Abstract
Background
Body dysmorphic disorder (BDD) is a debilitating disorder, characterised by obsessions and compulsions relating specifically to perceived appearance, newly classified within the DSM-5 Obsessive-Compulsive and Related Disorders grouping. Until now, little research has been conducted into the cognitive profile of this disorder.
Materials and Methods
Participants with BDD (n=12) and healthy controls (n=16) were tested using a computerised neurocognitive battery investigating attentional set-shifting (Intra/Extra Dimensional Set Shift Task), decision-making (Cambridge Gamble Task), motor response-inhibition (Stop-Signal Reaction Time Task) and affective processing (Affective Go-No Go Task). The groups were matched for age, IQ and education.
Results
In comparison to controls, patients with BDD showed significantly impaired attentional set shifting, abnormal decision-making, impaired response inhibition and greater omission and commission errors on the emotional processing task.
Conclusions
Despite the modest sample size, our results showed that individuals with BDD performed poorly compared to healthy controls on tests of cognitive flexibility, reward and motor impulsivity and affective processing. Results from separate studies in OCD patients suggest similar cognitive dysfunction. Therefore, these findings are consistent with the re-classification of BDD alongside OCD. These data also hint at additional areas of decision-making abnormalities that might contribute specifically to the psychopathology of BDD.
Keywords: Body Dysmorphic Disorder, Neurocognitive, Affective, Cognitive flexibility
Introduction
Individuals with Body Dysmorphic Disorder (BDD) are troubled by intrusive thoughts that they have a bodily imperfection that is visibly unsightly (Grant & Phillips, 2005). In some cases, they have a minor physical flaw that would not be regarded as abnormal or noticeable by most people; in other cases, the defect is imaginary. They fear showing the ‘imperfection’ in public (Rosen, 1995), leading to social avoidance and isolation (Goodman et al., 1989). They spend considerable time ruminating about the perceived defect, and engage in time consuming checking, camouflaging and reassurance-seeking rituals (Veale, 2001).
BDD has been relatively neglected by research, perhaps in part due to the assumption that it is a rare condition. However, extant epidemiological data contradict this perspective. In a German sample of approximately 2500 individuals, selected to be representative of the general population, the point prevalence of BDD was estimated at 1.2-2.1% (Rief et al., 2006). In a national household telephone survey conducted in approximately 2000 US citizens, the point prevalence was estimated at 2.4% (Koran et al., 2008). Other studies, mostly conducted in college student samples, suggest a point prevalence rates of around 2.5% or greater (Biby 1998; Bohne et al., 2002a,b; Sarwar et al., 2005). In addition to being relatively common, BDD is associated with profound impairment in quality of life and everyday functioning (Koran et al., 2008). Insight is frequently impaired and treatment-adherence is noted to be poor (Rashid et al., 2014). Furthermore, a prospective study conducted over four years in 185 subjects with BDD indicates that suicidality is a major concern. Each year, suicidal ideation occurred in more than 50% of individuals with BDD, 2.6% attempted suicide, and 0.3% completed suicide (Phillips and Menard, 2006).
In recognition of its nosological status as a compulsive disorder, the DSM-5 has moved BDD into the same category as obsessive compulsive disorder (OCD), under an expanded grouping of Obsessive Compulsive and Related Disorders (APA, 2013). Studies have demonstrated co-morbid and familial overlap between OCD and BDD (Thornton and Russell, 1997; Bienvenu et al, 2000). In those with OCD, co-morbid BDD has been reported in up to 37% of cases (Conceição Costa et al., 2012). Furthermore, in two seminal OCD family studies, the first-degree relatives of OCD probands were at significantly elevated risk for BDD, as well as trichotillomania, skin picking disorder and hypochondriasis, as compared to control relatives (Bienvenu et al., 2000, 2012). These findings are suggestive of a familial overlap between BDD and OCD on the one hand and between BDD and other putative obsessive compulsive and related disorders on the other, perhaps mediated by common genetic and/or cognitive predisposing factors.
Understanding of the neurobiology of BDD and related conditions can be informed by the use of well-validated cognitive tests that probe the integrity of the fronto-striatal neurocircuitry. Various cognitive impairments have been identified in OCD using computerised paradigms from the Cambridge Neuropsychological Test Automated Battery (CANTAB www.cambridgecognition.com), including in the domains of set-shifting (Extra-Dimensional Set-Shift (EDS)), inhibitory motor control (Stop-Signal Reaction Time (SSRT)), executive planning (Stockings of Cambridge (SOC) test), and affective bias toward negatively-valenced stimuli (for reviews see Chamberlain et al., 2005, Fineberg et al., 2010, Fineberg et al 2014). For some deficits (Extra-Dimensional Set-Shift, Stop-Signal Reaction Time, Stockings of Cambridge), similar cognitive dysfunction exists in unaffected first-degree relatives of patients with OCD and these therefore may represent predisposing or ‘vulnerability’ markers (e.g. Chamberlain et al., 2007a; Cavedini et al., 2010; Rajender et al., 2011; Vaghi et al., 2014). The findings are broadly consistent with current neurobiological models of OCD, which implicate not only dysfunction within the classical orbitofrontal circuitry but also the dorsolateral prefrontal cortical circuitry, which incorporate these cortical regions but also subcortical nodes including the ventral and dorsal striatum (Menzies et al., 2008; Fineberg et al., 2010, Vaghi et al., 2014).
There have been few published studies exploring neuropsychological function in BDD. Hanes and colleagues compared 14 subjects with BDD with 10 subjects with OCD and 24 controls, using a variety of non-computerised tests (Hanes, 1998). Both the BDD and OCD groups were similarly impaired, compared to controls, on tests of executive planning (Tower of London task) and colour-word interference (Stroop task), supporting the hypothesis that these two conditions are neurobiologically related. No significant deficits emerged in the BDD or OCD groups for category fluency and motor skill/speed on the Purdue Pegboard task, verbal learning on the Rey Auditory Verbal Learning task, or non-verbal learning/memory function on the Rey Complex Figures task (RCFT). In contrast, another study (Deckersbach et al., 2000), again using non-computerised tests, identified impairment in non-verbal learning/mnemonic domains (Rey Complex Figures Task), along with verbal learning impairment (California Verbal Learning Test), in 17 patients with BDD compared to 17 healthy controls. The authors postulated that the deficits were mediated by poor organisational strategy. Dunai and colleagues (2009) additionally explored cognitive functioning in 14 patients with BDD versus 14 healthy controls, using selected computerised paradigms from the CANTAB. Patients with BDD were impaired on spatial working memory (Spatial Working Memory test) and executive planning (SOC test); findings similar to those reported separately for OCD (Chamberlain et al., 2006). In a more recent study, executive dysfunction was investigated in 14 BDD participants, 14 matched (age and gender) healthy controls, and 23 participants with OCD. Similarities were seen in the BDD and OCD groups in spatial span, spatial working memory, pattern recognition and spatial planning (SOC) tasks compared with healthy controls. However, those with BDD were found to have relatively greater deficits in executive functioning, on the accuracy measure of the SOC Task, than those with OCD and compared with healthy controls (Labuschagne et al., 2013).
Based on the above limited evidence, the current study sought to explore specific aspects of cognitive functioning in BDD and healthy volunteers using relevant tests from the CANTAB. We focused on motor response inhibition (using the SSRT), cognitive flexibility (using the Intra-Extra Dimensional (IED) Set Shifting Task), and affective processing using the Affective Go/NoGo task (AGN). These three cognitive domains are linked to behavioural inhibition and have not previously been investigated in BDD, but have been found to be impaired in non-comorbid OCD (Chamberlain et al., 2006). We also included a test of decision-making (Cambridge Gambling Task- CGT), which tests aspects of reward-based impulse control, and which has previously been observed to be intact in OCD (Chamberlain, 2006), but which is impaired in patients with behavioural and substance addiction (Zois et al 2014, Fineberg et al 2014). It was hypothesised that BDD would be associated with a similar cognitive profile to that previously reported in OCD: namely, significantly impaired response inhibition and set-shifting, evidence of affective bias with increased sensitivity to negatively-valenced cues, but intact decision-making.
Materials and Methods
Participants
BDD patients, aged between 18 and 65 years of age, were recruited from the specialist OCD/BDD outpatient clinic of one of the authors (NAF). All had a DSM-IV diagnosis of BDD, ascertained by a detailed clinical assessment amplified by the Yale Brown Obsessive Compulsive Checklist and Scale for Body Dysmorphic Disorder (BDD-YBOCS, Goodman et al, 1989) to determine the degree of illness severity. In order to meet the inclusion criteria for the study, BDD was required to constitute the primary illness. All psychiatric comorbidity (such as OCD) as documented in the case notes was recorded.
Healthy, age, IQ and education-matched control participants were recruited from the University of Hertfordshire. Participants were approached within the University premises or via the university’s SONA system (an online computerised system by which students can indicate their interest in participating in research studies). Participants were screened to exclude the presence of Body Dysmorphic Disorder symptoms using the BDD-YBOCS using a cut off of >10. None of the participants in the control group scored more than 10 on this clinical rating scale. They were not formally screened for any other axis-I morbidity.
Clinical measures
Severities of depression and anxiety symptoms were quantified in all participants using the Montgomery-Åsberg Depression Rating Scale (MADRS, Montgomery and Ǻsberg, 1979) and the Hamilton Anxiety Scale (HAM-A, Hamilton, 1979).
Neuropsychological Measures
Participants completed the following paradigms from the CANTAB - see below. The tasks were administered in a fixed order (as below), in a quiet testing environment, supervised by a trained test administrator.
The intra/extra dimensional set-shift task (IED- http://www.cambridgecognition.com/tests/intra-extra-dimensional-set-shift-ied)
This is a nine-stage visual discrimination task and measures cognitive flexibility (Lawrence et al, 1998). Two stimuli are presented at a time, on a black screen and the task requires the participant to ascertain, by trial and error, and by computerised feedback, which of the stimuli is correct, and thus, the ‘rule’ of the game. At the start of each stage, the rule is altered. To successfully pass each stage, six consecutive indications of the correct stimulus must be achieved within 50 trials, otherwise, the task terminates. The extra dimensional shift (EDS) stage of the task is crucial for determining divergent thinking deficits as the participant is required to shift their attentional focus from the previously relevant stimulus dimension to a previously irrelevant stimulus dimension. Such set-shifting depends on the ventrolateral prefrontal cortex (vlPFC- e.g. Hampshire & Owen, 2006). The outcome measures of interest on this task include the total number of errors and total number of stages successfully completed. Where these global parameters differ significantly between groups, performance on individual stages of the task can be explored to account for the data.
The Cambridge Gambling Task (CGT- http://www.cambridgecognition.com/tests/cambridge-gambling-task-cgt)
This task assesses dissociable aspects of decision-making. Participants are asked to accumulate as many points as they can by making gambles across a range of different winning probabilities. Each trial shows 10 boxes (red or blue) across the top of a blank screen and participants are informed that a yellow token may be found under one of the blocks. Each trial has differing proportions of red and blue boxes. Participants are given 100 points to begin the gambling process and must choose which colour they think the token is under. Based on their confidence in their choice, they must place a bet on the location of the token. The bet amount either increases incrementally (5%, 25%, 50%, 75%, 95% of total collected points) or decreases (reverse order) over time. Outcome measures include mean percentage of points gambled (total proportion of overall bets), quality of decision-making (this measures rational decision making and is measured by the calculating the proportion of trials where the participant chose the more likely outcome (box colour), risk taking (the mean proportion of points bet on trials where the most likely outcome was chosen), deliberation time (how long it took to decide on which bet to choose) and delay aversion; this is measured as the tendency for participants to bet larger amounts due to an unwillingness, or inability, to wait for bets to decrease on trials where bet amounts are presented in descending order compared with when bets are presented in ascending order. The delay aversion variable is calculated by subtracting the risk taking measure for ascending trials from risk taking in descending trials. Decision-making tasks, such as the CGT, have been regarded as sensitive measures of orbitofrontal cortex (OFC) pathology in psychiatric disorders (Clark et al, 2004). For example, individuals with OFC damage have been found to show impaired functioning on quality of decision making, longer deliberation times and reduced betting amounts (Rogers et al., 2000).
The Stop Signal Task (SST- http://www.cambridgecognition.com/tests/stop-signal-task-sst)
This is a measure of pre-potent motor inhibition. On this computerised task, participants are required to respond rapidly to left or right oriented arrows, presented on a blank screen. When an audible sound emits (the ‘stop-signal’) from the task screen, participants are required to inhibit their response for that arrow and their degree of success is measured. Over the course of the test, the time between the presentation of the ‘go’ stimulus and the ‘stop-signal’ varies using a tracking algorithm. The main outcome measure on the task is the Stop-Signal Reaction Time (SSRT), which is an estimate of the time taken by the given individual to stop or suppress a response that would ordinarily be undertaken; longer SSRTs equate to poorer motor response inhibition, or greater ‘motor impulsivity’. The SSRT is thought to depend on the integrity of the right inferior frontal cortex and its subcortical connections, and is impaired in disorders associated with motor impulsivity such as attention deficit hyperactivity disorder and behavioural addiction (Chamberlain et al 2014) as well as OCD (Chamberlain et al., 2006; Menzies et al, 2007). The other outcome measure of interest is the median reaction time for ‘go’ trials; a generic measure of response speed not relating to inhibitory control.
The Affective Go/NoGo (AGN- http://www.cambridgecognition.com/tests/affective-go-no-go-agn)
This task assesses mood processing bias. A series of positive and negative words are presented on screen. The participant is required to respond to predetermined ‘target’ words by pressing a key pad when they see a target word. This target word will be ‘positive’ or ‘negative’ in valence. Other non-affective words are considered ‘distractor’ words and participants are required to avoid responding to these words and to only respond to the ‘target’ word. The outcome variables of interest include the mean correct latency representing the length of time each participant takes to respond to target words, as well as the total number of commission errors (pressing for a positive target word when it is a negative one or vice-versa) and omission errors (failing to respond when one should have done so) . This task is sensitive to abnormal affective processing bias in major depressive disorder, and is thought to be mediated by mood-cognition interactions, sub-served by the orbitofrontal cortex and associated neural regions (Murphy et al., 1999), including the cingulate gyrus (Elliott et al, 2000).
Statistical analysis
Between-group differences were investigated by conducting a multivariate Analysis of Variance (MANOVA) using IBM SPSS. Further exploratory analysis in SPSS included a test of covariance using anxiety (Ham-A) and depression (MADRS) scores as covariates. This being an exploratory study, statistical significance was defined as p<0.05 uncorrected.
Results
Demographic analysis
12 individuals with BDD (mean duration of illness 133.5 months [11.13 years]) and 16 healthy controls completed cognitive tasks and clinical questionnaires (Table 1). Importantly, the two groups did not differ significantly with regard to age, education and estimated IQ using the National Adult Reading Test (NART, Nelson, 1982: see Table 1).
Table 1.
Demographic analysis; BDD and control groups
| BDD (n=12) | Control (n=16) | |||||
|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | F | |
| Age (years) | 30.08 | (8.92) | 35.80 | (12.10) | 1.87 | .18 |
| Education (years) | 14.08 | (1.88) | 14.41 | (1.99) | 0.23 | .64 |
| NART (IQ) | 113.80 | (2.95) | 115.00 | (3.34) | 0.22 | .64 |
Clinical analysis
The BDD group showed a range of symptom-severity ranging from mild to moderately severe (BDD-YBOCS total range 7-24). The mean BDD Y-BOCS was 13.25 (SD 4.88), representing mild BDD. Control BDD-YBOCS scores ranged from 0-10 with an average score of 2.38 (SD= 3.40). None of the 16 control participants were taking prescribed medication, while all 12 of the BDD participants were taking prescribed medication (2 citalopram, 6 escitalopram, 3 fluvoxamine and 1 sertraline). Nine of the twelve BDD patients expressed symptoms of comorbid illnesses (all 9 patients showed comorbid OCD within the clinical range, 2 of those 9 were also diagnosed with social anxiety disorder and 1 patient exhibited gender identity disorder (GID)). Although both groups showed low levels of anxiety and depressive symptomatology, the BDD group showed significantly greater severity of symptoms of depression (MADRS, p=.01), and anxiety (Ham A p=.03) – see Table 2. Fifty per cent of the participants with BDD scored very low on the MADRS (‘normal or symptom absent’ with a MADRS score of less than 7 [Muller-Thompson 2005; McDowell, 2006]). The majority of the remainder (n=5) scored within the ‘mild depression’ with scores between 7 and 19, and 1 participant scored 24 representing ‘moderate depression’ (Muller-Thompson 2005; McDowell, 2006).
Table 2.
Clinical measures
| BDD (n=12) | Control (n=16) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | P | |
| HAM-A | 8.08 | (6.75) | 3.94 | (3.04) | 4.76 | .03* |
| MADRS | 7.50 | (5.98) | 2.50 | (4.29) | 6.66 | .01* |
| BDD-YBOCS | 13.25 | (4.88) | 2.38 | (3.40) | 48.40 | <.001* |
Note: HAM-A: Hamilton Anxiety Scale, MADRS: Montgomery-Åsberg Depression Rating Scale, BDD-YBOCS: Yale Brown Obsessive Compulsive Scale for Body Dysmorphic Disorder
Neurocognitive analysis
Intra/Extra Dimensional set shift task (IED)
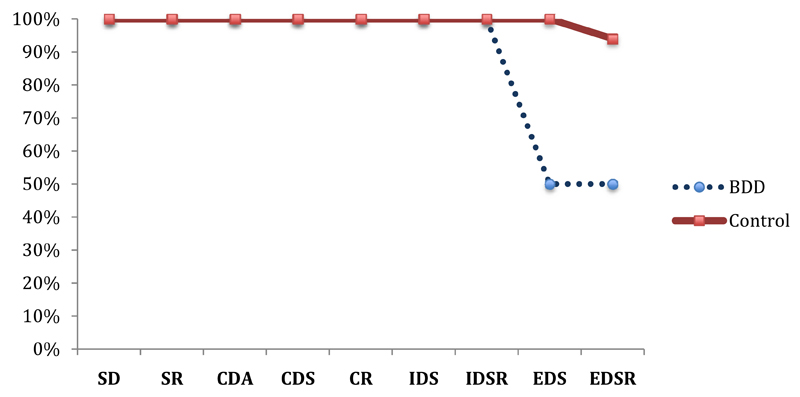
The BDD group made significantly more total errors (adjusted) on the task versus controls (Mean BDD 26.75 (SD 10.85) vs controls 13.18 (SD 4.98); F(1,26)= 14.27, p= .001; Cohen’s d= 1.54). These errors were specifically seen at the extra-dimensional shift (EDS) stage (stage 8) (Mean 17.25 [SD 12.10] vs 4.75[SD 3.92] (F(1,26)= 10.56, p= .003; Cohen’s d= 1.32)). All participants in both groups passed stages 1-7; however, only 50% (n=6) of the BDD group passed the EDS stage while all control participants (n=16) passed the EDS stage (see Figure 1). No notable changes to the significance of each variable were seen when results were co-varied for anxiety and depression.
Figure 1.
Percentage of BDD and control participants passing each stage on the IDED task
Note: SD = simple discrimination; SR = simple reversal; CDA = compound discrimination adjacent; CDS = compound discrimination superimposed; CR = compound reversal; ids = intra-dimensional shift; IDSR = intra-dimensional shift reversal; EDS = extra-dimensional shift; EDSR = extra-dimensional shift reversal
Stop Signal Task (SST)
The BDD group showed significantly longer stop-signal reaction times (SSRTs) than the controls (F(1,26)= 4.66, p= .04; Cohen’s d= .87). General psychomotor speed (measured as median ‘go’ reaction times) did not differ significantly between the groups (p= .77).
Cambridge Gambling Task (CGT)
The BDD group showed significantly more delay aversion than controls (F(1,26)= 5.22, p= .03, Cohen’s d=.94). However, the BDD group gambled a significantly smaller proportion of total points overall (F(1,26)= 63.16, p <.001, Cohen’s d= 3.24 [large effect size]). Between-group differences were also found in risk taking (measured by the proportion of total points bet over all trials), with the BDD group showing a significantly lower incidence of risk taking (F(1,26)= 4.72, p=.04, Cohen’s d=-1.25) than controls. Groups did not differ significantly in terms of the proportion of rational decisions made overall. (Rational decision making on ascending [p= .43] and descending [p= .93] trials). No significant differences were found with regard to the deliberation time when making bets (F(1,26)=1.84, p= .18, Cohen’s d= .55).
Affective Go/ No-Go (AGN)
Reaction time
Analysis of variance showed that the BDD group were slower to respond correctly to presented words than the controls (F(1,26)=4.85, p= .03, Cohen’s d= .90). Sub-analysis indicated that individuals with BDD took significantly longer to respond to positive words when compared to controls (Means: BDD 535; control 473.22; F(1,26)=19.77, p <.001, Cohen’s d= 1.81). The groups did not differ significantly for negative words.
Commissions
ANOVA showed that the number of commission errors differed significantly between the groups, due to higher errors in patients than controls overall (means BDD 10.17 (SD 7.66); control 4.62 (SD 2.09); F(1,26)=5.86, p= .02, Cohen’s d= .97). Sub-analysis indicated that there were significantly more commission errors in patients than controls for positively valenced words (Means: BDD 2.33; control .94; F(1,26)=5.85, p= .02, Cohen’s d= 1.39), and for negative words (Means: BDD 3.75; control .75; F(1,26)=10.78, p= .003, Cohen’s d= .79); but not neutral words (Means: BDD .00; control .18; F(1,26)= .50, p= .48, Cohen’s d= .29).
Omissions
More non-responses (omissions) were seen in the BDD group compared with controls overall (F(1,26)=24.44, p<.001, Cohen’s d= 2.00). When exploring emotional valence, the BDD group made statistically more omissions for positively valenced words (Means: BDD 1.33; control .13; F(1,26)= 7.11, p= .01, Cohen’s d= 1.09), and for negatively valenced words (Means: BDD 1.66; control .19; F(1,26)= 10.91, p= .003, Cohen’s d= 1.34); but not neutral words (Means: BDD .00; control .06; F(1,26)= .26, p= .61, Cohen’s d= .20).
Discussion
This study contributes to the body of research documenting impaired neurocognitive performance in BDD. Differences were seen between individuals with and without BDD and cognitive results generally appeared to be unaffected by severity of mood and anxiety symptoms.
Cognitive Inflexibility
The BDD group made significantly more errors on the IED task, with a significantly higher error rate at stage 8 of the task (the extra-dimensional shift stage- EDS). Only 50% of the BDD group progressed to stage 8 (EDS). Results from the IED task indicates significant attentional (or cognitive) inflexibility within the BDD group. A number of studies have found deficits in cognitive flexibility in OCD patients (Veale et al., 1996; Watkins et al., 2005; Chamberlain et al., 2006, 2007a), with the deficits appearing exclusively at the extra-dimensional stage (EDS), as was the case in the current study. The neurobiology of attentional shift flexibility has been the subject of translational study. Research into rodents (Dias et al 1996), primates (Brown & Bowman 2002 ; McAlonan & Brown 2003, Hornak et al., 2004) and humans (Rogers et al., 2000; Nagahama et al., 2001; Hampshire & Owen, 2006) implicate the ventro-lateral prefrontal cortex (or functionally homologous regions) as being required for intact cognitive flexibility.
The finding of cognitive inflexibility in the BDD group converges with published findings for OCD (Chamberlain, 2006) and with the clinical presentation of the disorder – specifically with the performance of compulsive (repetitive, urge-driven) behaviour. Individuals with BDD engage compulsively in thoughts or behaviours related to appearance and find difficulty diverting attention to non-image related thoughts or ‘purposeful’ forms of activity. However, we cannot exclude the possibility that the cognitive inflexibility found in the BDD group in this study is attributable to the presence of comorbid OCD, which was present in 9 of the participants. Indeed, significant differences were seen for completed stage errors (F(1,12)= 6.93, p=.03, Cohen’s d= 1.84), when comparing the participants in the BDD group who had a diagnosis of OCD with those who did not, suggesting that the presence of OCD may have had an influence upon cognitive flexibility. This may be clinically relevant, in that people with BDD comorbid with OCD may have a more rigid response style, which could impede ability to adjust behaviors in day-to-day life, and to engage with psychological treatments.
Decision Making
The Cambridge Gambling Task (CGT) is a measure of decision-making abilities with the advantage of assessing different aspects of decision-making separately (Rogers et al. 1999a,b; Deakin et al. 004). Individuals with OCD are usually unimpaired on the CGT (Chamberlain et al., 2007a), though abnormal performance on the task versus controls can be elicited in OCD with acute serotonergic challenge (Lochner et al., submitted). However, our results showed abnormal decision-making in a BDD sample. A higher incidence of delay aversion was seen in BDD patients (i.e. participants were unwilling to wait for bets to increase/decrease) suggesting an increased degree of impatience (decision-making impulsivity). Hollander and Wong (1995), in their investigation of gambling disorder and its associations with BDD, found that individuals with BDD showed an increased tendency for gambling. Studies have used the CGT to investigate decision-making in disordered gambling and substance addiction; in one recent study, participants with disordered gambling showed almost global impairments on the CGT including increased delay aversion as well as poorer quality of decision making, higher risk taking and a higher overall bet proportion, resembling the profile of an individual with ventromedial prefrontal cortex (vmPFC) damage. Individuals with disordered gambling and additional alcohol and smoking habits showed even higher levels of decision making impairment (Zois, 2014).
However, in contrast to the findings in disordered gambling, in the current study the BDD group demonstrated a significantly lower instance of risk taking (p=.04) and gambled a significantly smaller proportion of the total money gained (p <.001) than controls. Thus, unlike those with disordered gambling, the BDD group, despite their impatience to make bets, were if anything more conservative than controls in terms of other aspects of decision-making.
Motor Impulsivity
Significant differences in motor impulsivity were found between BDD patients and controls on the Stop Signal Reaction Time (SSRT) task. Impaired motor response inhibition has been proposed to represent an endophenotype of OCD, as studies have found that unaffected relatives are also impaired on the SSRT (Chamberlain, 2007a). Performance on the SSRT is dependent on an intact right inferior frontal gyrus (Aron et al., 2003; 2004). A number of further brain areas have been implicated in impaired response inhibition in OCD (Menzies et al brain 2007) including the orbitofrontal cortex, anterior cingulate, parietal cortex, caudate-putamen and cerebellum, suggesting involvement of circuits within and outside the orbitofrontal –striatal -thalamic loop.
Overall Impulse Control
Our data suggest that participants with BDD exhibit signs of both decision-making impulsivity and motor impulsivity. These findings align with the clinical phenomenology; many of the characteristic behavioural symptoms of BDD, e.g. being unable to resist the urge to undertake cosmetic, and even ’do it yourself (DIY)’ surgery to ‘correct’ perceived flaws, may be construed as poor impulse control. Indeed, Veale (2000) reported that of 25 patients he interviewed, nine (36%) had carried out their own DIY surgery in an attempt to dramatically alter their appearance. In addition, suicidal acts are common in patients with BDD. A large prospective study of suicide showed that in 185 BDD participants followed up over 4 years, for each year spent in the study an average of 57.8% reported suicidal urges, 2.6% attempted suicide and 0.3% (2 people) completed suicide.
While ‘impulsivity’ implies a predisposition toward performing rapid and unplanned reactions to stimuli and ‘compulsivity’ relates to the urge-driven performance of repetitive unwanted acts, both domains can be considered to represent a dysfunction in impulse control (Stein et al, 1996) and both may be represented in BDD. Separate cortico-striatal circuits are thought to sub serve impulsivity (ventral) and compulsivity (dorsal) (Fineberg et al., 2010). Hyperactivity of the striatal circuit (generation of activity) and hypoactivity of the prefrontal circuit (inhibition) may represent a common mechanism underpinning impulse control deficits in a range of obsessive-compulsive disorders such as OCD and BDD (Fineberg et al, 2010).
Affective Processing
On the AGN task, the BDD group showed a longer reaction time between the presentation of a target word and a correct response i.e. they took longer to respond to the target word, when a correct answer was given. In addition, individuals with BDD showed a higher instance of errors characterised by responding to distracter stimuli (non-target words) and also a higher instance of non-response on target stimuli compared with controls. These data mirror previous findings for OCD, in which disorder inappropriate motor responses to non-target stimuli were observed in comparison to those seen in healthy controls (Bannon et al, 2002; Aycicegi, 2003). Findings in OCD studies have been specific for word valance, with negative words being more difficult to forget in OCD groups a potential suggestion of incorrect processing of negative words (Wilhelm et al, 2006) but additional findings suggest that the type of word most difficult to forget in OCD groups is the type associated with their current OCD presentation (positive or negative- Tolin et al, 2002). In the current study, individuals with BDD showed a longer reaction time, more errors and non-responses for positive and negative target words, but not neutral target words. Previous OCD research revealed elevated commission errors for neutral words, compared with happy and sad words, in patients in one study (Johanssen & Dittrich, 2013); while another study found more omission errors for sad target words in OCD (Chamberlain et al., 2007). One interpretation for the current results in BDD patients is that the disorder is associated with more generalized dysregulation of emotional processing circuitry, with a global untoward impact of emotional information on attentional processing. Thus, the presentation of emotionally valenced stimuli (whether positive or negative) results in performance decrements that generalize across both commission and omission errors, with neutral stimuli not having such a pronounced effect.
Also, increased errors in the BDD group to positive and negative target and distractor words could result from individuals with BDD being unusually sensitive to emotional cues, i.e. stimuli that have some meaning to the BDD disorder. These could be negative words such as ‘ugly’ or even positive words such as ‘attractive’. Our findings revealed differences based on word valence, and not on neutral word trials, suggesting that the symptoms of BDD may rely on an inherent focus on both negatives and positives about appearance. Additionally, this bias within the BDD condition may result from cognitive inflexibility, in that individuals with BDD may become ‘stuck’ in a routine of thinking about positive and negative aspects of themselves.
The development of self - image and the role of appearance is thought to be influenced by environmental factors, including significant life events and shaped by memory (Bentall, 2003; McAdams, 1993; Osman et al., 2004). Individuals with BDD commonly report instances of bullying and teasing, potentially increasing their propensity for negative perception of themselves and of specific body parts (Osman et al., 2004, Silver et al., 2010). The finding of attentional bias toward affectively valenced words is consistent with this literature and may help explain how such experiences become overvalued and may result in an obsessive preoccupation with body image. Few studies have tested attention in BDD. Our findings suggest future research investigating the effect of BDD on attention to environmental cues, and the consequent impact on psychosocial function, is desirable.
Limitations
Our modest BDD sample may have had reduced statistical power to detect other potential differences of relevance. Other BDD studies of this type have also reported a small sample size and it may be that recruitment to BDD studies is particularly challenging (anecdotally, our perception was that BDD patients seemed reluctant to engage in research that focused attention on themselves). Nonetheless, replication in larger samples is required.
OCD and affective comorbidity could have had a confounding influence on the findings, considering 75% of our BDD group had comorbid OCD and 50% comorbid depressive symptomatology. On the other hand, the BDD cases were drawn from a well-defined clinical cohort, BDD was recognised by the patients and their clinicians as the primary disorder and constituted the focus for clinical treatment. BDD in clinical cohorts is almost always comorbid with disorders such as OCD and depression (Vinkers et al., 2007; Rashid et al., 2014) and by including patients with relevant comorbidity, the results may be generalised to BDD patients seen in the clinical setting.
Recognition of the influence that medication may have had on potentially changing the neurocognitive performance of BDD participants should be noted as all 12 of the BDD participants were taking medication (2 citalopram, 6 escitalopram, 3 fluvoxamine and 1 sertraline) at the time of testing. Certainly serotonin is known to play an important role in decision-making and emotional processing. Future research could be extended to investigate unaffected relatives, so as to avoid potential medication-related confounds. Research should also explore the functional impact of specific aspects of cognitive impairment on daily life, treatment-adherence, and suicidal activity.
Conclusion
Patients with BDD were impaired compared to healthy controls on tests of cognitive flexibility, reward and motor impulsivity and affective processing. Results from previous studies in the OCD population show similar deficits in cognitive flexibility and motor impulsivity; therefore our findings are consistent with the re-classification of BDD with OCD. However, the current study suggests that BDD may be characterized by additional abnormalities in domains of decision-making and emotional processing that differ from previous findings in OCD. Future work should explore the impact of these abnormalities on everyday functioning, ability to engage successfully with treatment and suicidality.
Table 3.
MANOVA results for CANTAB tasks
| BDD | Control | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | p | Cohen’s d | ||
| IED | Stages completed | 8.00 | 1.04 | 8.94 | .25 | 8.87 | .007* | 1.21 |
| Total Errors | 26.75 | 10.95 | 13.18 | 4.98 | 13.00 | .001* | 1.47 | |
| EDS Errors | 17.25 | 12.10 | 4.75 | 3.92 | 10.56 | .003* | 1.32 | |
| CGT | Delay Aversion | .47 | .17 | .28 | .19 | 5.22 | .03* | .94 |
| Deliberation Time (msec) | 1827.05 | 741.28 | 2133.09 | 533.32 | 1.84 | .20* | .55 | |
| Overall proportion of bet | .50 | .05 | .76 | .09 | 63.16 | <.001* | 3.24 | |
| Risk taking | .56 | .06 | .69 | .14 | 1.68 | .04* | 1.25 | |
| Quality of DM | .89 | .15 | .87 | .24 | .45 | .51 | .27 | |
| SST | Mean Reaction Time | 477.19 | 130.99 | 465.57 | 76.36 | .02 | .90 | .00 |
| Stop Signal Reaction Time | 182.64 | 74.84 | 137.81 | 52.62 | 4.66 | .04* | .87 | |
| AGN | Mean Correct Latency | 535.00 | 82.31 | 473.22 | 60.78 | 4.85 | .03* | .90 |
| POSITIVE | 545.46 | 90.80 | 418.74 | 49.73 | 19.76 | <.001* | 1.81 | |
| Total Omissions | 6.92 | 3.40 | 1.19 | 1.64 | 24.44 | <.001* | 2.00 | |
| POSITIVE | 1.33 | 1.37 | .13 | .34 | 7.11 | .01* | 1.09 | |
| NEGATIVE | 1.66 | 1.49 | .19 | .40 | 10.90 | .003* | 1.34 | |
| NEUTRAL | .00 | .00 | .06 | .25 | .26 | .61 | .20 | |
| Total Commissions | 10.17 | 7.66 | 4.62 | 2.09 | 5.86 | .02* | .96 | |
| POSITIVE | 3.75 | 3.13 | .75 | .85 | 10.78 | .003* | 1.34 | |
| NEGATIVE | 2.16 | 1.69 | 1.12 | .72 | 3.74 | .06 | .79 | |
| NEUTRAL | .19 | .54 | .10 | .42 | .50 | .49 | .29 | |
Note: * denotes a statistically significant result. IED (Intra/Extra Dimensional shift task), CGT (Cambridge Gambling Task), SST (Stop Signal Task), AGN (Affective Go/NoGo task)
References
- American Psychiatric Association. Highlights of changes from DSM-IV-TR to DSM-5. American Psychiatric Publishing; 2013. Author. Available from: http://www.dsm5.org/Documents/changes%20from%20dsm-iv-tr%20to%20dsm-5.pdf. [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aycicegi A, Dinn WM, Harris CL, Erkmen H. Neuropsychological function in obsessive–compulsive disorder: effects of comorbid conditions on task performance. Eur Psychiatry. 2003;8(5):241–248. doi: 10.1016/s0924-9338(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Bannon S, Gonsalvez CJ, Croft RJ, Boyce PM. Response inhibition deficits in obsessive–compulsive disorder. Psychiatry Res. 2002;110(2):165–174. doi: 10.1016/s0165-1781(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Bentall RP. Madness explained. London: Penguin; 2003. [Google Scholar]
- Biby EL. The relationship between body dysmorphic disorder and depression, self-esteem, somatization, and obsessive-compulsive disorder. J Clin Psychol. 1998 Jun;54:489–99. doi: 10.1002/(sici)1097-4679(199806)54:4<489::aid-jclp10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Samuels JF, Riddle MA, Hoehn-Saric R, Liang KY, Cullen BA, Nestadt G. The relationship of obsessive–compulsive disorder to possible spectrum disorders: results from a family study. Biological Psychiatry. 2000;48(4):287–293. doi: 10.1016/s0006-3223(00)00831-3. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Samuels JF, Wuyek LA, Liang KY, Wang Y, Grados MA, Cullen BA, Riddle MA, Greenberg BD, Rasmussen SA, Fyer AJ, et al. Is obsessive-compulsive disorder an anxiety disorder, and what, if, any, are spectrum conditions? A family study perspective. Psychol Med. 2012;42(1):1–13. doi: 10.1017/S0033291711000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne A, Wilhelm S, Keuthen NJ, Florin I, Baer L, Jenike MA. Prevalence of body dysmorphic disorder in a German college student sample. Psychiatry Res. 2002a Jan 31;109(1):101–4. doi: 10.1016/s0165-1781(01)00363-8. [DOI] [PubMed] [Google Scholar]
- Bohne A, Keuthen NJ, Wilhelm S, Deckersbach T, Jenike MA. Prevalence of symptoms of body dysmorphic disorder and its correlates: a cross-cultural comparison. Psychosomatics. 2002b Nov-Dec;43(6):486–90. doi: 10.1176/appi.psy.43.6.486. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2008;75:63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends in neurosciences. 2002;25(7):340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Zorzi C, Piccinni M, Cavallini MC, Bellodi L. Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: searching for a new intermediate phenotype. Biol Psychiatry. 2010 Jun 15;67(12):1178–84. doi: 10.1016/j.biopsych.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. Cognition is mania and depression: psychological models and clinical implications. Curr Psychiatry Rep. 2004;6(6):451–458. doi: 10.1007/s11920-004-0010-3. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 2005 May;29(3):399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor Inhibition and Cognitive Flexibility in Obsessive Compulsive Disorder (OCD) and Trichotillomania. American J Psychiatry. 2006 Jul;163(7):1282–1284. doi: 10.1176/ajp.2006.163.7.1282. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, Sahakian BJ. Impaired Cognitive Flexibility and Motor Inhibition in Unaffected First-Degree Relatives of Patients with Obsessive-Compulsive Disorder. Am J Psychiatry. 2007a;164(2):335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. A neuropsychological comparison of obsessive–compulsive disorder and trichotillomania. Neuropsychologia. 2007b;45:654–662. doi: 10.1016/j.neuropsychologia.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Derbyshire K, Leppink E, Grant JE. Is obsessive-compulsive disorder an anxiety disorder, and what, if, any, are spectrum conditions? A family study perspective. Impact of ADHD symptoms on clinical and cognitive aspects of problem gambling. Psychol Med. 2012 Jan;42(1):1–13. [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: Decision-making and reversal learning. Brain and Cognition. 2004;55(1):41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Conceição Costa DL, Chagas Assunção M, Arzeno Ferrão Y, Archetti Conrado L, Hajaj Gonzalez C, Franklin Fontenelle L, Fossaluza V, Constantino Miguel E, Rodrigues Torres A, Gedanke Shavitt R. Body dysmorphic disorder in patients with obsessive-compulsive disorder: prevalence and clinical correlates. Depress Anxiety. 2012 Nov;29(11):966–75. doi: 10.1002/da.21980. [DOI] [PubMed] [Google Scholar]
- Deakin J, Aitken M, Robbins T, Sahakian BJ. Risk taking during decision-making in normal volunteers changes with age. J Int Neuropsychol Soc. 2004;10:590–598. doi: 10.1017/S1355617704104104. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage CR, Phillips KA, Wilhelm S, Buhlmann U, Rauch SL, Jenike MA. Characteristics of memory dysfunction in body dysmorphic disorder. Journal of the International Neuropsychological Society. 2000;6(06):673–681. doi: 10.1017/s1355617700666055. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dingemans AE, van Rood YR, de Groot I, van Furth EF. Body dysmorphic disorder in patients with an eating disorder: prevalence and characteristics. Int J Eat Disord. 2012 May;45(4):562–9. doi: 10.1002/eat.20972. [DOI] [PubMed] [Google Scholar]
- Dunai J, Labuschagne I, Castle DJ, Kyrios M, Rossell SL. Executive function in body dysmorphic disorder. Psychol Med. 2010 Sep;40(9):1541–8. doi: 10.1017/S003329170999198X. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11(8):1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Robbins TW, Bullmore E, Potenza M, Menzies L, Chamberlain S, Sahakian B, Bechara A, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010 Feb;35(3):591–604. doi: 10.1038/npp.2009.185. Epub 2009 Nov 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJ, Gillan CM, Shekar S, Gorwood PA, Voon V, Morein-Zamir S, Denys D, et al. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr. 2014 Feb;19(1):69–89. doi: 10.1017/S1092852913000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Robbins TW, Bullmore E, Potenza M, Menzies L, Chamberlain S, Sahakian B, Bechara A, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010 Feb;35(3):591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler L, Blackwell A, Jaffa A, Palmer R, Robbins TW, Sahakian BJ, Dowson JH. Profile of neurocognitive impairments associated with female in-patients with anorexia nervosa. Psychological Medicine. 2006;36(04):517–527. doi: 10.1017/S0033291705006379. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, et al. The Yale–Brown Obsessive–Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Eckert ED. Body dysmorphic disorder in patients with anorexia nervosa: prevalence, clinical features, and delusionality of body image. Int J Eat Disord. 2002 Nov;32(3):291–300. doi: 10.1002/eat.10091. [DOI] [PubMed] [Google Scholar]
- Grant JE, Phillips KA. Recognizing and treating body dysmorphic disorder. Ann Clin Psychiatry. 2005 Oct-Dec;17(4):205–10. doi: 10.1080/10401230500295313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive- compulsive disorder. Neuron. 2000;28(2):343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Lhullier C, Rousseaux M. Non-spatial attention disorders in patients with frontal or posterior brain damage. Brain. 1996;119(Pt 1):191–202. doi: 10.1093/brain/119.1.191. [DOI] [PubMed] [Google Scholar]
- Hamilton MAX. The assessment of anxiety states by rating. British journal of medical psychology. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cerebral Cortex. 2006;16(12):1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Hanes KR. Neuropsychological performance in body dysmorphic disorder. J Int Neuropsychol Soc. 1998 Mar;4(2):167–71. doi: 10.1017/s1355617798001672. [DOI] [PubMed] [Google Scholar]
- Hollander E, Wong CM. Body dysmorphic disorder, pathological gambling, and sexual compulsions. Journal of Clinical Psychiatry. 1995 [PubMed] [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Humberstone M, Sawle GV, Clare S, Hykin J, Coxon R, Bowtell R, Macdonald IA, Morris PG. Functional magnetic resonance imaging of single motor events reveals human presupplementary motor area. Annals of Neurology. 1997;42:632–637. doi: 10.1002/ana.410420414. [DOI] [PubMed] [Google Scholar]
- Koran LM, Abujaoude E, Large MD, Serpe RT. The prevalence of body dysmorphic disorder in the United States adult population. CNS Spectr. 2008 Apr;13(4):316–22. doi: 10.1017/s1092852900016436. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Rossell SL, Dunai J, Castle DJ, Kyrios M. A comparison of executive function in Body Dysmorphic Disorder (BDD) and Obsessive-Compulsive Disorder (OCD) Journal of Obsessive-Compulsive and Related Disorders. 2013;2(3):257–262. [Google Scholar]
- Lavy E, van Oppen P, van den Hout M. Selective processing of emotional information in obsessive compulsive disorder. Behav Res Ther. 1994;32(2):243–246. doi: 10.1016/0005-7967(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Robbins TW. Cognitive functions and corticostriatal circuits: insights from Huntington’s disease. Trends Cogn Sci. 1998;2:379–388. doi: 10.1016/s1364-6613(98)01231-5. [DOI] [PubMed] [Google Scholar]
- McAdams D. The stories we live by. New York: Guildford Press; 1993. [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural brain research. 2003;146(1):97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, del Campo N, Sahakian BJ, Robbins TW, Bullmore E. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007 Dec;130(Pt 12):3223–36. doi: 10.1093/brain/awm205. Epub 2007 Sep 13. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32(3):525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg MARIE. A new depression scale designed to be sensitive to change. The British journal of psychiatry. 1979;134(4):382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, et al. Emotional bias and inhibitory control processes in mania and depression. Psychological Medicine. 1999;29(6):1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi J, Fukuyama H, Shibasaki H. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cerebral Cortex. 2001;11:85–92. doi: 10.1093/cercor/11.1.85. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National Adult Reading Test (NART): For the assessment of premorbid intelligence in patients with dementia: Test manual. NFER-Nelson; 1982. [Google Scholar]
- Osman S, Cooper M, Hackmann A, Veale D. Spontaneously occurring images and early memories in people with body dysmorphic disorder. Memory. 2004;12(4):428–436. doi: 10.1080/09658210444000043. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Menard W. Suicidality in body dysmorphic disorder: a prospective study. Am J Psychiatry. 2006 Jul;163(7):1280–2. doi: 10.1176/appi.ajp.163.7.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajender G, Bhatia MS, Kanwal K, Malhotra S, Singh TB, Chaudhary D. Study of neurocognitive endophenotypes in drug-naïve obsessive-compulsive disorder patients, their first-degree relatives and healthy controls. Acta Psychiatr Scand. 2011 Aug;124(2):152–61. doi: 10.1111/j.1600-0447.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- Rashid H, Khan AA, Fineberg NA. Adjunctive antipsychotic in the treatment of body dysmorphic disorder-A retrospective naturalistic case note study. International journal of psychiatry in clinical practice. 2014;(0):1–6. doi: 10.3109/13651501.2014.981546. [DOI] [PubMed] [Google Scholar]
- Rief W, Buhlmann U, Wilhelm S, Borkenhagen ADA, Brähler E. The prevalence of body dysmorphic disorder: a population-based survey. Psychological medicine. 2006;36(06):877–885. doi: 10.1017/S0033291706007264. [DOI] [PubMed] [Google Scholar]
- Roberts ME, Tchanturia K, Treasure JL. Exploring the neurocognitive signature of poor set-shifting in anorexia and bulimia nervosa. Journal of Psychiatric Research. 2010;44(1):964–970. doi: 10.1016/j.jpsychires.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. Journal of Cognitive Neuroscience. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci. 1999a;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999b;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rosen JC. The nature of body dysmorphic disorder and treatment with cognitive behavior therapy. Cognitive and Behavioral Practice. 1995;2(1):143–166. [Google Scholar]
- Rosen JC, Ramirez E. A comparison of eating disorders and body dysmorphic disorder on body image and psychological adjustment. Journal of Psychosomatic Research. 1998;44(3):441–449. doi: 10.1016/s0022-3999(97)00269-9. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Review. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, Taylor E, Andrew C, Giampietro V, Sharma T. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophrenia Research. 2001a;52:47–55. doi: 10.1016/s0920-9964(00)00173-0. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith AB, Oksanen H, Overmeyer S, Newman S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. British Journal of Psychiatry. 2001b;179:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- Sarwer DB, Cash TF, Magee L, Williams EF, Thompson JK, Roehrig M, Tantleff-Dunn S, Agliata AK, Wilfley DE, Amidon AD, Anderson DA, et al. Female college students and cosmetic surgery: an investigation of experiences, attitudes, and body image. Plast Reconstr Surg. 2005 Mar;115(3):931–8. doi: 10.1097/01.prs.0000153204.37065.d3. [DOI] [PubMed] [Google Scholar]
- Sarwer DB, Wadden TA, Foster GD. Assessment of body image dissatisfaction in obese women: specificity, severity, and clinical significance. Journal of Consulting and Clinical Psychology. 1998;66(4):651. doi: 10.1037//0022-006x.66.4.651. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Trestman RL, Mitropoulou V, et al. Impulsivity and serotonergic function in compulsive personality disorder. J Neuropsychiatry Clin Neurosci. 1996;8:393–398. doi: 10.1176/jnp.8.4.393. [DOI] [PubMed] [Google Scholar]
- Summerfeldt LJ, Endler NS. Examining the evidence for anxiety-related cognitive biases in obsessive-compulsive disorder. Journal of Anxiety Disorders. 1998;12(6):579–598. doi: 10.1016/s0887-6185(98)00035-8. [DOI] [PubMed] [Google Scholar]
- Tata PR, Leibowitz JA, Prunty MJ, Cameron M, Pickering AD. Attentional bias in obsessional compulsive disorder. Behaviour Research and Therapy. 1996;34(1):53–60. doi: 10.1016/0005-7967(95)00041-u. [DOI] [PubMed] [Google Scholar]
- Tavares JV, Drevets WC, Sahakian BJ. Cognition in mania and depression. Psychol Med. 2003;33(6):959–967. doi: 10.1017/s0033291703008432. [DOI] [PubMed] [Google Scholar]
- Thornton C, Russell J. Obsessive compulsive comorbidity in the dieting disorders. International Journal of Eating Disorders. 1997;21:83–87. doi: 10.1002/(sici)1098-108x(199701)21:1<83::aid-eat10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Veale DM, Sahakian BJ, Owen AM, Marks IM. Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive-compulsive disorder. Psychological Medicine. 1996;26:1261–1269. doi: 10.1017/s0033291700035984. [DOI] [PubMed] [Google Scholar]
- Veale D. Outcome of cosmetic surgery and ‘DIY’surgery in patients with body dysmorphic disorder. Psychiatric Bulletin. 2000;24(6):218–220. [Google Scholar]
- Veale D, Riley S. Mirror, mirror on the wall, who is the ugliest of them all? The psychopathology of mirror gazing in body dysmorphic disorder. Behaviour research and therapy. 2001;39(12):1381–1393. doi: 10.1016/s0005-7967(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Vinkers DJ, van Rood YR, van der Wee NJ. [Prevalence and comorbidity of body dysmorphic disorder in psychiatric outpatients] Tijdschrift voor psychiatrie. 2007;50(9):559–565. [PubMed] [Google Scholar]
- Watkins LH, Sahakian BJ, Robertson MM, Veale DM, Rogers RD, Pickard KM, Aitken MR, Robbins TW. Executive function in Tourette's syndrome and obsessive-compulsive disorder. Psychological Medicine. 2005;35:571–582. doi: 10.1017/s0033291704003691. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Campbell Z, Polsinelli A. Quantitative evidence for distinct cognitive impairment in anorexia nervosa and bulimia nervosa. Journal of neuropsychology. 2010;4(1):89–106. doi: 10.1348/174866409X459674. [DOI] [PubMed] [Google Scholar]
- Zois E, Kortlang N, Vollstädt-Klein S, Lemenager T, Beutel M, Mann K, Fauth-Bühler M. Decision-making deficits in patients diagnosed with disordered gambling using the CambridgeGambling task: the effects of substance use disorder comorbidity. Brain Behav. 2014;4:484–94. doi: 10.1002/brb3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]