Pigment cell & melanoma research. 2015;28(1):31–50. doi: 10.1111/pcmr.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hall A, Meyle KD, Lange MK, Klima M, Sanderhoff M, Dahl C, Abildgaard C, Thorup K, Moghimi SM, Jensen PB, Bartek J, Guldberg P, Christensen C. Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E)BRAF oncogene. Oncotarget. 2013;4(4):584–99. doi: 10.18632/oncotarget.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goodall ML, Wang T, Martin KR, Kortus MG, Kauffman AL, Trent JM, Gately S, MacKeigan JP. Development of potent autophagy inhibitors that sensitize oncogenic BRAF V600E mutant melanoma tumor cells to vemurafenib. Autophagy. 2014;10(6):1120–36. doi: 10.4161/auto.28594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xie X, White EP, Mehnert JM. Coordinate autophagy and mTOR pathway inhibition enhances cell death in melanoma. PloS one. 2013;8(1):e55096. doi: 10.1371/journal.pone.0055096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ma XH, Piao S, Wang D, McAfee QW, Nathanson KL, Lum JJ, Li LZ, Amaravadi RK. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(10):3478–89. doi: 10.1158/1078-0432.CCR-10-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Meierjohann S. Oxidative stress in melanocyte senescence and melanoma transformation. European journal of cell biology. 2014;93(1-2):36–41. doi: 10.1016/j.ejcb.2013.11.005. [DOI] [PubMed] [Google Scholar]
- [7].Godic A, Poljsak B, Adamic M, Dahmane R. The role of antioxidants in skin cancer prevention and treatment. Oxidative medicine and cellular longevity. 2014;2014:860479. doi: 10.1155/2014/860479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Denat L, Kadekaro AL, Marrot L, Leachman SA, Abdel-Malek ZA. Melanocytes as instigators and victims of oxidative stress. The Journal of investigative dermatology. 2014;134(6):1512–8. doi: 10.1038/jid.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].He C, Qureshi AA, Han J. Polymorphisms in genes involved in oxidative stress and their interactions with lifestyle factors on skin cancer risk. J Dermatol Sci. 2010;60(1):54–6. doi: 10.1016/j.jdermsci.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jablonska E, Gromadzinska J, Peplonska B, Fendler W, Reszka E, Krol MB, Wieczorek E, Bukowska A, Gresner P, Galicki M, Quispe O. Zambrano, Morawiec Z, Wasowicz W. Lipid peroxidation and glutathione peroxidase activity relationship in breast cancer depends on functional polymorphism of GPX1. BMC cancer. 2015;15:657. doi: 10.1186/s12885-015-1680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moretti D, Del Bello B, Allavena G, Corti A, Signorini C, Maellaro E. Calpain-3 impairs cell proliferation and stimulates oxidative stress-mediated cell death in melanoma cells. PloS one. 2015;10(2):e0117258. doi: 10.1371/journal.pone.0117258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ramprecht C, Jaritz H, Streith I, Zenzmaier E, Kofeler H, Hofmann-Wellenhof R, Schaider H, Hermetter A. Toxicity of oxidized phosphatidylcholines in cultured human melanoma cells. Chemistry and physics of lipids. 2015;189:39–47. doi: 10.1016/j.chemphyslip.2015.05.007. [DOI] [PubMed] [Google Scholar]
- [13].Ishaq M, Kumar S, Varinli H, Han ZJ, Rider AE, Evans MD, Murphy AB, Ostrikov K. Atmospheric gas plasma-induced ROS production activates TNF-ASK1 pathway for the induction of melanoma cancer cell apoptosis. Molecular biology of the cell. 2014;25(9):1523–31. doi: 10.1091/mbc.E13-10-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Corazao-Rozas P, Guerreschi P, Jendoubi M, Andre F, Jonneaux A, Scalbert C, Garcon G, Malet-Martino M, Balayssac S, Rocchi S, Savina A, Formstecher P, Mortier L, Kluza J, Marchetti P. Mitochondrial oxidative stress is the Achille's heel of melanoma cells resistant to Braf-mutant inhibitor. Oncotarget. 2013;4(11):1986–98. doi: 10.18632/oncotarget.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bravard A, Cherbonnel-Lasserre C, Reillaudou M, Beaumatin J, Dutrillaux B, Luccioni C. Modifications of the antioxidant enzymes in relation to chromosome imbalances in human melanoma cell lines. Melanoma research. 1998;8(4):329–35. doi: 10.1097/00008390-199808000-00006. [DOI] [PubMed] [Google Scholar]
- [16].Ibarrola-Villava M, Martin-Gonzalez M, Lazaro P, Pizarro A, Lluch A, Ribas G. Role of glutathione S-transferases in melanoma susceptibility: association with GSTP1 rs1695 polymorphism. The British journal of dermatology. 2012;166(6):1176–83. doi: 10.1111/j.1365-2133.2012.10831.x. [DOI] [PubMed] [Google Scholar]
- [17].Kasai S, Arakawa N, Okubo A, Shigeeda W, Yasuhira S, Masuda T, Akasaka T, Shibazaki M, Maesawa C. NAD(P)H:Quinone Oxidoreductase-1 Expression Sensitizes Malignant Melanoma Cells to the HSP90 Inhibitor 17-AAG. PloS one. 2016;11(4):e0153181. doi: 10.1371/journal.pone.0153181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bracalente C, Ibanez IL, Berenstein A, Notcovich C, Cerda MB, Klamt F, Chernomoretz A, Duran H. Reprogramming human A375 amelanotic melanoma cells by catalase overexpression: Upregulation of antioxidant genes correlates with regression of melanoma malignancy and with malignant progression when downregulated. Oncotarget. 2016 doi: 10.18632/oncotarget.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tong L, Chuang CC, Wu S, Zuo L. Reactive oxygen species in redox cancer therapy. Cancer letters. 2015;367(1):18–25. doi: 10.1016/j.canlet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- [20].Hambright HG, Meng P, Kumar AP, Ghosh R. Inhibition of PI3K/AKT/mTOR axis disrupts oxidative stress-mediated survival of melanoma cells. Oncotarget. 2015;6(9):7195–208. doi: 10.18632/oncotarget.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bernardes S. Santos, de Souza-Neto FP, Melo G. Pasqual, Guarnier FA, Marinello PC, Cecchini R, Cecchini AL. Correlation of TGF-beta1 and oxidative stress in the blood of patients with melanoma: a clue to understanding melanoma progression? Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016 doi: 10.1007/s13277-016-4967-4. [DOI] [PubMed] [Google Scholar]
- [22].Gadjeva V, Dimov A, Georgieva N. Influence of therapy on the antioxidant status in patients with melanoma. Journal of clinical pharmacy and therapeutics. 2008;33(2):179–85. doi: 10.1111/j.1365-2710.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- [23].Kirshner JR, He S, Balasubramanyam V, Kepros J, Yang CY, Zhang M, Du Z, Barsoum J, Bertin J. Elesclomol induces cancer cell apoptosis through oxidative stress. Molecular cancer therapeutics. 2008;7(8):2319–27. doi: 10.1158/1535-7163.MCT-08-0298. [DOI] [PubMed] [Google Scholar]
- [24].Berkenblit A, Eder JP, Jr., Ryan DP, Seiden MV, Tatsuta N, Sherman ML, Dahl TA, Dezube BJ, Supko JG. Phase I clinical trial of STA-4783 in combination with paclitaxel in patients with refractory solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(2 Pt 1):584–90. doi: 10.1158/1078-0432.CCR-06-0964. [DOI] [PubMed] [Google Scholar]
- [25].O'Day S, Gonzalez R, Lawson D, Weber R, Hutchins L, Anderson C, Haddad J, Kong S, Williams A, Jacobson E. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(32):5452–8. doi: 10.1200/JCO.2008.17.1579. [DOI] [PubMed] [Google Scholar]
- [26].Wangpaichitr M, Wu C, You M, Maher JC, Dinh V, Feun LG, Savaraj N. N',N'-Dimethyl-N',N'-bis(phenylcarbonothioyl) Propanedihydrazide (Elesclomol) Selectively Kills Cisplatin Resistant Lung Cancer Cells through Reactive Oxygen Species (ROS) Cancers. 2009;1(1):23–38. doi: 10.3390/cancers1010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Qu Y, Wang J, Sim MS, Liu B, Giuliano A, Barsoum J, Cui X. Elesclomol, counteracted by Akt survival signaling, enhances the apoptotic effect of chemotherapy drugs in breast cancer cells. Breast cancer research and treatment. 2010;121(2):311–21. doi: 10.1007/s10549-009-0470-6. [DOI] [PubMed] [Google Scholar]
- [28].Hedley D, Shamas-Din A, Chow S, Sanfelice D, Schuh AC, Brandwein JM, Seftel MD, Gupta V, Yee KW, Schimmer AD. A phase I study of elesclomol sodium in patients with acute myeloid leukemia. Leukemia & lymphoma. 2016:1–4. doi: 10.3109/10428194.2016.1138293. [DOI] [PubMed] [Google Scholar]
- [29].Farias CF, Massaoka MH, Girola N, Azevedo RA, Ferreira AK, Jorge SD, Tavares LC, Figueiredo CR, Travassos LR. Benzofuroxan derivatives N-Br and N-I induce intrinsic apoptosis in melanoma cells by regulating AKT/BIM signaling and display anti metastatic activity in vivo. BMC cancer. 2015;15:807. doi: 10.1186/s12885-015-1835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim JK, Kang KA, Ryu YS, Piao MJ, Han X, Oh MC, Boo SJ, Jeong SU, Jeong YJ, Chae S, Na SY, Hyun JW. Induction of Endoplasmic Reticulum Stress via Reactive Oxygen Species Mediated by Luteolin in Melanoma Cells. Anticancer research. 2016;36(5):2281–9. [PubMed] [Google Scholar]
- [31].Wang Y, Ma J, Yan X, Chen X, Si L, Liu Y, Han J, Hao W, Zheng Q. Isoliquiritigenin Inhibits Proliferation and Induces Apoptosis via Alleviating Hypoxia and Reducing Glycolysis in Mouse Melanoma B16F10 Cells. Recent patents on anti-cancer drug discovery. 2016;11(2):215–27. doi: 10.2174/1573406412666160307151904. [DOI] [PubMed] [Google Scholar]
- [32].Velatooru LR, Baggu CB, Janapala VR. Spatane diterpinoid from the brown algae, Stoechospermum marginatum induces apoptosis via ROS induced mitochondrial mediated caspase dependent pathway in murine B16F10 melanoma cells. Molecular carcinogenesis. 2016 doi: 10.1002/mc.22463. [DOI] [PubMed] [Google Scholar]
- [33].Genov M, Kreiseder B, Nagl M, Drucker E, Wiederstein M, Muellauer B, Krebs J, Grohmann T, Pretsch D, Baumann K, Bacher M, Pretsch A, Wiesner C. Tetrahydroanthraquinone Derivative (+/-)-4-Deoxyaustrocortilutein Induces Cell Cycle Arrest and Apoptosis in Melanoma Cells via Upregulation of p21 and p53 and Downregulation of NF-kappaB. Journal of Cancer. 2016;7(5):555–68. doi: 10.7150/jca.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wittgen HG, van Kempen LC. Reactive oxygen species in melanoma and its therapeutic implications. Melanoma research. 2007;17(6):400–9. doi: 10.1097/CMR.0b013e3282f1d312. [DOI] [PubMed] [Google Scholar]
- [35].Nath LR, Kumar SN, Das AA, Nambisan B, Shabna A, Mohandas C, Anto RJ. In Vitro Evaluation of the Antioxidant, 3,5-Dihydroxy-4-ethyl-trans-stilbene (DETS) Isolated from Bacillus cereus as a Potent Candidate against Malignant Melanoma. Frontiers in microbiology. 2016;7:452. doi: 10.3389/fmicb.2016.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mokhtari K, Rufino-Palomares EE, Perez-Jimenez A, Reyes-Zurita FJ, Figuera C, Garcia-Salguero L, Medina PP, Peragon J, Lupianez JA. Maslinic Acid, a Triterpene from Olive, Affects the Antioxidant and Mitochondrial Status of B16F10 Melanoma Cells Grown under Stressful Conditions. Evidence-based complementary and alternative medicine : eCAM. 2015;2015:272457. doi: 10.1155/2015/272457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Danciu C, Vlaia L, Fetea F, Hancianu M, Coricovac DE, Ciurlea SA, Soica CM, Marincu I, Vlaia V, Dehelean CA, Trandafirescu C. Evaluation of phenolic profile, antioxidant and anticancer potential of two main representants of Zingiberaceae family against B164A5 murine melanoma cells. Biological research. 2015;48:1. doi: 10.1186/0717-6287-48-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Uzarska M, Czajkowski R, Schwartz RA, Bajek A, Zegarska B, Drewa T. Chemoprevention of skin melanoma: facts and myths. Melanoma research. 2013;23(6):426–33. doi: 10.1097/CMR.0000000000000016. [DOI] [PubMed] [Google Scholar]
- [39].Hercberg S, Ezzedine K, Guinot C, Preziosi P, Galan P, Bertrais S, Estaquio C, Briancon S, Favier A, Latreille J, Malvy D. Antioxidant supplementation increases the risk of skin cancers in women but not in men. The Journal of nutrition. 2007;137(9):2098–105. doi: 10.1093/jn/137.9.2098. [DOI] [PubMed] [Google Scholar]
- [40].Mahabir S, Coit D, Liebes L, Brady MS, Lewis JJ, Roush G, Nestle M, Fry D, Berwick M. Randomized, placebo-controlled trial of dietary supplementation of alpha-tocopherol on mutagen sensitivity levels in melanoma patients: a pilot trial. Melanoma research. 2002;12(1):83–90. doi: 10.1097/00008390-200202000-00012. [DOI] [PubMed] [Google Scholar]
- [41].Papa L, Manfredi G, Germain D. SOD1, an unexpected novel target for cancer therapy. Genes & cancer. 2014;5(1-2):15–21. doi: 10.18632/genesandcancer.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Glasauer A, Chandel NS. Targeting antioxidants for cancer therapy. Biochemical pharmacology. 2014;92(1):90–101. doi: 10.1016/j.bcp.2014.07.017. [DOI] [PubMed] [Google Scholar]
- [43].Yeramian A, Vea A, Benitez S, Ribera J, Domingo M, Santacana M, Martinez M, Maiques O, Valls J, Dolcet X, Vilella R, Cabiscol E, Matias-Guiu X, Marti RM. 2-phenylethynesulphonamide (PFT-mu) enhances the anticancer effect of the novel hsp90 inhibitor NVP-AUY922 in melanoma, by reducing GSH levels. Pigment cell & melanoma research. 2016;29(3):352–71. doi: 10.1111/pcmr.12472. [DOI] [PubMed] [Google Scholar]
- [44].Vene R, Castellani P, Delfino L, Lucibello M, Ciriolo MR, Rubartelli A. The cystine/cysteine cycle and GSH are independent and crucial antioxidant systems in malignant melanoma cells and represent druggable targets. Antioxidants & redox signaling. 2011;15(9):2439–53. doi: 10.1089/ars.2010.3830. [DOI] [PubMed] [Google Scholar]
- [45].Mayola E, Gallerne C, Esposti DD, Martel C, Pervaiz S, Larue L, Debuire B, Lemoine A, Brenner C, Lemaire C. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis : an international journal on programmed cell death. 2011;16(10):1014–27. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- [46].Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407(6802):390–5. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- [47].Peiris-Pages M, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Metastasis and Oxidative Stress: Are Antioxidants a Metabolic Driver of Progression? Cell metabolism. 2015;22(6):956–8. doi: 10.1016/j.cmet.2015.11.008. [DOI] [PubMed] [Google Scholar]
- [48].Vinceti M, Dennert G, Crespi CM, Zwahlen M, Brinkman M, Zeegers MP, Horneber M, D'Amico R, Del Giovane C. Selenium for preventing cancer. The Cochrane database of systematic reviews. 2014;3 doi: 10.1002/14651858.CD005195.pub3. CD005195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Halliwell B. Cell culture, oxidative stress, and antioxidants: avoiding pitfalls. Biomed J. 2014;37(3):99–105. doi: 10.4103/2319-4170.128725. [DOI] [PubMed] [Google Scholar]
- [50].Liu-Smith F, Krasieva TB, Liu J, Liu J, Meyskens FL., Jr. Measuring Redox Status of Melanoma Cells. Methods in molecular biology. 2016 doi: 10.1007/7651_2016_352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochimica et biophysica acta. 2009;1792(1):3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- [52].Fitzwalter BE, Thorburn A. Recent insights into cell death and autophagy. The FEBS journal. 2015;282(22):4279–88. doi: 10.1111/febs.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fulda S, Kogel D. Cell death by autophagy: emerging molecular mechanisms and implications for cancer therapy. Oncogene. 2015;34(40):5105–13. doi: 10.1038/onc.2014.458. [DOI] [PubMed] [Google Scholar]
- [54].Liu H, He Z, Simon HU. Protective role of autophagy and autophagy-related protein 5 in early tumorigenesis. Journal of molecular medicine. 2015;93(2):159–64. doi: 10.1007/s00109-014-1241-3. [DOI] [PubMed] [Google Scholar]
- [55].Setaluri V. Autophagy as a melanocytic self-defense mechanism. The Journal of investigative dermatology. 2015;135(5):1215–7. doi: 10.1038/jid.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Reiners JJ, Jr., Kleinman M, Joiakim A, Mathieu PA. The chemotherapeutic agents XK469 (2-{4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy}propionic acid) and SH80 (2-{4-[(7-bromo-2-quinolinyl)oxy]phenoxy}propionic acid) inhibit cytokinesis and promote polyploidy and induce senescence. The Journal of pharmacology and experimental therapeutics. 2009;328(3):796–806. doi: 10.1124/jpet.108.144808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Xie X, Koh JY, Price S, White E, Mehnert JM. Atg7 Overcomes Senescence and Promotes Growth of BrafV600E-Driven Melanoma. Cancer discovery. 2015;5(4):410–23. doi: 10.1158/2159-8290.CD-14-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Elgendy M, Ciro M, Abdel-Aziz AK, Belmonte G, Dal Zuffo R, Mercurio C, Miracco C, Lanfrancone L, Foiani M, Minucci S. Beclin 1 restrains tumorigenesis through Mcl-1 destabilization in an autophagy-independent reciprocal manner. Nature communications. 2014;5:5637. doi: 10.1038/ncomms6637. [DOI] [PubMed] [Google Scholar]
- [59].Yun WJ, Kim EY, Park JE, Jo SY, Bang SH, Chang EJ, Chang SE. Microtubule-associated protein light chain 3 is involved in melanogenesis via regulation of MITF expression in melanocytes. Scientific reports. 2016;6:19914. doi: 10.1038/srep19914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Han C, Sun B, Wang W, Cai W, Lou D, Sun Y, Zhao X. Overexpression of microtubule-associated protein-1 light chain 3 is associated with melanoma metastasis and vasculogenic mimicry. The Tohoku journal of experimental medicine. 2011;223(4):243–51. doi: 10.1620/tjem.223.243. [DOI] [PubMed] [Google Scholar]
- [61].Ellis RA, Horswell S, Ness T, Lumsdon J, Tooze SA, Kirkham N, Armstrong JL, Lovat PE. Prognostic impact of p62 expression in cutaneous malignant melanoma. The Journal of investigative dermatology. 2014;134(5):1476–8. doi: 10.1038/jid.2013.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Miracco C, Cevenini G, Franchi A, Luzi P, Cosci E, Mourmouras V, Monciatti I, Mannucci S, Biagioli M, Toscano M, Moretti D, Lio R, Massi D. Beclin 1 and LC3 autophagic gene expression in cutaneous melanocytic lesions. Human pathology. 2010;41(4):503–12. doi: 10.1016/j.humpath.2009.09.004. [DOI] [PubMed] [Google Scholar]
- [63].Liu H, He Z, Simon HU. Autophagy suppresses melanoma tumorigenesis by inducing senescence. Autophagy. 2014;10(2):372–3. doi: 10.4161/auto.27163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Manca A, Lissia A, Capone M, Ascierto PA, Botti G, Caraco C, Stanganelli I, Colombino M, Sini M, Cossu A, Palmieri G. Activating PIK3CA mutations coexist with BRAF or NRAS mutations in a limited fraction of melanomas. Journal of translational medicine. 2015;13:37. doi: 10.1186/s12967-015-0401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Maes H, Martin S, Verfaillie T, Agostinis P. Dynamic interplay between autophagic flux and Akt during melanoma progression in vitro. Experimental dermatology. 2014;23(2):101–6. doi: 10.1111/exd.12298. [DOI] [PubMed] [Google Scholar]
- [66].Tsao H, Chin L, Garraway LA, Fisher DE. Melanoma: from mutations to medicine. Genes & development. 2012;26(11):1131–55. doi: 10.1101/gad.191999.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tsao H, Mihm MC, Jr., Sheehan C. PTEN expression in normal skin, acquired melanocytic nevi, and cutaneous melanoma. Journal of the American Academy of Dermatology. 2003;49(5):865–72. doi: 10.1016/s0190-9622(03)02473-3. [DOI] [PubMed] [Google Scholar]
- [68].Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA, Schuchter LM, Torigian DA, Panosian JT, Troxel AB, Tan KS, Heitjan DF, DeMichele AM, Vaughn DJ, Redlinger M, Alavi A, Kaiser J, Pontiggia L, Davis LE, O'Dwyer PJ, Amaravadi RK. Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy. 2014;10(8):1391–402. doi: 10.4161/auto.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Egger ME, Huang JS, Yin W, McMasters KM, McNally LR. Inhibition of autophagy with chloroquine is effective in melanoma. The Journal of surgical research. 2013;184(1):274–81. doi: 10.1016/j.jss.2013.04.055. [DOI] [PubMed] [Google Scholar]
- [70].Xia Y, Li Y, Westover KD, Sun J, Chen H, Zhang J, Fisher DE. Inhibition of Cell Proliferation in an NRAS Mutant Melanoma Cell Line by Combining Sorafenib and alpha-Mangostin. PloS one. 2016;11(5):e0155217. doi: 10.1371/journal.pone.0155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Aliwaini S, Swarts AJ, Blanckenberg A, Mapolie S, Prince S. A novel binuclear palladacycle complex inhibits melanoma growth in vitro and in vivo through apoptosis and autophagy. Biochemical pharmacology. 2013;86(12):1650–63. doi: 10.1016/j.bcp.2013.09.020. [DOI] [PubMed] [Google Scholar]
- [72].Sharma K, Ishaq M, Sharma G, Khan MA, Dutta RK, Majumdar S. Pentoxifylline triggers autophagy via ER stress response that interferes with Pentoxifylline induced apoptosis in human melanoma cells. Biochemical pharmacology. 2016;103:17–28. doi: 10.1016/j.bcp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- [73].Das A, Pushparaj C, Herreros J, Nager M, Vilella R, Portero M, Pamplona R, Matias-Guiu X, Marti RM, Canti C. T-type calcium channel blockers inhibit autophagy and promote apoptosis of malignant melanoma cells. Pigment cell & melanoma research. 2013;26(6):874–85. doi: 10.1111/pcmr.12155. [DOI] [PubMed] [Google Scholar]
- [74].Junco JJ, Mancha-Ramirez A, Malik G, Wei SJ, Kim DJ, Liang H, Slaga TJ. Ursolic acid and resveratrol synergize with chloroquine to reduce melanoma cell viability. Melanoma research. 2015;25(2):103–12. doi: 10.1097/CMR.0000000000000137. [DOI] [PubMed] [Google Scholar]
- [75].Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nature reviews. Molecular cell biology. 2008;9(12):1004–10. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ghosh S, Bishayee K, Khuda-Bukhsh AR. Graveoline isolated from ethanolic extract of Ruta graveolens triggers apoptosis and autophagy in skin melanoma cells: a novel apoptosis-independent autophagic signaling pathway. Phytotherapy research : PTR. 2014;28(8):1153–62. doi: 10.1002/ptr.5107. [DOI] [PubMed] [Google Scholar]
- [77].Tomic T, Botton T, Cerezo M, Robert G, Luciano F, Puissant A, Gounon P, Allegra M, Bertolotto C, Bereder JM, Tartare-Deckert S, Bahadoran P, Auberger P, Ballotti R, Rocchi S. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell death & disease. 2011;2:e199. doi: 10.1038/cddis.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chen Y, Liersch R, Detmar M. The miR-290-295 cluster suppresses autophagic cell death of melanoma cells. Scientific reports. 2012;2:808. doi: 10.1038/srep00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Goodall ML, Fitzwalter BE, Zahedi S, Wu M, Rodriguez D, Mulcahy-Levy JM, Green DR, Morgan M, Cramer SD, Thorburn A. The Autophagy Machinery Controls Cell Death Switching between Apoptosis and Necroptosis. Developmental cell. 2016;37(4):337–49. doi: 10.1016/j.devcel.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Matsuzawa Y, Oshima S, Nibe Y, Kobayashi M, Maeyashiki C, Nemoto Y, Nagaishi T, Okamoto R, Tsuchiya K, Nakamura T, Watanabe M. RIPK3 regulates p62-LC3 complex formation via the caspase-8-dependent cleavage of p62. Biochemical and biophysical research communications. 2015;456(1):298–304. doi: 10.1016/j.bbrc.2014.11.075. [DOI] [PubMed] [Google Scholar]
- [81].Huang S, Okamoto K, Yu C, Sinicrope FA. p62/sequestosome-1 up-regulation promotes ABT-263-induced caspase-8 aggregation/activation on the autophagosome. The Journal of biological chemistry. 2013;288(47):33654–66. doi: 10.1074/jbc.M113.518134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Young MM, Takahashi Y, Khan O, Park S, Hori T, Yun J, Sharma AK, Amin S, Hu CD, Zhang J, Kester M, Wang HG. Autophagosomal membrane serves as platform for intracellular death-inducing signaling complex (iDISC)-mediated caspase-8 activation and apoptosis. The Journal of biological chemistry. 2012;287(15):12455–68. doi: 10.1074/jbc.M111.309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hammerova J, Uldrijan S, Taborska E, Vaculova AH, Slaninova I. Necroptosis modulated by autophagy is a predominant form of melanoma cell death induced by sanguilutine. Biological chemistry. 2012;393(7):647–58. doi: 10.1515/hsz-2011-0279. [DOI] [PubMed] [Google Scholar]
- [84].Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26(9):1021–32. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Rangwala R, Leone R, Chang YC, Fecher LA, Schuchter LM, Kramer A, Tan KS, Heitjan DF, Rodgers G, Gallagher M, Piao S, Troxel AB, Evans TL, DeMichele AM, Nathanson KL, O'Dwyer PJ, Kaiser J, Pontiggia L, Davis LE, Amaravadi RK. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy. 2014;10(8):1369–79. doi: 10.4161/auto.29118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. The Journal of biological chemistry. 2010;285(14):10850–61. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lazova R, Klump V, Pawelek J. Autophagy in cutaneous malignant melanoma. Journal of cutaneous pathology. 2010;37(2):256–68. doi: 10.1111/j.1600-0560.2009.01359.x. [DOI] [PubMed] [Google Scholar]
- [88].Liu H, He Z, von Rutte T, Yousefi S, Hunger RE, Simon HU. Down-regulation of autophagy-related protein 5 (ATG5) contributes to the pathogenesis of early-stage cutaneous melanoma. Science translational medicine. 2013;5(202):202ra123. doi: 10.1126/scitranslmed.3005864. [DOI] [PubMed] [Google Scholar]
- [89].Lazova R, Camp RL, Klump V, Siddiqui SF, Amaravadi RK, Pawelek JM. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(2):370–9. doi: 10.1158/1078-0432.CCR-11-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Smith JW, Koshoffer A, Morris RE, Boissy RE. Membranous complexes characteristic of melanocytes derived from patients with Hermansky-Pudlak syndrome type 1 are macroautophagosomal entities of the lysosomal compartment. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2005;18(6):417–26. doi: 10.1111/j.1600-0749.2005.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Beitner H, Nakatani T, Hedblad MA. A transmission electron microscopical study of dysplastic naevi. Acta dermato-venereologica. 1990;70(5):411–6. [PubMed] [Google Scholar]
- [92].Kraya AA, Piao S, Xu X, Zhang G, Herlyn M, Gimotty P, Levine B, Amaravadi RK, Speicher DW. Identification of secreted proteins that reflect autophagy dynamics within tumor cells. Autophagy. 2015;11(1):60–74. doi: 10.4161/15548627.2014.984273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Martin S, Dudek-Peric AM, Maes H, Garg AD, Gabrysiak M, Demirsoy S, Swinnen JV, Agostinis P. Concurrent MEK and autophagy inhibition is required to restore cell death associated danger-signalling in Vemurafenib-resistant melanoma cells. Biochemical pharmacology. 2015;93(3):290–304. doi: 10.1016/j.bcp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- [94].Chen JJ, Chou CW, Chang YF, Chen CC. Proteasome inhibitors enhance TRAIL-induced apoptosis through the intronic regulation of DR5: involvement of NF-kappa B and reactive oxygen species-mediated p53 activation. Journal of immunology. 2008;180(12):8030–9. doi: 10.4049/jimmunol.180.12.8030. [DOI] [PubMed] [Google Scholar]
- [95].Lu Y, Zhang R, Liu S, Zhao Y, Gao J, Zhu L. ZT-25, a new vacuolar H(+)-ATPase inhibitor, induces apoptosis and protective autophagy through ROS generation in HepG2 cells. European journal of pharmacology. 2016;771:130–8. doi: 10.1016/j.ejphar.2015.12.026. [DOI] [PubMed] [Google Scholar]
- [96].Liu B, Cheng Y, Zhang B, Bian HJ, Bao JK. Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS-p38-p53 pathway. Cancer letters. 2009;275(1):54–60. doi: 10.1016/j.canlet.2008.09.042. [DOI] [PubMed] [Google Scholar]
- [97].Del Bello B, Toscano M, Moretti D, Maellaro E. Cisplatin-induced apoptosis inhibits autophagy, which acts as a pro-survival mechanism in human melanoma cells. PloS one. 2013;8(2):e57236. doi: 10.1371/journal.pone.0057236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].He H, Feng YS, Zang LH, Liu WW, Ding LQ, Chen LX, Kang N, Hayashi T, Tashiro S, Onodera S, Qiu F, Ikejima T. Nitric oxide induces apoptosis and autophagy; autophagy down-regulates NO synthesis in physalin A-treated A375-S2 human melanoma cells. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2014;71:128–35. doi: 10.1016/j.fct.2014.06.007. [DOI] [PubMed] [Google Scholar]
- [99].Syed DN, Lall RK, Chamcheu JC, Haidar O, Mukhtar H. Involvement of ER stress and activation of apoptotic pathways in fisetin induced cytotoxicity in human melanoma. Archives of biochemistry and biophysics. 2014;563:108–17. doi: 10.1016/j.abb.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zugic A, Jeremic I, Isakovic A, Arsic I, Savic S, Tadic V. Evaluation of Anticancer and Antioxidant Activity of a Commercially Available CO2 Supercritical Extract of Old Man's Beard (Usnea barbata) PloS one. 2016;11(1):e0146342. doi: 10.1371/journal.pone.0146342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. The EMBO journal. 2007;26(7):1749–60. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Son YO, Pratheeshkumar P, Roy RV, Hitron JA, Wang L, Divya SP, Xu M, Luo J, Chen G, Zhang Z, Shi X. Antioncogenic and Oncogenic Properties of Nrf2 in Arsenic-induced Carcinogenesis. The Journal of biological chemistry. 2015;290(45):27090–100. doi: 10.1074/jbc.M115.675371. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [103].Park JS, Kang DH, Bae SH. p62 prevents carbonyl cyanide m-chlorophenyl hydrazine (CCCP)-induced apoptotic cell death by activating Nrf2. Biochemical and biophysical research communications. 2015;464(4):1139–44. doi: 10.1016/j.bbrc.2015.07.093. [DOI] [PubMed] [Google Scholar]
- [104].Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137(6):1062–75. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. The Journal of biological chemistry. 2010;285(29):22576–91. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Wu H, Che X, Zheng Q, Wu A, Pan K, Shao A, Wu Q, Zhang J, Hong Y. Caspases: a molecular switch node in the crosstalk between autophagy and apoptosis. International journal of biological sciences. 2014;10(9):1072–83. doi: 10.7150/ijbs.9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Li X, Su J, Xia M, Li H, Xu Y, Ma C, Ma L, Kang J, Yu H, Zhang Z, Sun L. Caspase-mediated cleavage of Beclin1 inhibits autophagy and promotes apoptosis induced by S1 in human ovarian cancer SKOV3 cells. Apoptosis : an international journal on programmed cell death. 2016;21(2):225–38. doi: 10.1007/s10495-015-1197-y. [DOI] [PubMed] [Google Scholar]
- [108].Du Y, Wooten MC, Wooten MW. Oxidative damage to the promoter region of SQSTM1/p62 is common to neurodegenerative disease. Neurobiology of disease. 2009;35(2):302–10. doi: 10.1016/j.nbd.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ci Y, Shi K, An J, Yang Y, Hui K, Wu P, Shi L, Xu C. ROS inhibit autophagy by downregulating ULK1 mediated by the phosphorylation of p53 in selenite-treated NB4 cells. Cell death & disease. 2014;5:e1542. doi: 10.1038/cddis.2014.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wani WY, Boyer-Guittaut M, Dodson M, Chatham J, Darley-Usmar V, Zhang J. Regulation of autophagy by protein post-translational modification. Lab Invest. 2015;95(1):14–25. doi: 10.1038/labinvest.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, Hirao A, Takagi K, Mizushima T, Motohashi H, Lee MS, Yoshimori T, Tanaka K, Yamamoto M, Komatsu M. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51(5):618–31. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- [112].Michaud M, Xie X, Bravo-San Pedro JM, Zitvogel L, White E, Kroemer G. An autophagy-dependent anticancer immune response determines the efficacy of melanoma chemotherapy. Oncoimmunology. 2014;3(7):e944047. doi: 10.4161/21624011.2014.944047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].O'Day SJ, Eggermont AM, Chiarion-Sileni V, Kefford R, Grob JJ, Mortier L, Robert C, Schachter J, Testori A, Mackiewicz J, Friedlander P, Garbe C, Ugurel S, Collichio F, Guo W, Lufkin J, Bahcall S, Vukovic V, Hauschild A. Final results of phase III SYMMETRY study: randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(9):1211–8. doi: 10.1200/JCO.2012.44.5585. [DOI] [PubMed] [Google Scholar]
- [114].Hossain A, Radwan FF, Doonan BP, God JM, Zhang L, Bell PD, Haque A. A possible cross-talk between autophagy and apoptosis in generating an immune response in melanoma. Apoptosis : an international journal on programmed cell death. 2012;17(10):1066–78. doi: 10.1007/s10495-012-0745-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Li H, Li Y, Jiao J, Hu HM. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nature nanotechnology. 2011;6(10):645–50. doi: 10.1038/nnano.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]