Abstract
Antiretroviral therapy is not curative. Given the challenges in providing life-long therapy to a global population of over 35 million people living with HIV, there is intense interest in developing a cure for HIV infection. The International AIDS Society convened a group of international experts to develop a scientific strategy for research towards an HIV cure. This Perspective summarizes the group's strategy.
INTRODUCTION
The HIV/AIDS pandemic represents the most important global health challenge in modern history. Fortunately, when used optimally, combination antiretroviral therapy (ART) can effectively control HIV replication, prevent the development of AIDS, prolong life and reduce transmission risk. Despite this unquestioned success, there are limitations with current treatment strategies. The operational and logistical challenges in delivering life-long treatment are daunting and the economic costs of providing ART to over 35 million people currently living with HIV may be unsustainable1. Life-long adherence treatment is challenging for many. Antiretroviral drug resistance remains a problem, particularly in those individuals who are unable to fully adhere to treatment. Drug toxicities and the persistence of immune dysfunction during ART have significant health consequences. These factors highlight the urgency of identifying an effective means to control the virus in the absence of ART, or a cure (Text Box 1). The search for a curative strategy for HIV is now a key priority for the HIV community2 and encouraging results have already been reported (Figure 1 and Text 2).
TEXT BOX 1. Defining cure in HIV disease.
The definition of cure is important to clarify for researchers, clinicians, and people living with HIV. The optimal outcome would be the complete eradication within an individual of all replication-competent HIV. Such a sterilising cure will be challenging to achieve and will be impossible to prove with the current technologies111. A more feasible outcome will be the achievement of a long-term remission. Remission is likely a necessary precursor towards the development of an HIV cure, and is increasingly utilised in the field to indicate the goal of long-term undetectable viremia for an as-yet undefined period (likely of several years) in the absence of ART112. The concept of disease remission denotes improvement albeit with some uncertainty and is already well-entrenched in medical settings113.
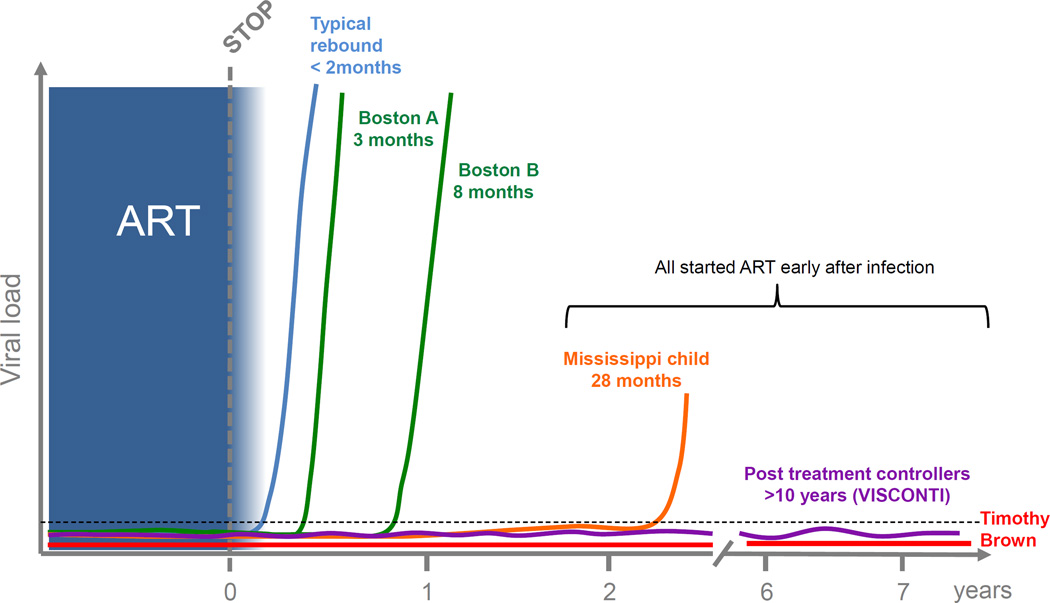
Figure 1. Cases of post-treatment control and resission.
Viral rebound following cessation of ART usually occurs within 2–3 weeks. In some circumstances viral rebound has been significantly delayed in the setting of stem cell transplantation (Boston patients) or very early ART in an infant (Mississippi child). In some individuals, long term post treatment control (PTC) off ART has been achieved. In these PTC, ART was nearly always initiated in acute infection and virus is usually detected at low levels in plasma. Timothy Brown remains the only HIV-infected individual off ART with no virus detected in blood or tissue. He received a stem cell transplant from a donor who was CCR5D32 negative and remains off ART for over 7 years.
The International AIDS Society (IAS) established the “Towards an HIV Cure” initiative in 2010. A major outcome of this initiative was the development in 2012 of a long-term scientific strategy by a large, multi-disciplinary group of scientists3. Given the evolving nature of HIV cure research (Text Box 2), the initiative expanded its scope by adding new members with unique expertise relevant to the emerging agenda, broadening the strategy beyond biomedical research to include social and behavioural sciences (Text Box 3). This second edition of the Global Scientific Strategy: Towards an HIV Cure 2016 describes the critical knowledge gaps and research questions in the field (Table).
TEXT BOX 2. Progress in HIV Cure Research 2012–2016.
There have been a number of significant advances since the publication of the first 2012 Global Scientific Strategy Towards an HIV Cure3. Sustained periods of aviremia in the absence of therapy was achieved in an aggressively treated infant, in at least two individuals who have received allogeneic stem cell transplant, and adults who received several years of ART that was initiated soon after infection (Figure 1)62,94,114,115. Non-human primate and humanized mouse models of well-treated SIV/HIV disease have been validated and used to advance the scientific agenda63,116. Most HIV in blood was found to be replication-incompetent and most of the apparent replication-competent virus was found to be non-inducible ex vivo8. Early initiation of ART limits the establishment of the reservoir and prevents the generation of immune escape in latently infected cells63,117,118. New tools that can quantify the frequency of a cell that carries replication competent virus have been developed88,119, and some biomarkers have been shown to predict the time to viral rebound following a treatment interruption92,93,120,121. The central role of the T follicular helper cells and the B cell follicle in supporting SIV/HIV replication was established35. The central role of long-lived self-renewing memory CD4+ T cells as a reservoir during sustained ART (> 10 years) was established122, while the role of monocytes/macrophages as a stable reservoir during ART has been challenged36,123. Homeostatic proliferation induced by cytokines or HIV integration events as a mechanism of persistence was demonstrated25,39,40,124. Evidence was presented suggesting that HIV continues to replicate and evolve during the first 6 months of ART125 but not necessarily during long-term ART13. New latency reversing agents and combination approaches were identified in vitro21,126–128 and the capacity of more established LRAs to disrupt latency was demonstrated in a series of phase I/II clinical trials15–17,22,129. Novel vaccines were developed that contained and possibly cured SIV infection when administered before infection46, and the safety and potential efficacy of broadly neutralizing antibodies and bi-specific antibodies demonstrated47,51,52,54,130,131. The safety of gene therapy with CCR5 modification was found to be feasible and safe10,132.
TEXT BOX 3. Methodology.
This second edition of the Global Scientific Strategy: Towards an HIV Cure was developed under the auspices of the International AIDS Society (IAS) to revise and update the original strategy released in 2012. The strategy was discussed and developed by the International Scientific Working Group, a global team of leading stakeholders, including basic scientists, clinical physicians, social scientists, ethicists, and community leaders from around the world. The International Scientific Working Group was composed of seven multidisciplinary subgroups, including input from medical ethicists in each subgroup.
The Global Scientific Strategy (GSS) was discussed, developed and finalised at a series of in-person workshops and electronic discussions from autumn 2014 to February 2016. In addition to the discussions with the International Scientific Working Group, the GSS underwent broad dissemination for a peer-review process incorporating comments and edits from a broad range of stakeholders, in keeping with the values of the International AIDS Society. These research recommendations represent the culmination of hundreds of hours of online and in-person meetings with community leaders, pharmaceutical company representatives, funders and regulatory agency representatives, as well as HIV researchers from low-income, middle-income, and high-income country contexts. A number of non-HIV researchers were consulted on specific scientific issues.
TABLE.
| Molecular biology of HIV latency and reversal strategies |
Viral reservoirs, immunology of HIV persistence and 'kill' strategies |
Models for HIV cure or sustainable remission |
Research in neonates, infants and children |
Gene and cell therapy |
Novel biomarkers and technologies to quantify HIV reservoirs |
Social sciences and health systems research |
|---|---|---|---|---|---|---|
| Further develop and refine resting CD4 T cell models that reflect the diversity of proviral latency, to decipher the molecular mechanism of HIV latency, ensuring they are sufficiently tractable for use in therapeutic testing and development |
Characterize in a diverse population of HIV-infected children and adults the distribution of the replication- competent virus in all tissues |
Establish an ethical balance between scientific gain and study participants’ interests in testing invasive or potentially risky interventions considering medical and non- medical risks and benefits for individual participants, and the broader societal benefits. |
Understand the development of the innate and adaptive immune system in the systemic and mucosal compartments. |
Explore the potential of engineered T-cells to target HIV- infected cells |
Systematically and impartially determine the performance characteristics of all assays of putative biomarkers |
Promote patient-focused research to understand the perceptions, attitudes, and beliefs of HIV- infected individuals, their partners, and communities towards HIV cure. |
| Apply new tools, including single cell analyses to explore the diversity of proviral latency, and identify pathways and factors that can be potentially targeted in therapeutic testing and development |
Characterize the impact of host immune environment on size, distribution and inducibility of the reservoir, focusing on most relevant tissues |
Develop iterative animal and clinical studies to understand the anatomic and cellular components of the reservoirs, the responses to novel interventions and the utility of candidate biomarkers for predicting viral recrudescence |
Understand latent reservoir dynamics and factors associated with establishing and maintaining latency, including the central nervous system as a reservoir. |
Develop methods to deliver gene therapies to reservoir cells |
Develop non- human primate and human sample repositories and clinical databases to validate single or composite biomarkers of the duration of ART- free remission. |
Measure and promote stakeholder engagement in HIV cure research among a diverse group of individuals from a range of local contexts. |
| Explore the contribution of HIV restriction factors in controlling the establishment and the maintenance of HIV latency |
Determine in animal models, and ultimately humans, the impact of immune- modifying drugs on latency reversal |
Understand the impact of timing of ART on HIV persistence and remission by in depth characterization of the immunologic and virologic profiles at the time of ART initiation. |
Understand pre- existing immune responses against HIV and the role of immunotherapeu tics in eliminating latently infected cells. |
Develop methods to boost immune responses in combination with cell and gene therapies. |
Assess biomarkers of HIV persistence in different populations including children, adolescents, the elderly, women and transgender populations |
Use decision analysis and related modelling strategies to optimize clinical trials, enhance HIV cure strategies, and demonstrate budgetary impact. |
| Develop assay systems in patient cells to assess a range of responses: enforcement of latency, RNA expression, antigen expression, virion production |
Develop therapies that enhance the capacity of the immune system to target and eliminate virus- producing cells during ART and to control residual HIV in the absence of ART |
Construct an operational definition for HIV remission, for use in both clinical research and community communications |
Identify ways to appropriately conduct HIV cure research in children, including studies that involve new interventions, invasive procedures and ATI. |
Understand the consequences of myeloablative conditioning to enhance HSC engraftment and develop alternatives approaches. |
Leverage technologic advances in genomics, metabolomics, proteomics, imaging and single cell analyses to identify new, potential biomarkers of the persistence of replication- competent proviruses in blood and tissue and identify a phenotypic marker of a latently infected cell. |
Determine optimal health systems and policy strategies to promote HIV cure research. |
MOLECULAR BIOLOGY OF HIV LATENCY
Background
A major barrier to curing HIV is latency, which is defined as the persistence of integrated viral DNA that is replication competent but transcriptionally silent. HIV persists primarily as a latent genome in long-lived memory CD4+ T cells and to a lesser degree in naive CD4+ T cells. Whether latency occurs in myeloid cells remains controversial. Multiple cellular mechanisms have been defined that contribute to the establishment and maintenance of latency (Figure 2). A repressive chromatin environment is actively maintained at the HIV long terminal repeat (LTR) by the activity of histone deacetylases and other regulatory proteins and by the absence of host factors needed to support viral transcription, including nuclear factor kappa B (NF-Kb) and positive transcription elongation factor b (PTEF-b)4–6. Transcriptional interference by host promoter activities also prevents successful HIV proviral transcription7. These multiple levels of transcriptional control confer an apparent stochastic nature to HIV proviral DNA expression, whereby even in the face of multiple activating signals, latency cannot be instantaneously disrupted in all proviral genomes8.
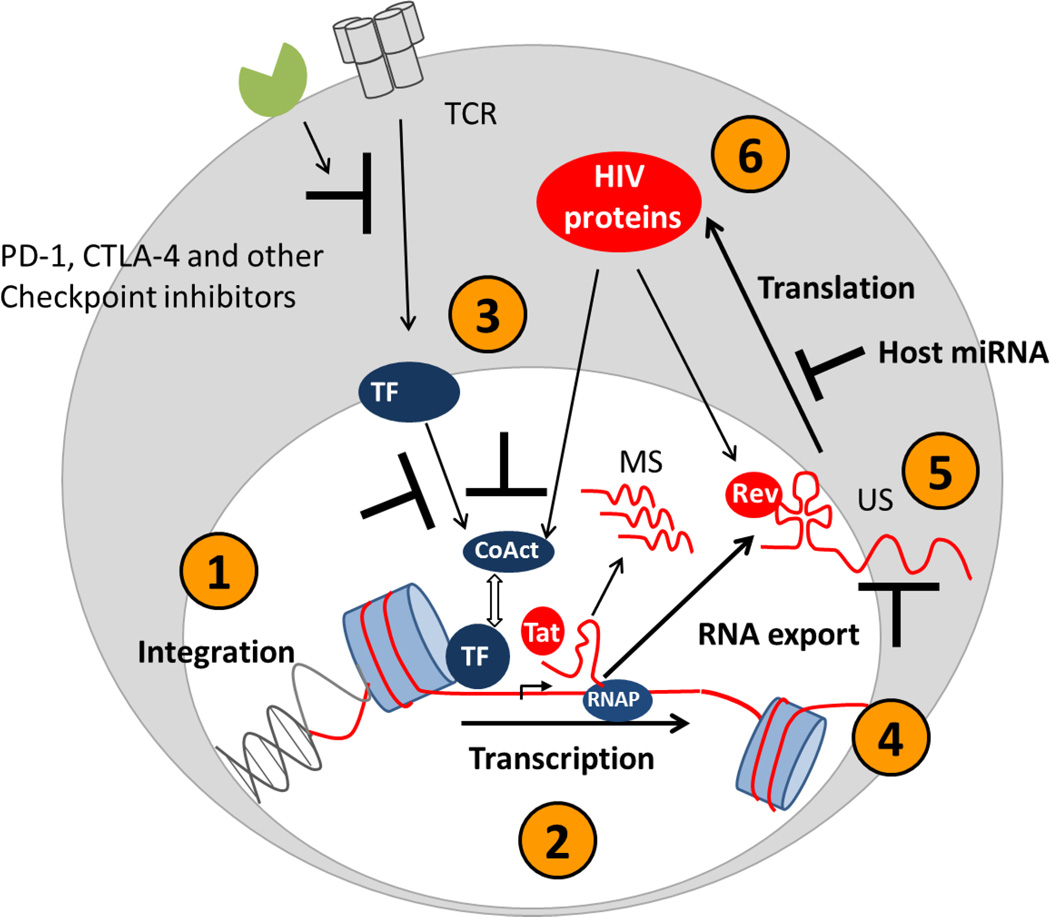
Figure 2. Mechanisms that maintain HIV latency in resting CD4+ T-cells.
There are multiple blocks to viral production in latently infected resting CD4+ T-cells including the site of integration (1), epigenetic silencing (2), lack of cellular transcription factors (3), incomplete elongation of transcripts (4), nuclear retention of transcripts (5) and micro RNAs limiting translation of viral proteins (6). TCR = T cell receptor; TF = transcription factors; co-Act = co-activators; MS = multiply spliced; US = unspliced; miRNA = microRNA.
The contribution of residual low-level (or “cryptic”) virus replication and spread during ART to HIV persistence is unresolved. Two controlled studies of ART intensification with the integrase inhibitor, raltegravir, found evidence of persistent cycles of virus replication in a subset of participants9,10. A study of HIV sequence evolution within lymph node tissue found evidence of viral evolution within the first six 6 months of initiating ART, consistent with low-level replication11,12, but other studies of long-term ART failed to find any evidence of evolution13, even in tissues14.
The current most-studied approach for eliminating latently infected T cells is based on the hypothesis that HIV latency can be reversed, leading to the clearance of these cells through virus or immune mediated cytolysis (“shock and kill”). Although the administration of LRAs, including histone deacetylase inhibitors (HDACi) and disulfiram to HIV-infected individuals on ART have demonstrated an increase in both cell associated and plasma HIV RNA, these interventions had no apparent effect on the frequency of latently infected cells15–18. It is unknown whether current approaches have failed because they are insufficiently potent or because cells induced to produce virus are not cleared19.
Understanding blocks in HIV transcription and virus production in latently infected resting cells
Basic research on the cellular mechanisms that constitute the rate-limiting steps controlling gene expression in resting CD4+ T-cells may be informative. Indeed, a better understanding of factors that allow or restrict transcriptional initiation and RNA processing at the HIV LTR, and how to manipulate these factors therapeutically is needed (Figure 2).
HIV RNA transcription is not the only event that is required to effectively disrupt latent infection. HIV mRNA export, splicing and translation, viral antigen expression, and/or processing and presentation have been relatively under-studied, especially within resting memory CD4+ T-cell populations. A full understanding of the steps and processes that allow the rare latently infected cell to be revealed to the immune system, or to be targeted, is still lacking.
Development of in vitro models of HIV latency
The field currently lacks validated systems with which to test and compare different “latency-reversing agents” (LRAs)20. The development of in vitro cellular models based on resting CD4+ T-cells is critically needed. These models will need to reiterate the biology critical for the establishment and maintenance of latency.
Development of more selective and effective LRAs
While it is clear that LRAs can stimulate the production and release of virions from a small subset of infected cells in vitro, more potent LRAs are needed. This will likely require the redistribution or upregulation of several key cellular factors with combination therapies21. Identifying such combinations with current in vitro systems will be challenging as these systems fail to capture the complex in vivo signalling networks that maintain memory cells in a resting state (and hence maintain latent infection). One new approach under study may function indirectly by activating antigen-presenting cells to signal and activate memory cells (e.g., toll-like receptor agonists). Current systems are also unable to study LRA activity over prolonged periods, which will likely be needed given the stochastic nature of latency reversal, and highlight the role of animal models in this research.
All LRAs target host cellular pathways and hence might have untoward effects on multiple other host genes22. LRAs that broadly activate all T cells will likely reverse latency, but the inflammatory consequences will almost certainly prove risky23. LRAs that alter the epigenetic environment that silences DNA transcription could increase the risk of malignant transformation; indeed, the first generation of LRAs studied in the clinic (vorinostat and panobinostat) had mutagenic potential in common screening assays. Given non-specific effects on the activation status of memory cells, some LRAs have the potential to stimulate cell proliferation, which could lead to expansion of an infected cell population24,25. Immune modifying drugs that mitigate this response may need to be developed along with LRAs. Finally, these approaches will have to be carefully vetted for their potential to dampen the very immune responses needed for viral clearance26.
Strategies to permanently silence the HIV provirus
An alternative approach that represents a significant departure from the “shock and kill” paradigm is to fully and irreversibly suppress HIV transcription, leading to permanent silencing and the lack of virus production when ART is discontinued. This strategy will likely require a greater degree of specificity with respect to targeting the HIV LTR27,28. We lack an understanding of the molecular mechanisms involved in pharmacologically-mediated “deep” latency, and the durability of this state.
Defining the role of HIV replication as a cause of persistence
The role of ongoing HIV replication in maintaining HIV persistence remains controversial. HIV may continue to spread despite ART as a consequence of insufficient drug potency, pharmacologic barriers that prevent distribution of treatment to all tissue reservoirs and/or lack of effective immunity, particularly in potential immune-privileged sanctuaries. Assays that can assess new infection events rather than simply the production of virions will need to be developed; these assays will presumably need to be validated using lymphoid tissues. Novel strategies that overcome potential mechanisms for persistent replication (e.g., more potent ART, enhanced T cell immunity) will need to be studied in animal models and eventually people to determine if replication persists and if it can be inhibited.
THE IMMUNOLOGY OF HIV PERSISTENCE
Background
Untreated HIV causes irreversible harm to the immune system. Effective ART reverses many of these abnormalities, but a state of persistent inflammation and immune dysfunction typically persists. This immune state during ART is characterized by chronic low level inflammation within the adaptive and innate immune systems, elevated immunoregulatory responses, and CD4+ and CD8+ T cell dysfunction. It is expected that this compromised immune state contributes to HIV persistence on ART and that efforts to control HIV in the absence of ART may require interventions that reverse some or all of these immunologic abnormalities29.
Characterizing and quantifying the total body burden of replication-competent HIV is challenging as most of the virus resides in difficult-to-access lymphoid issues. In blood and tissues, HIV primarily persists in either latently or productively infected memory CD4+ T cells30–32. Over time, the virus may become enriched in memory cells with self-renewing stem-cell like capacity33. T follicular helper (Tfh) may also be highly enriched for replication-competent virus, perhaps because they largely reside in B cell follicles, which are relatively inaccessible to HIV-specific CD8+ T cells34,35. Although macrophages are productively infected during untreated HIV infection, it has proven difficult to demonstrate conclusively that replication-competent HIV persists indefinitely in these cells during ART36 or that HIV persists in those tissues that are rich in macrophages, particularly the central nervous system37,38.
It is generally accepted that the biology of CD4+ T cell memory determines the fate of latently infected cells. Major knowledge gaps exist in this area. The life span of these cells in vivo is unknown. Clonal expansion of HIV in memory CD4+ T cells is apparently common, perhaps because the site where HIV integrates enhances cell proliferative or survival capacity14,39–42. The relative contributions of cytokine-mediated T cell turnover (T cell homeostasis) versus antigen-mediated T cell activation on the persistence of the replication-competent reservoir is unknown, but massive and sustained clonal expansion of cells containing an intact provirus capable of sustaining infectious viremia has been reported42.
Defining the distribution of HIV during ART
We need to better define and quantitate the anatomical and cellular sites of HIV persistence on ART, and assess their evolution over time. For accessible tissue sites (e.g., lymph node and gut), the spatial distribution of the replication-competent virus should be defined and the degree that active replication persists within putative “sanctuaries” (e.g., the B cell follicle or variable sites of antiretroviral penetration) determined12,35. For tissue sites that are less accessible (e.g., spleen, brain, genital tract, and thymus), tissue banks should be accessed as these contain samples both from biopsies and autopsies of HIV-infected people who died while on ART. It is expected that further validation of non-human primate models will complement these human studies.
A number of questions persist regarding the memory CD4+ T cell populations that harbour HIV during ART. Is HIV enriched in cells with certain antigen-specificity during ART? Can replication-competent HIV be readily found in all CD4+ T cell populations, including T regulatory cells, Th1, Th2, Th17, and Tfh cells? Does the distribution of virus across these populations change over time? What is the nature of the tissue-resident CD4+ T cells that harbour latent HIV and cannot be readily sampled in humans? Does signalling between antigen-presenting cells (e.g., dendritic cells, macrophages) and neighbouring infected cells in lymphoid tissues contribute to latency43?
An emphasis should be placed on exploring how human variability (e.g., host genetics, age, gender, co-morbidities, co-infections, HIV disease progression state, and the microbiome) affects HIV persistence on ART. Since the majority of those infected with HIV are chronically co-infected with other pathogens such as malaria, Mycobacterium tuberculosis, hepatitis B and C virus and helminthic worms, the impact of such co-infections on the persistence of HIV should also be studied. Also, as the epidemic matures, more people will have been on suppressive ART for up to two decades. The long-term (decades) stability of the reservoir in terms of size, distribution, response to activation and replication-competence should be defined.
Defining the biology of the reservoir
How CD4+ T cell memory is established and maintained in humans has not been fully characterized. These efforts will likely involve transcriptomic and proteomic analysis of infected cells as well as analysis of the transcriptional state of the virus. The impact of anatomical site, microenvironment, cellular metabolism, and microbiome on T cell dynamics should be defined.
Enhancing capacity of the immune system to clear or control HIV
Most cure strategies will require active elimination of infected cells or control of HIV persistence by T cells, antibodies, NK cells and/or macrophages. With regard to enhancing T cell function, a number of approaches should be pursued. T cell vaccines that are able to enhance HIV-specific immunity remain a priority. There is specific interest in approaches that stimulate responses against novel, non-dominant epitopes, given that cytotoxic T lymphocyte (CTL) escape to standard (canonical) epitopes likely exist in the majority of individuals and that most vaccines appear to stimulate pre-existing memory responses44. A potent cytomegalovirus (CMV) vector reengineered to stimulate sustained responses to novel, non-immunodominant epitopes has shown promise in nonhuman primate models45–47.
Broadly neutralizing antibodies (bNabs) may also play a role48–52. The degree to which such antibodies might target latently infected cells that go on to express viral antigens spontaneously or following induction by a LRA, or are able to overcome the sequence diversity which emerges in chronic infection needs to be defined. The optimal effector pathway (e.g., natural killer (NK) cells, macrophages, complement) for clearing infected cells is unknown53. Therapies that target effector cells that home to lymphoid tissue, including chemokine blockade and/or disruption of B-cell follicles, should also be pursued. Bi-specific antibodies that enhance virus production and simultaneously recognize and eliminate virus-expressing cells have shown promise in pre-clinical models and should be advanced into the clinic, recognizing that potentially harmful off-target effects may occur54,55.
Targeting T cell homeostasis and T cell dysfunction
Therapies aimed at reversing chronic inflammation could contribute to cure or remission by (1) altering the chronically dysfunctional immunoregulatory environment with an aim at enhancing T cell function, (2) reducing T cell proliferation (homeostatic and antigen-driven) and (3) reducing virion production and the generation of new target cells, thereby reducing virus spread. Proof-of-concept for these approaches was recently demonstrated in non-human primates56. Potentially targetable pathways that could be informative if blocked or enhanced include immune checkpoints (PD-1, CLTA-4, TIGIT, others), IDO, IL-18, mTOR, JAK/STAT, IL-10, and TGF-beta, among others.
Many of these approaches have significant risks, which makes studies in generally healthy HIV-infected adults on ART challenging from an ethical perspective. More interactions between HIV specialists and other disciplines working with these approaches are urgently needed. There may be particular synergies between HIV and those working in oncology, autoimmune diseases, and transplantation. Indeed, careful studies of HIV-infected adults undergoing transplantation have revealed novel insights regarding HIV persistence on ART and the potential role of immune-modifying therapies57,58. Careful studies of HIV-infected adults with cancer receiving immune checkpoint blockers and other emerging immunotherapies should prove to be particularly informative and may lead to identification of novel curative strategies for HIV infection59.
Developing and maintaining well-characterized cohorts
Studies of those who control HIV naturally (“elite controllers”) have provided the strongest evidence to date that HIV-specific CD8+ T cell immunity can contribute to virus control60. More recently, a group of individuals who might have been destined for poorly controlled HIV was possibly turned into long-term controllers by the early introduction of ART that was subsequently discontinued61,62. The development of larger cohorts of these “post-treatment controllers” will be needed to confirm these provocative findings. Other cohorts that will likely prove valuable include those of individuals undergoing solid organ transplantation58 or of individuals receiving immunotherapy and other treatments for the management of cancer and other chronic disease59.
MODELS FOR HIV CURE OR SUSTAINABLE REMISSION
Background
Animal models involving experimental AIDS virus infection of non-human primates and humanized mice provide numerous important experimental advantages, including the ability to control experimental variability, definition of the identity, size, timing, and route of the virus inoculum, and flexibility of experimental and therapeutic interventions. The ability to perform extensive tissue sampling in animal models, including elective necropsy, is a key advantage as the vast majority of virus that persists on ART resides in tissues that are difficult to impossible to sample in a clinical setting. These models are important contributors to the overall research effort aimed at achieving HIV remission. Studies utilizing animal models have informed the field about how quickly latency is established, the anatomic and cellular components of the reservoirs, and the responses to novel interventions35,63,64. ART regimens have recently been developed that can achieve and maintain clinically relevant levels of viral suppression in nonhuman primate models, and encouragingly with regard to the potential predictive value of such studies, results in nonhuman primate studies of latency reactivating agents have paralleled results from clinical studies of the same agents65.
Humanized mouse models have also been used for studies in this area, but limitations on sampling from individual animals, the relatively short duration of feasible studies (weeks) and graft vs host disease in many models restrict their utility to addressing only certain questions64,66. However, such models permit the rapid evaluation of selected strategies in the context of HIV infection of human immune cells.
Development of models
Due to the high bar to clinical testing of unproven and potentially hazardous interventions in a population doing well with standard of care ART, animal models provide an important pathway for the evaluation of novel strategies to achieve cure or sustained off ART remission of HIV infection, serving as a key step for in vivo proof of concept study and safety testing. Key research questions in this setting include further efforts to establish whether these models recapitulate known and newly discovered features of HIV persistence in humans on ART, including cellular and anatomic sites of residual virus, cellular and molecular factors influencing latency, the nature of immune dysregulation, and responses of the infected hosts to experimental interventions. Results to date are encouraging, although some important aspects, such as potential differences in the biology of HIV persistence in HIV-infected humans that have been on ART for decades, can be effectively modelled in studies of shorter duration. Iterative studies in which cycles of preclinical experiments in animal models are informed by emerging clinical data offer great promise to provide insights into the most relevant and effective approaches.
Identity viable combination regimens
It is unlikely that any single intervention will result in a durable remission or cure. Most long-term strategies now being pursued involve combinations of various approaches. Examples include combinations of two or more latency reversing agents with therapies aimed to enhance clearance of virus-producing cells (“shock and kill”) or combinations of therapeutic vaccines with adjuvants and/or immune modifying agents (e.g., immune checkpoint inhibitors). Identifying, developing and optimizing such combinations will be challenging in humans, given that many combinations will need to be tested, and that characterizing the safety and pharmacology of individual interventions will likely need to be completed before such combinations can even be considered. Once animal models are fully optimized, well-resourced, iteratively designed and adequately powered studies of single and combination approaches should be performed, with the most promising combinations advanced into humans.
HIV REMISSION IN THE PEDIATRIC POPULATION
Background
Worldwide there are almost 4 million children living with HIV and 250,000 are infected every year61. Children face a prospect of life-long ART and the added challenges associated with ART through childhood and adolescence (e.g. limited appropriate drug formulations, poor adherence). As such, HIV remission represents an especially desirable goal for the pediatric population.
Perinatal HIV-infection offers a unique opportunity to assess prompt control of HIV replication because of the known timing of HIV exposure through maternal infection. Other factors that may potentially further reduce the frequency of latently infected cells include immune tolerance in infancy, lower immune activation compared to adults, and the slower pace of T cell memory development67–69.
Characterize mechanisms for persistence
The major knowledge gaps for perinatal HIV infection are in understanding the mechanisms of latency in infants and children. The dynamics of HIV persistence in children are probably different than those in adults, owing to a number of factors such as the types and numbers of target cells, efficiency in clearing HIV-infected cells, and pharmacokinetics of ART in blood and tissues. Little is known about the development of the newborn and infant innate and adaptive immune system, and about the role of immune activation, homeostasis, inflammation, and viral and host factors in the establishment and maintenance of HIV latency70. Early ART can preserve normal development of B and T cells, as demonstrated by the ability to mount immune responses against childhood vaccines. However, there is limited understanding about the development of HIV-specific B and T cell immunity including neutralizing and non-neutralizing antibodies, and effector and polyfunctional T cell responses. Development of techniques for virologic and immunologic characterization that require small blood volumes is critical to advancing paediatric cure research. Infant animal models could be used to fill gaps and limitations in pediatric HIV pathogenesis and mechanisms of interventions.
CELL AND GENE THERAPY
Background
There is growing interest in the potential of gene and cell therapies to treat HIV infection (Figure 3). This has been driven in part by recent technological advances and successes in other disease areas, especially inherited immune deficiencies and cancer, and the successful outcome for Timothy Brown71. This individual received a hematopoietic stem cell (HSC) transplantation as part of treatment for acute myeloid leukemia, from a donor who was homozygous for the CCR5Δ32 deletion and therefore resistant to HIV infection. Mimicking this approach, most gene therapies to date for HIV have been based on engineering a patient’s own (autologous) cells to confer HIV-resistance, either by removing CCR572 or introducing genes that encode anti-HIV proteins73,74.
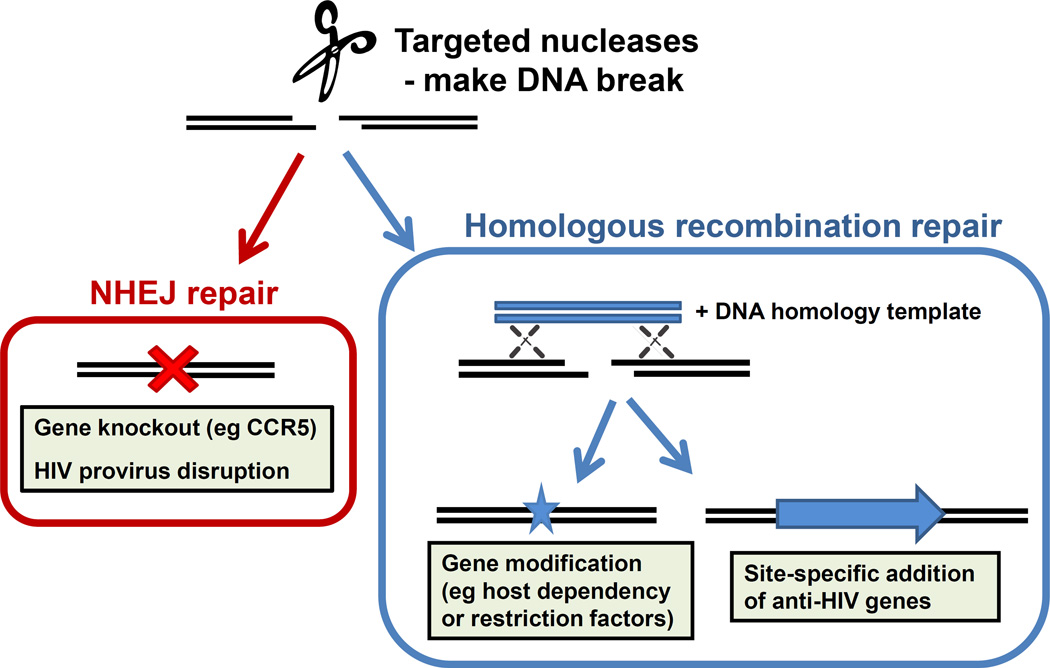
Figure 3. Using targeted nucleases against HIV.
Targeted nucleases, such as zinc finger nucleases and CRISPR/Cas9, provide more precise methods of gene therapy. They create site-specific DNA breaks, whose subsequent repair by the non-homologous end joining (NHEJ) pathway can be exploited to disrupt a gene, such as CCR5, or even an integrated HIV genome. Alternatively, repair can occur through homologous recombination, and a co-introduced DNA homology template can be designed to create small mutations in host genes, or direct the site-specific insertion of an anti-HIV gene.
Gene therapies are also being considered as a way to directly remove HIV-infected cells on ART, although daunting technical barriers exist. Other approaches seek to target integrated HIV genomes for inactivation using engineered nucleases such as zinc finger nucleases (ZFNs), TAL effector nucleases (TALENs) or CRISPR/Cas975 although directing such treatments to latently infected cells in vivo poses substantial challenges, including the potential off-target mutations. More practically, immune cells could be modified to recognize and destroy HIV-infected cells that express HIV antigens76 in the same way that engineered T-cell receptors or chimeric antigen receptor (CAR) T cells have proven successful against certain cancers77,78. Finally, cells could be turned into factories for the long-term production of anti-HIV molecules, such as broadly neutralizing antibodies79 or CD4/CCR5 mimetics80.
Since fully myeloablative conditioning will not be acceptable in non-cancer settings, the percentage of gene-modified cells immediately post-transplant will represent a minority of the cells in the body. Although it is assumed that there could be some selection for gene modified HIV-resistant cells as the virus depletes those that remain gene unmodified and hence HIV-susceptible, this may not be effective if ART is maintained and there is little/no replicating virus in the individual. Such selection could be temporarily induced with a treatment interruption, although how long a period of uncontrolled virus replication would be required to select for a sufficient number of gene modified cells is unknown, and there will be limited support for studies that require prolonged periods of viremia.
Explore the potential of engineered T cells to eliminate HIV-infected cells
The field of T cell engineering is rapidly moving, with great successes in the area of immunotherapies for cancer. In a similar approach, it is possible that engineering T cells to express modified TCRs, or CARs recognizing HIV antigens, could provide control of HIV or eliminate infected cells. Other immune effector cells are also being developed as candidates for these methods81.
Develop an HIV-resistant T cell population
There are a number of gene modifications that might render a T cell resistant to HIV infect. Achieving a life-long remission will likely require protecting all possible target cells, including CD4+ T cells and perhaps monocyte/macrophages. The ideal population to target are hematopoietic stem cells (HSCs) as they are long-lived precursors for multiple cell types, but these cells are rare and technically challenging to isolate, gene-modify and engraft. As reengineered stem cells have the potential for malignant transformation, the use of “kill switches” (genes which can be activated to cause cell death) will likely need to be considered.
Develop methods to deliver targeted nucleases to latently infected cells
Targeted nucleases such as ZFNs and CRISPR/Cas9 can disrupt HIV proviral DNA in cell culture models, but their application in HIV-infected individuals will require enhanced methods of delivery. This includes the challenge of delivery to the rare, latently infected cells that may not express HIV antigens. Achieving in vivo delivery is a challenge for the field of gene therapy in general.
Apply methods to boost immune responses in combination with cell and gene therapies
It is uncertain if genetically modified HSC or T cells, even if they are resistant to HIV infection, could mediate eradication of other infected cells. Thus some additional mechanisms will likely be required to control or eliminate persistent HIV, while the gene-modified HIV-resistant cells protect against re-infection. Cell and gene therapies could therefore be combined with other treatments that boost HIV-specific immune responses, including novel therapeutic vaccine strategies, drugs modulating T cell responses such as PD-1 inhibitors, and LRAs. There are already some indications that engineered HSC- derived CD4 T cells or peripheral T cells can boost the endogenous immune system to control HIV74,82 but this needs to be understood better.
Development of less toxic immunosuppressive conditioning regimens
While the efficiency of rendering T cells or HSCs resistant ex vivo has improved substantially over the past years, there is still an enormous problem in getting these cells to engraft without the use of toxic conditioning regimens. Although chemoablation increases the efficiency of engraftment of gene-modified HSCs and T cells, there are concerns about its toxicity. One mitigating approach is to do such studies first in HIV-infected individuals with cancer, but this patient population is small and getting smaller. More investigation is needed into the long-term effects of such treatments since the risks of ablation in the autologous gene therapy setting are unknown. Thus, novel less toxic regimens need to be developed. These include safer (non-mutagenic) methods of conditioning and the possibility of positive selection for engineered cells in vivo, post-transplantation83.
NOVEL BIOMARKERS TO ANALYSE/QUANTIFY HIV RESERVOIRS
Background
The quantitative viral outgrowth assay (QVOA) has long been considered the gold-standard for measuring the size of the replication-competent reservoir84–86. The assay is labour intensive, expensive and requires large numbers of cells. Recent advances in measuring inducible virus have included a similar limiting dilution format but measuring production of cell associated RNA or release of viral RNA in supernatant87,88 and amplification of latent infectious virus using a humanized mouse model – the murine viral outgrowth assay (MVOA)89.
HIV-infected cells can also be quantified using PCR based assays. Total or integrated HIV DNA are both high throughput assays that are more easily standardised; however, they over estimate the number of latently infected cells because most proviruses that persist during ART have lethal mutations or deletions8,90. Quantification of low-level plasma viremia by single copy assay is useful in studies of latency reversal but its relationship with the frequency of latently infected cells is unclear (Figure 4)90.
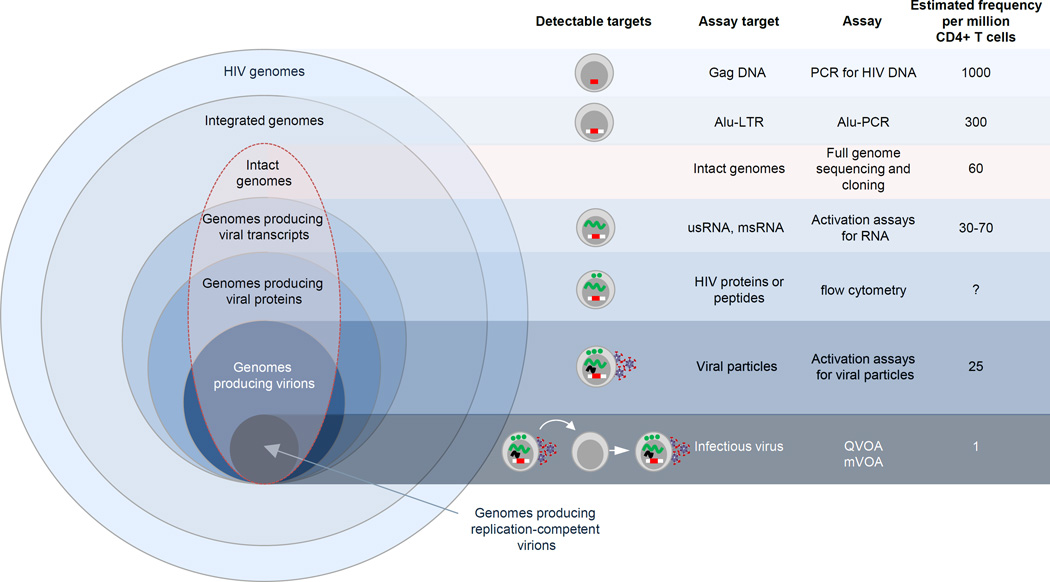
Figure 4. Assays used to quantify HIV persistence on ART.
The frequency of cells that produce infectious virus is only a subset of cells that are infected with intact (highlighted in a red line) and defective genomes (total pool of infected cells). US = unspliced; MS = multiply spliced; QVOA = quantitative viral outgrowth assay; MVOA = murine viral outgrowth assay
It is possible that measuring the immune response to HIV could be a more sensitive strategy to detect residual virus than measuring the virus itself. The avidity and concentration of HIV antibodies appears to change with declining numbers of latently infected cells91 and markers of T cell activation and proliferation have been shown to correlate with the number of latently infected cells in multiple studies29.
No biomarker has been identified that can accurately and consistently predict time to viral rebound following ART discontinuation or the duration of ART-free remission, although progress is being made92,93. In the absence of predictable biomarkers of virologic rebound, assessment of HIV remission requires interruption of ART.
Define the performance characteristics of existing and evolving biomarkers
The performance characteristics of putative biomarker assays need to be much better characterized through unbiased assessment of sensitivity, specificity, precision and accuracy through testing of blinded panels of clinical samples that are prepared and distributed by a central organization. Impartial, centralized distribution of test panels and data analysis are critical components of the research infrastructure needed to accurately determine biomarker assay performance. These efforts should include both blood-based and tissue-based biomarkers.
Develop highly sensitive biomarkers of HIV persistence
Biomarkers in blood may be too insensitive to adequately represent the extent of HIV persistence in tissue35. Indeed, in the previous case reports of prolonged remission following HSC transplantation in Boston94 and very early ART administration to a child born to an untreated HIV-infected mother in Mississippi95 provide evidence that blood sampling alone, perhaps because there is a limit to how much blood can be collected, will be inadequate as a measure of HIV persistence. Less cumbersome and more scalable means of sampling tissue reservoirs need to be achieved as a potential means of increasing the sensitivity of biomarkers of HIV persistence. Advances in whole body imaging technologies such as immune-PET scanning96 and stereotactic guided tissue sampling hold promise but reduction of risk and simplification of the sampling process to that equivalent to phlebotomy are formidable challenges. Animal models, such as humanized mice and the MVOA can enhance the lower limit of sensitivity to detect infectious HIV allowing for the use of large numbers of human cells that exceed what is currently possible in vitro, although these models will be limited by amount of cells which can be infused and by costs.
Identify specific markers of an infected cell
There is a compelling need to identify phenotypic markers for latently infected cells in vivo. It has been argued that HIV is enriched in cells that express markers of T cell activation and function, including HLA-DR, CCR5, CCR6, CXCR3 and PD-130,97–99 although it is likely that only a fraction of cells expressing such markers harbour latent HIV. Whether persistent virus in activated cells differs from resting cells remains unclear. The identification of markers for the infected population could allow more targeted therapies.
Develop methods for detection of replication-competent proviruses
These methods may involve nucleic acid detection of signature proviral sequences that are present only in intact proviruses and not in defective ones; high throughput, full-length single genome sequencing to identify intact proviruses; or simplified virus outgrowth assays that induce the complete reactivation of latent but intact proviruses, potentially using additional stimuli other than activation of the T-cell receptor100. Recent innovations in high-throughput analyses of single cells should be applied with the goal of quantifying rare cells with inducible, intact proviruses.
Develop non-virologic biomarkers that quantify the total-body reservoir
Potential biomarkers of this type include the levels of antibody to specific HIV proteins, the affinity of antibodies for such proteins, or the frequency of B-cells responsive to specific HIV-1 antigens. Similarly, assays to assess the frequency of CD4+ or CD8+T-cells that are responsive to specific HIV-1 antigens should be sought as markers of HIV-1 persistence. Host transcriptional or metabolic signatures of continued innate or adaptive immune response to HIV-1 nucleic acids or proteins may also prove to be sensitive markers of HIV-1 persistence.
Validate biomarkers for use in studies of HIV cure/remission
Any putative biomarker needs to be validated as a predictor of the duration of ART-free remission. A major repository of biologic samples of various types (blood, body fluids and tissues) and a robust clinical database of individuals who suspend ART in a controlled manner, are essential for validation of candidate biomarkers.
Characterize and validate biomarkers for all HIV subtypes
An infrastructure to support biomarker development on a global scale will be needed to ensure that assays are optimised to detect common circulating HIV clades, in addition to subtype B.
SOCIAL SCIENCES AND HEALTH SYSTEMS RESEARCH
Background
Given the complexity of cure science outlined in the preceding sections, research on science translation and public engagement is critical. Social science research on HIV cure has the potential to guide meaningful community engagement, ensure ethical conduct of research, mitigate the risks of behavioural disinhibition and therapeutic misconception, enhance patient-physician communication, engage global key populations, ensure economic viability, and reduce pervasive HIV and sexual stigma. Health systems research can facilitate policy-relevant research synergies, assist with health systems preparedness, spur public-private collaboration, and inform effective community engagement strategies.
Identify HIV-infected individuals’ perceptions
The voices of HIV-infected individuals always have been central to the HIV response, and this must extend to cure research101. For many individuals with HIV infection, their serostatus has been the basis for making a number of decisions that influence health (e.g., serosorting and other sexual behaviours) and wellbeing (e.g., participation in community groups and advocacy). How they understand the meaning of HIV cure is important, because these beliefs may influence participation in clinical trials, trust in HIV service delivery systems, engagement in care and treatment, serostatus disclosure, and ongoing risk and protective behaviours.
The personal, behavioural, ethical and social implications of participating in HIV cure clinical research warrant greater attention in the context of clinical research. Examples of this type of research include the following: research about how to effectively communicate the science, benefits and risks of HIV cure trials as part of informed consent102,103; qualitative research among HIV-infected trial participants and their partners about how participation in cure studies affect the participant’s HIV identity, sexual behaviours, and social relationships; and research on how HIV-infected individuals understand (or misunderstand) ongoing HIV cure research104,105.
Measuring and increasing stakeholder engagement
There are multiple stakeholders in cure research, including HIV-infected individuals, key affected populations, health professionals, scientists, funding agencies, international agencies, public health and regulatory authorities, pharmaceutical industries, and civil society organizations. The history of HIV intervention research shows how early stakeholder engagement along multiple levels can help increase the likelihood of success and mitigate failure106. However, there are many key research areas that require further investigation, including the following: better and more standardized tools for measuring stakeholder engagement and its downstream effects (e.g., changes in retention rates and recruitment pace in cure research projects); optimal timing and substance of HIV community and non-community stakeholder engagement, including investigation of the role of community advisory boards in representing the community research interests; ensuring affordability of community engagement strategies, especially in resource-constrained contexts.
Clinical trial equity and inclusiveness
In order to ensure an equitable distribution of cure strategies, it is essential to incorporate a diverse group of participants in terms of sex, ethnicity/nationality, location, age, and other characteristics. However, optimal mechanisms and tools for achieving this inclusiveness are unclear. This field could benefit from understanding the experience of the HIV vaccine research field over the past several decades.107 Research opportunities in this realm include community-based research on how to ensure inclusiveness and representativeness in HIV cure studies and approaches to improve the likelihood of equitable implementation of and access to efficacious HIV cure strategies, especially in resource limited settings.
Health systems research
Modelling research could help researchers understand which individual cure strategy or group of strategies would be optimal for achieving population-level effects108. Both cost-effectiveness (i.e., will the strategy be worth paying for?) and budgetary impact research (i.e., will the strategy be affordable?) will be important, especially in resource-limited settings109. Identifying who will pay for a cure and how prices will be established is also critical. Studies on how best to enhance public-private collaboration towards an HIV cure could alleviate some of the regulatory and logistical challenges associated with drug development.
Global perspectives
The local context of HIV cure research will likely prove to be critical. For example, previous examples of ineffective HIV "cures" in Sub-Saharan Africa110 may influence how HIV-infected individuals perceive HIV cure research moving forward. The cost of delivering a cure would likely be different in these resource-constrained contexts. Yet, to date, none of the existing literature has focused on low-income country contexts that have a higher burden of HIV and would potentially have the greatest to benefit from a cure.
CONCLUSION
The development of a safe, affordable and scalable strategy that results in complete eradication of HIV or sustained virus control in absence of therapy is a key priority of the IAS, funders, and the broader HIV community. Once considered aspirational, there are now a number of potential therapeutic strategies that could conceivably achieve this goal. The challenges, however, remain significant. A central premise of the IAS Global Scientific Strategy is that a multi-disciplinary, collaborative and sustained effort will be needed to overcome these challenges. The strategy outlined here highlight the priority areas for research and will hopefully guide a global strategic research effort and inspire new investigators to engage in the challenge.
Acknowledgments
Other contributing authors are as follows: Galit Alter1, Judith Auerbach2, Brigitte Autran3,4,5, Dan H. Barouch6,1, Georg Behrens7, Marina Cavazzana8, Zhiwei Chen9, Éric A. Cohen10, Giulio Maria Corbelli11, Serge Eholié12, Nir Eyal13, Sarah Fidler14, Laurindo Garcia15, Cynthia Grossman16, Gail Henderson17, Timothy J. Henrich2,18, Richard Jefferys19, Hans-Peter Kiem20, Joseph McCune2, Keymanthri Moodley21, Peter A. Newman22, Monique Nijhuis23, Moses Supercharger Nsubuga24, Melanie Ott25, Sarah Palmer26, Douglas Richman27, Asier Saez-Cirion28, Matthew Sharp29, Janet Siliciano30, Guido Silvestri31, Jerome Singh32, Bruno Spire33, Jeffrey Taylor34, Martin Tolstrup35, Susana Valente36, Jan van Lunzen37, Rochelle Walensky38, Ira Wilson39, Jerome Zack40.
1Ragon Institute of MGH, MIT and Harvard, Boston, Massachusetts, USA, 2University of California, San Francisco, San Francisco, California, USA, 3Sorbonne Universités, UPMC Univ Paris 06, CIMI – Paris, France, 4Inserm U1135, CIMI – Paris, Paris, France, 5AP-HP, Hôpital Pitié-Salpêtrière, Département d’Immunologie, Paris, France 6Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA, 7Hannover Medical School, Hanover, Germany, 8Hôpital Necker-Enfants Malades, Paris, France, 9The University of Hong Kong, Pok Fu Lam, Hong Kong, 10Institut de Recherches Cliniques de Montréal, Université de Montréal, Montréal, Quebec, Canada, 11European AIDS Treatment Group, Italy, 12Centre Hospitalier Universitaire de Treichville, Abidjan, Côte d’Ivoire, 13Harvard T. H. Chan School of Public Health, Department of Global Health and Population, Boston, Massachusetts, USA, 14Imperial College London, London, United Kingdom, 15The B-Change Group, Manila, Philippines, 16National Institute of Mental Health, NIH, Bethesda, Maryland, USA, 17University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA, 18Brigham & Women’s Hospital, Boston, Massachusetts, USA, 19Treatment Action Group, New York, New York, USA, 20Fred Hutchinson Cancer Research Center, Seattle, Washington, USA, 21Centre for Medical Ethics and Law, Department of Medicine, Stellenbosch University, Western Cape, South Africa, 22University of Toronto, Toronto, Ontario, Canada, 23University Medical Center Utrecht, Utrecht, Netherlands, 24Joint Clinical Research Centre, Kampala, Uganda, 25Gladstone Institutes, University of California, San Francisco, San Francisco, California, USA, 26Westmead Millennium Institute, Westmead, Australia, 27VA San Diego Healthcare System and University of California, San Diego, San Diego, California, USA, 28Institut Pasteur, Paris, France, 29Independent HIV Education and Advocacy Consultant, San Francisco, California, USA, 30Johns Hopkins University School of Medicine, Baltimore, Maryland, USA, 31Yerkes National Primate Research Centre, Emory University, Atlanta, Georgia, USA, 32Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa, 33INSERM UMR_S912, Marseille, France, 34CARE Collaboratory Community Advisory Board, Palm Springs, California, USA, 35Aarhus University, Aarhus, Denmark, 36The Scripps Research Institute, Jupiter, Florida, USA, 37ViiV Healthcare, London, United Kingdom, 38Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA, 39Department of Health Services, Policy & Practice, Brown University School of Public Health, Providence, Rhode Island, USA, 40David Geffen School of Medicine at UCLA, Los Angeles, California, USA.
Footnotes
Disclaimer: The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defence or the National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the DHHS, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Ying-Ru Lo is a WHO staff. The opinions herein are those of the authors and should not be construed as official or representing the views of the World Health Organization.
REFERENCES
- 1.UN Joint Programme on HIV/AIDS (UNAIDS) The Gap Report, 2014. [accessed 23 April 2015]; available at: http://www.refworld.org/docid/53f1e1604.html. [Google Scholar]
- 2.Barre-Sinoussi F, Ross AL, Delfraissy JF. Past, present and future: 30 years of HIV research. Nature reviews. Microbiology. 2013;11:877–883. doi: 10.1038/nrmicro3132. [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012 doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Lint C, Emiliani S, Ott M, Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. Embo J. 1996;15:1112–1120. [PMC free article] [PubMed] [Google Scholar]
- 5.Coull JJ, et al. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol. 2000;74:6790–6799. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyagi M, Pearson RJ, Karn J. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J Virol. 2010;84:6425–6437. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han Y, et al. Orientation-dependent regulation of integrated HIV-1 expression by host gene transcriptional readthrough. Cell host & microbe. 2008;4:134–146. doi: 10.1016/j.chom.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho YC, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzon MJ, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 10.Hatano H, et al. Increase in 2-Long Terminal Repeat Circles and Decrease in D-dimer After Raltegravir Intensification in Patients With Treated HIV Infection: A Randomized, Placebo-Controlled Trial. J Infect Dis. 2013;208:1436–1442. doi: 10.1093/infdis/jit453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher CV, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. 2014;111:2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzo-Redondol R, et al. Persistent HIV-1 replication maintains the HIV-1 reservoir during therapy. Nature. 2016 doi: 10.1038/nature16933. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearney MF, et al. Lack of detectable HIV-1 molecular evolution during suppressive antiretroviral therapy. PLoS Pathog. 2014;10:e1004010. doi: 10.1371/journal.ppat.1004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josefsson L, et al. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1308313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archin NM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sogaard OS, et al. The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. PLoS Pathog. 2015;11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott J, et al. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV. 2015 doi: 10.1016/S2352-3018(15)00226-X. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen TA, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014;1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 19.Shan L, et al. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spina CA, et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 2013;9:e1003834. doi: 10.1371/journal.ppat.1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laird GM, et al. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest. 2015;125:1901–1912. doi: 10.1172/JCI80142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott JH, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Praag RM, et al. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J Clin Immunol. 2001;21:218–226. doi: 10.1023/a:1011091300321. [DOI] [PubMed] [Google Scholar]
- 24.Bui JK, Mellors JW, Cillo AR. HIV-1 Virion Production from Single Inducible Proviruses following T-Cell Activation Ex Vivo. J Virol. 2015;90:1673–1676. doi: 10.1128/JVI.02520-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandergeeten C, et al. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood. 2013;121:4321–4329. doi: 10.1182/blood-2012-11-465625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones RB, et al. Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes. PLoS Pathog. 2014;10:e1004287. doi: 10.1371/journal.ppat.1004287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mousseau G, Mediouni S, Valente ST. Targeting HIV transcription: the quest for a functional cure. Current topics in microbiology and immunology. 2015;389:121–145. doi: 10.1007/82_2015_435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mousseau G, et al. The Tat Inhibitor Didehydro-Cortistatin A Prevents HIV-1 Reactivation from Latency. mBio. 2015;6:e00465. doi: 10.1128/mBio.00465-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barouch DH, Deeks SG. Immunologic strategies for HIV-1 remission and eradication. Science. 2014;345:169–174. doi: 10.1126/science.1255512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yukl SA, et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. The Journal of infectious diseases. 2013;208:1212–1220. doi: 10.1093/infdis/jit308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheret A, et al. Combined ART started during acute HIV infection protects central memory CD4+ T cells and can induce remission. The Journal of antimicrobial chemotherapy. 2015;70:2108–2120. doi: 10.1093/jac/dkv084. [DOI] [PubMed] [Google Scholar]
- 33.Buzon MJ, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014;20:139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connick E, et al. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol. 2007;178:6975–6983. doi: 10.4049/jimmunol.178.11.6975. [DOI] [PubMed] [Google Scholar]
- 35.Fukazawa Y, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calantone N, et al. Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells. Immunity. 2014;41:493–502. doi: 10.1016/j.immuni.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Churchill MJ, Cowley DJ, Wesselingh SL, Gorry PR, Gray LR. HIV-1 transcriptional regulation in the central nervous system and implications for HIV cure research. J Neurovirol. 2015;21:290–300. doi: 10.1007/s13365-014-0271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honeycutt JB, et al. Macrophages sustain HIV replication in vivo independently of T cells. J Clin Invest. 2016 doi: 10.1172/JCI84456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner TA, et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345:570–573. doi: 10.1126/science.1256304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maldarelli F, et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345:179–183. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imamichi H, et al. Lifespan of effector memory CD4+ T cells determined by replication-incompetent integrated HIV-1 provirus. Aids. 2014;28:1091–1099. doi: 10.1097/QAD.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 42.Simonetti FR, et al. Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1522675113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans VA, et al. Myeloid dendritic cells induce HIV-1 latency in non-proliferating CD4+ T cells. PLoS pathogens. 2013;9:e1003799. doi: 10.1371/journal.ppat.1003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casazza JP, et al. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. The Journal of infectious diseases. 2013;207:1829–1840. doi: 10.1093/infdis/jit098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen SG, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340:1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen SG, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen SG, et al. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science. 2016 doi: 10.1126/science.aac9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halper-Stromberg A, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch RM, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caskey M, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chun TW, et al. Broadly neutralizing antibodies suppress HIV in the persistent viral reservoir. Proc Natl Acad Sci U S A. 2014;111:13151–13156. doi: 10.1073/pnas.1414148111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Euler Z, Alter G. Exploring the potential of monoclonal antibody therapeutics for HIV-1 eradication. AIDS Res Hum Retroviruses. 2015;31:13–24. doi: 10.1089/aid.2014.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pegu A, et al. Activation and lysis of human CD4 cells latently infected with HIV-1. Nature communications. 2015;6:8447. doi: 10.1038/ncomms9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sung JA, et al. Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J Clin Invest. 2015;125:4077–4090. doi: 10.1172/JCI82314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Micci L, et al. Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. J Clin Invest. 2015;125:4497–4513. doi: 10.1172/JCI81400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henrich TJ, et al. Antiretroviral-Free HIV-1 Remission and Viral Rebound After Allogeneic Stem Cell Transplantation: Report of 2 Cases. Ann Intern Med. 2014 doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stock PG, et al. Reduction of HIV Persistence Following Transplantation in HIV-Infected Kidney Transplant Recipients. Am J Transplant. 2014 doi: 10.1111/ajt.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wightman F, et al. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. Aids. 2015;29:504–506. doi: 10.1097/QAD.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.International HIVCS, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science (New York, N.Y. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saez-Cirion A, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS pathogens. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frange P, et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV. 2016;3:e49–e54. doi: 10.1016/S2352-3018(15)00232-5. [DOI] [PubMed] [Google Scholar]
- 63.Whitney JB, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Denton PW, et al. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog. 2014;10:e1003872. doi: 10.1371/journal.ppat.1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del Prete GQ, et al. Elevated plasma viral loads in romidepsin treated SIV-infected rhesus macaques on suppressive combination antiretroviral therapy. Antimicrob Agents Chemother. 2015 doi: 10.1128/AAC.02625-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marsden MD, et al. HIV latency in the humanized BLT mouse. J Virol. 2012;86:339–347. doi: 10.1128/JVI.06366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Persaud D, et al. Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA pediatrics. 2014;168:1138–1146. doi: 10.1001/jamapediatrics.2014.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ananworanich J, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014;28:1015–1020. doi: 10.1097/QAD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 69.Uprety P, et al. Cell-Associated HIV-1 DNA and RNA Decay Dynamics During Early Combination Antiretroviral Therapy in HIV-1-Infected Infants. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 doi: 10.1093/cid/civ688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muenchhoff M, Prendergast AJ, Goulder PJ. Immunity to HIV in Early Life. Frontiers in immunology. 2014;5:391. doi: 10.3389/fimmu.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hutter G, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 72.Cannon P, June C. Chemokine receptor 5 knockout strategies. Current opinion in HIV and AIDS. 2011;6:74–79. doi: 10.1097/COH.0b013e32834122d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DiGiusto DL, et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med. 2010;2:36ra43. doi: 10.1126/scitranslmed.3000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Younan PM, et al. Positive selection of mC46-expressing CD4+ T cells and maintenance of virus specific immunity in a primate AIDS model. Blood. 2013;122:179–187. doi: 10.1182/blood-2013-01-482224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu W, et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci U S A. 2014;111:11461–11466. doi: 10.1073/pnas.1405186111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kitchen SG, et al. In vivo suppression of HIV by antigen specific T cells derived from engineered hematopoietic stem cells. PLoS pathogens. 2012;8:e1002649. doi: 10.1371/journal.ppat.1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balazs AB, et al. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med. 2014;20:296–300. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gardner MR, et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015;519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith DJ, et al. Genetic engineering of hematopoietic stem cells to generate invariant natural killer T cells. Proc Natl Acad Sci U S A. 2015;112:1523–1528. doi: 10.1073/pnas.1424877112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tebas P, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370:901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beard BC, et al. Efficient and stable MGMT-mediated selection of long-term repopulating stem cells in nonhuman primates. J Clin Invest. 2010;120:2345–2354. doi: 10.1172/JCI40767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laird GM, et al. Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS Pathog. 2013;9:e1003398. doi: 10.1371/journal.ppat.1003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lehrman G, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crooks AM, et al. Precise Quantitation of the Latent HIV-1 Reservoir: Implications for Eradication Strategies. The Journal of infectious diseases. 2015 doi: 10.1093/infdis/jiv218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cillo AR, et al. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1402873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Procopio FA, et al. A Novel Assay to Measure the Magnitude of the Inducible Viral Reservoir in HIV-infected Individuals. EBioMedicine. 2015;2:872–881. doi: 10.1016/j.ebiom.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Metcalf Pate KA, et al. A Murine Viral Outgrowth Assay to Detect Residual HIV Type 1 in Patients With Undetectable Viral Loads. J Infect Dis. 2015 doi: 10.1093/infdis/jiv230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eriksson S, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burbelo PD, et al. HIV antibody characterization as a method to quantify reservoir size during curative interventions. The Journal of infectious diseases. 2014;209:1613–1617. doi: 10.1093/infdis/jit667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williams JP, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. eLife. 2014;3:e03821. doi: 10.7554/eLife.03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li JZ, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. Aids. 2016;30:343–353. doi: 10.1097/QAD.0000000000000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Henrich TJ, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161:319–327. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Persaud D, Luzuriaga K. Absence of HIV-1 after treatment cessation in an infant. The New England journal of medicine. 2014;370:678. doi: 10.1056/NEJMc1315498. [DOI] [PubMed] [Google Scholar]
- 96.Santangelo PJ, et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods. 2015;12:427–432. doi: 10.1038/nmeth.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hatano H, et al. Cell-Based Measures of Viral Persistence Are Associated With Immune Activation and Programmed Cell Death Protein 1 (PD-1)-Expressing CD4+ T cells. The Journal of infectious diseases. 2012 doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murray JM, et al. HIV DNA subspecies persist in both activated and resting memory CD4+ T cells during antiretroviral therapy. Journal of virology. 2014;88:3516–3526. doi: 10.1128/JVI.03331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khoury G, et al. Persistence of integrated HIV DNA in CXCR3+CCR6+ memory CD4+ T-cells in HIV-infected individuals on antiretroviral therapy. AIDS. 2016 doi: 10.1097/QAD.0000000000001029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Sluis RM, et al. Dendritic cell type-specific HIV-1 activation in effector T-cells: implications for latent HIV-1 reservoir establishment. AIDS. 2015 doi: 10.1097/QAD.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 101.Tucker JD, Rennie S Social & Ethical Working Group on, H.I.V.C. Social and ethical implications of HIV cure research. AIDS. 2014;28:1247–1250. doi: 10.1097/QAD.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henderson GE. The ethics of HIV "cure" research: what can we learn from consent forms? AIDS Res Hum Retroviruses. 2015;31:56–63. doi: 10.1089/aid.2014.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peay HL, Henderson GE. What motivates participation in HIV cure trials? A call for real-time assessment to improve informed consent. Journal of virus eradication. 2015;1:51–53. doi: 10.1016/S2055-6640(20)31143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moodley K, Staunton C, de Roubaix M, Cotton M. HIV cure research in South Africa: a preliminary exploration of stakeholder perspectives. AIDS Care. 2015:1–4. doi: 10.1080/09540121.2015.1112351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chu CE, et al. Exploring the social meaning of curing HIV: a qualitative study of people who inject drugs in Guangzhou, China. AIDS Res Hum Retroviruses. 2015;31:78–84. doi: 10.1089/aid.2014.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lo Y, Chu C, Ananworanich J, Excler J, Tucker JD. Stakeholder engagement in HIV cure research: Lessons learned from other HIV interventions and the way forward. AIDS Patient Care & STDs. 2015;29 doi: 10.1089/apc.2014.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Newman PA, Rubincam C. Advancing community stakeholder engagement in biomedical HIV prevention trials: principles, practices and evidence. Expert review of vaccines. 2014;13:1553–1562. doi: 10.1586/14760584.2014.953484. [DOI] [PubMed] [Google Scholar]
- 108.Sax PE, et al. HIV cure strategies: how good must they be to improve on current antiretroviral therapy? PloS one. 2014;9:e113031. doi: 10.1371/journal.pone.0113031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Freedberg KA, et al. The HIV cure research agenda: the role of mathematical modelling and cost-effectiveness analysis. Journal of virus eradication. 2015;1:245–249. doi: 10.1016/S2055-6640(20)30929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Amon JJ. Dangerous medicines: unproven AIDS cures and counterfeit antiretroviral drugs. Global Health. 2008;4:5. doi: 10.1186/1744-8603-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yukl SA, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9:e1003347. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fauci AS, Marston HD, Folkers GK. An HIV cure: feasibility, discovery, and implementation. Jama. 2014;312:335–336. doi: 10.1001/jama.2014.4754. [DOI] [PubMed] [Google Scholar]
- 113.Tucker JD, Volberding PA, Margolis DM, Rennie S, Barre-Sinoussi F. Words matter: Discussing research towards an HIV cure in research and clinical contexts. Journal of acquired immune deficiency syndromes. 2014;67:e110–e111. doi: 10.1097/QAI.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Persaud D, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. The New England journal of medicine. 2013;369:1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saez-Cirion A, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Denton PW, et al. Generation of HIV latency in humanized BLT mice. J Virol. 2012;86:630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deng K, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517:381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Archin NM, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci U S A. 2012;109:9523–9528. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Metcalf Pate KA, et al. A Murine Viral Outgrowth Assay to Detect Residual HIV Type 1 in Patients With Undetectable Viral Loads. J Infect Dis. 2015;212:1387–1396. doi: 10.1093/infdis/jiv230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hurst J, et al. Immunological biomarkerspredict HIV-1 viral rebound after treatment interruption. Nature communications. 2015;6:8495. doi: 10.1038/ncomms9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Assoumou L, et al. A low HIV-DNA level in peripheral blood mononuclear cells at antiretroviral treatment interruption predicts a higher probability of maintaining viral control. AIDS. 2015;29:2003–2007. doi: 10.1097/QAD.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 122.Buzon MJ, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014;20:139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Josefsson L, et al. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc Natl Acad Sci U S A. 2013;110:E4987–E4996. doi: 10.1073/pnas.1308313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Katlama C, et al. Treatment intensification followed by interleukin-7 reactivates HIV without reducing total HIV DNA: a randomized trial. AIDS. 2016;30:221–230. doi: 10.1097/QAD.0000000000000894. [DOI] [PubMed] [Google Scholar]
- 125.Lorenzo-Redondo R, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530:51–56. doi: 10.1038/nature16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wightman F, et al. Entinostat is a histone deacetylase inhibitor selective for class 1 histone deacetylases and activates HIV production from latently infected primary T cells. Aids. 2013;27:2853–2862. doi: 10.1097/QAD.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jiang G, et al. Reactivation of HIV latency by a newly modified Ingenol derivative via protein kinase Cdelta-NF-kappaB signaling. AIDS. 2014;28:1555–1566. doi: 10.1097/QAD.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boehm D, et al. BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. Cell cycle. 2013;12:452–462. doi: 10.4161/cc.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rasmussen T, et al. Panobinostat. A histone deacetylase inhibitor for latent virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase I/II single group clinical trial. Lancet HIV. 2014 doi: 10.1016/S2352-3018(14)70014-1. Sept 23rd, 2014 epub. [DOI] [PubMed] [Google Scholar]
- 130.Hansen SG, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hansen SG, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tebas P, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. The New England journal of medicine. 2014;370:901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]