Abstract
The transmission of classical bovine spongiform encephalopathy (C-BSE) through contaminated meat product consumption is responsible for variant Creutzfeldt-Jakob disease (vCJD) in humans. More recent and atypical forms of BSE (L-BSE and H-BSE) have been identified in cattle since the C-BSE epidemic. Their low incidence and advanced age of onset are compatible with a sporadic origin, as are most cases of Creutzfeldt-Jakob disease (CJD) in humans. Transmissions studies in primates and transgenic mice expressing a human prion protein (PrP) indicated that atypical forms of BSE may be associated with a higher zoonotic potential than classical BSE, and require particular attention for public health. Recently, methods designed to amplify misfolded forms of PrP have emerged as promising tools to detect prion strains and to study their diversity. Here, we validated real-time quaking-induced conversion assay for the discrimination of atypical and classical BSE strains using a large series of bovine samples encompassing all the atypical BSE cases detected by the French Centre of Reference during 10 years of exhaustive active surveillance. We obtained a 100% sensitivity and specificity for atypical BSE detection. In addition, the assay was able to discriminate atypical and classical BSE in non-human primates, and also sporadic CJD and vCJD in humans. The RT-QuIC assay appears as a practical means for a reliable detection of atypical BSE strains in a homologous or heterologous PrP context.
Introduction
Prion diseases are fatal transmissible disorders affecting humans and animals. These neurodegenerative diseases are characterized by brain vacuolization, neuronal loss and accumulation of PrPSc, an abnormal isoform of the host-encoded cellular prion protein (PrPc). The infectious agent is mainly, if not solely, composed of abnormal PrPSc and is capable of converting cellular PrPc into PrPSc in an autocatalytical manner [1]. After proteinase K digestion of PrPSc, molecular features of the protease resistant fragment (PrPres) can be evidenced by Western blot.
Among animals, the classical bovine spongiform encephalopathy (C-BSE) affects cattle. The C-BSE epidemic in the 1980s became a major matter of concern for human health when the variant of Creutzfeldt-Jakob disease (vCJD) appeared as the result of a C-BSE foodborne transmission to humans [2–4]. Since the C-BSE epidemic, atypical forms of BSE have been reported in cattle [5]. They present a biochemical signature distinct from C-BSE, with a higher (H-BSE) or lower (L-BSE) apparent molecular weight of unglycosylated PrPres observed in Western blot [6]. The annual incidence (1 case per million) and the old age of atypical BSE-affected animals are compatible with a sporadic origin [5]. When transmitted to primates [7–9] and transgenic mice expressing a human PrP [10, 11], L-BSE showed an apparent higher pathogenicity than C-BSE. Pathological and biochemical similarities have been observed between L-BSE in cattle or primates and certain sCJD subtype [7, 12]. When L-BSE was transmitted to sheep [13] and transgenic mice expressing ovine PrP [14, 15], specific strain properties were retained, yet important PrPres molecular changes could be observed in some conditions [16]. Taken together, these studies highlight the importance of a rapid and proper identification of atypical BSE strains in cattle and other species.
Among recent methods developed to amplify prions in vitro, the real-time quaking-induced conversion (RT-QuIC) assay allows the sensitive detection of prion seeding activity in numerous tissues of animal and human origins [17–26]. The RT-QuIC assay relies upon the conversion of recombinant prion protein (recPrP) by prion-associated seeds into amyloid fibrils in presence of an amyloid-sensitive dye, thioflavine T (ThT). The incorporation of ThT within elongating fibrils is monitored in a multiwell plate in real time.
In two recent studies, this test was used successfully for the discrimination of a few C-BSE and L-BSE samples of cattle [27], and for the discrimination of C-, L-, and H-BSE samples of experimentally inoculated cattle [28]. Here, we took advantage of the extensive collection of classical and atypical BSE isolates identified over a 10 year-period of active surveillance in France to validate the rapid discrimination of classical and atypical BSE samples of cattle using RT-QuIC. Furthermore, we were also able to discriminate sporadic CJD and vCJD in humans as reported previously [24, 29], and also classical BSE and atypical L-BSE in primates.
Materials and methods
Ethic statement
A written informed consent for autopsy and research use was provided by patient’s relatives, according to the French regulation (L.1232-1 to L.1232-3, Code de la Santé Publique). The brain tissues with the corresponding written informed consent are referred for postmortem diagnosis and research to the French National Neuropathological Network for CJD (funded by the French Government) and to the French National Centre of Reference for prions (funded by the French Institute for Public Health Surveillance). No approval by local ethics committee is required during this procedure.
Sources of tissues
Samples from primates and cattle existed before the study began. BSE brainstem samples were collected during the active surveillance in France and confirmed by discriminatory western blot by the Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail (ANSES, Lyon, France). In this study, 13 H-BSE and 14 L- BSE isolates, encompassing all the atypical BSE cases collected during the period 2000–2010, were analyzed by RT-QuIC. Fifteen C-BSE cases collected during this period were also included. Among samples collected from fallen stock or after several freeze-and-thaw cycles, autolysis was often observed, affecting 10 out of 13 H-BSE samples, 11 out of 14 L-BSE samples and 6 out of 15 C-BSE samples (Table 1). Brainstems from 8 negative BSE animals collected in 2010 were analyzed as negative controls. Brain tissue was taken from 3 non-CJD patients, 3 iatrogenic CJD (iCJD-hGH) patients resulting from an infection with contaminated growth hormone of human origin, 2 vCJD cases (MM2b) and 4 sCJD cases (MM1, MV1, MV2, and VV2) as defined by their PRNP codon 129 genotype (MM, MV or VV), their PrPres type (type 1 or 2), and according to the migration pattern of PrPres on Western blot. Patients were referred to the French National Reference Center for Unconventional Transmissible Agents for CJD, and the diagnosis was confirmed biochemically and neuropathologically. Brain samples from cynomolgus macaques previously inoculated via intracerebral and oral routes with classical and atypical L-BSE isolates [7, 30] were analyzed blindly by RT-QuIC.
Table 1. Collection of the atypical and classical BSE samples analyzed by RT-QuIC.
| Cattle ID | Result of molecular typing by Western blot | Sample shown in Fig 3 | Age (year) |
|---|---|---|---|
| 01–2437 | C-BSE | 1 | 6 |
| 01–2579 | C-BSE | 6 | |
| 02–2263 | C-BSE | 2 | 7 |
| 02–2715 | C-BSE | 8 | |
| 02–2811 | C-BSE | 3 | 6 |
| 02–2872 | C-BSE | 9 | |
| 02–2992 | C-BSE | 4 | 8 |
| 04–0881 | C-BSE | 9 | |
| 05–0294 | C-BSE | 9 | |
| 06–1164 | C-BSE | 5 | 12 |
| 07–0324 | C-BSE | 6 | 12 |
| 07–0453 | C-BSE | 12 | |
| 09–0170 | C-BSE | 7 | 15 |
| 09–0335 | C-BSE | 15 | |
| 10–0015 | C-BSE | 6 | |
| 00–2549 | H-BSE | 13 | |
| 01–2604 | H-BSE | 8 | |
| 02–0558 | H-BSE | 8 | 13 |
| 02–2695 | H-BSE | 11 | |
| 03–0440 | H-BSE | 9 | 16 |
| 03–1928 | H-BSE | 8 | |
| 03–2095 | H-BSE | 12 | |
| 07–0644 | H-BSE | 10 | 11 |
| 08–0257 | H-BSE | 18 | |
| 08–0498 | H-BSE | 11 | 8 |
| 09–0169 | H-BSE | 16 | |
| 09–0497 | H-BSE | 12 | 13 |
| 10–0161 | H-BSE | 13 | |
| 02–2528 | L-BSE | 8 | |
| 10–0075 | L-BSE | 13 | 15 |
| 03–2052 | L-BSE | 14 | 13 |
| 04–0824 | L-BSE | 15 | 19 |
| 05–0009 | L-BSE | 12 | |
| 06–0931 | L-BSE | 16 | 13 |
| 07–0012 | L-BSE | 10 | |
| 07–1136 | L-BSE | 17 | 10 |
| 08–0074 | L-BSE | 11 | |
| 08–0374 | L-BSE | 18 | 15 |
| 09–0007 | L-BSE | 12 | |
| 09–0397 | L-BSE | 19 | 14 |
| 09–0481 | L-BSE | 18 | |
| 10–0409 | L-BSE | 20 | 9 |
Recombinant prion protein preparation
Human recombinant full length PrP (codon 129M) (recHuPrP; aa 23–231; GenBank accession number no. M13899) and bovine recombinant full length PrP (recBovPrP; aa 25–242; GenBank accession number no. NP_851358) were purified according to the protocol published previously [31]. Protein concentrations were determined by measuring the absorbance at 280 nm, and aliquots were stored at -80°C until use.
Brain homogenate preparation
The tissues were prepared in PBS (Sigma-Aldrich) containing 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, and Complete Protease Inhibitor Cocktail (Roche) to give a final tissue concentration of 10% (w/v). The homogenization was performed in 2ml centrifuge tubes containing ceramic beads using a FastPrep® 24 instrument (MP Biomedical) for 45 s at speed 6.5. Gross cellular debris were removed from brain homogenates (BH) after a centrifugation at 2000 g for 2 min. Supernatants were collected and stored at -80°C until use.
Normalization of brain homogenates from affected individuals
To assess the analytical sensitivity of our RT-QuIC assay for the detection of sCJD and determine the minimal amount of detectable PrPSc, brain homogenates (BH) from CJD patients were normalized using known concentrations of recHuPrP. The samples containing PrPres were digested with PK 100μg/ml for 1h at 37°C and loaded onto Novex 4–12% Bis-Tris acrylamide gels (Life Technologies), alongside dilutions of recHuPrP (ranging from 10 to 1.25 ng). To compare the detection of the studied prion strains in the different groups of affected individuals (iCJD patients, non-human primates), brain homogenates were analyzed by Western blot (WB) as previously described [32]. The amount of PrPres in samples was adjusted with a further dilution to match the amount detected in the sample with the lowest PrPres signal at the determined dilution.
After electrophoresis, the separated proteins were transferred to nitrocellulose membrane and immunoblotted with anti-PrP mAb 3F4 (Eurogentec) for human samples. It was followed by an incubation with a secondary antibody coupled with horseradish peroxidase (HRP). The HRP activity was revealed using ECL (GE Biosciences), and the blots were exposed to ECL Hyperfilms (GE Biosciences). The films were scanned using a Bio-Rad GS800 densitometer and analyzed using Bio-Rad Quantity One software. The densities of the single Western blot band corresponding to recHuPrP were compared with the combined densities of the 3 bands corresponding to PrPres. To obtain an estimated amount of PrPres in 2 μl equivalent to 100 fg of recHuPrP, the corresponding 100% brain tissue dilutions varied from 10−6 (MM1, MV1, MV2) to 5x10-7 (MM2b, VV2). For BSE isolates, all the samples were tested by RT-QuIC at the same dilution (10−4), regardless of the PrPres level in the sample. A Western blot analysis with TeSeE confirmatory Western blot kit (Bio-Rad) was done to compare relative quantity of PrPres between groups (H-, C- and L-BSE) and to confirm the presence of PrPres when no seeding activity was detected by RT-QuIC.
RT-QuIC method
The RT-QuIC assay was prepared as described previously [26, 33]. Samples were serially diluted 10-fold in PBS containing 1x N2 media supplement (Life Technologies) and 0.1% SDS. The RT-QuIC reaction mixture was prepared in 1X PBS with final concentration of 300 mM NaCl, 1 mM EDTA, 10 μM thioflavin T, 0.1 mg/ml full length recombinant human (23–231) or bovine (25–241) recPrP, and 98 μl of this mixture were distributed in 96-well black bottom optic plates (Nalgene Nunc). Each reaction was seeded in triplicates with 2 μl of tissue dilution and plates were placed in a BMG Fluostar Omega plate reader (BMG Labtech) at 42°C for 70h (280 cycles, each consisting of 1 min shaking at 600 rpm and 1 min at rest, with ThT fluorescence measurement taken every 15-min with a gain setting of 1000).
Data analysis
All RT-QuIC experiments were performed at least three times and produced comparable results. At pertinent time points, the statistical significance of the difference between mean fluorescence or lag phases of analyzed groups was assessed using the non-parametric, unpaired t- test with unequal variance (Welch correction), using GraphPad Prism software v6.0 (San Diego, USA).
Results
Atypical BSE strains seeded more efficiently the conversion of recombinant PrP than classical BSE strain
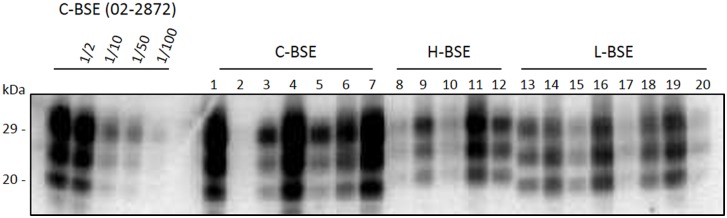
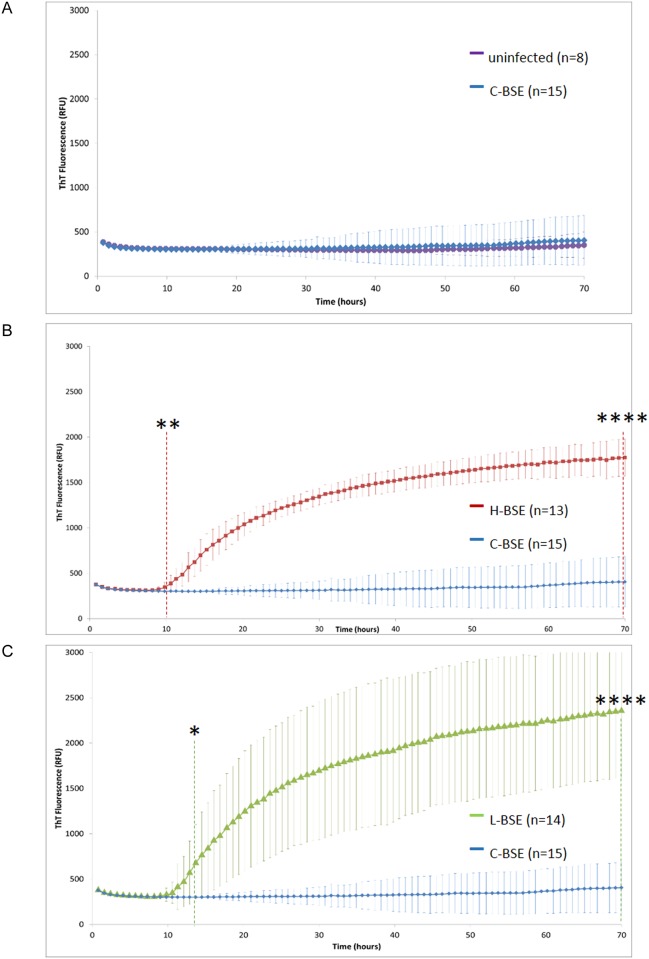
The seeding efficiency of brain homogenates prepared from cattle affected by atypical and classical BSE was investigated. A total of 15 C-BSE, 14 L-BSE and 13 H-BSE isolates (see details in Table 1), along with 8 negative bovine cases, were analyzed by RT-QuIC. PrPres Western blot results for each BSE group are illustrated in Fig 1. Although a substantial inter-individual variability was observed within each group, a tendency to a higher PrPres level was observed in C-BSE samples. A typical run was performed using 2 plates, in which were included the 8 negative samples and a half of each BSE group. In our conditions, negative and classical BSE samples could not be differentiated using recHuPrP (average data, Fig 2A; individual data, panels A & D in S1 Fig). On the contrary, an efficient amplification of atypical BSE samples was obtained, along with a remarkable homogeneity of the lag phase (~10h) for animals of each group (H-BSE and L-BSE) (Fig 2B and 2C; panels B, C, E, F in S1 Fig). Statistical analyses were performed to determine the time at which the increase in fluorescence becomes significant for each BSE group. No difference was observed between uninfected and C-BSE samples (Fig 2A). The difference of fluorescence signal between C-BSE and H-BSE or L-BSE was statistically significant as early as 10h (p<0.01) (Fig 2B) and 12h (p<0.05) (Fig 2C), respectively, and at terminal 70h time point (p<0.0001).
Fig 1. Western blot analysis of PrPres levels in brainstem homogenates from cattle affected by classical or atypical BSE.
Homogenates were subjected to proteinase K digestion followed by immunoblotting using Sha31 monoclonal antibody. Dilutions of a C-BSE sample (02–2872) are indicated, along with samples from each C-, H- and L-BSE group. The numbers 1 to 20 refer to cattle identity indicated in Table 1.
Fig 2. Conversion of human recombinant PrP by atypical BSE strains.
Average data and statistical significance of the individual results shown in S1 Fig are represented here. (A), classical BSE isolates and uninfected bovine samples. (B), classical BSE and atypical H-BSE isolates. (C), classical BSE and atypical L-BSE isolates. Each point represents the mean value of 3 replicate relative fluorescence unit readings, which were averaged over the number of animals in each group. Error bars represent the mean standard deviation (SD). Vertical dashed lines indicate a statistically significant difference of signal between the test groups. *, p<0,05; **, p<0,01; ****, p<0,0001.
Experiments were repeated using a different recombinant PrP protein. Although homology of sequence between seed and substrate was described as being not mandatory with RT-QuIC method, we used bovine PrP (recBovPrP) to ascertain that the lack of C-BSE amplification we observed was not due to the use of recHuPrP. Similar results were obtained with recBovPrP. While C-BSE and negative samples remained undistinguishable (panel A in S2 Fig, panels A and D in S3 Fig), H-BSE and L-BSE groups were clearly distinct from C-BSE group (panels B and C in S2 Fig; panels B, C, E and F in S3 Fig). The lag phase was in the same range as that obtained with recHuPrP. The difference of fluorescence signal between C-BSE and H-BSE or L-BSE was statistically significant as early as 11h (p<0.05) (panel B in S2 Fig) and 14h (p<0.05) (panel C in S2 Fig), respectively, and at terminal 70h time point (p<0.0001). We next investigated whether such properties of differential amplification between atypical and classical BSE strains were preserved after passage to non-human primates.
Efficient amplification of atypical and classical BSE strains after transmission to non-human primates
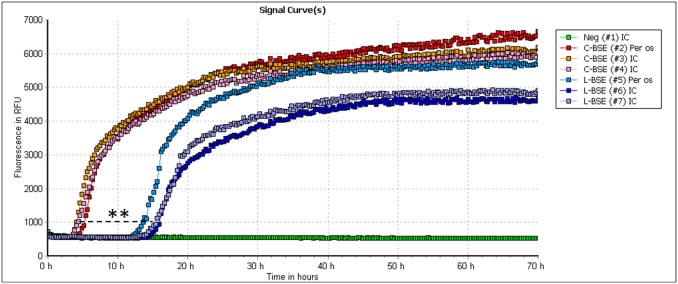
Western blot showing the PrPres content in brain homogenates from macaques with C-BSE and L-BSE are illustrated in S4 Fig (panel A). Both L-BSE and C-BSE strains, which have been transmitted to non-human primates by intracerebral and oral routes [7,31], seeded efficiently RT-QuIC reactions with human recombinant PrP (Fig 3). An efficient reaction, although with a lower maximum fluorescence level, was also achieved with bovine recombinant PrP (not shown). In both cases, classical BSE samples provided a more efficient seeding material than L-BSE, with a lag phase shorter than 10h. A remarkable homogeneity of the samples within each group was observed, and it was possible to blindly identify 2 groups of samples. No apparent effect of the inoculation route was evidenced. The difference of lag phases (when RFU > 1000) between the 2 groups was statistically significant (p<0.005).
Fig 3. Conversion of human recombinant PrP by C- and L- BSE transmitted to macaques.
RT-QuIC reactions were seeded with 10−4 and 2.5x10-5 dilutions of brain tissue from macaques that had been inoculated by oral (per os) or intracranial (IC) routes with C-BSE and L-BSE, respectively. Each point represents the mean value of 3 replicate relative fluorescence unit readings. Horizontal dashed line indicates a statistically significant difference of lag phase between the L-BSE and C-BSE groups. **, p<0.01.
Brain homogenates from sCJD patients seeded more efficiently the conversion of human recPrP than vCJD brain homogenates
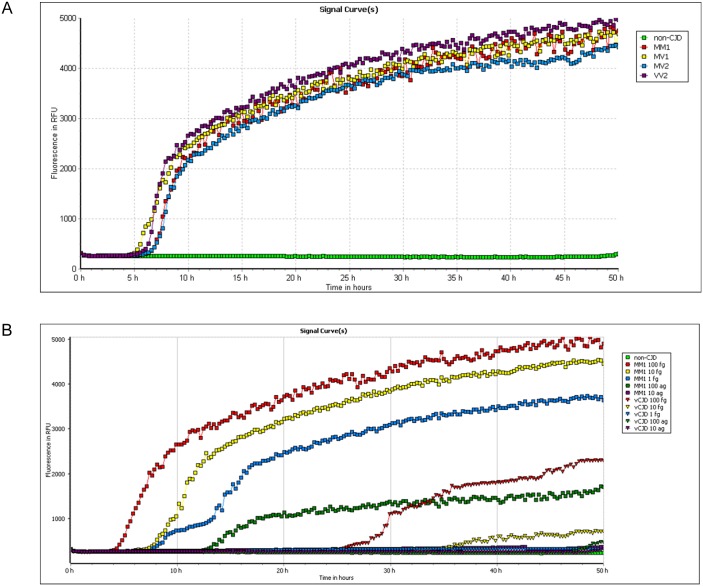
We next assessed whether differential seeding activity can be observed in humans using seeds from patients infected with the C-BSE agent and from patients with sporadic CJD. Western blot results showing the PrPres content in brain homogenates from sCJD and vCJD patients are illustrated in S4 Fig (panel B). Brain homogenates from sCJD patients with different molecular subtypes (MM1, MV1, MV2 and VV2) seeded with the same efficiency RT-QuIC reactions using full-length recHuPrP (Fig 4A), after the normalization of the PrPres levels. The assay was able to detect the molecular subtype MM1 down to a 10−9 dilution of 100% brain tissue, corresponding to 100 ag of PrPres (Fig 4B). In contrast, and despite equivalent amounts of PrPres (100 fg) used to seed the reaction, vCJD brain homogenates proved less efficient to initiate RT-QuIC reactions, and only a sensitivity down to a 10−7 brain dilution (equivalent to 10 fg of PrPres) could usually be achieved in our conditions (Fig 4B). Replacing human recPrP with bovine recPrP did not improve the detection of vCJD (data not shown). Negative results were obtained with normal brain homogenates (NBH), which were diluted 106 fold starting from 100% brain tissue.
Fig 4. Conversion of human recombinant PrP by sCJD seeds.
(A) RT-QuIC was seeded with an estimated amount of 100 fg PrPres from MM1, MV1, MV2 and VV2 brain homogenates. Non-CJD brain homogenate was diluted at an equivalent dilution (10−6). (B) Serial dilutions of sCJD MM1, vCJD MM2b and non-CJD seeds were submitted to RT-QuIC. Amounts of PrPres detected in brain homogenate ranged from the equivalent of 100 fg to 10 ag. Each point represents the mean value of 3 replicate relative fluorescence unit readings.
Brain homogenates from iCJD-hGH patients seeded efficiently the conversion of human recPrP
We also studied the efficiency of our RT-QuIC assay with iatrogenic CJD, an additional infectious form of human prion disease due to a contamination by the peripheral route, after cadaver–derived human growth hormone treatment. Brain homogenates from 3 French iatrogenic cases seeded as efficiently as sCJD MM1 the conversion of human recPrP, as sporadic and iatrogenic seeds were indistinguishable and showed similar lag-phases (<10h) (Fig 5).
Fig 5. Conversion of human recombinant PrP by iCJD seed.
RT-QuIC reactions were seeded with 10−6 and 8x10-7 dilutions of brain tissues from one sCJD MM1 and three iCJD-hGH patients (iCJD #1, #2 and #3), respectively. Each point represents the mean value of 3 replicate relative fluorescence unit readings.
Discussion
In this study, we assessed the power of discrimination of BSE strains from cattle using RT-QuIC. Indeed, in two recent studies, this assay was used successfully for the discrimination of a few C-BSE and L-BSE samples from cattle [27], and for the discrimination of C-, L-, and H-BSE samples from experimentally inoculated cattle [28]. Here, we took advantage of the extensive collection of classical and atypical BSE isolates identified over a 10 year-period of active surveillance in France to validate RT-QuIC performances through the analysis of 15 classical and 27 atypical BSE samples from cattle. Classical BSE, from which human vCJD is derived, was particularly inefficient to seed recombinant PrP and could not be differentiated from negative bovine samples. On the contrary, all the atypical BSE samples were readily detected, with the same lag phase, despite very different PrPres content. Thus, our study confirms previous results obtained with 5 L-BSE and 4 C-BSE natural cases by Orru et al [27] and with 3 experimental cases of each BSE subtype by Masujin et al [28], using large series of natural cases (15 C-BSE, 14 L-BSE and 13 H-BSE cases). In addition, we further tested the detection of L-BSE and C-BSE after transmission to macaques and compared the seeding properties of vCJD samples to those obtained with sCJD and French iCJD after GH treatment.
Atypical cases of BSE putatively represent sporadic forms of prion disease in cattle, with PrPsc glycotypes, neuropathology and PrPsc deposition different from those observed in classical BSE [5, 12]. In humans, we also observed a differential amplification between seeds from sCJD and vCJD, as previously reported [24]. Using sCJD brain homogenates, we achieved a sensitivity in a similar range to that reported by others using hamster [24] or human recPrP substrates [18], without false positive reaction, contrasting with the experience from other laboratories [21]. Likewise, vCJD homogenates were less efficient to seed reactions than sCJD homogenates. Possible explanations have been discounted, such as case-to-case variations, brain region variation, or inhibitors present in vCJD brain [24]. In our study, we tested different regions, from 2 different vCJD patients, without any improvement of the detection.
RT-QuIC discrimination may rely on differences in abnormal PrP assemblies sustaining distinct seeding activities that may vary with several factors (sporadic or infectious origin of the disease, affected species, route of infection). We were able to discriminate brain samples from macaques inoculated with C-BSE and with L-BSE. Surprisingly, in macaques, C-BSE showed the most efficient seeding activity while it was relatively inefficient in humans. Differences in PrP amino acid sequence might contribute to this discrepancy. Mature forms of bovine and human PrP share 91.3% of amino acid sequence, and bovine and cynomolgus macaques PrP only 88.7%. Different regions of PrP have been proposed as key domains for fibrillization, such as the S1H1S2 region [34, 35] or the H2H3 domain [36]. It was suggested that single amino acid variations in the H2 and H3 domains trigger different oligomerization pathways [37]. A sequence alignment of bovine and macaque PrP amino acids shows variations at residues 100 and 108 in macaque (and not in human PrP). This central region of PrP contains an amyloidogenic sequence AGAAAAGA that appears critical for PrPres formation [38] and because corresponding synthetic peptides are able to specifically inhibit in vitro formation of PrPres [39]. Other variations exist in macaque PrP at residue 143 (H1 domain), residue 155 (6 amino acids before S2 domain), and residue 220 (H3 domain). Hence, it is possible that such amino acid variations in critical regions of the macaque PrP has an impact on the quaternary structure of PrPsc assemblies and therefore on the related seeding activity. For example, the H187R mutation in the human PrP has a dramatic effect on the protein folding, resulting in a markedly increased propensity to oligomerize [40].
However, the unpredictable manner of the biological changes of prion strain properties during interspecies transmission has been extensively described [41–44]. In more recent studies, when L-BSE isolates were propagated in wild type mice [45] and transgenic mice expressing ovine PrP [15], strain features closely similar to those of C-BSE agent were observed. In addition, a shift in the biochemical signature of the C-BSE agent was observed in the spinal cord of orally-infected macaques at the preclinical stage [46]. Altogether, these data highlight the fact that the properties of BSE strains may evolve during interspecies transmission, notably in macaques. Our results suggest that such a strain variation also has an impact on seeding activity.
A main feature of C-BSE-infected brain tissue from various animal models including macaques is the presence of amyloid plaques [47–52] composed of PrPSc fibers which could modulate the seeding activity of these tissues. However, plaques are also observed in the brain of vCJD patients, and vCJD brain homogenates show a poor seeding activity. Another characteristic of C-BSE in macaques is the high proportion of diglycosylated PrPres, but C-BSE or vCJD PrPres share the same feature, and yet are not associated with an efficient seeding activity. Likewise, the Western blot type of PrPres as defined by the molecular mass of the unglycosylated PrPres after proteinase K digestion cannot account for these different seeding activities, since PrPres type 2 is distributed in both groups with efficient (sCJD MV2, VV2, C-BSE in macaques) and inefficient (vCJD) seeding properties. It was intriguing that PrPsc from all the studied natural diseases with a sporadic or presumably sporadic origin (all sCJD subtypes, all atypical BSE isolates) showed a high level of seeding activity, unlike peripherally acquired diseases due to the C-BSE agent (vCJD, C-BSE). To investigate the role of the peripheral route on the selection of PrPSc species with seeding activity, we analyzed brain homogenates from iatrogenic CJD cases secondary to growth hormone treatment, which are acquired prion diseases and result from a human-to-human CJD transmission. We found similar and efficient seeding activities for sCJD MM1 and iCJD-hGH cases using RT-QuIC. Moreover, we also found similar and efficient seeding activities in brain homogenates from non-human primates inoculated intracerebrally and via the oral route with C-BSE or L-BSE. Altogether, our data do not support the peripheral route as a main factor influencing the selection of PrPsc species with low seeding activity. In our hands, it appears that this poorly efficient RT-QuIC profile was limited to the C-BSE agent, which propagated in cattle and humans.
To conclude, we showed that RT-QuIC detects PrPSc from a large series of 27 atypical BSE isolates within hours, and provides a promising tool for the diagnosis of these natural diseases and for their discrimination from C-BSE in a homologous and heterologous PrP context.
Supporting information
RT-QuIC reactions were seeded with 10−4 dilutions of bovine tissue (brainstem), using human recombinant protein. (A) and (D), classical BSE and uninfected bovine tissues. (B) and (E), classical BSE and atypical H-BSE. (C) and (F), classical BSE and atypical L-BSE. Each point represents the mean value of 3 replicate relative fluorescence unit readings.
(PDF)
Average data and statistical significance of the individual results presented in S3 Fig are represented here. (A), classical BSE isolates and uninfected bovine samples. (B), classical BSE and atypical H-BSE isolates. (C), classical BSE and atypical L-BSE isolates. Each point represents the mean value of 3 replicate relative fluorescence unit readings, which were averaged over the number of animals in each group. Error bars represent the mean standard deviation (SD). Vertical dashed lines indicate a statistically significant difference of signal between the test groups. *, p<0,05; ****, p<0,0001.
(PDF)
RT-QuIC reactions were seeded with 10−4 dilutions of bovine tissue (brainstem), using bovine recombinant protein. (A) and (D), classical BSE isolates and uninfected bovine samples. (B) and (E), classical BSE and atypical H-BSE isolates. (C) and (F), classical BSE and atypical L-BSE isolates. Each point represents the mean value of 3 replicate relative fluorescence unit readings.
(PDF)
Homogenates were subjected to proteinase K digestion and serial dilutions were detected by immunoblotting using Sha31 (primates) or 3F4 (CJD patients) monoclonal antibodies.
(PDF)
Acknowledgments
The research leading to these results has received funding from the program “Investissements d’avenir” ANR-10-IAIHU-06. This study was funded in part by LFB Biomédicaments and Institut de Veille Sanitaire (InVS).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by “Investissements d’avenir” ANR-10-IAIHU-06; LFB Biomédicaments; Institut de Veille Sanitaire (InVS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Prusiner SB. Prions. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(23):13363–83. Epub 1998/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandel JP, Heath CA, Head MW, Levavasseur E, Knight R, Laplanche JL, et al. Variant Creutzfeldt-Jakob disease in France and the United Kingdom: Evidence for the same agent strain. Annals of neurology. 2009;65(3):249–56. Epub 2009/04/01. 10.1002/ana.21583 [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB. Prion diseases and the BSE crisis. Science. 1997;278(5336):245–51. Epub 1997/10/10. [DOI] [PubMed] [Google Scholar]
- 4.Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, et al. The same prion strain causes vCJD and BSE. Nature. 1997;389(6650):448–50, 526 Epub 1997/10/23 22:27. 10.1038/38925 [DOI] [PubMed] [Google Scholar]
- 5.Biacabe AG, Morignat E, Vulin J, Calavas D, Baron TG. Atypical bovine spongiform encephalopathies, France, 2001–2007. Emerging infectious diseases. 2008;14(2):298–300. 10.3201/eid1402.071141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs JG, Langeveld JP, Biacabe AG, Acutis PL, Polak MP, Gavier-Widen D, et al. Molecular discrimination of atypical bovine spongiform encephalopathy strains from a geographical region spanning a wide area in Europe. Journal of clinical microbiology. 2007;45(6):1821–9. Epub 2007/04/20. 10.1128/JCM.00160-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comoy EE, Casalone C, Lescoutra-Etchegaray N, Zanusso G, Freire S, Marce D, et al. Atypical BSE (BASE) transmitted from asymptomatic aging cattle to a primate. PloS one. 2008;3(8):e3017 10.1371/journal.pone.0003017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mestre-Frances N, Nicot S, Rouland S, Biacabe AG, Quadrio I, Perret-Liaudet A, et al. Oral transmission of L-type bovine spongiform encephalopathy in primate model. Emerging infectious diseases. 2012;18(1):142–5. Epub 2012/01/21. 10.3201/eid1801.111092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono F, Tase N, Kurosawa A, Hiyaoka A, Ohyama A, Tezuka Y, et al. Atypical L-type bovine spongiform encephalopathy (L-BSE) transmission to cynomolgus macaques, a non-human primate. Japanese journal of infectious diseases. 2011;64(1):81–4. Epub 2011/01/27. [PubMed] [Google Scholar]
- 10.Beringue V, Herzog L, Reine F, Le Dur A, Casalone C, Vilotte JL, et al. Transmission of atypical bovine prions to mice transgenic for human prion protein. Emerging infectious diseases. 2008;14(12):1898–901. Epub 2008/12/03. 10.3201/eid1412.080941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong Q, Zheng M, Casalone C, Qing L, Huang S, Chakraborty B, et al. Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. Journal of virology. 2008;82(7):3697–701. 10.1128/JVI.02561-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casalone C, Zanusso G, Acutis P, Ferrari S, Capucci L, Tagliavini F, et al. Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3065–70. 10.1073/pnas.0305777101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuura Y, Iwamaru Y, Masujin K, Imamura M, Mohri S, Yokoyama T, et al. Distribution of abnormal prion protein in a sheep affected with L-type bovine spongiform encephalopathy. Journal of comparative pathology. 2013;149(1):113–8. Epub 2013/01/01. 10.1016/j.jcpa.2012.11.231 [DOI] [PubMed] [Google Scholar]
- 14.Baron T, Bencsik A, Biacabe AG, Morignat E, Bessen RA. Phenotypic similarity of transmissible mink encephalopathy in cattle and L-type bovine spongiform encephalopathy in a mouse model. Emerging infectious diseases. 2007;13(12):1887–94. Epub 2008/02/09. 10.3201/eid1312.070635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beringue V, Andreoletti O, Le Dur A, Essalmani R, Vilotte JL, Lacroux C, et al. A bovine prion acquires an epidemic bovine spongiform encephalopathy strain-like phenotype on interspecies transmission. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(26):6965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicot S, Bencsik A, Migliore S, Canal D, Leboidre M, Agrimi U, et al. L-type bovine spongiform encephalopathy in genetically susceptible and resistant sheep: changes in prion strain or phenotypic plasticity of the disease-associated prion protein? The Journal of infectious diseases. 2014;209(6):950–9. Epub 2013/11/13. 10.1093/infdis/jit596 [DOI] [PubMed] [Google Scholar]
- 17.Atarashi R, Sano K, Satoh K, Nishida N. Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion. 2011;5(3):150–3. Epub 2011/07/23. 10.4161/pri.5.3.16893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nature medicine. 2011;17(2):175–8. Epub 2011/02/01. 10.1038/nm.2294 [DOI] [PubMed] [Google Scholar]
- 19.Atarashi R, Wilham JM, Christensen L, Hughson AG, Moore RA, Johnson LM, et al. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nature methods. 2008;5(3):211–2. 10.1038/nmeth0308-211 [DOI] [PubMed] [Google Scholar]
- 20.Elder AM, Henderson DM, Nalls AV, Wilham JM, Caughey BW, Hoover EA, et al. In vitro detection of prionemia in TSE-infected cervids and hamsters. PloS one. 2013;8(11):e80203 Epub 2013/11/14. 10.1371/journal.pone.0080203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuire LI, Peden AH, Orru CD, Wilham JM, Appleford NE, Mallinson G, et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Annals of neurology. 2012;72(2):278–85. 10.1002/ana.23589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orru CD, Bongianni M, Tonoli G, Ferrari S, Hughson AG, Groveman BR, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. The New England journal of medicine. 2014;371(6):519–29. Epub 2014/08/08. 10.1056/NEJMoa1315200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orru CD, Wilham JM, Hughson AG, Raymond LD, McNally KL, Bossers A, et al. Human variant Creutzfeldt-Jakob disease and sheep scrapie PrP(res) detection using seeded conversion of recombinant prion protein. Protein engineering, design & selection: PEDS. 2009;22(8):515–21. Epub 2009/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peden AH, McGuire LI, Appleford NE, Mallinson G, Wilham JM, Orru CD, et al. Sensitive and specific detection of sporadic Creutzfeldt-Jakob disease brain prion protein using real-time quaking-induced conversion. The Journal of general virology. 2012;93(Pt 2):438–49. Epub 2011/10/28. 10.1099/vir.0.033365-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sano K, Satoh K, Atarashi R, Takashima H, Iwasaki Y, Yoshida M, et al. Early detection of abnormal prion protein in genetic human prion diseases now possible using real-time QUIC assay. PloS one. 2013;8(1):e54915 Epub 2013/02/02. 10.1371/journal.pone.0054915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilham JM, Orru CD, Bessen RA, Atarashi R, Sano K, Race B, et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS pathogens. 2010;6(12):e1001217 Epub 2010/12/15. 10.1371/journal.ppat.1001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orru CD, Favole A, Corona C, Mazza M, Manca M, Groveman BR, et al. Detection and discrimination of classical and atypical L-type bovine spongiform encephalopathy by real-time quaking-induced conversion. Journal of clinical microbiology. 2015;53(4):1115–20. Epub 2015/01/23. 10.1128/JCM.02906-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masujin K, Orru CD, Miyazawa K, Groveman BR, Raymond LD, Hughson A, et al. Detection of atypical H-type bovine spongiform encephalopathy and the discrimination of bovine prion strains by RT-QuIC. Journal of clinical microbiology. 2016. Epub 2016/01/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orru CD, Groveman BR, Raymond LD, Hughson AG, Nonno R, Zou W, et al. Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains. PLoS pathogens. 2015;11(6):e1004983 Epub 2015/06/19. 10.1371/journal.ppat.1004983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lasmezas CI, Comoy E, Hawkins S, Herzog C, Mouthon F, Konold T, et al. Risk of oral infection with bovine spongiform encephalopathy agent in primates. Lancet. 2005;365(9461):781–3. Epub 2005/03/01. 10.1016/S0140-6736(05)17985-9 [DOI] [PubMed] [Google Scholar]
- 31.Rezaei H, Marc D, Choiset Y, Takahashi M, Hui Bon Hoa G, Haertle T, et al. High yield purification and physico-chemical properties of full-length recombinant allelic variants of sheep prion protein linked to scrapie susceptibility. European journal of biochemistry / FEBS. 2000;267(10):2833–9. Epub 2000/05/12. [DOI] [PubMed] [Google Scholar]
- 32.Levavasseur E, Laffont-Proust I, Morain E, Faucheux BA, Privat N, Peoc'h K, et al. Regulating factors of PrP glycosylation in Creutzfeldt-Jakob disease—implications for the dissemination and the diagnosis of human prion strains. PloS one. 2008;3(7):e2786 Epub 2008/07/31. 10.1371/journal.pone.0002786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGuire LI, Poleggi A, Poggiolini I, Suardi S, Grznarova K, Shi S, et al. Cerebrospinal fluid real-time quaking-induced conversion is a robust and reliable test for sporadic creutzfeldt-jakob disease: An international study. Annals of neurology. 2016;80(1):160–5. Epub 2016/05/01. 10.1002/ana.24679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Govaerts C, Wille H, Prusiner SB, Cohen FE. Evidence for assembly of prions with left-handed beta-helices into trimers. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(22):8342–7. Epub 2004/05/25. 10.1073/pnas.0402254101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wille H, Michelitsch MD, Guenebaut V, Supattapone S, Serban A, Cohen FE, et al. Structural studies of the scrapie prion protein by electron crystallography. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):3563–8. Epub 2002/03/14. 10.1073/pnas.052703499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cobb NJ, Sonnichsen FD, McHaourab H, Surewicz WK. Molecular architecture of human prion protein amyloid: a parallel, in-register beta-structure. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(48):18946–51. Epub 2007/11/21. 10.1073/pnas.0706522104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakroun N, Prigent S, Dreiss CA, Noinville S, Chapuis C, Fraternali F, et al. The oligomerization properties of prion protein are restricted to the H2H3 domain. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24(9):3222–31. Epub 2010/04/23. [DOI] [PubMed] [Google Scholar]
- 38.Holscher C, Delius H, Burkle A. Overexpression of nonconvertible PrPc delta114-121 in scrapie-infected mouse neuroblastoma cells leads to trans-dominant inhibition of wild-type PrP(Sc) accumulation. Journal of virology. 1998;72(2):1153–9. Epub 1998/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chabry J, Caughey B, Chesebro B. Specific inhibition of in vitro formation of protease-resistant prion protein by synthetic peptides. The Journal of biological chemistry. 1998;273(21):13203–7. Epub 1998/05/28. [DOI] [PubMed] [Google Scholar]
- 40.Hosszu LL, Tattum MH, Jones S, Trevitt CR, Wells MA, Waltho JP, et al. The H187R mutation of the human prion protein induces conversion of recombinant prion protein to the PrP(Sc)-like form. Biochemistry. 2010;49(40):8729–38. Epub 2010/08/20. 10.1021/bi100572j [DOI] [PubMed] [Google Scholar]
- 41.Bartz JC, Bessen RA, McKenzie D, Marsh RF, Aiken JM. Adaptation and selection of prion protein strain conformations following interspecies transmission of transmissible mink encephalopathy. Journal of virology. 2000;74(12):5542–7. Epub 2000/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimberlin RH, Cole S, Walker CA. Temporary and permanent modifications to a single strain of mouse scrapie on transmission to rats and hamsters. The Journal of general virology. 1987;68 (Pt 7):1875–81. Epub 1987/07/01. [DOI] [PubMed] [Google Scholar]
- 43.Scott MR, Groth D, Tatzelt J, Torchia M, Tremblay P, DeArmond SJ, et al. Propagation of prion strains through specific conformers of the prion protein. Journal of virology. 1997;71(12):9032–44. Epub 1997/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wadsworth JD, Asante EA, Desbruslais M, Linehan JM, Joiner S, Gowland I, et al. Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science. 2004;306(5702):1793–6. Epub 2004/11/13. 10.1126/science.1103932 [DOI] [PubMed] [Google Scholar]
- 45.Capobianco R, Casalone C, Suardi S, Mangieri M, Miccolo C, Limido L, et al. Conversion of the BASE prion strain into the BSE strain: the origin of BSE? PLoS pathogens. 2007;3(3):e31 10.1371/journal.ppat.0030031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holznagel E, Yutzy B, Schulz-Schaeffer W, Kruip C, Hahmann U, Bierke P, et al. Foodborne transmission of bovine spongiform encephalopathy to nonhuman primates. Emerging infectious diseases. 2013;19(5):712–20. Epub 2013/05/08. 10.3201/eid1905.120274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bencsik A, Baron T. Bovine spongiform encephalopathy agent in a prion protein (PrP)ARR/ARR genotype sheep after peripheral challenge: complete immunohistochemical analysis of disease-associated PrP and transmission studies to ovine-transgenic mice. The Journal of infectious diseases. 2007;195(7):989–96. Epub 2007/03/03. 10.1086/512087 [DOI] [PubMed] [Google Scholar]
- 48.Bencsik A, Debeer S, Petit T, Baron T. Possible case of maternal transmission of feline spongiform encephalopathy in a captive cheetah. PloS one. 2009;4(9):e6929 Epub 2009/09/10. 10.1371/journal.pone.0006929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castilla J, Gutierrez Adan A, Brun A, Pintado B, Ramirez MA, Parra B, et al. Early detection of PrPres in BSE-infected bovine PrP transgenic mice. Archives of virology. 2003;148(4):677–91. 10.1007/s00705-002-0958-4 [DOI] [PubMed] [Google Scholar]
- 50.Cordier C, Bencsik A, Philippe S, Betemps D, Ronzon F, Calavas D, et al. Transmission and characterization of bovine spongiform encephalopathy sources in two ovine transgenic mouse lines (TgOvPrP4 and TgOvPrP59). The Journal of general virology. 2006;87(Pt 12):3763–71. Epub 2006/11/14. 10.1099/vir.0.82062-0 [DOI] [PubMed] [Google Scholar]
- 51.Crozet C, Bencsik A, Flamant F, Lezmi S, Samarut J, Baron T. Florid plaques in ovine PrP transgenic mice infected with an experimental ovine BSE. EMBO reports. 2001;2(10):952–6. Epub 2001/09/26. 10.1093/embo-reports/kve204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lasmezas CI, Deslys JP, Demaimay R, Adjou KT, Lamoury F, Dormont D, et al. BSE transmission to macaques. Nature. 1996;381(6585):743–4. Epub 1996/06/27. 10.1038/381743a0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RT-QuIC reactions were seeded with 10−4 dilutions of bovine tissue (brainstem), using human recombinant protein. (A) and (D), classical BSE and uninfected bovine tissues. (B) and (E), classical BSE and atypical H-BSE. (C) and (F), classical BSE and atypical L-BSE. Each point represents the mean value of 3 replicate relative fluorescence unit readings.
(PDF)
Average data and statistical significance of the individual results presented in S3 Fig are represented here. (A), classical BSE isolates and uninfected bovine samples. (B), classical BSE and atypical H-BSE isolates. (C), classical BSE and atypical L-BSE isolates. Each point represents the mean value of 3 replicate relative fluorescence unit readings, which were averaged over the number of animals in each group. Error bars represent the mean standard deviation (SD). Vertical dashed lines indicate a statistically significant difference of signal between the test groups. *, p<0,05; ****, p<0,0001.
(PDF)
RT-QuIC reactions were seeded with 10−4 dilutions of bovine tissue (brainstem), using bovine recombinant protein. (A) and (D), classical BSE isolates and uninfected bovine samples. (B) and (E), classical BSE and atypical H-BSE isolates. (C) and (F), classical BSE and atypical L-BSE isolates. Each point represents the mean value of 3 replicate relative fluorescence unit readings.
(PDF)
Homogenates were subjected to proteinase K digestion and serial dilutions were detected by immunoblotting using Sha31 (primates) or 3F4 (CJD patients) monoclonal antibodies.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.