Abstract
Background
The aim of this study was to evaluate the antiemetic effect of aprepitant and to determine how to provide triple combination therapy (aprepitant/azasetron/dexamethasone) to women receiving paclitaxel/carboplatin moderately emetogenic chemotherapy (MEC).
Material/Methods
The current study was a prospective study of 163 women with gynecologic cancers. We compared the digestive symptoms scores (nausea, vomiting, appetite loss, and dietary intake) of 37 women with ovarian cancers before and after aprepitant administration. We also compared these symptoms in women who underwent 193 cycles of triple combination therapy with symptoms of women who underwent 226 cycles of double combination therapy. For triple combination therapy, azasetron, dexamethasone (reduced dose: 40% of 20 mg), and aprepitant (125 mg) were administered on Day 1, followed by only aprepitant (80 mg) administration on Days 2 and Day 3.
Results
In 37 women with ovarian cancer, three symptoms, nausea, appetite loss, and dietary intake, were significantly improved by primarily adding aprepitant to double combination therapy in the delayed phase of MEC. Upon comparing their digestive symptoms in all cycles, however, these three symptoms were not significantly different in the delayed phase. Furthermore, all four symptoms in all cycles were worse following triple combination therapy than following double combination therapy in the acute phase (p<0.02). The control of digestive symptoms was generally insufficient without the administration of dexamethasone.
Conclusions
Primary aprepitant as an addition to MEC demonstrated efficacy in improving digestive symptoms in the delayed phase. However, its effect may decrease with repeated use. To improve the antiemetic effect, the dose reduction of dexamethasone should be restricted on Day 1 and dexamethasone should be used throughout the delayed phase as well.
MeSH Keywords: Antiemetics, Carboplatin, Dexamethasone, Endometrial Neoplasms, Ovarian Neoplasms, Uterine Cervical Neoplasms
Background
Chemotherapy-induced nausea and vomiting (CINV) is one of the most feared and distressing adverse events in cancer treatment [1,2]. Uncontrolled CINV can limit the dose intensity of chemotherapy and compromise a patient’s quality of life (QOL) [3]. Low-toxicity chemotherapy is important for maintaining a good performance status and enabling repeat chemotherapy for cancers, including gynecologic cancers. Thus, the prevention of CINV remains the most important issue in supportive cancer care.
Azasetron is a potent and selective first-generation 5-HT3 receptor antagonist developed and sold only in Japan [4–6]. A 5-HT3 receptor antagonist plus dexamethasone is the conventional antiemetic therapy for gynecologic cancers. However, using this double combination therapy still results in CINV in approximately 25% and 50% of women treated with highly emetogenic antitumor agents in the acute and delayed phases, respectively [7].
Aprepitant is a neurokinin-1 (NK1) receptor antagonist that was developed as a treatment for both acute and delayed CINV. It acts by inhibiting the binding of substance P to the NK1 receptor in the vomiting center [8–10]. Some reports have found that the addition of an NK1 receptor antagonist to conventional antiemetic therapy appears to have a significant effect in controlling highly emetogenic chemotherapy (HEC), such as cisplatin-induced emesis. In these studies, the comparative benefit of the aprepitant regimen was more pronounced in the delayed phase [11–13]. However, the Multinational Association of Supportive Care in Cancer (MASCC) and the American Society of Clinical Oncology (ASCO) guidelines restrict the recommendation of aprepitant use in moderately emetogenic chemotherapy (MEC) [14]. In contrast, the National Comprehensive Cancer Network (NCCN) guidelines recommended aprepitant use in MEC in select patients according the HEC regimen [15].
Carboplatin is categorized as a MEC agent that induces emesis in the delayed phase [15]. Despite being used widely in the treatment of gynecologic cancers, few studies have investigated the emetic potential of carboplatin-containing therapies such as paclitaxel/carboplatin therapy, and the benefit of adding aprepitant to such regimens is unknown [16,17].
We conducted a prospective study to investigate aprepitant use as part of combination therapy and compared the efficacy of triple combination therapy with aprepitant, azasetron, and dexamethasone with that of double combination therapy (conventional therapy) with a 5-HT3 receptor antagonist and dexamethasone in women who received paclitaxel/carboplatin therapy for gynecologic cancers, thus allowing us to reconsidered how triple combination therapy should be administered.
Material and Methods
Study design
The present study was a prospective, non-randomized, single institution study of triple combination therapy (aprepitant/azasetron/dexamethasone) for the prevention of CINV in women with gynecologic cancers treated with paclitaxel/carboplatin. We compared the effects of triple combination therapy with those of double combination therapy to evaluate the antiemetic effect of the addition of aprepitant, and to determine how triple combination therapy should be administered to women with gynecologic cancer receiving multicycles of paclitaxel/carboplatin chemotherapy (which is considered a MEC). The study protocol was approved by the ethics committee of Kyoto University Hospital (registration number/C515) on April 21, 2011, and all of the women provided their written informed consent prior to study entry.
Eligibility
All women with gynecologic cancers were treated with paclitaxel/carboplatin therapy at Kyoto University Hospital from April 2011 to April 2015. A total of 163 women who had gynecologic cancers were include: 62 women with endometrial cancers, 27 women with cervical cancers, and 74 women with ovarian cancers (Table 1). None of the 163 women received radiation therapy while receiving double or triple combination therapy.
Table 1.
Patient characteristics.
| Stage | No. aprepitant (+) (n=78) | No. aprepitant (−) (n=85) | |
|---|---|---|---|
| Endometrial cancer | I | 5 | 13 |
| II | 5 | 5 | |
| III | 9 | 7 | |
| IV | 11 | 7 | |
| Cervical cancer | I | 0 | 6 |
| II | 5 | 6 | |
| IV | 6 | 4 | |
| Ovarian cancer | I | 1 | 6 |
| II | 7 | 5 | |
| III | 21 | 20 | |
| IV | 8 | 6 |
The total number of women receiving triple combination therapy was 78 (30 endometrial, 11 cervical, and 37 ovarian cancers), and those receiving double combination therapy was 85 (32 endometrial, 16 cervical, and 37 ovarian cancers). The ratios of endometrial cancer, cervical cancer, and ovarian cancer cases were roughly the same between the two groups.
Of the 78 women using triple combination therapy, 37 had primary ovarian cancers. These 37 women initially received double combination therapy in the first cycle of chemotherapy and then their treatment was changed to triple combination therapy in the second cycle. In the first cycle, we did not inform them that they would be additionally receiving aprepitant during the next cycle of chemotherapy. We compared the digestive symptoms (nausea, vomiting, appetite loss, and dietary intake) in the 37 women before and after aprepitant administration following the first and second chemotherapy treatments, respectively. The remaining 41 women (30 endometrial cancers, 11 cervical cancers) in the triple therapy group received triple combination therapy (including aprepitant) from start to finish. We compared symptoms in the 78 women who underwent 193 cycles using triple combination therapy with the symptoms in the 85 women who underwent 226 cycles using double combination therapy.
Eligible women with gynecologic cancers were treated with paclitaxel (175 mg/m2 intravenous) and carboplatin (dose targeted by the Calvert equation for a target AUC of 5 or 6) after primary surgery or at recurrence.
Regimens
In the double combination therapy regimen, azasetron (10 mg) was administered intravenously 30 minutes prior to chemotherapy, and dexamethasone (20 mg diluted in 50 mL of 0.9% saline) was administered over 30 minutes prior to chemotherapy on Day 1. The dexamethasone dose was fixed at 20 mg (maximum dose). Dexamethasone was not administered after Day 2 in any of these patients.
In the triple combination therapy regimen, azasetron (10 mg) was administered intravenously 30 minutes prior to chemotherapy, and dexamethasone (8 mg; 40% of maximum dosage, occasionally 4 mg diluted in 50 mL of 0.9% saline) was administered over 30 minutes prior to chemotherapy on Day 1. Aprepitant was administered as follows: a 125 mg capsule was administered orally one hour before chemotherapy on Day 1 followed by an 80 mg capsule on Days 2 and Day 3 (Table 2). Dexamethasone was not administered after Day 2 in any of the women. The antiemetic therapy regimens at the first and second chemotherapy sessions in the 37 women with ovarian cancers receiving triple therapy are shown in Table 3.
Table 2.
The double combination regimen and the triple combination regimen.
| Double combination therapy | Day 1 (before chemotherapy) | Day 2 | Day 3 |
|---|---|---|---|
| Azasetron | 10 mg | ||
| Dexamethasone | 20 mg | ||
| Triple combination therapy | Day 1 (before chemotherapy) | Day 2 | Day 3 |
| Aprepitant | 125 mg | 80 mg | 80 mg |
| Azasetron | 10 mg | ||
| Dexamethasone | (4)–8 mg |
Table 3.
The regimens of antiemetic therapy at first chemotherapy and second chemotherapy in the 37 patients with ovarian cancers.
| Conventional therapy | Day 1 (before chemotherapy) | Day 2 | Day 3 | Addition of aprepitant | Day 1 (before chemotherapy) | Day 2 | Day 3 |
|---|---|---|---|---|---|---|---|
| ⇒ | Aprepitant | 125 mg | 80 mg | 80 mg | |||
| Azasetron | 10 mg | Azasetron | 10 mg | ||||
| Dexamethasone | 20 mg | Dexamethasone | (4)–8mg |
Endpoint
The primary endpoint of this study was the change in the degree of four digestive symptoms: nausea, vomiting, appetite loss, and dietary intake.
Evaluation and statistical analysis
We evaluated the symptoms of nausea, vomiting, and appetite loss using a scoring system as follows: not present=0 points, present=1 point, and strongly present=2 points. Regarding the dietary intake, we evaluated the food consumed using a 0–10 point scale for staple foods and 0–10 point scale for side dishes, with a score of 20 points considered a perfect score.
Acute CINV occurs within 24 hours after chemotherapy infusion, while delayed CINV begins 24 hours or longer after infusion. We compared the four digestive symptoms between Day 1 (acute phase; approximately 10 hours after carboplatin administration) and Day 5 (delayed phase; approximately 105 hours after carboplatin administration).
The required sample size was calculated based on an analyses of the mean±standard deviation (SD) dietary intake indices before and after aprepitant administration on Day 5. With a power of 90% and a two-tailed alpha (α) of 0.05, we calculated the sample size. The required population of women with ovarian cancer before and after aprepitant administration was thus calculated to be 24.
The statistical analyses were performed using the paired Student’s t-test (before and after the administration of aprepitant in each woman), Student’s t-test (comparing the average), and the χ2 test.
Results
Comparison of the symptoms between cycle 1 (without aprepitant) and cycle 2 (with aprepitant)
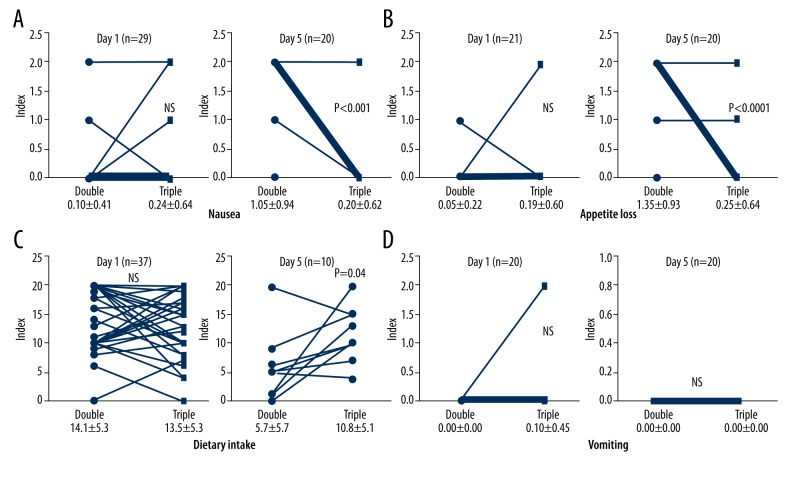
In 37 women with ovarian cancer, we noted no significant difference in nausea, appetite loss, or dietary intake indices before and after the administration of aprepitant on Day 1 (0.10±0.41 vs. 0.24±0.64, 0.05±0.22 vs. 0.19±0.60, and 14.2±5.3 vs. 13.5±5.3, respectively) (Figure 1A–1C). However, the nausea, appetite loss, and dietary intake indices after the administration of aprepitant were significantly improved on Day 5 (1.05±0.94 vs. 0.20±0.62, p<0.001; 1.35±0.93 vs. 0.25 ±0.64, p=0.0001 and 5.7±5.7 vs. 10.8±5.1, p=0.04, respectively). Accordingly, the symptoms of nausea, appetite loss, and dietary intake in the woman who received primary treatment with aprepitant in addition to double combination therapy were improved in the delayed phase.
Figure 1.
(A–C) In the 37 patients with ovarian cancer, a comparison of the patients’ nausea, appetite loss, and dietary intake indices before and after the administration of aprepitant on Day 1 were not significantly different (0.10±0.41 vs. 0.24±0.64, 0.05±0.22 vs. 0.19±0.60 and 14.2±5.3 vs. 13.5±5.3, respectively). Nausea, appetite loss and dietary intake indices after the administration of aprepitant were significantly improved on Day 5 (1.05±0.94 vs. 0.20±0.62, p<0.001; 1.35±0.93 vs. 0.25 ±0.64, p=0.0001 and 5.7±5.7 vs. 10.8±5.1, p=0.04, respectively). (D) A comparison of the patients’ vomiting indices before and after the administration of aprepitant on Day 1 and Day 5 were not significantly different (vomiting: Day 1: 0.00±0.00 vs. 0.10±0.45, Day 5: 0.00±0.00 vs. 0.00±0.00).
Conversely, a comparison of the vomiting indices between cycle 1 (without aprepitant) and cycle 2 (with aprepitant) on Days 1 and 5 showed no significant difference between the sessions (Day 1: 0.00±0.00 vs. 0.10±0.45, Day 5: 0.00±0.00 vs. 0.00±0.00) (Figure 1D). The greater decrease in the values of the observed women on Day 5 than on Day 1 was due to observation errors or noncoordination.
Comparison of the symptoms between double and triple combination therapy in all cycles
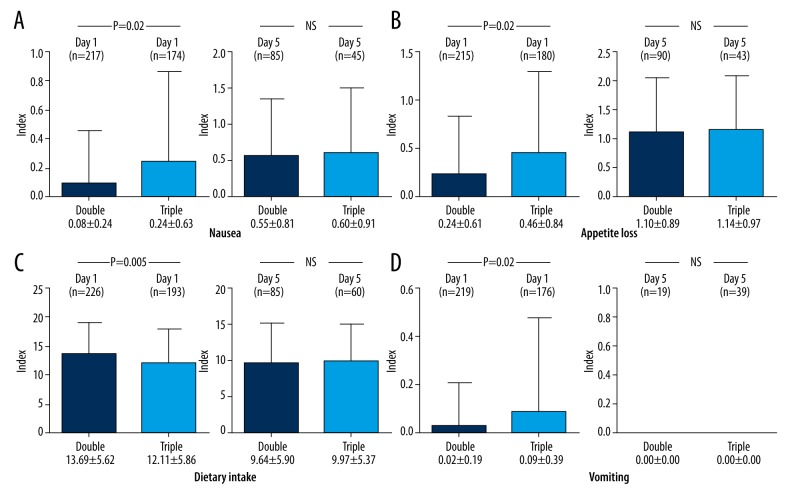
We next compared the symptoms in the women after 193 cycles of triple combination therapy and after 226 cycles of double combination therapy (Figure 2). Regarding the comparison of the women’s nausea, appetite loss, and vomiting indices between double and triple combination therapy on Day 1, the symptoms in the latter group were significantly higher than in the former group (0.08±0.24 vs. 0.24 ±0.63, p=0.002; 0.24±0.61 vs. 0.46±0.84, p=0.002; 0.02±0.19 vs. 0.09±0.39, p=0.02, respectively) (Figure 2A, 2B, 2D). However, no marked differences were noted between the symptoms on Day 5 (0.55±0.81 vs. 0.60±0.91, 1.10±0.89 vs. 1.14±0.97, 0.00±0.00 vs. 0.00±0.00, respectively). The dietary intake index was significantly lower on Day 1 in the triple combination therapy group than in the double combination therapy group (13.69±5.62 vs. 12.11±5.86, p=0.005) (Figure 2C), although no marked differences were noted on Day 5 (9.64±5.90 vs. 9.97±5.37).
Figure 2.
(A–D) The symptoms of the patients’ nausea, appetite loss, dietary intake and vomiting following 193 cycles of triple combination therapy were significantly worse than following 226 cycles of double combination therapy on Day 1 (0.08±0.24 vs. 0.24±0.63, p=0.002; 0.24±0.61 vs. 0.46±0.84, p=0.002; 13.69±5.62 vs. 12.11±5.86, p=0.005; 0.02±0.19 vs. 0.09±0.39, p=0.02, respectively). However, there were no differences between the symptoms and treatment regimens on Day 5 (0.55±0.81 vs. 0.60±0.91, 1.10±0.89 vs. 1.14±0.97, 9.64±5.90 vs. 9.97±5.37, 0.00±0.00 vs. 0.00±0.00, respectively). Three symptoms (except for vomiting) were worse in the delayed phase (Day 5) compared with the acute phase (Day 1) regardless of aprepitant administration.
Accordingly, all four symptoms were worse after 193 cycles of triple combination therapy than after 226 cycles using double combination therapy in the acute phase (Day 1) (Figure 2A–2D), possibly due to the dose reduction of dexamethasone from 20 mg to 8 mg.
Furthermore, absolute indices of three symptoms (except for vomiting) were worse in the delayed phase (Day 5) compared with the acute phase (Day 1) regardless of aprepitant administration in all cycles (Figure 2A–2C). Only vomiting seemed to disappear in the delayed phase (Day 5) with or without aprepitant administration (Figure 2D).
Discussion
It is well known that the combination of aprepitant, a 5-HT3 receptor antagonist, and dexamethasone is recommended for the prophylaxis of nausea and vomiting in the acute phase of HEC, and aprepitant plus dexamethasone is recommended in the delayed phase of HEC [11–13,18]. However, despite the widespread use of carboplatin in cancer therapy, only a few studies have evaluated the risk of emesis with carboplatin-based therapies. In one report, aprepitant added to a 5-HT3 receptor antagonist and dexamethasone improved the overall and delayed complete response (no vomiting and no rescue therapy) rates in carboplatin and pemetrexed chemotherapy [16]. In another study, the combination of aprepitant, ramosetron, and high-dose dexamethasone demonstrated efficient CINV prevention in women with ovarian cancer receiving paclitaxel and carboplatin [17]. Recently, Rapoport et al. performed a large phase III randomized double-blind trial that compared a triple regimen with a control regimen of a broad range of MEC regimens (anthracycline/cyclophosphamide (AC) or non-AC) [19]. Significantly, more women treated with triple combination therapy (5-HT3 receptor antagonist, dexamethasone, and aprepitant) achieved no vomiting and a complete response (CR), regardless of whether they received the AC or non-AC regimen within 120 hours after chemotherapy. However, due to the heterogeneity of chemotherapy in the non-AC MEC population and the use of post-hoc analyses, the data were not sufficient for the recommendation of aprepitant for standard use with initial non-AC MEC chemotherapy.
For acute CINV, the ASCO and MASCC guidelines restrict the recommendation of aprepitant use with MEC, such as a carboplatin-based regimens, but not with an AC-based regimen [20,21]. The NCCN guidelines, however, broaden the spectrum of the use of aprepitant in this setting and advocate its use in select patients receiving other MECs (e.g., carboplatin, epirubicin, ifosfamide, or irinotecan) [15]. For delayed CINV with MEC, the ASCO and MASCC guidelines state that when aprepitant is used for the prevention of acute CINV then it should also be used for the prophylaxis of delayed CINV as monotherapy. The NCCN guidelines suggest the use of aprepitant with or without dexamethasone [14]. Thus, no standard consensus has yet been established regarding the use of aprepitant in patients receiving MECs.
In the present study, we examined the changes in the symptoms of 37 ovarian cancer patients between cycle 1 (without aprepitant) and cycle 2 (with aprepitant). The nausea, appetite loss, and dietary intake were significantly improved in the delayed phase (Day 5) by first aprepitant addition, but not vomiting. In addition, we administered no dexamethasone in the delayed phase (Days 2–5) in order to investigate the effects of aprepitant alone. Furthermore, no antiemetic medicines were used in the delayed phase of double combination therapy, although aprepitant was administered in the delayed phase (Days 2–3) of triple combination therapy. We hypothesized that the digestive symptoms would be improved with the first addition of aprepitant to double combination therapy. We analyzed these effects on Day 5 when two days had passed after we discontinued aprepitant use. Therefore, we consider that the antiemetic effects of aprepitant may present for a while after discontinuation. After comparing 193 cycles of triple combination therapy with 226 cycles of double combination therapy, however, these digestive symptoms were not significantly different in the delayed phase. This suggests that the effect of aprepitant in the delayed phase was initially good, but its effect gradually might weaken when administered repeatedly.
Aprepitant inhibits CYP3A4, which in turn inhibits the metabolism of dexamethasone, a substrate of CYP3A4 [23]. One article reported that the area under the concentration-time curve (AUC) of dexamethasone was increased approximately 2-fold after the administration of aprepitant (120 mg/day on Day 1 and 80 mg/day on Days 2–5) in adults. Therefore, the dose of dexamethasone is generally understood to be reduced by 50% when used with aprepitant [22]. Although the theoretical half-dose of 20 mg (maximum dose) is 10 mg, the dexamethasone dose was fixed at 8 mg on Day 1 with 120 mg of aprepitant in MEC at our institute. According to all guidelines, the dexamethasone dose with aprepitant is not set at half dosage without aprepitant. A dose of 8 mg of dexamethasone on Day 1 for MEC is in line with the NCCN guidelines [15]. As a result, all four symptoms in all cycles were worse following triple combination therapy than following double combination therapy on Day 1. We believe that the symptoms were influenced by the dose reduction of dexamethasone from 20 mg to 8 mg, which might have eclipsed those induced by the addition of 125 mg of aprepitant; this dose reduction may therefore have been too drastic on Day 1. Recent studies have reported no marked difference in the risk of infection-related serious adverse events between the base and reduced dosages of dexamethasone [17]. Therefore, we concluded that the Day 1 use of 20 mg dexamethasone would be well tolerated and more effective in patients receiving MEC than a reduces dose [17]. Our findings suggest that the suitable range of dexamethasone on Day 1 in MEC is roughly 10 mg to 20 mg, but further safety studies are necessary for women receiving triple combination therapy.
The antiemetic effects may have been underestimated in the delayed phase of CINV, although such effects occur more frequently than in the acute phase [10,12]. The delayed phase of CINV has a stronger negative impact on the QOL than the acute phase [23]. In the present study, all of the symptoms (except for vomiting) were worse in all cycles regardless of aprepitant use on Day 5 compared to Day 1. We believe that this finding was due to the discontinuation of dexamethasone in the delayed phase (Days 2–5). It therefore appears that aprepitant alone is not sufficient to maintain QOL in the delayed phase. The symptoms (except for vomiting) might be improved if we administer additional dexamethasone in the delayed phase together with aprepitant. Chawla et al. suggested that prolonged dexamethasone (8 mg) be used on Days 2–5. Additional prolonged dexamethasone use might rescue the delayed worsening symptoms [24]. As aforementioned, the ASCO and MASCC guidelines do not include any recommendations for dexamethasone use in the delayed phase and the NCCN guidelines suggest aprepitant use with or without dexamethasone [15,20,21]. However, we propose that the prolonged use of dexamethasone on Days 2–5 with aprepitant (Days 2–3) may result in a greater improvement of the symptoms of nausea, appetite loss, and dietary intake in the delayed phase.
The cellular mechanisms of the antiemetic action of dexamethasone were recently reported [25]. These mechanisms might include the following: anti-inflammatory effect; direct central action at the solitary tract nucleus; interaction with the enzymes such as the neurotransmitter serotonin and receptor protein tachykinin NK1 and NK2; regulation of the hypothalamic-pituitary-adrenal axis; and reducing pain with the concomitant use of opioids and opioid-related nausea and vomiting.
Conclusions
In summary, primary additional use of aprepitant with double combination therapy was effective for improving nausea, appetite loss, and dietary intake in the delayed phase. However, repeated use of aprepitant for MEC did not demonstrate marked efficacy for the digestive symptoms in the delayed phase. To improve the QOL of women with gynecologic cancers, the dose reduction of dexamethasone should be restricted on Day 1 and continued throughout the delayed phase. Further studies with continuous administration of aprepitant and dexamethasone in MEC regimens should be performed. Some guidelines recommend palonosetron as the preferred 5-HT3 receptor antagonist in women undergoing MEC [26,27] instead of the first-generation 5-HT3 receptor antagonist used in this study. Therefore, further studies of combination therapy with aprepitant, palonosetron, and dexamethasone in MEC regimens for patients with gynecologic cancers should also be performed in the future.
Acknowledgements
We would like to thank Dr Brian Quinn for the English proofreading and ONO Pharmaceutical Co. (Osaka, Japan) for providing the supporting data.
Footnotes
Conflict of interest
The authors have no financial conflicts of interest relevant to the present work to declare. The authors declare no conflicts of interest in association with this study.
Source of support: Departmental sources
References
- 1.Roscoe JA, Morrow GR, Hickok JT, et al. Nausea and vomiting remain a significant clinical problem: trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in community clinical practices. J Pain Symptom Manage. 2000;20:113–21. doi: 10.1016/s0885-3924(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 2.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–94. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 3.Oo TH, Hesketh PJ. Drug insight: new antiemetics in the management of chemotherapy-induced nausea and vomiting. Nat Clin Pract Oncol. 2005;2:196–201. doi: 10.1038/ncponc0132. [DOI] [PubMed] [Google Scholar]
- 4.Inaba K, Morimoto Y, Fukuda T, et al. Inhibition by Y-25130 of the von Bezold-Jarisch effect evoked by 5-HT or 2-methyl-5-HT in anesthetized rats. Folia Pharmacol Jpan. 1991;98:293–99. doi: 10.1254/fpj.98.4_293. [Abstract in English] [DOI] [PubMed] [Google Scholar]
- 5.Sato N, Sakamori M, Haga K, et al. Antagonistic activity of Y-25130 on 5-HT3 receptors. Jpan J Pharmacol. 1992;59:443–48. doi: 10.1254/jjp.59.443. [DOI] [PubMed] [Google Scholar]
- 6.Yakushiji T, Akaike N. Blockade of 5-HT3 receptor mediated currents in dissociated frog sensory neurons by benzoxazine derivative, Y-25130. Br J Pharmacol. 1992;107:853–57. doi: 10.1111/j.1476-5381.1992.tb14536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gralla RJ, Osoba D, Kris MG, et al. Recommendations for the Use of Antiemetics: Evidence-based, clinical practice guidelines. J Clin Oncol. 1999;17(9):2971–94. doi: 10.1200/JCO.1999.17.9.2971. [DOI] [PubMed] [Google Scholar]
- 8.Tattersall FD, Rycroft W, Cumberbatch M, et al. The novel NK1 receptor antagonist MK-0869 (L-754,030) and its water soluble phosphoryl prodrug, L-758, 298, inhibit acute and delayed cisplatin-induced emesis in ferrets. Neuropharmacology. 2000;39:652–63. doi: 10.1016/s0028-3908(99)00172-0. [DOI] [PubMed] [Google Scholar]
- 9.Warr DG, Grunberg SM, Gralla RJ, et al. The oral NK1 antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: Pooled data from 2 randomised, double-blind, placebo controlled trials. Eur J Cancer. 2005;41:1278–85. doi: 10.1016/j.ejca.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23:2822–30. doi: 10.1200/JCO.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 11.Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin – the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–19. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 12.Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090–98. doi: 10.1002/cncr.11433. [DOI] [PubMed] [Google Scholar]
- 13.Schmoll HJ, Aapro MS, Poli-Bigelli S, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol. 2006;17:1000–6. doi: 10.1093/annonc/mdl019. [DOI] [PubMed] [Google Scholar]
- 14.Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: Past present and future recommendations. Oncologist. 2007;12:1143–50. doi: 10.1634/theoncologist.12-9-1143. [DOI] [PubMed] [Google Scholar]
- 15.Ettinger DS, Armstrong DK, Barbour S, et al. Antiemesis. JNCCN Netw. 2012;10:456–85. doi: 10.6004/jnccn.2012.0047. [DOI] [PubMed] [Google Scholar]
- 16.Ito Y, Karayama M, Inui N, et al. Aprepitant in patients with advanced non-small-cell lung cancer receiving carboplatin-based chemotherapy. Lung Cancer. 2014;84:259–64. doi: 10.1016/j.lungcan.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Choi CH, Kim MK, Park JY, et al. Safety and efficacy of aprepitant, ramosetron, and dexamethasone for chemotherapy-induced nausea and vomiting in patients with ovarian cancer treated with paclitaxel/carboplatin. Suppor Care Cancer. 2014;22:1181–87. doi: 10.1007/s00520-013-2070-6. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T, Hoshi E, Takagi M, et al. Multicenter, phase II, placebo-controlled, double-blind, randomized study of aprepitant in Japanese patients receiving high-dose cisplatin. Cancer Sci. 2010;101:2455–61. doi: 10.1111/j.1349-7006.2010.01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: A randomized, double-blind study. Support Care Cancer. 2010;18:423–31. doi: 10.1007/s00520-009-0680-9. [DOI] [PubMed] [Google Scholar]
- 20.Kris MG, Hesketh PJ, Somerfiiela MR, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: Update 2006. J Clin Oncol. 2006;24:2932–47. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]
- 21.Roila F, Hesketh PJ, Herrstedt J, et al. Prevention of chemotherapy- and radiotherapy-induced emesis: Results of the 2004 Pergia International Antiemetic Consensus Conference. Ann Oncol. 2006;17:20–28. doi: 10.1093/annonc/mdj078. [DOI] [PubMed] [Google Scholar]
- 22.McCrea JB, Majumdar AK, Goldberg MR, et al. Effects of the neurokinin1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisolone. Clin Pharmacol Ther. 2003;74:17–24. doi: 10.1016/S0009-9236(03)00066-3. [DOI] [PubMed] [Google Scholar]
- 23.Bloechl-Daum B, Deuson RR, Mavros P, et al. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–78. doi: 10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 24.Chawla SP, Grunberg SM, Gralla RJ, et al. Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting. Cancer. 2003;97:2290–300. doi: 10.1002/cncr.11320. [DOI] [PubMed] [Google Scholar]
- 25.Chu CC, Hsing CH, Shieh JP, et al. The cellular mechanisms of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur J Pharmacol. 2014;722:48–54. doi: 10.1016/j.ejphar.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: Results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–43. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 27.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189–98. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]