Abstract
Background
Inferior vena caval filters (IVCFs) may prevent recurrent pulmonary embolism (PE). Despite uncertainty about their net benefit, patterns of use and outcomes of this device in contemporary practice are unknown.
Objectives
We determined the trends in utilization rates and outcomes of IVCF placement in patients with PE, and explored regional variations in use in the United States.
Methods
In a National cohort study of all Medicare Fee-For-Service beneficiaries aged ≥65 years with a principal discharge diagnosis of PE between 1999–2010, we determined the Rates of IVCF placement per 100,000 beneficiary-years, and per 1,000 patients with PE. We also investigated the 30-day and 1-year mortality rates after IVCF placement.
Results
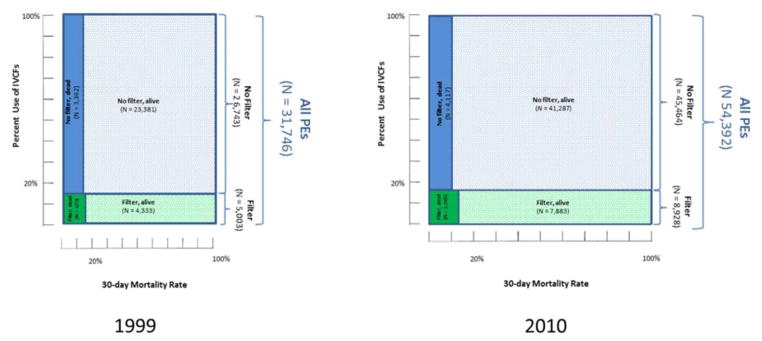
Among 556,658 patients hospitalized with PE, 94,427 underwent IVCF placement. Between 1999 and 2010, the number of PE hospitalizations with IVCF placement increased from 5,003 to 8,928, representing an increase in the rate per 100,000 beneficiary-years from 19.0 to 32.5 (P <0.001 for both). As the total number of PE hospitalizations increased (from 31,746 in 1999 to 54,392 in 2010), the rate of IVCF placement per 1,000 PE hospitalizations did not change significantly (157.6 to 164.1, P = 0.11). Results were consistent across demographic subgroups, although IVCF use was higher in blacks and patients aged ≥85 years. IVCF utilization varied widely across regions, with the highest rate in the South Atlantic region and the lowest rate in the Mountain region.
Conclusions
In a period of increasing PE hospitalizations among Medicare Fee-For-Service beneficiaries, IVCF placement increased as utilization rates in patients with PE remained above 15%. Mortality associated with PE hospitalizations is declining, regardless of IVCF use.
Keywords: deep vein thrombosis, trends, mortality
Introduction
Inferior vena caval filters (IVCFs) are an advanced therapy for pulmonary embolism (PE) with uncertain net benefit. IVCFs can prevent recurrent PE (1); yet, this benefit might be offset by procedural and longer-term device-related complications such as recurrent deep vein thrombosis (DVT) and post-thrombotic syndrome. The available randomized controlled trials have not shown a mortality benefit associated with use of IVCFs (1–4). Expert guidelines recommend the use of IVCFs for cases with contraindications to anticoagulation or with recurrent PE despite receiving anticoagulation (5–7). Given the equivocal data for risks and benefits, clinical equipoise for use of IVCFs has persisted (8–11).
The older adults may represent a population in whom IVCF utilization is common despite the uncertainties around clinical benefit. Age and medical comorbidities place older adults at higher risk for the development of PE, as well as its complications, including right ventricular dysfunction (12) and death (12–15). Meanwhile, older adults are less likely to receive alternative therapies such as thrombolytic therapy (12,16,17) due to concerns for hemorrhagic complications, or surgical thrombectomy due to a high prevalence of multiple comorbidities. These factors, as well as recent technological advances in IVCF design (18) may have increased the use of IVCFs over time. Accordingly, we assessed the utilization rates and outcomes of IVCF placement among all Medicare Fee-For-Service beneficiaries aged ≥65 years in the United States, from 1999 to 2010 and also examined regional variations in IVCF use.
Methods
Data Source
We used the 100% Medicare enrollment file from the Centers for Medicare & Medicaid Services (CMS) to identify all Medicare Fee-For-Service (FFS) beneficiaries aged ≥65 years from 1999 through 2010 with at least 1 month of enrollment who resided in and were hospitalized in the United States. For each year, we counted the total number of beneficiaries and calculated person-years for beneficiaries to account for new enrollment, disenrollment, or death during the study period. We then linked the person-years beneficiary data with the inpatient claims data to identify all FFS beneficiaries with a principal discharge diagnosis of PE who underwent IVCF placement from January 1, 1999, through December 31, 2010. The Medicare inpatient claims data encompass procedural and diagnostic information for hospitalizations based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), as well as demographics, and dates of hospital admission and discharge. Death was determined through the Medicare enrollment file, which includes information on out-of-hospital mortality.
Patients
We included patients with principal ICD-9-CM discharge diagnosis of PE using the following codes: 415.1X (pulmonary embolism and infarction), 415.11 (iatrogenic pulmonary embolism and infarction), 415.13 (saddle embolus of pulmonary artery), or 415.19 (other pulmonary embolism and infarction). We excluded patients with principal discharge diagnosis of septic pulmonary embolism (415.12). For patients with multiple hospitalizations (5.7% in 1999 and 2.5% in 2010) for pulmonary embolism in each given year, we randomly selected one hospitalization. Among patients with principal ICD-9-CM discharge diagnosis of PE, we used ICD-9-CM procedure codes to identify those who received IVCFs (38.7) during the index PE hospitalization.
Outcome Measures
We determined the number of hospitalized patients with PE who received IVCFs in each year during the study period and reported the rates of PE hospitalizations that underwent IVCF placement per 100,000 person-years of Medicare FFS beneficiaries. Further, to provide a clinically meaningful denominator for use of IVCFs, we determined the number of patients with principal discharge diagnosis of PE in each year. Using the PE hospitalizations that underwent IVCF placement as the numerator, we calculated the rate of IVCF use per 1,000 patients with principal discharge diagnoses of PE. Among patients receiving IVCFs, we determined the rates of in-hospital, 30-day, 6-month, and 1-year all-cause death. The time zero for all deaths was the date of IVCF placement, and the mortality rates are reported as percentages. We determined the hospital length of stay and trends in utilization rate and outcomes of IVCF placement from 1999 to 2010. We also determined the utilization of IVCFs across 9 U.S. Census regions.
Statistical Analysis
We used the Mantel-Haenszel Chi-squared test to assess the temporal trends in the procedure utilization rates and mortality rates for IVCF placement. To obtain adjusted 30-day, 6-month, and 1-year mortality rates, we fitted separate linear mixed-effects models with logit link functions and hospital-specific random intercepts. We used the data from 1999 as the referent and indicator variables for each subsequent year to estimate the likelihood of mortality for each subsequent year adjusted for comorbidities (19,20). We converted the odds ratio values to risk ratio estimates (21) and multiplied the risk ratio for each year by the mortality rate of the baseline year (1999) to calculate adjusted mortality rates for subsequent years. We performed all analyses with SAS 9.3 (SAS Institute, Cary, North Carolina). All tests were 2-sided, and a p-value <0.05 was considered significant. The Human Investigation Committee at Yale University exempted this study from additional review since all data were de-identified.
Results
Patient Characteristics
Among 335,302,975 beneficiary-years between January 1, 1999, and December 31, 2010, there were 556,658 hospitalizations with the principal discharge diagnosis of PE. Among the patients with PE, 94,427 (16.9%) underwent IVCF placement. Compared with the entire cohort of patients with PE, those undergoing IVCF placement had an overall greater frequency of comorbidities (including cancer, heart failure, atherosclerotic and vascular diseases, and functional disability, Table 1). Within the cohort of patients with PE that received IVCFs, the demographic characteristics remained relatively unchanged from 1999 to 2010. Some comorbidities remained stable during the study period; some were less frequent (such as atherosclerotic disease and heart failure, P <0.05 for both); and some were more frequent (such as respiratory failure, renal failure, and hypertension, P <0.01 for all).
Table 1.
Patient Characteristics
| Patients with PE | PE + IVCF Placement | |
|---|---|---|
| Total | 556,658 | 94,427 |
| Demographics | ||
| Age (y) | 77.9 | 78.2 |
| Female (%) | 60.6 | 58.7 |
| Race | ||
| White (%) | 85.9 | 83.9 |
| Black (%) | 11.3 | 13.2 |
| Other (%) | 2.8 | 2.9 |
| Comorbidities | ||
| Cancer (%) | 22.6 | 29.4 |
| Heart Failure (%) | 15.2 | 18.4 |
| Myocardial Infarction (%) | 3.1 | 3.7 |
| Unstable Angina (%) | 2.8 | 2.8 |
| Peripheral Vascular Disease (%) | 10.7 | 16.9 |
| Atherosclerotic Disease (%) | 29.8 | 31.2 |
| Stroke (%) | 3.3 | 6.0 |
| CVD Other than Stroke (%) | 4.6 | 5.7 |
| Hypertension (%) | 63.4 | 62.6 |
| Respiratory Failure (%) | 6.2 | 7.9 |
| COPD (%) | 28.0 | 30.0 |
| Pneumonia (%) | 20.1 | 22.3 |
| Renal failure (%) | 8.3 | 10.6 |
| Liver Disease (%) | 0.7 | 1.1 |
| Diabetes (%) | 22.1 | 22.6 |
| Other Conditions | ||
| Trauma (%) | 10.9 | 13.5 |
| Malnutrition (%) | 5.8 | 8.3 |
| Other psychiatric disorder (%) | 2.9 | 3.2 |
| Depression (%) | 9.6 | 9.0 |
| Dementia (%) | 12.7 | 15.0 |
| Functional Disability (%) | 3.9 | 6.2 |
COPD: chronic obstructive pulmonary disease, CVD: cerebrovascular disease, IVCF: inferior vena caval filter, PE: pulmonary embolism
IVCF Utilization Rates
The number of patients with PE undergoing IVCF placement increased annually from 5,003 in 1999 to 8,928 in 2010 (P <0.001 for trend). The rate of PE hospitalizations undergoing IVCF placement increased from 19.0 to 32.5 per 100,000 beneficiary-years (P <0.001). The number of hospitalizations with principal discharge diagnosis of PE increased annually from 31,746 in 1999 to 54,392 in 2010. Therefore, the rate of PE hospitalizations undergoing IVCF placement per 1,000 patients with PE did not change significantly (from 157.6 in 1999 to 164.1 in 2010, P = 0.11 for trend).
Outcomes
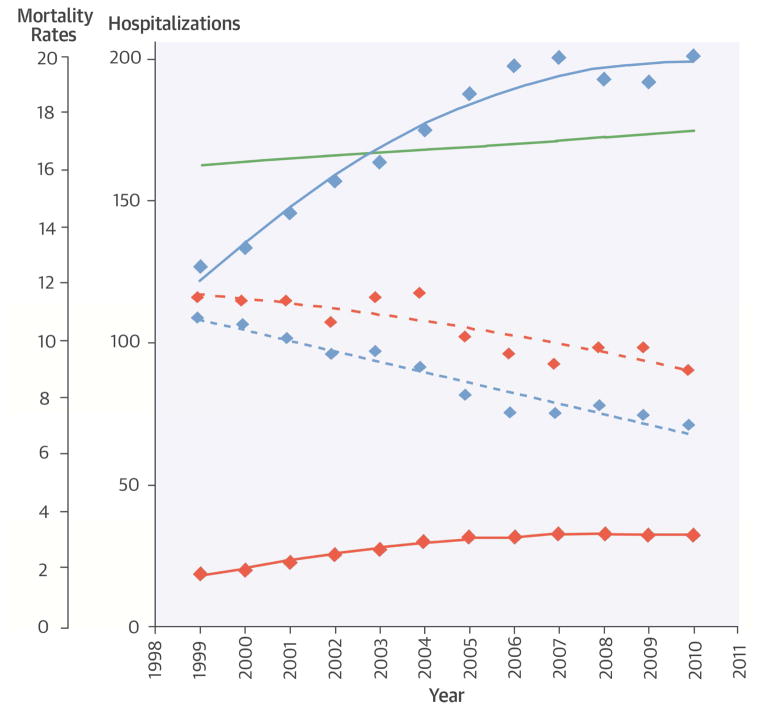
Short and long-term mortality rates declined in all subgroups with PE from 1999 to 2010. Adjusted mortality rates in the entire cohort of patients with principal discharge diagnosis of PE declined from 12.7% to 9.0% at 30 days, and from 26.3% to 22.4% at 1 year (P <0.001 for both time periods). Among patients with PE undergoing IVCF placement, there were significant declines in in-hospital mortality (8.2% to 4.3%), and in post-procedural adjusted 30-day (13.4% to 10.9), 6-month mortality (28.8% to 26.7%) and 1-year mortality (33.4% to 30.6%; P for trend ≤0.001 for all, Table 2). A similar trend but with more pronounced decline in mortality rates was also observed in the cohort of patients with PE that did not undergo IVCF placement (Figure 1; Table 3).
Table 2.
Number, Rate, and Outcomes for Patients Undergoing IVCF Placement
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 5003 | 5339 | 6157 | 7096 | 7815 | 8835 | 9348 | 8976 | 9128 | 8962 | 8840 | 8928 |
| Rate per 1,000 PEs | 157.6 | 156.8 | 161.8 | 167.6 | 173.3 | 181.8 | 178.4 | 166.9 | 170.1 | 174.4 | 172.8 | 164.1 |
| Length of stay (d) | 10.3 | 9.9 | 9.8 | 9.9 | 9.6 | 9.3 | 8.9 | 8.7 | 8.6 | 8.6 | 8.3 | 8.1 |
| In-hospital mortality rate (%) | 8.2 | 7.9 | 7.4 | 7.1 | 6.8 | 6.9 | 5.5 | 5.0 | 5 | 5 | 5.1 | 4.3 |
| Adjusted 30-day mortality rate (%)† | 13.4 | 13.3 | 13.3 | 12.6 | 13.4 | 13.6 | 12.1 | 11.5 | 11.1 | 11.7 | 11.7 | 10.9 |
| Adjusted 6-month mortality rate (%)† | 28.8 | 30.2 | 30.0 | 29.6 | 29.2 | 30.0 | 28.6 | 27.8 | 27.6 | 28.6 | 27.3 | 26.7 |
| Adjusted 1-year mortality rate (%)† | 33.4 | 33.8 | 34.5 | 33.6 | 33.4 | 34.1 | 32.0 | 31.6 | 31.6 | 32.7 | 31.6 | 30.6 |
adjusted for demographics and comorbidities (reference: 1999).
IVCF: inferior vena caval filter, PE: pulmonary embolism.
Figure 1. Proportion of Medicare FFS Patients with PE with and without IVCF Placement in 1999 and 2010.
Shown are the number of patients with PE, those that received IVCFs, and the fatalities in those who did and did not receive IVCFs. The size of each rectangle is proportionate to the number of patients in each subgroup. Note that the number of deaths in the entire cohort, as well as among those with and without IVCFs increased from 1999 to 2010. However, in the context of increasing PE hospitalizations (denominator), the mortality rates declined in all cohorts, with most notable decline in the subgroup that did not receive an IVCF. The fatalities in the figure represent 30-day unadjusted deaths. Adjusted 30-day and 1-year fatalities follow a similar pattern. FFS: Fee-For-Service, IVCF: inferior vena caval filter PE: pulmonary embolism.
Table 3.
Temporal Change in Medicare FFS Patients with PE with and without IVCF Placement in 1999 and 2010.
| 1999 | 2010 | Change (N, %) | |
|---|---|---|---|
| Hospitalizations | |||
| All Patients with PE | 31,746 | 54,392 | ↑ 22646 ↑ (71%) |
| Receiving an IVCF | 5,003 | 8,928 | ↑ 3925 ↑ (78%) |
| Not receiving an IVCF | 26,743 | 45,464 | ↑ 18721 ↑ (70%) |
| 30-day Mortality | |||
| All Patients with PE (N for mortality) | 4,032 | 5,222 | ↑ 1190 ↑ (29.5%) |
| All Patients with PE (Mortality Rate [%]) | 12.7 | 9.6 | ↓ (3.1%) |
| Receiving an IVCF (N for mortality) | 670 | 1,045 | ↑ 375 ↑ (59.7%) |
| Receiving an IVCF (Mortality Rate) | 13.4 | 11.7 | ↓ (1.7%) |
| Not receiving an IVCF (N for mortality) | 3,362 | 4,177 | ↑ 815 ↑ (24.2%) |
| Not receiving an IVCF (Mortality Rate) | 12.6 | 9.2 | ↓ (3.2%) |
Note that fatalities in the table represent 30-day unadjusted deaths. Adjusted 30-day and 1-year fatalities follow a similar pattern. FFS: Fee-For-Service, IVCF: IVCF: inferior vena cava filter, PE: pulmonary embolism.
Findings across Demographic Subgroups
The oldest patients (aged ≥85 years) had the greatest relative increase in rate of IVCF placement over time (from 150.0 in 1999 to 194.6 in 2010 per 1,000 patients with PE, P <0.001). Men had higher procedure rates compared with women throughout the study period. Across the racial subgroups, blacks had a significant decline in IVCF utilization rates over time (P <0.05), nevertheless, blacks had a persistently higher IVCF placement rate compared with the other races. Adjusted 30-day and 1-year mortality rates declined over time among all age, sex, and race subgroups. Across the age subgroups, higher 30-day and 1-year mortality rates were observed in the oldest old (≥85 years), with 1-year mortality rate exceeding 33% throughout the study period (range: 33.2%–37.9%). Compared with women, men had higher 30-day and 1-year mortality rates over time (Table 4).
Table 4.
Procedure Utilization Rates and Outcomes Across Age, Sex, and Racial Subgroups
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospitalization rate per 1000 Patients with PE | ||||||||||||
| Age | ||||||||||||
| 65–74 | 157.0 | 155.0 | 155.6 | 159.6 | 166.0 | 174.3 | 164.2 | 155.8 | 158.0 | 161.2 | 156.0 | 149.6 |
| 75–84 | 161.0 | 158.1 | 166.2 | 172.4 | 177.3 | 185.6 | 184.3 | 172.6 | 173.2 | 177.2 | 176.5 | 164.1 |
| ≥85 | 150.0 | 158.0 | 167.0 | 176.0 | 181.0 | 189.9 | 196.5 | 178.4 | 187.9 | 194.8 | 200.1 | 194.6 |
| Sex | ||||||||||||
| Female | 152.3 | 150.0 | 155.1 | 162.1 | 167.8 | 177.2 | 174.9 | 161.8 | 165.1 | 168.7 | 167.3 | 159.6 |
| Male | 166.6 | 168.7 | 173.2 | 176.9 | 182.0 | 189.0 | 183.7 | 174.7 | 177.3 | 182.7 | 180.8 | 170.5 |
| Race | ||||||||||||
| Black | 204.7 | 195.7 | 208.3 | 204.1 | 197.6 | 219.7 | 200.7 | 188.8 | 192.4 | 195.3 | 193.8 | 182.3 |
| White | 152.0 | 152.4 | 155.7 | 162.9 | 169.5 | 176.6 | 174.8 | 163.7 | 166.5 | 171.3 | 169.1 | 161.5 |
| Other | 159.1 | 151.7 | 193.3 | 171.3 | 197.4 | 182.6 | 194.7 | 177.1 | 188.1 | 184.0 | 199.3 | 166.6 |
| Adjusted 30-day mortality rate (%)* | ||||||||||||
| 65–74 | 12.4 | 12.3 | 13.2 | 11.4 | 12.7 | 12.4 | 11.5 | 12.0 | 10.9 | 11.1 | 11.6 | 9.6 |
| 75–84 | 13.3 | 13.5 | 13.0 | 13.1 | 13.1 | 13.9 | 12.1 | 11.4 | 11.5 | 11.8 | 12.5 | 11.7 |
| ≥85 | 15.8 | 14.9 | 14.2 | 14.4 | 15.3 | 14.9 | 13.9 | 11.8 | 12.3 | 13.5 | 11.8 | 12.8 |
| Sex | ||||||||||||
| Female | 12.3 | 12.5 | 11.8 | 11.4 | 12.2 | 12.9 | 11.4 | 10.5 | 10.5 | 11.1 | 11.2 | 10.1 |
| Male | 15.1 | 14.6 | 15.7 | 14.7 | 15.1 | 14.3 | 13.5 | 13.5 | 12.9 | 13.1 | 13.0 | 12.9 |
| Race | ||||||||||||
| Black | 14.2 | 14.9 | 11.6 | 13.5 | 13.1 | 13.7 | 12.5 | 11.4 | 10.3 | 10.8 | 10.6 | 11.1 |
| White | 13.2 | 13.2 | 13.7 | 12.6 | 13.4 | 13.4 | 12.3 | 11.7 | 11.7 | 12.0 | 12.2 | 11.1 |
| Other | 18.0 | 11.6 | 11.5 | 11.5 | 12.7 | 15.1 | 9.2 | 11.3 | 9.5 | 10.6 | 11.6 | 11.3 |
| Adjusted 1-year mortality rate (%)* | ||||||||||||
| 65–74 | 32.2 | 33.5 | 34.1 | 31.5 | 31.3 | 32.6 | 31.1 | 29.9 | 28.5 | 29.9 | 28.8 | 29.0 |
| 75–84 | 32.7 | 33.4 | 34.0 | 34.8 | 33.4 | 34.0 | 31.4 | 32.0 | 32.3 | 32.5 | 32.0 | 30.7 |
| ≥85 | 37.7 | 35.8 | 36.7 | 36.0 | 37.4 | 37.0 | 36.3 | 33.2 | 35.8 | 37.9 | 35.0 | 33.7 |
| Sex | ||||||||||||
| Female | 30.5 | 32.2 | 32.4 | 32.4 | 32.0 | 32.6 | 30.9 | 29.4 | 29.9 | 31.5 | 30.3 | 29.0 |
| Male | 37.9 | 36.7 | 38.1 | 36.0 | 35.5 | 36.2 | 33.9 | 34.4 | 33.9 | 34.7 | 33.4 | 33.4 |
| Race | ||||||||||||
| Black | 34.4 | 34.2 | 34.8 | 37.2 | 34.6 | 34.4 | 34.6 | 31.8 | 31.6 | 34.4 | 32.8 | 31.1 |
| White | 33.1 | 34.2 | 34.9 | 33.1 | 32.9 | 33.8 | 31.7 | 31.3 | 31.5 | 32.5 | 31.3 | 30.6 |
| Other | 36.9 | 24.3 | 26.9 | 33.7 | 37.6 | 34.7 | 29.9 | 34.2 | 31.9 | 31.0 | 33.5 | 29.6 |
Adjusted for demographics and comorbidities (reference: 1999).
IVCF: inferior vena caval filter, PE: pulmonary embolism.
Regional Variation
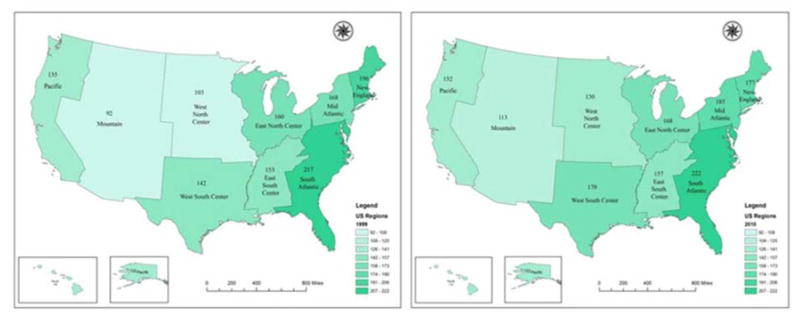
The utilization of IVCFs varied across regions and states. From 1999 to 2010, IVCF use was consistently highest in the South Atlantic region (from 217 to 222 per 1,000 patients with PE) and lowest in the Mountain region (from 92 to 113 per 1,000 patients with PE). While the utilization rates increased in the West North Central and Mid-Atlantic regions over time, New England was the only region wherein the rates of IVCF placement declined throughout the study period (P <0.001 for all comparisons, Figure 2).
Figure 2. Rates of Inferior Vena Caval Filter Placement Across 9 U.S. Regions in 1999 and 2010.
Note the changes in 2010 compared with 1999, including the decline in the New England and the increase in most other regions. Rates are reported per 1,000 patients with PE. South Atlantic: Delaware, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, Washington D.C., and West Virginia. New England: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont. Mid Atlantic: New Jersey, New York, and Pennsylvania. East North Center: Illinois, Indiana, Michigan, Ohio, and Wisconsin. East South Center: Alabama, Kentucky, Mississippi, and Tennessee. West South Center: Arkansas, Louisiana, Oklahoma, and Texas. Pacific: Alaska, California, Hawaii, Oregon, and Washington. West North Center: Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota and South Dakota. Mountains: Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, and Wyoming
Discussion
Among Medicare FFS beneficiaries, >15% of patients with PE received an IVCF in each year from 1999 to 2010. In the context of a significant 71% relative increase in hospitalizations with PE, we observed a significant 78% relative increase in the number of IVCFs during the study period. Collectively, these 2 translated into a modestly increased IVCF use rate per 1,000 hospitalizations with PE which was not statistically significant. In the setting of increasing PE hospitalizations and associated decline in mortality rates of the entire cohort of patients with PE (including those who did not receive IVCFs), we observed an increase in utilization of IVCFs that coincided with a gradual decline in overall mortality rates, including among the patients with PE that underwent IVCF placement (Central Illustration; Figure 1). The trends were consistent across various age, sex, and race subgroups, and across different regions. The rates of IVCF placement were consistently higher among blacks and the oldest patients (≥85 years). Wide regional variation in IVCF use persisted from 1999 to 2010.
Central Illustration.
IVC Filters in Older Adults with Pulmonary Embolism: Mortality and Hospitalization Rates from 1999 to 2010.
Our results are consistent with several theories that could explain the increasing utilization of IVCFs and decreased mortality rates in the cohort with PE, including those receiving IVCFs, over time. In addition to recent technological advancements such as availability of retrievable IVCFs that made them more palatable for referring physicians (18), the increased use of IVCFs may reflect increased PE diagnoses (22). Such temporal increase in the pool of patients with PE could, in turn, explain the increase in number of IVCFs placed over time. With regard to decreased mortality rates over time, it is possible that IVCFs are effective in preventing fatal PE, and their increased use over time averted some fatal PEs. This would be in line with our observation that the most notable change has occurred in short-term mortality rates. Observational studies by Stein et al. (10,23,24) with limited adjustments (8,9) showed lower inhospital mortality rates among elderly patients with PE who received an IVCF compared with those who did not. Likewise, in a recent propensity-matched analysis from a large registry of patients with venous thromboembolism, use of IVCFs was associated with a non-significant reduction in all-cause death among patients at high risk of bleeding (25). However, our study was not designed for comparative effectiveness. Further, as discussed before, mortality rates similarly declined among patients that did not receive an IVCF. Another explanation for decreased mortality rates over time could be that procedure-related mortality for IVCF placement may have declined due to improvements in technique, equipment, and operator proficiency (18). It is also possible that improved utilization rates of routine anticoagulants have occurred over time and have contributed to reduced mortality rates over time, although we did not have access to data to investigate such patterns.
Alternatively, the constellation of increasing number of hospitalizations with PE in the Medicare FFS population, increasing IVCF utilization, and reduced mortality rates, including among those that did not receive IVCFs from 1999 to 2010, may indicate inclusion of potentially less sick patients in the PE cohort over time, as well as more permissive use of IVCF placement among potentially less sick patients with PE. More frequent diagnosis of PE among potentially less sick patients, in an era of more sensitive diagnostic tools for PE, may help explain lower mortality rates in the entire cohort of hospitalized patients with PE (26). Further, more widespread use of IVCFs among less critically-ill patients might have limited impact on fatalities, but show reduced mortality rates among recipients of IVCFs, reflected by a dilution effect. This would be also in line with the findings of the only three available randomized trials of IVCF placement, which did not show a mortality benefit with use of IVCFs (1–4). Finally, it is possible that the observed trends in IVCF utilization and outcomes are multifactorial, with contribution from each of the above explanations.
Our results are consistent with a recent study that showed marked variations in IVCF utilization across the US states, which was not entirely explained by differences in rates of DVT or PE hospitalizations (27). Similarly, a study in 263 California hospitals showed high hospital-level variation in utilization of IVCFs within one state (28). While the uncertainty around risks and benefits of IVCF utilization may play a role, other reasons behind such markedly different practice patterns, as well as changes in observed trends (such as increase in IVCF utilization rates in West North Central and Mid-Atlantic regions but a decline New England) need further investigation.
We provide a contemporary national perspective about IVCF utilization among older adults with PE, as well as short-term and long-term outcome data. Our study, however, has several limitations. First, we only studied Medicare FFS beneficiaries, and findings might not be generalizable to the uninsured, Medicare Advantage patients, or younger patients with PE. Second, interpretations related to appropriateness of IVCF placement are limited. Although we did not have access to coexisting therapies, or indications for treatment, such as contraindications to antithrombotic therapy or recurrence of PE despite adequate anticoagulation, some studies suggest that IVCFs have been used more frequently than guidelines recommendations (29). Likewise, we did not have access to information on patient preferences regarding IVCF placement. Finally, although the comorbidities were relatively stable over time and adjusted using our mixed-effects models, we did not have access to reliable metrics for determining PE disease severity.
Conclusions
In this study of Medicare FFS beneficiaries between 1999 and 2010, we demonstrated frequent and increasing use of IVCFs in patients with PE over time. This occurred in the context of increasing PE hospitalizations and declining mortality rates in all patients with PE, including those who did and did not receive IVCFs. Collectively, our results suggest that more permissive use of technology has occurred over time in the setting of persistent controversy for net benefit. The optimal use of this technology remains uncertain. Further investigations should identify the subgroups that will benefit most from this procedure.
Perspectives.
Competency in Medical Knowledge
Use of inferior vena cava (IVC) filters has increased among Medicare fee-for-service beneficiaries with pulmonary thromboembolism (PE), but mortality rates have declined in patients with PE with and without IVC filters.
Translational Outlook
Further studies are required to define patient subgroups that gain the most benefit from IVC filters.
Acknowledgments
The authors would like to thank Abraham Kaleo Parrish from Yale Map Department for his help for preparation of Figure 2.
Funding Source: This study was supported by grant number U01HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsor or of CMS. The funding sources did not have a role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or the preparation and approval of the manuscript.
Abbreviations
- CMS
Centers for Medicare & Medicaid Services
- DVT
Deep vein thrombosis
- FFS
Fee-For-Service
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IVCF
Inferior vena caval filter
- PE
Pulmonary embolism
Footnotes
Disclosures: Krumholz is a recipient of research grants from Medtronic and from Johnson & Johnson, through Yale University, and is chair of a cardiac scientific advisory board for UnitedHealth. Spertus and Nallamothu are members of cardiac scientific advisory board for UnitedHealth. The other authors do not report any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338:409–15. doi: 10.1056/NEJM199802123380701. [DOI] [PubMed] [Google Scholar]
- 2.Group PS. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112:416–22. doi: 10.1161/CIRCULATIONAHA.104.512834. [DOI] [PubMed] [Google Scholar]
- 3.Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA. 2015;313:1627–35. doi: 10.1001/jama.2015.3780. [DOI] [PubMed] [Google Scholar]
- 4.Barginear MF, Gralla RJ, Bradley TP, et al. Investigating the benefit of adding a vena cava filter to anticoagulation with fondaparinux sodium in patients with cancer and venous thromboembolism in a prospective randomized clinical trial. Support Care Cancer. 2012;20:2865–72. doi: 10.1007/s00520-012-1413-z. [DOI] [PubMed] [Google Scholar]
- 5.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 6.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–69. 3069a–3069k. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 8.Girard P, Meyer G, Mismetti P. The pseudo-effect of vena cava filters. Am J Med. 2014;127:e21. doi: 10.1016/j.amjmed.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 9.Prasad V. Observational studies cannot justify the inferior vena cava filter. Am J Med. 2014;127:e15. doi: 10.1016/j.amjmed.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Stein PD, Matta F. Vena cava filters in unstable elderly patients with acute pulmonary embolism. Am J Med. 2014;127:222–5. doi: 10.1016/j.amjmed.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Bikdeli B. Therapies for venous thromboembolism. JAMA. 2014;311:2543. doi: 10.1001/jama.2014.6114. [DOI] [PubMed] [Google Scholar]
- 12.Berman AR, Arnsten JH. Diagnosis and treatment of pulmonary embolism in the elderly. Clin Geriatr Med. 2003;19:157–75. viii. doi: 10.1016/s0749-0690(02)00055-1. [DOI] [PubMed] [Google Scholar]
- 13.Spencer FA, Gore JM, Lessard D, et al. Venous thromboembolism in the elderly. A community-based perspective. Thromb Haemost. 2008;100:780–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Piazza G, Goldhaber SZ, Kroll A, et al. Venous thromboembolism in patients with chronic obstructive pulmonary disease. Am J Med. 2012;125:1010–8. doi: 10.1016/j.amjmed.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–9. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 16.Krumholz HM, Pasternak RC, Weinstein MC, et al. Cost effectiveness of thrombolytic therapy with streptokinase in elderly patients with suspected acute myocardial infarction. N Engl J Med. 1992;327:7–13. doi: 10.1056/NEJM199207023270102. [DOI] [PubMed] [Google Scholar]
- 17.Punukollu H, Khan IA, Punukollu G, Gowda RM, Mendoza C, Sacchi TJ. Acute pulmonary embolism in elderly: clinical characteristics and outcome. Int J Cardiol. 2005;99:213–6. doi: 10.1016/j.ijcard.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Kinney TB. Inferior vena cava filters. Semin Intervent Radiol. 2006;23:230–9. doi: 10.1055/s-2006-948760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 20.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–92. doi: 10.1161/CIRCULATIONAHA.105.611186. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 22.Minges KE, Bikdeli B, Wang Y, et al. National Trends in Pulmonary Embolism Hospitalization Rates and Outcomes for Adults Aged >/=65 Years in the United States (1999 to 2010) Am J Cardiol. 2015;116:1436–42. doi: 10.1016/j.amjcard.2015.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein PD, Matta F, Hull RD. Increasing use of vena cava filters for prevention of pulmonary embolism. Am J Med. 2011;124:655–61. doi: 10.1016/j.amjmed.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med. 2012;125:478–84. doi: 10.1016/j.amjmed.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Muriel A, Jimenez D, Aujesky D, et al. Survival effects of inferior vena cava filter in patients with acute symptomatic venous thromboembolism and a significant bleeding risk. J Am Coll Cardiol. 2014;63:1675–83. doi: 10.1016/j.jacc.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 26.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831–7. doi: 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meltzer AJ, Graham A, Kim JH, et al. Clinical, demographic, and medicolegal factors associated with geographic variation in inferior vena cava filter utilization: an interstate analysis. Surgery. 2013;153:683–8. doi: 10.1016/j.surg.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 28.White RH, Geraghty EM, Brunson A, et al. High variation between hospitals in vena cava filter use for venous thromboembolism. JAMA Intern Med. 2013;173:506–12. doi: 10.1001/jamainternmed.2013.2352. [DOI] [PubMed] [Google Scholar]
- 29.Spencer FA, Bates SM, Goldberg RJ, et al. A population-based study of inferior vena cava filters in patients with acute venous thromboembolism. Arch Intern Med. 2010;170:1456–62. doi: 10.1001/archinternmed.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]