Abstract
Background and Objectives
Induction is a crucial period of opioid addiction treatment. This study aimed to identify buprenorphine/naloxone (BUP) induction patterns and examine their association with outcomes (opioid use, retention, and related adverse events [AEs]).
Methods
The secondary analysis of a study of opioid-dependent adults seeking treatment in eight treatment settings included 740 participants inducted on BUP with flexible dosing.
Results
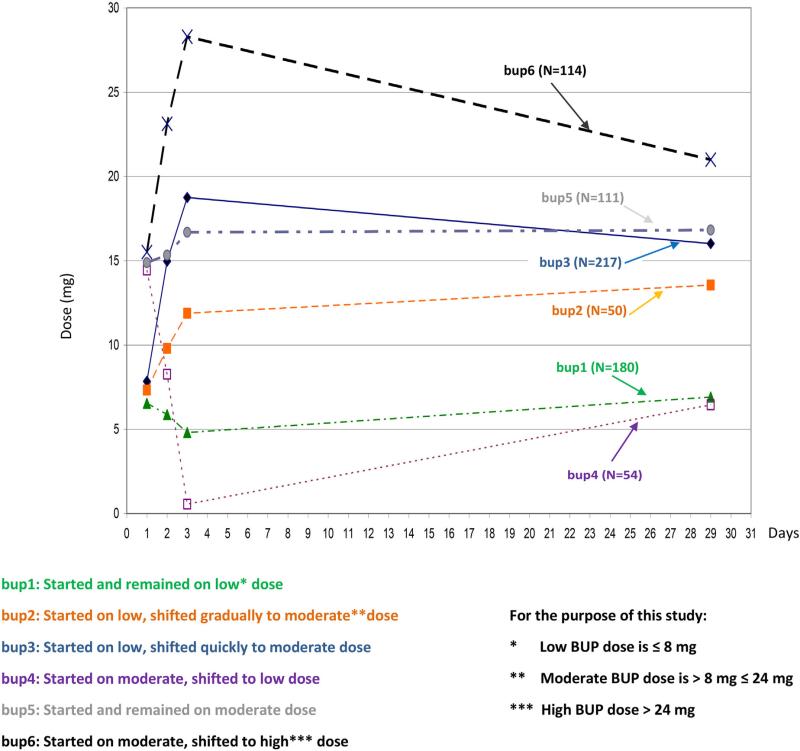
Latent class analysis models detected six distinctive induction trajectories: bup1-started and remained on low; bup2-started low, shifted slowly to moderate; bup3-started low, shifted quickly to moderate; bup4-started high, shifted to low; bup5-started and remained on moderate; bup6-started moderate, shifted to high dose (Fig. 1). Baseline characteristics, including Clinical Opioid Withdrawal Scale (COWS), were important predictors of retention. When controlled for the baseline characteristics, bup6 participants were three times less likely to drop out the first 7 days than bup1 participants (adjusted hazard ratio (aHR) = .28, p = .03). Opioid use and AEs were similar across trajectories. Participants on ≥16 mg BUP compared to those on <16 mg at Day 28 were less likely to drop out (aHR = .013, p = .001) and less likely to have AEs during the first 28 days (aOR = .57, p = .03).
Discussion and Conclusions
BUP induction dosing was guided by an objective measure of opioid withdrawal. Participants with higher baseline COWS whose BUP doses were raised more quickly were less likely to drop out in the first 7 days than those whose doses were raised slower.
Scientific Significance
This study supports the use of an objective measure of opioid withdrawal (COWS) during BUP induction to improve retention early in treatment.
INTRODUCTION
Research has demonstrated that the partial opioid agonist BUP is effective in treating opioid addiction.1 A recent systematic review found that in fixed dose studies, low doses of BUP were less effective than high doses.2 Adequate doses of BUP decrease opioid use by eliminating craving and withdrawal symptoms and blocking the reinforcing effects of opioids.3 The current combination product Suboxone package insert states that BUP induction should not start until “objective signs of withdrawal are evident;” and an adequate maintenance dose should be subsequently achieved to prevent withdrawal symptoms. The induction schedules used vary by clinician, setting, and country.4–6 The Center for Substance Abuse Treatment suggested using a maximum dose of 8 mg BUP on the first day of treatment.7 With increasing clinical experience, changes in guidelines have occurred and some clinical and research protocols incorporated a maximum of 16 mg dose of BUP on the first day.8,9 Low first BUP dose (2 mg), recent use of methadone (MET) or benzodiazepines, lack of prior experience with BUP, and lower initial withdrawal scores were found to be associated with precipitated or protracted withdrawal during induction.10,11
The National Drug Abuse Treatment Clinical Trials Network multi-site “Starting Treatment with Agonist Replacement Therapies” (START) trial provided an opportunity to examine induction practices of eight community treatment providers in different geographical locations, most of whom were familiar with MET but new to the practice of BUP dosing. START is an open-label, phase IV study of liver function in opioid dependent adults randomized to treatment with BUP or MET. The findings showed no evidence of liver damage during the initial 6 months of treatment with either BUP or MET.9 This secondary analysis of START aims to characterize a range of induction practices by deriving induction trajectories and to determine whether variations in induction practices are associated with different treatment outcomes. The specific parameters of induction explored are higher versus lower dose trajectories during the first three days of induction (Hypothesis 1), and latency to achieve a maintenance dose (Hypothesis 3). To replicate previous findings in the literature, the association between the dose at Day 28 and subsequent treatment outcomes were analyzed (Hypothesis 2).
METHODS
Medication and Dosing
Participants randomized in START were treatment-seeking adults who met DSM-IV criteria for opioid dependence and eligibility criteria for MET and BUP treatment.9 This analysis included all 740 participants randomized to the BUP arm. Participants were instructed to abstain from opioids for at least 12 hours and to come to the clinic in mild to moderate withdrawal (COWS> 8). Dosing, including induction, was flexible and based on clinicians’ judgment of participants’ craving, withdrawal symptoms, and medication side effects, with the first dose ranging from 2 to 8 mg. Participants were observed within 2 hours and if withdrawal was present (COWS greater than 5), a second dose was given up to total maximum 16 mg on the first day. The maximum BUP dose allowed on Day 2 and 3 was 32 mg.
Identifying BUP Trajectories to Test Hypothesis 1
To develop BUP trajectories, Longitudinal Exploratory Analysis plotted the dose trajectory for BUP participants across time. A series of trajectory model plots were examined to cover all possible combinations based on plausible theoretical and clinical dosing practice eg, low, moderate, and high dose. Group-based trajectory modeling (ie, finite mixture modeling), was employed to examine trajectories using the SAS Proc Traj group-based modeling procedure.12 The best model selected was based on the smallest absolute value of the Bayesian Information Criterion (BIC).
Outcome Measures and Covariates
The following outcome measures were included. (1) Opioid use: number of days of opioid use measured by Timeline Follow Back (TLFB), self-reported opioid use during the prior 4 weeks collected throughout the study, and Urine Drug Screens (UDS) collected weekly. (2) Retention: number of days receiving the study medication. (3) Safety: number of study-related AEs including related serious adverse events (SAEs). Symptoms associated with withdrawal were not classified as AEs. For each of the outcome measures, three time periods were considered: the first 7 days to reflect the early induction, the first 28 days to reflect the BUP stabilization, and the last 28 days of the 6 month treatment to reflect the end of treatment. Another measure of interest was COWS (score range = 0–48). COWS was measured at the original study only at two time-points, baseline (prior to BUP dose), and 2 hours post-BUP initiation. Two hours post-BUP initiation COWS was another way to describe each group, as a measure of the individual's response to the initial BUP dose during the induction.
Covariates included participants’ baseline characteristics of age, gender, race, opioid use (baseline UDS positive vs. negative), route of drug administration (intravenous or not), depression, pain, baseline COWS, and site.
Hypotheses
All hypotheses were determined before conducting outcome analyses.
Hypothesis 1
Higher Dose Induction (Day 1–3) Will Have Better Outcomes than Lower Dose Induction Reflected by Different Trajectories (Fig. 1). Participants given higher doses in the first three days of treatment will have fewer days of opioid use and higher retention, than those given lower doses while having a similar safety profile. The first three days of treatment were chosen, because they were viewed as the most crucial and varied across different practices. Accordingly, the START protocol described how to dose study participants during the first three days.
FIGURE 1.
Six BUP Induction Trajectories identified by longitudinal exploratory analysis & group based trajectory modeling. These trajectories used to test Hypothesis 1.
Hypothesis 2
Participants given ≥16 mg BUP Dose on Day 28 Will Have Better Outcomes than Participants Given <16 mg BUP Dose on Day 28. Participants on a BUP dose ≥16 mg/day were going to be compared to participants who received <16 mg/day on Day 28. These two dose groups were determined based on following: (1) according to the START protocol, all participants should have been stabilized by Day 28, and (2) 16 mg/day is considered a minimally effective BUP dose suppressing illicit opioid use.2 It was expected that participants on ≥16 mg BUP will have better outcomes without any increase in related AEs compared to participants on <16 mg BUP on Day 28.
Hypothesis 3
Participants Who Reach Their BUP Maintenance Dose More Quickly Will Have Better Outcomes than Those Who Reach Their BUP Maintenance Dose Slowly. Induction speed was defined as the number of days to reach a maintenance dose, where the maintenance dose was defined as the highest BUP dose that a participant took for > 4 or more weeks. It was assumed that the longer time to reach a maintenance dose, after controlling for other factors, may provide the greater opportunity to experience craving which in-turn may be associated with tendency to use opioids.
Data Analysis
Analysis for Hypothesis 1 included only participants whose data were available on Day 1, 2, 3, and 28 (N = 726). Hypothesis 2 included all participants with known BUP dose on Day 28 (N = 733). Hypothesis 3 included all participants in the parent study randomized into the BUP arm (N = 740).
For hypothesis 1, latent class analysis models were fit to the longitudinal data13 in order to identify BUP trajectories. The model with the best fit based on the smallest absolute value of BIC was selected as “best.” Propensity score matching was also used in order to obtain covariate balance among the trajectories.14 The propensity score was estimated as the predicted probability of belonging to a BUP trajectory from logistic regression, using relevant participant baseline characteristics as predictors. The use of these characteristics was based on previous research, which indicates that BUP dose might be predictable for some patients and associated with opioid use severity as measured by route of administration.15 In other studies, race,16 gender,17 psychiatric symptoms,18 and chronic pain19 were found to be associated with BUP dose. These variables were included in estimating the propensity score in Hypothesis 1 to assure balanced covariates among the trajectories, since participants were not randomized to these trajectories. Baseline COWS was included in the models as a separate covariate. The distributions of the outcomes were examined prior to analyses. Because the distribution for number of days of opioid use and UDS were skewed, negative binomial models were used. These models had a better fit based on the Akaike information criterion (AIC) and BIC, compared to Poisson and linear models. A logistic regression model was used to examine AEs since they are binary outcomes (1 = AE, 0 = no AE). For retention (the number of dosing days before dropout), the semi-parametric Cox regression survival analysis was used. Finally, to examine withdrawal (COWS), linear regression was used since the distribution was essentially normal. In all models, mixed effects were used with site as a random factor to control for possible site effects.
For Hypotheses 2 and 3, the trajectories were not included in the models. Potential confounders in Hypothesis 2 and 3 were controlled by adjusting for the covariates (the participants’ baseline characteristics and site) in the models. For hypothesis 2, participants who were given ≥16 mg BUP on Day 28 were compared to those who were given <16 mg BUP on Day 28 in terms of retention, days of opioid use and the occurrence of AEs. For hypothesis 3, the association between the length of time to reach BUP maintenance dose and retention, days of opioid use and the occurrence of AEs was examined. For hypotheses 2 and 3, Cox regression survival analyses were used to examine retention; negative binomial regression models were used to examine days of opioid use; and logistic regression models were used to examine the occurrence of AEs.
In all the models used for hypothesis 1, 2, and 3, model diagnostics and sensitivity analyses were performed. Based on the propensity score matching analysis (Table 1), the distribution of the covariates by BUP trajectories were examined. The unadjusted baseline characteristics by trajectory group show that none of the covariates have sparse data or empty cells, which could have potentially bias the results. After propensity score matching, the difference in the means were closer to zero indicating improved covariate balance. The intent-to-treat (ITT) analysis strategy retains all participants for the entire treatment period, independent of treatment response. Missing data items were handled using multiple imputations by the Markov Chain Monte Carlo approach. Sensitivity analyses were performed by comparing results from the complete-case and ITT analyses. In the final analysis, diagnostic examination of the final models was performed. Results showed model improvement in the fit indices for the selected model as measured by AIC and BIC. A sensitivity analysis was also conducted using the full model with all the covariates explicitly included in the model, and the results corroborated the findings of propensity score matching.
TABLE 1.
Diagnostic tables: covariate distributions and propensity score matching
| Unadjusted baseline demographics by trajectory groups |
Propensity score matching |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| bup1 | bup2 | bup3 | bup4 | bup5 | bup6 | p-value | Difference in Means before & after matching | T Statistic | p-value | ||
| Age (sd) | 39.3 (11.5) | 37.6 (11.9) | 38.3 (10.8) | 37.1 (11.2) | 37.5 (10.9) | 35.5 (10.9) | .11 | Unmatched | .405 | −.35 | .73 |
| Matched | .059 | .68 | .50 | ||||||||
| Females (%) | 21.5 | 25.2 | 31.4 | 34.5 | 33.3 | 37.7 | .06 | Unmatched | −.015 | .49 | .63 |
| Matched | .011 | −.42 | .67 | ||||||||
| African-American (%) | 11.5 | 8.8 | 11.0 | 7.2 | 7.6 | 7.5 | .07 | Unmatched | .046 | −1.62 | .11 |
| Matched | −.001 | −.08 | .94 | ||||||||
| Hispanic (%) | 22.8 | 13.7 | 15.3 | 15.5 | 21.4 | 13.1 | .09 | Unmatched | .052 | −1.65 | .11 |
| Matched | −.006 | −.1 | .9 | ||||||||
| Other Race (%) | 10.2 | 7.7 | 3.4 | 11.2 | 8.6 | 8.9 | .33 | Unmatched | .012 | −.42 | .68 |
| Matched | −.001 | −.07 | .95 | ||||||||
| Opiate use at baseline (%) | 14.7 | 16.0 | 16.1 | 16.4 | 16.6 | 20.2 | .03 | Unmatched | −.039 | 1.6 | .10 |
| Matched | −.002 | −.09 | .92 | ||||||||
| Depression at baseline (%) | 7.5 | 8.2 | 12.7 | 18.2 | 19.3 | 20.4 | .02 | Unmatched | −.083 | 1.88 | .07 |
| Matched | −.016 | .33 | .74 | ||||||||
| Pain (sd) | 2.3 (1.3) | 2.5 (1.3) | 3.2 (1.2) | 3.4 (1.2) | 3.7 (1.2) | 4.1 (1.1) | .03 | Unmatched | −.110 | .58 | .59 |
| Matched | −.088 | .66 | .51 | ||||||||
| Route IV (%) | 57.5 | 64.9 | 65.6 | 69.7 | 75.9 | 79.8 | .01 | Unmatched | −.049 | .99 | .32 |
| Matched | .005 | −.11 | .91 | ||||||||
The following descriptive statistics were obtained to assess the clinical significance of study findings: (1) percentages of participants retained in each BUP trajectory; (2) average number of days of opioid use in the first 7, first 28 and last 28 days, by BUP trajectories; (3) the mean number of AEs and SAEs; (4) COWS scores by BUP trajectory; and (5) mean and median number of days to reach the maintenance dose.
RESULTS
Participants Characteristics
Participants’ mean age was 37 years, with 66% male, and about 70% white. On average, opioid use was approximately 27 days in the 30 days prior to randomization. See Table 1, Table 2 (baseline COWS), and the START article20 for baseline characteristics.
TABLE 2.
Hypothesis 1: Dropout rates, average number of opioid using days and study related AEs during first 7, first 28 and last 28 days: BUP trajectory groups compared to bup1 (reference group)
| BUP Trajectory | DROP OUT | |||||
|---|---|---|---|---|---|---|
| First 7 days |
First 28 days |
Last 28 days |
||||
| Hazard Ratioa [95%CI] | p-value | Hazard Ratioa [95%CI] | p-value | Hazard Ratioa [95%CI] | p-value | |
| bup 1: low stays the same (reference group) | 1 | 1 | 1 | |||
| bup 2: low, increases | .69 [.22,2.19] | .53 | .91 [.51,1.62] | .74 | .91 [.42,1.97] | .80 |
| bup3: low, shifts to moderate | .32 [.08,1.24] | .10 | .86 [.49,1.52] | .61 | .77 [.34,1.74] | .54 |
| bup4: high, shifts to low | .27 [.07,1.06] | .06 | .62 [.34,1.15] | .13 | 1.8 [.91,3.56] | .09 |
| bup5: moderate, stays the same | .33 [.10,1.06] | .06 | .67 [.36,1.23] | .19 | .80 [.32,2.0] | .63 |
| bup6: moderate to high | .28 [.09,.89] | .03 | .54 [.28,1.05] | .07 | .76 [.27,2.18] | .61 |
| OPIOID USE | ||||||
|---|---|---|---|---|---|---|
| First 7 days |
First 28 days |
Last 28 days |
||||
| # of days (N) | p-valueb | # of days (N) | p-valueb | # of days (N) | p-valueb | |
| bup 1: low stays the same (reference group) | 3.3 (98) | 10.8 (97) | 3.8 (42) | |||
| bup 2: low, increases | 3.4 (47) | .83 | 10.3 (47) | .83 | 3.1 (37) | .18 |
| bup3: low, shifts to moderate | 3.7 (97) | .67 | 12.2 (97) | .58 | 3.8 (38) | .57 |
| bup4: high, shifts to low | 2.9 (51) | .70 | 9.6 (50) | .62 | 4.4 (47) | .40 |
| bup5: moderate, stays the same | 2.2 (69) | .14 | 7.7 (69) | .14 | 2.5 (35) | .02 |
| bup6: moderate to high | 2.3 (87) | .20 | 9.3 (85) | .48 | 2.6 (54) | .17 |
| STUDY RELATED AEsc | ||||||
|---|---|---|---|---|---|---|
| First 7 days |
First 28 days |
Last 28 days |
||||
| Odds Ratio [95%CI] | p-value | Odds Ratio [95%CI] | p-value | Odds Ratio [95%CI] | p-value | |
| bup 1: low stays the same (reference group) | 1 | 1 | 1 | |||
| bup 2: low, increases | .91 [.45,1.83] | .79 | .75 [.40,1.39] | .36 | 1.09 [.15,7.84] | .93 |
| bup3: low, shifts to moderate | .66 [.31,1.40] | .28 | .78 [.42,1.44] | .43 | 1.08 [.15,7.77] | .94 |
| bup4: high, shifts to low | 1.40 [.72,2.69] | .32 | 1.40 [.79,2.47] | .25 | 2.23 [.40,12.42] | .36 |
| bup5: moderate, stays the same | .91 [.45,1.83] | .79 | .83 [.45,1.53] | .56 | 2.21 [.40,12.31] | .37 |
| bup6: moderate to high | 1.11 [.59,2.12] | .74 | 1.07 [.61,1.86] | .82 | 1.32 [.22,8.03] | .76 |
| BASELINE COWS |
2 HOURS POST-BUP INITIATION COWS |
|||||
|---|---|---|---|---|---|---|
| Mean(sd) | Coefficientd [95%CI] | p-value | Mean(sd) | Coefficientd [95%CI] | p-value | |
| bup 1: low stays the same (reference group) | 8.98 (3.59) | 0 (reference) | 4.36 (2.04) | 0 (reference) | ||
| bup 2: low, increases | 10.90 (1.77) | 1.91 [1.08, 2.74] | .001 | 5.02 (3.67) | .66 [.46,1.79] | .25 |
| bup3: low, shifts to moderate | 11.82 (2.52) | 2.84 [2.00, 3.67] | <.001 | 5.49 (4.39) | 1.12 [.00,2.24] | .05 |
| bup4: high, shifts to low | 12.61 (2.76) | 3.63 [2.79,4.46] | <.001 | 6.28 (4.26) | 1.92 [.78,3.05] | .001 |
| bup5: moderate, stays the same | 14.03 (3.69) | 5.04 [4.21,5.87] | <.001 | 6.92 (4.45) | 2.55 [1.42,3.69] | .001 |
| bup6: moderate to high | 17.50 (4.42) | 8.51 [8.41,9.56] | <.001 | 8.01 (5.08) | 3.64 [2.56,4.73] | .001 |
A hazard ratio below 1 represents a dropout rate below bup1 trajectory.
p-value of coefficient from negative binomial model, based on comparison with bup1 (reference).
Outcomes are binary (1=yes, 0=no adverse events in a given period of time) by group trajectory characteristics, logistic regression.
A positive coefficient represents the number of COWS score points above that of the bup1 trajectory (reference group).
Models a, b, c include propensity score, baseline COWS, and site as covariates; model d includes propensity score and site as covariates.
Hypothesis 1
BUP Trajectories. Six trajectories were chosen as the improvement in BIC began to level off after six groups. These BUP trajectories (Fig. 1) were described as: bup1 (n = 180) participants started and remained on low dose; bup2 (n = 50) participants started on low dose and shifted gradually to a moderate dose; bup3 (n = 217) participants started on low dose and shifted quickly to a moderate dose; bup4 (n = 54) participants started on moderate dose and shifted to a low dose; bup5 (n = 111) participants started and remained on a moderate dose, and; bup6 (n = 114) participants started on a moderate dose and shifted to high dose. The definition of low, moderate and high dose is included in Figure 1. None of the participants were found to lie exactly in or near the midpoint between any two BUP trajectories. This was tested within ± 5% of the midpoint dose for the first 3 days. Wald test showed that the six BUP trajectories are significantly different.
Hypothesis 1: Higher Dose Induction (Day 1–3) Will Be Associated With Better Outcomes than Lower Dose Induction Reflected by Different Trajectories (Fig. 1)
The survival model with covariate adjustment indicates that participants in higher dose trajectories (bup2, 3, 4, 5, 6) were less likely to drop out from treatment during the first 7 days compared to bup1 participants. This difference was statistically significant only between bup6 and bup1 participants (aHR = .28, 95%CI = .09–.89). Dropout during the first 28 days was the highest in bup1, however the differences between bup1 and all other trajectories (bup2–6) were not statistically significant (Table 2). Adjusted HR for dropout during the last 28 days in bup2, 3, 5, and 6 was lower than bup1, however, none were statistically significant. The actual dropout in each trajectory (descriptive statistics, data not shown), suggested that dropout during the first 28 days was the highest in bup6 (25.4%). This finding was explored further by a survival model without adjusting for baseline characteristics, which revealed no statistically significant differences in retention between bup6 and bup1 at this time point.
The numbers of opioid-using days during the first 7 days and first 28 days of treatment measured by TLFB were comparable across all 6 BUP trajectories. During the last 28 days of treatment, participants in the higher dose BUP trajectories (bup2-bup6) had fewer opioid using days than bup1, however, only the difference between bup5 and bup1 reached statistical significance (p = .02) (Table 2). The analyses based on UDS (data not shown) revealed similar results as TLFB.
There were no statistically significant differences among the six BUP trajectories in related AEs within the first 7, first 28 and last 28 days of treatment (Table 2). The mean number of AEs during the first 28 days was .13 (SD = .47). The mean number of SAEs during the first 28 days was .01 (SD = .09).
The bup1 group had in 2 hours post-BUP initiation lower withdrawal scores (COWS) than bup3 (p = .05), bup4 (p = .001), bup5 (p = .001), and bup6 (p = .001), (Table 2). Participants with more severe opioid use disorder (reflected by opiate use, depression, pain, intravenous use, and higher COWS at the baseline) were more likely to be placed in higher dose trajectories (Table 1, 2). The Table 2 also shows mean (sd) of 2 hours post-BUP initiation COWS, which is about 50% of the baseline COWS in each trajectory.
Hypothesis 2: Participants Given ≥16 mg BUP Dose on Day 28 Will Have Better Outcomes than Participants Given <16 mg BUP Dose on Day 28
Compared to participants dosed at <16 mg on Day 28, participants who received ≥16 mg on Day 28 had significantly higher 2 hour post-initiation dose COWS score (p = .002), were less likely to drop out in the first 28 days (aHR = .013, p = .001), and were less likely to have medication-related AEs during the first 28 days (aOR = .57, p = .03). There was no significant difference between dose groups in number of opioid use days during the first and last 28 days, retention in the last 28 days, and number of AEs in the last 28 days (Table 3).
TABLE 3.
Hypothesis 2: Predictor = dummy variable for BUP dose at Day 28 ≥16mg vs. BUP dose at Day 28 <16mg Hypothesis 3: Predictor = speed (number of days) at which BUP participants reach their maintenance dose
| HYPOTHESIS 2 | HYPOTHESIS 3 | |||
|---|---|---|---|---|
| Hazard Ratio 1) ≥16 mg BUP at Day 28 compared to 2)<16 mg BUP at Day 28 |
Hazard Ratio |
|||
| Outcomes* | [95% CI] | p | [95% CI] | p |
| Dropout first 28 days | .013 [.004,.038] | .001 | .99 [.89,1.12] | .31 |
| Dropout last 28 days | .58 [.34,1.006] | .053 | .99 [.85,1.15] | .55 |
| Differences in groups** 1)≥16mg BUP at Day 28 vs. 2)<16 mg BUP at Day 28 |
Coefficient of days to reach maintenance dose |
|||
|---|---|---|---|---|
| [95% CI] | p | [95% CI] | p | |
| 2 hours post-BUP initiation COWS | 1.34 [.50,2.18] | .002 | −.64 [−1.32,.036] | .06 |
| Opioid use first 28 days (TLFB) | .02 [−.32,.352] | .92 | .28 [−.99,1.57] | .44 |
| Opioid use last 28 days (TLFB) | .17 [−.39,.72] | .55 | .10 [.02,.19] | .02 |
| UDS first 28 days | −.07 [−.32,.17] | .56 | .10 [−.09,.29] | .29 |
| UDS last 28 days | .003 [−.39,.40] | .98 | −.26 [−.56,.05] | .10 |
| Odds Ratio 1) ≥16 mg BUP at Day 28 vs. 2)<16 mg BUP at Day 28 |
Odds Ratio |
|||
|---|---|---|---|---|
| [95% CI] | p | [95% CI] | p | |
| AE first 28 days (yes/no) | .57 [.34,.96] | .03 | 1.03 [.66,1.59] | .89 |
| AE last 28 days (yes/no) | .81 [.21,3.05] | .75 | .53 [.14,2.01] | .35 |
The model for 2 hour post-BUP initiation COWS adjusts for baseline characteristics and site, all the other models in addition to baseline characteristics and site adjust also for the baseline COWS.
The reference group is the group of participants that are treated with less than 16 mg of BUP on Day 28.
Hypothesis 3: For each extra day of reaching maintenance dose, COWS goes down by .64, opioid use goes up by log count of .1 opioid using day.
Hypothesis 3: Participants Who Reach Their BUP Maintenance Dose More Quickly Will Have Better Outcomes than Those Who Reach Their BUP Maintenance Dose Slowly
There was a positive association between the number of days to reach the maintenance dose and number of opioid-using days in the last 28 days of treatment (p = .02), i.e. the longer to reach maintenance dose, the more days of opioid use during the last 28 days of treatment. The speed of BUP induction was not associated with having related AEs during the first and last 28 days of treatment or retention during the first and last 28 days of treatment (Table 3). The mean number of days to reach the maintenance dose was 23.22 (sd = 8.54), the median was 24.
DISCUSSION
This exploratory study attempts to delineate possible BUP induction patterns in opioid-dependent study participants in outpatient treatment programs (OTPs). BUP was relatively new to OTPs in the study, and the START protocol allowed a flexible approach to dosing recommending addressing withdrawal symptoms, instructions to clinicians included a maximum upper limit of BUP 16 mg on Day 1, and 32 mg on Days 2–168.The findings indicate that participants were inducted in a wide range of ways, but within the dosing recommendations and requirements of maximum doses as described in the protocol. The lowest dose induction trajectory (bup1) was found to be associated with lower retention (after adjusting for baseline characteristics, including differing levels of opioid withdrawal as measured with COWS). Similar numbers of AEs were observed across trajectory groups. Interestingly, days of opioid use did not differ across BUP trajectories during the first 7 and first 28 days of the study. This may be because it takes several weeks or months to stop or minimize habitual drug use, even when a participant is not receiving the reinforcing effects of illicit opioids while in BUP treatment.
The baseline and 2 hours post-BUP initiation COWS was strongly associated with the dose determination. Table 2 shows that participants with higher COWS received higher initial BUP dosing. This finding is consistent with the dosing recommendations described in the START protocol. A higher COWS score means that a participant experienced more severe withdrawal, which is found in patients with a higher level of physical dependence. The finding that baseline COWS was a statistically significant covariate indicates that it has an important influence on retention. It is consistent with some previous studies that showed that higher withdrawal is associated with poorer retention.21 The survival model that controls for the baseline COWS shows that the effect of the medication dose is more prominent when controlled for the baseline characteristics, especially baseline COWS. This study included COWS at 2 hours post-BUP initiation because this is an important measure to reflect persistent opioid withdrawal after medication commencement. Mean COWS in each trajectory decreased in the 2 hours following BUP administration by about 50%, which demonstrates that the BUP dosing reduced the withdrawal symptoms. Based upon baseline and 2 hours post-initiation COWS and BUP dose requirements, the bup6 group had participants with more severe physical dependence on opioids. This characteristic probably drove the more rapid dose escalation.
The induction trajectory of participants in bup4 is difficult to explain. These participants were inducted with medium to high level of withdrawal symptoms, dosed first day at the higher dose trajectories, but their dosing dropped the second and the third day, and remained low at Day 28. We can confirm that the dose reduction is not explained by a higher number of AEs in this dosing group. Other possible explanations are lower mean doses or missed doses for various reasons such as desire to use illicit opioids mentioned by some study participants during additional qualitative interviews.22 However, we were not able to confirm this conjecture from our data. Participant and clinician related reasons for reducing BUP doses early in treatment are an important area for future investigation.
Participants dosed with ≥16 mg BUP at Day 28 were less likely to drop out in the first 28 days. This result is consistent with other secondary analysis of START that was looking at predictors of retention during the entire study (24 weeks)20 and other studies, including recent meta-analysis.2,23 Hypothesis 2 results add to the previous findings20 that these participants were also less likely to have medication-related AEs during the first 28 days than participants dosed with <16 mg BUP at Day 28 and more likely to have more severe opioid withdrawal at 2 hours post-BUP initiation.
Participants who achieved their maintenance dose quickly had less opioid use in the last 28 days of treatment than those achieving their maintenance dose slowly, without an increase in AEs in the first or last 28 days of treatment. The speed of BUP induction has not been addressed in the previous literature and deserves more research attention in the future.
There are some caveats to be aware of when interpreting these findings. Participants for this study were not randomized to different (predetermined) dose trajectories. These trajectories were derived from data using a statistical approach. Therefore, one cannot rule out other unexplored confounding factors that may have influenced results or reach definitive conclusions about causal effects of BUP dose/induction patterns on outcomes. To decrease the risk of confounding, propensity scoring was calculated in Hypothesis 1 testing. The analyses in Hypotheses 2 and 3 have been adjusted to the main covariates. Matching was performed based on propensity scores to eliminate systematic biases due to imbalance in observed covariates. The matched sample was evaluated in terms of covariate balance. These analyses suggest that matching was successful in balancing the measured covariates. Although an extensive search of the literature for potentially confounding covariates was conducted, it is possible that some other relevant covariates may not have been identified.
The low rate of AEs may have decreased the ability to detect differences between the groups on this measure. However, the finding of few AEs also demonstrates that BUP is generally well tolerated, including during induction.
Other considerations include the fact that the study was implemented in OTPs with daily observed BUP dosing. Therefore, the results might not be fully generalizable to BUP treatment in physicians’ offices with less directly supervised dosing requirements. Finally, as multiple analyses were performed, p values close to .05 should be questioned due to multiplicity.
Acknowledgments
We acknowledge the National Drug Abuse Treatment Clinical Trials Network for implementation of the original START study, and assistance with this secondary analysis.
Declaration Of Interest
The original START study was funded by the National Institute on Drug Abuse, where the buprenorphine/naloxone for START was provided by Reckitt Benckiser Pharmaceuticals. The authors of this secondary analysis collaborated on the implementation of START and received the dataset. Dr. Saxon has grant support from the Clinical Trials Network: Pacific Northwest Node. 5 U10 DA013714-08, NIH/NIDA, served on the Pharmaceutical, Scientific Advisory Board, Alkermes, Inc. and has been a presenter for Reckitt-Benckiser, Inc. He has no publishing constraints. Dr. Nielsen is supported by a NHMRC Research Fellowship (#1013803), has been an investigator on united educational grants from Reckitt-Benckiser, completely unrelated to this work, no publishing constraints. Dr. Blaine has been a treatment advocate for Reckitt Benckiser Pharmaceuticals, Inc., with no publishing constraints. The authors alone are responsible for the content and writing of this paper.
Footnotes
Conflicts of Interests: None.
REFERENCES
- 1.Ducharme S, Fraser R, Gill K. Update on the clinical use of buprenorphine: In opioid-related disorders. Can Fam Physician. 2012;58:37–41. [PMC free article] [PubMed] [Google Scholar]
- 2.Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. 2014 Published Online: methadone-maintenance-for-opioid-dependence#s-thash.INOx5xNh.dpuf. [Google Scholar]
- 3.Kleber HD. Pharmacologic treatments for opioid dependence: Detoxification and maintenance options. Dialogues Clin Neurosci. 2007;9:455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quaglio G, Pattaro C, Gerra G, et al. Buprenorphine in maintenance treatment: Experience among Italian physicians in drug addiction centers. Am J Addict. 2010;19:222–230. doi: 10.1111/j.1521-0391.2010.00040.x. [DOI] [PubMed] [Google Scholar]
- 5.Vignau J, Duhamel A, Catteau J, et al. Practice-based buprenorphine maintenance treatment (BMT): How do French healthcare providers manage the opiate-addicted patients?. J Subst Abuse Treat. 2001;21:135–144. doi: 10.1016/s0740-5472(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 6.Auriacombe M, Fatséas M, Dubernet J, et al. French Field Experience with Buprenorphine. Am J Addict. 2004;13:S17–S28. doi: 10.1080/10550490490440780. [DOI] [PubMed] [Google Scholar]
- 7.Center for Substance Abuse Treatment (CSAT) Medication-assisted treatment for opioid addiction in opioid treatment programs. U. S. Dept. of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Treatment; Rockville, MD: 2008. [Google Scholar]
- 8.Gunderson EW, Levin FR, Rombone MM, et al. Improving temporal efficiency of outpatient buprenorphine induction. Am J Addict. 2011;20:397–404. doi: 10.1111/j.1521-0391.2011.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/Naloxone and methadone effects on laboratory indices of liver health: A randomized trial. Drug Alcohol Depend. 2013;128:71–76. doi: 10.1016/j.drugalcdep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitley SD, Sohler NL, Kunins HV, et al. Factors associated with complicated buprenorphine inductions. J Subst Abuse Treat. 2010;39:51–57. doi: 10.1016/j.jsat.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen S, Hillhouse M, Weiss R, et al. The relationship between primary prescription opioid and buprenorphine-naloxone induction outcomes in a prescription opioid dependent sample. Am J Addict. 2014;23:343–348. doi: 10.1111/j.1521-0391.2013.12105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagin DS. Group-based modeling of development. Harvard University Press; Cambridge, MA: 2005. [Google Scholar]
- 13.Muthen B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Sage handbook of quantitative methodology Thousand Oaks. Sage; CA: 2004. pp. 345–368. [Google Scholar]
- 14.Haviland A, Nagin DS, Rosenbaum PR. Combining propensity score matching and group-based trajectory modeling in an observational study. Psychological Methods. 2007;12:247–267. doi: 10.1037/1082-989X.12.3.247. [DOI] [PubMed] [Google Scholar]
- 15.Gossop M, Griffiths P, Powis B, et al. Severity of opioid dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992;87:1527–36. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 16.Kosten TR, Rayford BS. Effects of ethnicity on low-dose opiate stabilization. J Subst Abuse Treat. 1995;12:111–116. doi: 10.1016/0740-5472(94)00069-4. [DOI] [PubMed] [Google Scholar]
- 17.Schottenfeld RS, Pakes JR, Kosten TR. Prognostic factors in Buprenorphine-versus methadone-maintained patients. J Nerv Ment Dis. 1998;186:35–43. doi: 10.1097/00005053-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Wedekind D, Jacobs S, Karg I, et al. Psychiatric comorbidity and additional abuse of drugs in maintenance treatment with L- and D,L-methadone. World J Biol Psychiatry. 2010;11:390–399. doi: 10.3109/15622970802176487. [DOI] [PubMed] [Google Scholar]
- 19.Chakrabarti A, Woody GE, Griffin ML, et al. Predictors of buprenorphine-naloxone dosing in a 12-week treatment trial for opioid-dependent youth: Secondary analyses from a NIDA Clinical Trials Network study. Drug Alcohol Depend. 2010;107:253–256. doi: 10.1016/j.drugalcdep.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hser Y, Saxon A, Huang D, et al. Treatment Retention among Patients Randomized to Buprenorphine/Naloxone Compared to Methadone in Multi-site Trial. Addiction. 2014;109:79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soyka M, Zingg CH, Koller G, et al. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: Results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641–653. doi: 10.1017/S146114570700836X. [DOI] [PubMed] [Google Scholar]
- 22.Teruya Ch, Mitchell SG, et al. Patient perspectives on buprenorphine/naloxone: A qualitative study of retention during the Starting Treatment with Agonist Replacement Therapies (START) study. J Psychoactive Drugs. 2014;46:412–426. doi: 10.1080/02791072.2014.921743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fareed A, Vazalapalli S, Casarella J, et al. Effect of buprenorphine dose on treatment outcome. J Addict Dis. 2012;31:8–18. doi: 10.1080/10550887.2011.642758. [DOI] [PubMed] [Google Scholar]