Abstract
Objectives
We sought to determine hospital patterns of change in use of nesiritide over a 6-year period following publications of safety concerns in 2005, and to identify hospital characteristics associated with these patterns.
Background
The changing nature of medical evidence often requires a change in practice. Nesiritide was commercialized in 2001 for early relief of dyspnea in patients with decompensated heart failure. In 2005 concerns about its safety led to recommendations to restrict its use. Little is known about how hospitals responded to this information.
Methods
We analyzed data from the Premier database including 403 hospitals contributing 813,783 hospitalizations with heart failure, spanning 2005–2010. We applied a growth mixture modeling approach to hospital-level, risk-standardized, quarterly utilization rates of nesiritide to distinguish hospital groups based on their patterns of change in utilization.
Results
Proportion of hospitalizations using nesiritide declined from 15.4% in 2005 to 1.2% in 2010. The level and speed of change varied markedly among hospitals. After adjusting for differences in patient characteristics across hospitals and years, we identified three distinct groups of hospitals: “low utilizers”, “fast de-adopters”, and “slow de-adopters”. In multivariate regression analysis, these groups did not differ in traditional hospital characteristics such as size, urban setting, or teaching status.
Conclusions
We identified three distinct hospital groups characterized by their patterns of change in nesiritide utilization. These trajectory curves can provide hospitals with an important feedback on how fast and effectively they react to new information compared with other hospitals. Uncovering factors that promote organizational learning requires further research.
Keywords: heart failure, drug utilization, hospital, practice patterns, response to new evidence, organizational learning
The changing nature of medical evidence often requires a change in practice. Studies have described the challenges of translating new information into practice which may take decades, as it did with the beta-Blocker Heart Attack Trial (BHAT).(1) No studies, to our knowledge, have evaluated longitudinal patterns of change in practice at the hospital level. Nesiritide (Natrecor®) provides a good case study of how hospitals changed practices in response to new information. Nesiritide was approved by the Food and Drug Administration (FDA) in 2001 for early relief of dyspnea in patients with acutely decompensated heart failure, but once on the market, it was widely prescribed and used beyond its original indication.(2) In spring 2005, two meta-analyses of small randomized trials raised concerns regarding renal toxicity(3) and higher mortality associated with nesiritide.(4) These publications resulted in an FDA-mandated revision of prescribing information in the “Adverse Reactions/Effects on Mortality” section. A panel of experts recommended in June 2005 that nesiritide be used only in patients with acutely decompensated heart failure who had dyspnea at rest and not to be used for improvement of renal function, enhancement of diuresis, intermittent outpatient infusion, or scheduled repetitive use.(5) To physicians planning the use of nesiritide to relieve symptoms, the panel recommended considering the use of alternative therapies. In 2011, the results of a large randomized trial, i.e., the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF), showed that nesiritide had no effect on dyspnea, renal function, mortality or readmission; but was associated with increased rates of hypotension, and it was concluded that nesiritide could not be recommended for routine use in patients with acute heart failure.(6)
Prior work by Hauptman and collaborators has shown that between March and December 2005, the overall use of nesiritide decreased by 66% (from 16.6% to 5.6%).(7) Their study focused on overall change in utilization immediately before and after the publications of safety concerns. Our current study was designed to extend prior work by evaluating the patterns of change among hospitals between 2005 and 2010. We hypothesized that amid a continuing general decrease in nesiritide use, there would be marked heterogeneity in level and speed of de-adoption across hospitals, revealing various institutional responses to new information. We also sought to determine what hospital characteristics would be associated with these distinct hospital groups.
METHODS
Data Source
We used data from a voluntary, fee-supported database developed by Premier, Inc. Charlotte, NC, for measuring quality and health care utilization. Containing over 330 million discharges from 620 geographically diverse hospitals, the database represents one in every five discharges from U.S. hospitals. In addition to the information available in the standard hospital discharge file, the Premier database contains a date-stamped log of all billed items at the individual patient level including medications and laboratory, diagnostic, and therapeutic services. We used data from calendar years 2005–2010 for our analysis.
Patient data are de-identified in accordance with the Health Insurance Portability and Accountability Act and a random hospital identifier assigned by Premier is used to identify individual hospitals. The Yale University Human Investigation Committee determined that this study is not considered to be Human Subjects Research as defined by the Office of Human Research Protections.
Heart Failure cohort
We included in the study cohort, all hospitalizations from January 1, 2005 to December 31, 2010 with a principal diagnosis of heart failure as defined by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx or a principal diagnosis of respiratory failure (ICD-9-CM code 518.81) with a secondary diagnosis of congestive heart failure (ICD-9-CM code 428.0). We excluded patients who were less than 18 years of age at the time of admission or those whose physicians were pediatricians, since our focus was not on congenital disease. A patient could contribute more than one hospitalization to the study cohort.
Patient and hospital characteristics
Patient characteristics available in our dataset included age, sex, race/ethnicity, insurance status, and comorbidities. We used the Healthcare Costs and Utilization Project software provided by the Agency for Healthcare Research and Quality (AHRQ) to classify comorbidities from the standard hospital discharge file based on methods described by Elixhauser and Steiner.(8)
For each hospital, Premier database contains information, collected from the American Hospital Association database, on bed count, teaching status, geographic location, and whether it serves an urban or rural population. In addition, we derived the following measures about each hospital’s characteristics by pooling its patient-level hospitalization data across 2005–2010: average number of HF hospitalizations each year, proportion of the attending physicians being a cardiologist, proportion of patients with Medicaid as the primary payer, whether the hospital had any cardiology intensive care unit, and capability of performing a number of procedures including ventricular assist device (VAD) or heart transplant, percutaneous coronary intervention (PCI), and implantable cardioverter defibrillator (ICD).
Statistical Analysis
Descriptive statistics (frequencies and percentages) were calculated to assess sample characteristics and drug use. We assessed the proportion of nesiritide use at hospitalization-level (denominator being all hospitalizations with HF across all hospitals) and compared it with the use of potential alternative therapies including other vasodilators (intravenous (IV) nitroglycerin and sodium nitroprusside), and positive inotropic agents (dobutamine, dopamine, and milrinone).
We also assessed nesiritide use at hospital-level (denominator being all hospitalizations with HF in a given hospital). Hierarchical generalized linear modeling (HGLM) was used to calculate hospital-level risk-standardized utilization rates of nesiritide.(9) The model included patient demographic characteristics (age groups, sex, race / ethnicity), comorbidities, and a hospital random effect for each calendar quarter. This model specification takes into account within hospital correlation of utilization patterns while adjusting for differences in case mix both across hospitals and over time. The full list of risk-variables included in the HGLM models with their estimated odds ratios and corresponding 95% confidence intervals are reported in Appendix Table
Appendix Table.
Fixed effects estimates of the hierarchical logistic regression model used for calculating hospital risk-standardized utilization rates of nesiritide
| Variables | OR (95% CI) | p-value |
|---|---|---|
| Time points (quarter) | ||
| 1 | 21.1 (16.4–27.1) | <.0001 |
| 2 | 15.4 (11.9–19.8) | <.0001 |
| 3 | 8.7 (6.7–11.3) | <.0001 |
| 4 | 6.3 (4.8–8.1) | <.0001 |
| 5 | 4.8 (3.7–6.2) | <.0001 |
| 6 | 4.6 (3.5–5.9) | <.0001 |
| 7 | 3.8 (2.9–4.9) | <.0001 |
| 8 | 3.6 (2.7–4.6) | <.0001 |
| 9 | 3.3 (2.6–4.3) | <.0001 |
| 10 | 3.5 (2.7–4.6) | <.0001 |
| 11 | 3.2 (2.5–4.2) | <.0001 |
| 12 | 3.1 (2.4–4.1) | <.0001 |
| 13 | 2.6 (2.0–3.4) | <.0001 |
| 14 | 2.6 (2.0–3.4) | <.0001 |
| 15 | 2.5 (1.9–3.2) | <.0001 |
| 16 | 2.4 (1.8–3.1) | <.0001 |
| 17 | 1.9 (1.5–2.5) | <.0001 |
| 18 | 1.8 (1.4–2.4) | <.0001 |
| 19 | 1.6 (1.2–2.2) | 0.0006 |
| 20 | 1.4 (1.1–1.9) | 0.01 |
| 21 | 1.3 (1.0–1.8) | 0.0424 |
| 22 | 1.3 (1.0–1.7) | 0.101 |
| 23 | 1.0 (0.8–1.4) | 0.7969 |
| 24 | 1.0 (.–.) | . |
| Age Group | ||
| 18 – 24 | 2.1 (1.6–2.6) | <.0001 |
| 25 – 34 | 1.5 (1.3–1.7) | <.0001 |
| 35 – 44 | 1.4 (1.3–1.5) | <.0001 |
| 45 – 54 | 1.3 (1.2–1.3) | <.0001 |
| 55 – 64 | 1.3 (1.2–1.3) | <.0001 |
| 65 – 74 | 1.2 (1.1–1.2) | <.0001 |
| 75 – 99 | 1.0 (.–.) | . |
| Gender | ||
| Female | 0.8 (0.8–0.8) | <.0001 |
| Male | 1.0 (.–.) | . |
| Race | ||
| White | 1.1 (1.1–1.2) | 0.0001 |
| Black | 1.2 (1.2–1.3) | <.0001 |
| Hispanic | 1.1 (1.0–1.2) | 0.1077 |
| Other | 1.0 (.–.) | . |
| Elixhauser Comorbidity | ||
| Valvular disease | 1.2 (1.1–1.3) | 0.0002 |
| Pulmonary circulation disease | 0.8 (0.8–0.9) | 0.0005 |
| Peripheral vascular disease | 1.0 (0.9–1.1) | 0.3963 |
| Hypertension | 0.9 (0.8–0.9) | <.0001 |
| Paralysis | 0.8 (0.7–0.9) | <.0001 |
| Other neurological disorders | 0.8 (0.7–0.8) | <.0001 |
| Chronic pulmonary disease | 0.9 (0.9–1.0) | <.0001 |
| Diabetes w/o chronic complications | 1.2 (1.1–1.2) | <.0001 |
| Diabetes w/chronic complications | 1.2 (1.2–1.3) | <.0001 |
| Hypothyroidism | 1.1 (1.0–1.1) | 0.001 |
| Renal failure | 1.4 (1.3–1.4) | <.0001 |
| Liver disease | 1.0 (0.9–1.1) | 0.9721 |
| Peptic ulcer Disease x bleeding | 0.7 (0.4–1.4) | 0.361 |
| Acquired immune deficiency syndrome | 0.6 (0.4–0.8) | 0.0002 |
| Lymphoma | 0.9 (0.8–1.1) | 0.3999 |
| Metastatic cancer | 0.7 (0.6–0.8) | <.0001 |
| Solid tumor w/out metastasis | 0.8 (0.7–0.9) | <.0001 |
| Rheumatoid arthritis/collagen vas | 0.9 (0.8–1.0) | 0.0095 |
| Coagulopthy | 1.2 (1.2–1.3) | <.0001 |
| Obesity | 1.1 (1.0–1.1) | <.0001 |
| Weight loss | 1.2 (1.1–1.3) | <.0001 |
| Fluid and electrolyte disorders | 1.4 (1.3–1.4) | <.0001 |
| Chronic blood loss anemia | 0.9 (0.8–1.0) | 0.0686 |
| Deficiency Anemias | 1.0 (1.0–1.1) | 0.0256 |
| Alcohol abuse | 1.1 (1.0–1.1) | 0.1291 |
| Drug abuse | 1.0 (0.9–1.1) | 0.6205 |
| Psychoses | 0.8 (0.8–0.9) | <.0001 |
| Depression | 0.9 (0.8–0.9) | <.0001 |
| Other AHRQ Comorbidity | ||
| Disorders of lipid metabolism | 1.0 (1.0–1.1) | 0.0003 |
| Coronary atherosclerosis and other heart disease | 1.4 (1.4–1.5) | <.0001 |
| Acute myocardial infarction | 1.4 (1.3–1.5) | <.0001 |
| Peripheral and visceral atherosclerosis | 1.1 (1.0–1.3) | 0.0307 |
| Aortic; peripheral; and visceral artery aneurysms | 1.1 (1.0–1.2) | 0.1274 |
| Aortic and peripheral arterial embolism or thrombosis | 1.1 (0.9–1.4) | 0.254 |
| Transient cerebral ischemia | 0.8 (0.6–1.0) | 0.0568 |
| Cardiac dysrhythmias | 1.3 (1.3–1.3) | <.0001 |
| Cardiac arrest and ventricular fibrillation | 1.1 (1.0–1.3) | 0.0023 |
We applied a growth mixture modeling approach to hospital risk-standardized utilization rates via a SAS macro Proc Traj.(10) This approach assumes there are clusters or groupings of distinctive patterns of change in a population.(11) All hospitals that contributed HF hospitalizations in at least one calendar quarter were included in the analysis. Models with different number of trajectory groups were estimated and the optimal number of distinct trajectory groups was determined by comparing the Bayesian Information Criteria (BIC) index across these models. Our final analysis used a three-group model which had the most favorable BIC index. Each hospital was assigned to a trajectory group based on the estimated posterior probability of its group membership (i.e., following a maximum posterior probability assignment rule).(11)
Chi-square tests and Kruskal-Wallis tests were used to assess whether there were any significant associations between individual hospital characteristics and identified trajectory groups. Multivariate multinomial logistic regression analysis was also performed to examine the association between hospital characteristics and trajectory group membership. Stepwise selection algorithm was used to choose the variables included in the final multivariate model. Estimates with P<0.05 were considered statistically significant.
Analyses were conducted with SAS version 9.2 (SAS Institute Inc., Cary, NC), and figures were created with R version 2.11.1.(12)
RESULTS
Use of nesiritide at hospitalization-level
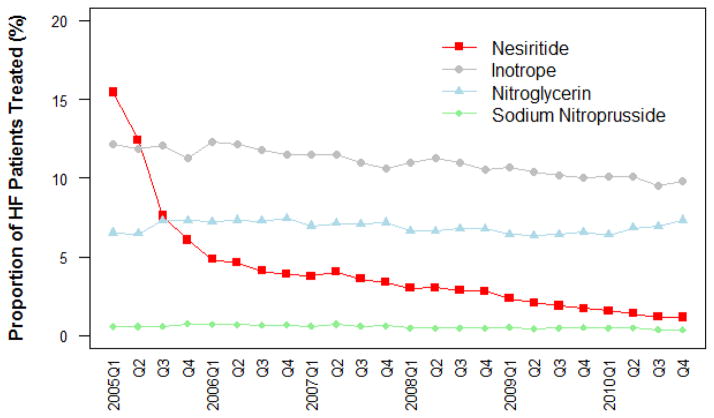
Between 2005 and 2010, there were 813,783 hospitalizations with heart failure. Among these hospitalizations, the proportion using nesiritide decreased from 15.4% (5508/35,769) in the first quarter of 2005 to 1.2% (429/35,872) in the last quarter of 2010 (Figure 1). The sharpest drop in use occurred between second and third quarters of 2005 when the odd ratio (95% CI) for being treated by nesiritide (compared with the last quarter of 2010) dropped from 15.4 (11.9–19.8) to 8.7 (6.7–11.3) Appendix Table. Over the same period, the proportion of hospitalizations including IV nitroglycerin remained stable between 6% and 8%: 6.5% (2,325/35,769) in the first quarter of 2005, and 7.3% (2,612/35,872) in last quarter of 2010. The proportion of hospitalizations using sodium nitroprusside was less than 1% throughout the 6-year period: 0.6% (208/35,769) in the first quarter of 2005 and 0.4% (136/35,872) at the last quarter of 2010. The proportion of hospitalizations with a positive inotropic agent was 12.1% (4,328/35,769) in the first quarter of 2005, 12.3% (4,787/38,978) in the first quarter of 2006, and then decreased progressively to 9.8% (3,516/35,872) in the last quarter of 2010. (Figure 1)
Figure 1. Trends in use of IV vasodilators and inotropes among patients hospitalized with heart failure.
The figure shows the proportion of hospitalizations including an IV vasodilator (nesiritide, nitroglycerin, sodium nitroprusside) or a positive inotropic agent among all heart failure hospitalizations between the first quarter of 2005 and the last quarter of 2010.
Use of nesiritide at hospital-level
Between 2005 and 2010, a total of 403 hospitals contributed data on heart failure patients to the database. These were mainly urban, non-teaching, small and medium size hospitals. Key characteristics of these hospitals are summarized in Table 1.
Table 1.
Sample characteristics
| Hospitals N (%) |
Hospitalizations N (%) |
|
|---|---|---|
| Total | 403* | 813,783 |
| Number of beds | ||
| <200 | 146 (36) | 120,033 (15) |
| 200 – 400 | 155 (38) | 303,521 (37) |
| > 400 | 100 (25) | 390,229 (48) |
| Teaching status | ||
| NO | 292 (73) | 491,719 (60) |
| YES | 109 (27) | 321,500 (40) |
| Region | ||
| Midwest | 88 (22) | 166,989 (21) |
| Northeast | 63 (16) | 169,392 (21) |
| South | 169 (42) | 365,294 (45) |
| West | 81 (20) | 111,544 (14) |
| Population served | ||
| Rural | 86 (21) | 89,642 (11) |
| Urban | 315 (79) | 723,577 (89) |
| Average annual HF volume | ||
| < 25 | 9 (2) | 336 (0) |
| 26 – 200 | 128 (32) | 67,638 (8) |
| 201 – 500 | 152 (38) | 261,071 (32) |
| 501 – 1000 | 91 (23) | 330,976 (41) |
| 1001 – 1500 | 23 (6) | 153,762 (19) |
: 2 hospitals were missing general characteristics including number of beds, teaching status, area served, and geographic location.
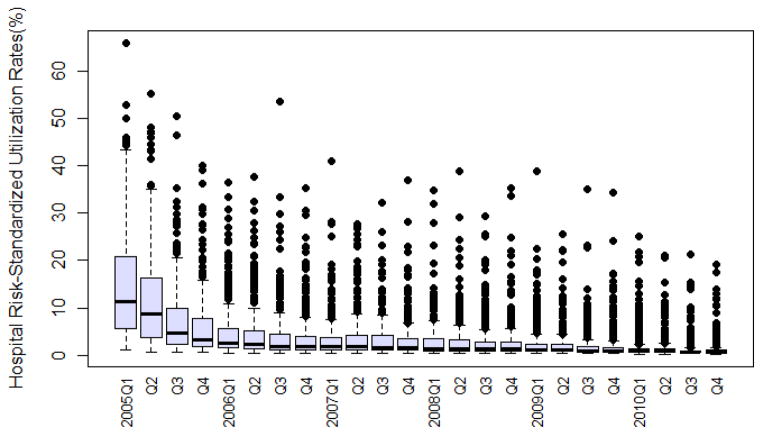
There was a wide variation across hospitals in the proportion of HF patients treated with nesiritide. In the first quarter of 2005, the risk-standardized rates ranged from a minimum of 1.0% to a maximum of 65.9% (median: 11.4%, IQR: 5.6%–20.8%). In the last quarter of 2010, the adjusted rates ranged from a minimum of 0.3% to a maximum of 19.2% (median: 0.7%, IQR: 0.5%–1.0%). (Figure 2)
Figure 2. Distributions of hospital risk-standardized rates of nesiritide utilization.
The figure shows the distribution across hospitals of nesiritide risk-standardized utilization rates for each quarter of calendar year from 2005 through 2010.
Hospital groups based on patterns of change in nesiritide use
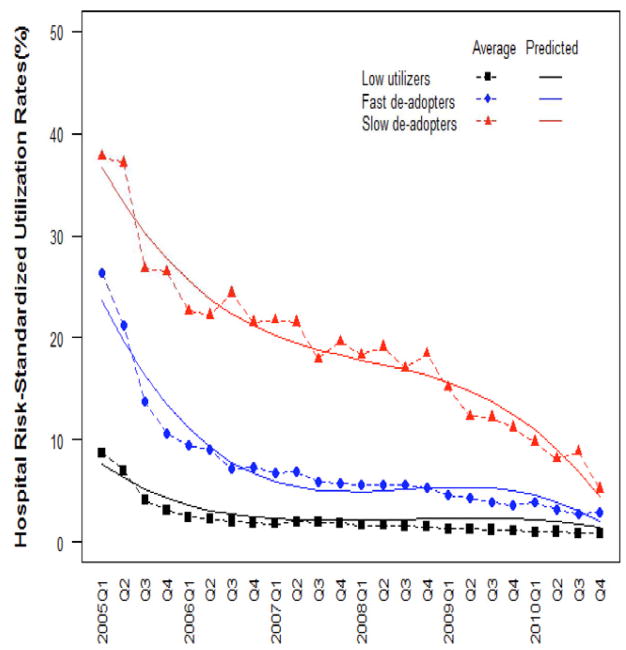
Application of the growth mixture modeling to hospital risk-standardized utilization rates led to the emergence of three distinct groups of hospitals based on their patterns of change in utilization over time: “low utilizers”, “fast de-adopters” and “slow de-adopters” (Figure 3). The approach took into account both level and speed of change in utilization over the entire 6-year period, however for the sake of simplicity, only the most dominant attribute was used to name the groups. The “low-utilizer” group included 302 hospitals (75% of hospitals, together accounting for 69% of all hospitalizations) with an average risk-standardized rate of 9% in the first quarter of 2005 which decreased to almost 2% at the beginning of 2006 and plateaued at around 1% from 2009. The “fast de-adopter” group included 82 hospitals (20% of hospitals, together accounting for 25% of all hospitalizations) with an average initial risk-standardized utilization rate of 26% that decreased to 10% at the beginning of 2006, 5% at 2009, and 3% at the end of 2010. The remaining 19 hospitals (5% of hospitals, together encompassing 6% of all hospitalizations) were classified as the “slow de-adopters”. They had the highest initial risk-standardized utilization rates and a slower rate of decrease in use over time than the other hospitals. They started with an average utilization rate of 38% which decreased to 26% at the end of 2005, then 20% at 2007 and were still at 10% at the beginning of 2010 (Figure 3).
Figure 3. Distinct groups of hospitals based on their patterns of change in nesiritide use over the 6-year period 2005–2010.
Three distinct groups of hospitals were identified. The figure shows the average and predicted group trajectories using hospital-level risk-standardized rates of nesiritide utilization between the first quarter of 2005 and the last quarter of 2010.
The average posterior probability of group membership was greater than 0.98 for each of the groups indicating excellent performance of the model in distinguishing the different trajectory patterns.
Association between hospital characteristics and distinct hospital groups
We investigated what hospital characteristics were associated with different nesiritide de-adoption trajectory groups. Table 2 shows the hospital characteristics by trajectory group. The three groups differed significantly in hospital size, annual volume of heart failure hospitalizations, regional location, PCI and ICD capability, proportion of cardiologist as attending physician, and proportion of Medicaid patients.(Table 2) However, in multivariate regression analysis, none of the hospitals characteristics differed significantly between the slow de-adopters group and the other two groups. The fast de-adopters were more likely to be located in the Midwest and the South, to have ICD capability and a higher proportion of Medicaid patients in comparison with low utilizers.(Table 3)
Table 2.
Hospital characteristics by nesiritide use trajectory groups
| Low Utilizers (N=302) | Fast De-adopters (N=82) | Slow De-adopters (N=19) | p-value | |
|---|---|---|---|---|
| Number of beds | ||||
| < 200 | 41.4 | 20.7 | 31.6 | 0.0048 |
| 200–400 | 37.4 | 43.9 | 31.6 | |
| > 400 | 21.2 | 35.4 | 36.8 | |
| Teaching status | ||||
| NO | 72.4 | 72 | 83.3 | 0.5886 |
| YES | 27.6 | 28.1 | 16.7 | |
| Region | ||||
| MIDWEST | 20.9 | 28.1 | 11.1 | <0.0001 |
| NORTHEAST | 18.3 | 7.3 | 11.1 | |
| SOUTH | 36.2 | 58.5 | 66.7 | |
| WEST | 24.6 | 6.1 | 11.1 | |
| Population served | ||||
| RURAL | 23.9 | 12.2 | 22.2 | 0.0719 |
| URBAN | 76.1 | 87.8 | 77.8 | |
| Heart failure volume | ||||
| < 25 | 3 | 0 | 0 | 0.0005 |
| 26 – 200 | 37.1 | 17.1 | 10.5 | |
| 201 – 500 | 33.8 | 48.8 | 52.6 | |
| 501 – 1000 | 20.2 | 31.7 | 21.1 | |
| 1001 – 1500 | 6 | 2.4 | 15.8 | |
| Procedure performed | ||||
| LVAD/Transplant | 8.6 | 10.8 | 15.8 | 0.5638 |
| PCI | 55 | 76.8 | 84.2 | 0.0002 |
| ICD | 61.9 | 86.6 | 84.2 | <0.0001 |
| Use of CCU | 47.4 | 48.8 | 63.2 | 0.4076 |
| Percent (%) of Medicaid patients, median (IQR) | 3.2 (1.6–6.0) | 4.5 (2.8–6.6) | 3.3 (2.1–6.6) | 0.0066 |
| Percent (%) of cardiologist as attending, median (IQR) | 7.7 (0.4–19.1) | 16.5 (6.1–29.6) | 17.5 (3.1–31.8) | 0.0075 |
Table 3.
Relationship between hospital characteristics and patterns of nesiritide use
| Hospital characteristics | Fast de-adopters vs. Low utilizers | Slow de-adopters vs. Low utilizers | Slow de-adopters vs. Fast de-adopters | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Midwest vs. West | 7.2 | 2.4–22.4 | 0.0005 | 1.4 | 0.2–19.6 | 0.7720 | 0.2 | 0.0–1.8 | 0.1465 |
| Northeast vs. West | 2.8 | 0.7–10.8 | 0.1246 | 1.9 | 0.2–15.6 | 0.5441 | 0.7 | 0.1–7.5 | 0.7462 |
| South vs. West | 9.5 | 3.3–27.4 | <0.0001 | 5.0 | 1.0–24.9 | 0.0518 | 0.5 | 0.1–3.4 | 0.4976 |
| Percent (%) of Medicaid patients1 | 2.2 | 1.3–3.8 | 0.0036 | 1.7 | 0.6–4.7 | 0.3164 | 0.8 | 0.3–2.2 | 0.6126 |
| ICD performed (yes vs. no) | 3.6 | 1.8–7.2 | 0.0003 | 3.1 | 0.9–11.0 | 0.0839 | 0.9 | 0.2–3.5 | 0.8298 |
:The unit for OR effect is 10%.
ICD: Implantable Cardioverter Defibrillator
DISCUSSION
In this study, we used data from a large network of hospitals to characterize longitudinal patterns of change in nesiritide use following publications raising concerns about its safety. The results showed a continued reduction in the use of this medication between 2005 and 2010, with an initial sharp decrease immediately after the publications followed by a more gradual decrease between 2006 and 2010. The overall average change however, obscures that there was marked variation in nesiritide utilization across hospitals. When taking into account both level and speed of change in utilization over the 6-year period, the hospital trajectories coalesced around some specific patterns leading to the emergence of 3 distinct groups of hospitals. Since the utilization rates already adjusted for differences in case mix across hospitals and across years, these 3 groups depict mainly the heterogeneity of organizational response to new information. These trajectory curves can provide crucial feedback to hospitals about how fast and effectively they react to new information in comparison with other hospitals.
We chose to use hospitals as our unit of analysis for several reasons. First, heart failure patients are usually seen by multiple physicians and it’s not always possible to identify the prescribing physician. Second, revealing variation at the hospital level rather than individual physician level is consistent with an emerging appreciation of team-based care, systems of care, and the impact of hospital internal environment on performance.(13–16) Third, medical decision making is influenced by various organizational characteristics such as team composition (number and type of specialists on the team, inclusion of a pharmacist), internal culture (quality and frequency of communication and collaboration between team members), regulatory context (drug formularies), availability and use of clinical decision support systems for the practice of evidence-based medicine. (13,17,18) However, one of the limitations of our study and the currently available healthcare databases in general is the lack of information on these characteristics.
Our results suggested that there may be common underlying factors among hospitals within each trajectory group. However, when we examined the association between hospital characteristics available in our database and various trajectory groups, none was significantly associated with a hospital’s likelihood of being in the slow de-adopter group compared with the other two groups. This could be due to small number of hospitals in this group, or to the data limitations (i.e., lack of measures reflecting team composition, communication, internal culture, regulations and restrictions). There is a need for further qualitative and mixed method research to identify additional factors, both internal to the organization and external. For example, one of the unmeasured factors that may explain the significant difference in regional location observed between hospital groups could be the prevalence of pharmaceutical marketing across regions.
Before its safety concerns were published in 2005, nesiritide was widely prescribed.(7,19) The proportion of HF hospitalizations using nesiritide almost doubled those with the main alternative vasodilator, IV nitroglycerin, despite the fact that nesiritide was only approved for very specific indication and was much more expensive. Following the publications, the rate of nesiritide use declined dramatically but we did not observe a “substitution” effect such as a sudden or substantial increase in use of other vasodilators, or of positive inotropic agents. These results could suggest a case of nesiritide overuse before spring 2005.
Our study further revealed that this initial, short-term strong response to new information was followed by a steady decrease in use over subsequent years although at a much more gradual level and speed. This pattern is consistent with what has been observed in many other studies of the adoption of innovations. Those studies have suggested that adoption decisions of organizations are a function of both internal factors as well as external and social factors, but the relative importance of these factors changes over time as information diffuses among potential adopters.(20–22)
There are several limitations to this study. First, hospitals included may not be a representative sample of all hospitals in the United States. Nevertheless, the Premier database contains approximately 20% of annual nationwide acute care hospitalizations. Second, a patient could contribute more than one hospitalization to the study cohort, introducing correlation in data between the multiple hospitalizations. However the impact was likely small since only 9% of patients had more than one hospitalization per quarter, of which the majority had only two hospitalizations (median: 2; IQR: 2–2). Third, our risk-adjustment model relied on claims data only. However, our earlier work of profiling hospital performance for the Centers for Medicare and Medicaid Services has demonstrated that administrative data can provide estimates similar to models employing richer clinical data.(23–24) Finally, as previously mentioned, we lack data on a number of characteristics that might have affected drug utilization such as formularies, other hospital restrictions, and marketing factors.
In conclusion, this study establishes that amid a general decrease in nesiritide use, there were important variations across hospitals revealing distinct hospital groups based on their patterns of change in practice in response to new information. These trajectory curves can provide hospitals with an important feedback on their “learning rates” or how fast and effectively they react to new information. The study also highlights the need for additional mixed-methods research to uncover the factors that foster or impede organizational learning.
Supplementary Material
Acknowledgments
Funding: This work was supported by grant DF10-301 from the Patrick and Catherine Weldon Donaghue Medical Research Foundation in West Hartford, Connecticut and by grant UL1 RR024139-06S1 from the National Center for Advancing Translational Sciences in Bethesda, Maryland. Dr. Krumholz is supported by grant U01 HL105270-02 (Center for Cardiovascular Outcomes Research at Yale University).
ABBREVIATIONS AND ACRONYMS
- FDA
Food and Drug Administration
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- AHRQ
Agency for Healthcare Research and Quality
- VAD
Ventricular Assist Device
- PCI
Percutaneous Coronary Intervention
- ICD
Implantable Cardioverter Defibrillator
- HGLM
Hierarchical Generalized Linear Model
- BIC
Bayesian Information Criteria
- OR
Odds Ratio
- IQR
Inter-quartile Range
Footnotes
Relationships with Industry: Dr. Krumholz reports that he is the recipient of a research grant from Medtronic, Inc. through Yale University and is chair of a cardiac scientific advisory board for United Health. The other authors report no relationships with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bradford WD, Chen J, Krumholz HM. Under-utilisation of beta-blockers after acute myocardial infarction. Pharmacoeconomic implications Pharmacoeconomics. 1999;15:257–68. doi: 10.2165/00019053-199915030-00005. [DOI] [PubMed] [Google Scholar]
- 2.Peacock WF, Holland R, Gyarmathy R, et al. Observation unit treatment of heart failure with nesiritide: results from the proaction trial. J Emerg Med. 2005;29:243–52. doi: 10.1016/j.jemermed.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–91. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 4.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–5. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E, Burnett JC, Colucci WS, et al. Natrecor Advisory Panel Report. Panel of cardiology experts provides recommendations to Scios regarding Natrecor. 2005 [Google Scholar]
- 6.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 7.Hauptman PJ, Schnitzler MA, Swindle J, Burroughs TE. Use of nesiritide before and after publications suggesting drug-related risks in patients with acute decompensated heart failure. JAMA. 2006;296:1877–84. doi: 10.1001/jama.296.15.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Normand SLT, Shahian DM. Statistical and clinical aspects of hospital outcomes profiling. Statistical Science. 2007;22:206–226. [Google Scholar]
- 10.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociological Methods & Research. 2007;35:542–571. [Google Scholar]
- 11.Nagin DS, editor. Group-Based Modeling of Development. Cambridge, MA, USA: Harvard University Press; 2005. [Google Scholar]
- 12.Team R. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- 13.Bradley EH, Curry LA, Spatz ES, et al. Hospital strategies for reducing risk-standardized mortality rates in acute myocardial infarction. Ann Intern Med. 2012;156:618–26. doi: 10.1059/0003-4819-156-9-201205010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curry LA, Spatz E, Cherlin E, et al. What distinguishes top-performing hospitals in acute myocardial infarction mortality rates? A qualitative study. Ann Intern Med. 2011;154:384–90. doi: 10.7326/0003-4819-154-6-201103150-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krumholz HM, Curry LA, Bradley EH. Survival after acute myocardial infarction (SAMI) study: the design and implementation of a positive deviance study. Am Heart J. 2011;162:981–987. e9. doi: 10.1016/j.ahj.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson EC, Batalden PB, Huber TP, et al. Microsystems in health care: Part 1. Learning from high-performing front-line clinical units. Jt Comm J Qual Improv. 2002;28:472–93. doi: 10.1016/s1070-3241(02)28051-7. [DOI] [PubMed] [Google Scholar]
- 17.Bradley EH, Herrin J, Wang Y, et al. Door-to-drug and door-to-balloon times: where can we improve? Time to reperfusion therapy in patients with ST-segment elevation myocardial infarction (STEMI) Am Heart J. 2006;151:1281–7. doi: 10.1016/j.ahj.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Bradley EH, Holmboe ES, Mattera JA, Roumanis SA, Radford MJ, Krumholz HM. A qualitative study of increasing beta-blocker use after myocardial infarction: Why do some hospitals succeed? JAMA. 2001;285:2604–11. doi: 10.1001/jama.285.20.2604. [DOI] [PubMed] [Google Scholar]
- 19.Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2007;153:1021–8. doi: 10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Burns LR, Wholey DR. Adoption and abandonment of matrix management programs: effects of organizational characteristics and interorganizational networks. Academy of Management Journal. 1993;36:106–138. [PubMed] [Google Scholar]
- 21.Rogers E. Diffusion of Innovations. New York: Free Press; 1995. [Google Scholar]
- 22.Young GJ, Charns MP, Shortell SM. Top manager and network effects on the adoption of innovative management practices: A study of TQM in a public hospital system. Strategic Management Journal. 2001;22:935–951. [Google Scholar]
- 23.Keenan PS, Normand SL, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 24.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.