ABSTRACT
Targeted therapeutics is used as an alternative treatment of non-small cell lung cancer (NSCLC); however, treatment effect is far from being satisfactory, and therefore identification of new targets is needed. We have previously shown that metuzumab inhibit tumor growth in vivo. The present study was performed to investigate the anti-tumor efficacy of metuzumab combined with gemcitabine and cisplatin (GP), paclitaxel and cisplatin (TP) or navelbine and cisplatin (NP) regimens in multiple NSCLC cell lines. Our results demonstrate that, in comparison to single agent metuzumab or GP treated cells, metuzumab combined with GP display inhibitory effects on tumor growth. Furthermore, we found that metuzumab elevated the sensitivity of cell lines to gemcitabine, which was identified by MTT assay. Flow cytometric analysis showed that metuzumab combined with gemcitabine (GEM) treatment led to an obvious G1 arrest and an elevated apoptosis in A549, NCI-H460 and NCI-H520 cells. Western blot analysis also demonstrated a significantly reduced level of cyclin D1, Bcl-2, and an obviously increase level of Bax and full-length caspase-3 in A549, NCI-H460 and NCI-H520 cells treated with metuzumab/gemcitabine combination in comparison with single agent treated cells. In addition, metuzumab/gemcitabine treated A549, NCI-H460 and NCI-H520 cells also demonstrated a significantly increase in deoxycytidine kinase (dCK) protein level compared with single agent metuzumab or gemcitabine treated cells. Xenograft models also demonstrated that this metuzumab/gemcitabine combination led to upregulation of dCK. Taken together, the mechanisms of metuzumab combined with GP repress tumor growth were that the combined treatment significantly inhibited the tumor cell proliferation, apoptosis and cell cycle in vitro and in vivo and at least partially by induction of dCK expression. Our results suggested that metuzumab could significantly enhance chemosensitivity of human NSCLC cells to gemcitabine. Metuzumab/gemcitabine combination treatment may be a potentially useful therapeutic regimen for NSCLC patients.
KEYWORDS: CD147, chemosensitivity, gemcitabine, metuzumab, monoclonal antibody, non-small-cell lung cancer, targeted therapy
Abbreviations
- NSCLC
non-small cell lung cancer
- GP
gemcitabine and cisplatin
- TP
paclitaxel and cisplatin
- NP
navelbine and cisplatin
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- GEM
gemcitabine
- dCK
deoxycytidine kinase
- MMPs
matrix metalloproteinases
- ADCC
antibody-dependent cell-mediated cytotoxicity
- CFDA
China Food and Drug Administration
- dFdCTP
gemcitabine triphosphate
- hIgG1
human IgG1
- STR
short tandem repeat
- TUNEL
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling
- IOD
integrated optical density
- dFdCDP
gemcitabine diphosphate
- dCTP
deoxycytidine triphosphate
- NOAEL
no observed adverse effect level
Introduction
Lung cancer is one of the leading cause of cancer morbidity and mortality worldwide especially in China.1 Approximately 80–85% of lung cancers are non-small cell lung cancers (NSCLCs) and the majority of patients with NSCLC have a poor prognosis.2 The main treatments of lung cancer include surgical resection,3-5 radiofrequency ablation,6 radiotherapy,7 platinum-based chemotherapies,8-11 and targeted therapy.10,12-14 Despite progress in the development for the treatment of NSCLC, only 15% of patients are expected to be alive at 5 years,7 and has necessitated the development of targeted therapies.
CD147 is a highly glycosylated transmembrane protein that belongs to the immunoglobulin superfamily. Previously studies had shown that many malignant neoplasms highly express CD147,15 especially in NSCLC.16 Since aberrant expression of CD147 is associated with matrix metalloproteinases (MMPs) production,17 tumorigenesis,18 clinicopathological features,16,19 invasion and metastasis,16 poor prognosis19,20 and chemoresistence,21 it can be a potential candidate for target therapy.
Metuzumab is an affinity-optimized and nonfucosylated anti-CD147 human-mouse chimeric IgG1 monoclonal antibody designed to reduce immunoreactivity and enhance antibody-dependent cell-mediated cytotoxicity (ADCC).22 It has been approved to perform phase I clinical trial for NSCLC in China by China Food and Drug Administration (CFDA). Earlier pre-clinical studies of metuzumab combined with cisplatin and gemcitabine in xenograft mouse have demonstrated that this combination therapy was safe, tolerable and effectiveness. Additional studies evaluating the safety and efficacy of metuzumab in combination with various chemotherapeutic agents are ongoing.
In this study, we assess to the efficacy of metuzumab in combination with cisplatin dependent chemotherapy consisting of paclitaxel, navelbine and gemcitabine (GEM) as compared with chemotherapy alone in NSCLC xenograft mouse. Collectively, our results showed that metuzumab, in combination with cisplatin and gemcitabine, significantly enhanced the therapeutic efficacy in human NSCLC cells compared with single treatment, which may be associated with apoptosis induction and cell cycle arrest at G1 phase. Our results also indicated that metuzumab binding to CD147 promote the deoxycytidine kinase (dCK) expression level and may contribute to the gemcitabine metabolism to gemcitabine triphosphate (dFdCTP). Our results may support further evaluation of metuzumab in NSCLC patients, especially if subsets of patients more likely to benefit can be identified based on tumor molecule phenotype. However, the mechanism of metuzumab enhanced chemosensitivity of gemcitabine should be further studied.
Results
Anti-proliferation activity of metuzumab combined with chemotherapy drugs in human NSCLC xenograft mice NSCLC cells
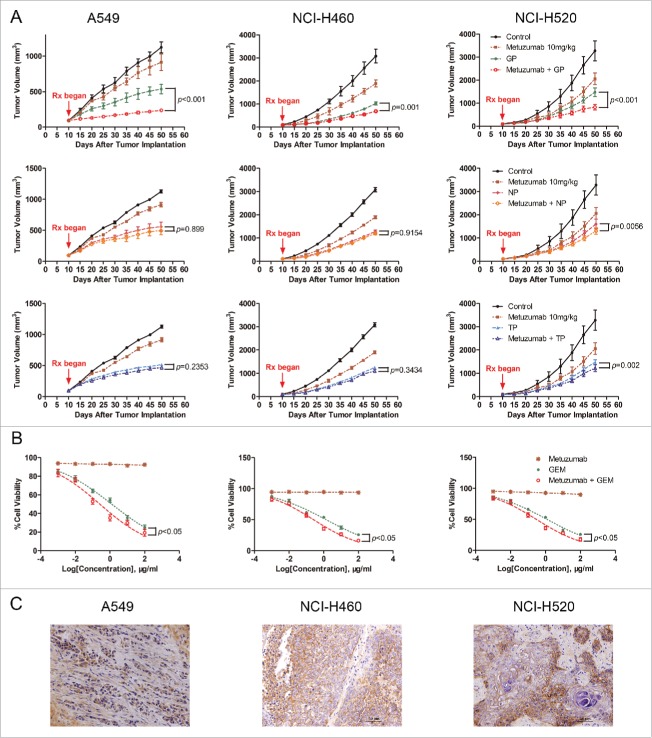
To test efficacy of metuzumab combined with gemcitabine and cisplatin (GP), navelbine and cispaltin (NP) or paclitaxel and cispaltin (TP) in human NSCLC cell lines A549, NCI-H460 and NCI-H520 tumor xenograft model in nude mice, respectively. We treated the mice with saline, metuzumab, GP/TP/NP, or metuzumab combined with GP/TP/NP, and observed the development of tumors over a defined period. The tumor progression curves were shown in Fig. 1A. At the end of treatment, the average tumor volume of mice treated with the vehicle control increased by about 11.9-fold. The mean volume of tumors of the NSCLC cell xenografts treated with metuzumab or chemical drug alone were significantly smaller than those in the control groups (Table 1). We also found that metuzumab combined with NP did not significantly enhanced tumor toxicity compared with NP in A549 (54.40% vs. 53.01%, p = 0.8990)and NCI-H460 (57.22% vs. 57.58%, p = 0.9154) xenografts nude mice, metuzumab combined with TP neither enhanced tumor toxicity compared with TP in A549 (53.31% vs. 58.93%, p = 0.2353) and NCI-H460 (58.80% vs. 57.28%, p = 0.3434) xenografts nude mice, however, in NCI-H520 xenografts nude mice, a slight regression was found in metuzumab combined with NP or TP group compared with NP or TP treatment alone (57.62% vs. 47.89%, p = 0.0056 and 61.16% vs. 50.63%, p = 0.002), respectively. Importantly, we noted that combination treatment with metuzumab and GP completely inhibited tumor growth in A549 xenograft model (91.89% vs. 71.01%, p < 0.001 vs. GP), and a modest and statistically significant tumor growth inhibition compared with GP treated mice were found in NCI-H460 and NCI-H520 xenograft model (73.99% vs. 67.67%, p = 0.0001 and 69.74% vs. 52.60%, p < 0.001, respectively), especially, in A549 xenografts nude mice, the mean tumor volume of metuzumab combined with GP even smaller than that of pre-treatment. Moreocer, the level of metuzumab detected by immunohistochemistry staining represent the continued exposure of tumors to metuzumab at the end of experiments (Fig. 1C). All together, the antitumor activity of metuzumab combined with GP is better than those of metuzumab combined with TP or NP, and indicated that metuzumab could significantly improve the chemosensitivity of NSCLC cells to GP in vivo.
Figure 1.
The antitumor efficacy of metuzumab combined with GP, TP or NP in vitro and in vivo. (A) Tumor growth inhibition with metuzumab alone or combined with GP, TP or NP in Balb/c nude mice bearing A549 (left), NCI-H460 (middle) and NCI-H520 (right) human lung cancer cell line xenografts. Data are presented as tumor volume (mm3) in the means ± SD (n = 10). (B) Analysis of cell-growth rate by modified MTT assay. (C) Representative images of metuzumab distribution in nude mice bearing A549, NCI-H460 and NCI-H520 cell xenograft. Each experiment was repeated for at least 3 times.
Table 1.
The IRTV of metuzumab, GP, NP and TP alone or combination in xenografts nude mice.
| A549 | NCI-H460 | NCI-H520 | |
|---|---|---|---|
| metuzumab | 39.34% | 36.39% | 35.18% |
| GP | 71.01% | 67.67% | 52.60% |
| NP | 53.01% | 57.58% | 47.89% |
| TP | 58.93% | 57.28% | 50.63% |
| metuzumab combined with GP | 91.89% | 73.99% | 69.74% |
| metuzumab combined with NP | 54.40% | 57.22% | 57.62% |
| metuzumab combined with TP | 53.31% | 58.80% | 61.16% |
IRTV, inhibition ratio of tumor volume.
To determine the relationship between the level of CD147 and the anti-tumor efficacy, the CD147 expression level of the 3 NSCLC cell lines used in this work was determined by western blot (Fig. 2A), there were a greatest level of CD147 expression in NCI-H460 cells compared with A549 cells and NCI-H520 cells. The CD147 expression level was lowest in A549 cells among the 3 cell lines. The results was further confirmed by flow cytometry (Fig. 2B). However, these results showed the anti-tumor growth efficacy of metuzumab is independent of CD147 express level in these cell lines.
Figure 2.
Metuzumab combined with GP represses cell proliferation and induces apoptosis in vivo. (A) Western blot analysis on the level of CD147 expression in A549, NCI-H460 or NCI-H520 cells. β-actin was used as the internal control. The experiments were performed triplicate. Gray-scale value was analyzed by Image J software for 3 times. The numbers indicate relative protein expression normalized to β-actin. (B) FACS analysis was used to examine the level of CD147 expression in A549, NCI-H460 or NCI-H520 cells. (C) Representative images of immunohistochemical staining for Ki-67, TUNEL, Bax and Bcl-2 in nude mice bearing A549 cell xenograft received metuzumab, GP or metuzumab combined with GP treatment. Scale bar in Ki-67, Bax and Bcl-2 represents 50 μm, scale bar in TUNEL represents 20μm. Histogram represents the integrated optical density (IOD) of Ki-67, Bax and Bcl-2 expression and percentage of TUNEL positive nuclei from the studied group. The bars represent each sample performed in triplicate, and the error bars indicate mean ± SD. **p < 0.01. ***p < 0.001.
Metuzumab promoted GP-induced apoptosis and restrained tumor proliferation in vivo
To elucidate the mechanism of metuzumab combined with GP repress tumor growth, the tissue sections from each were collected, and assayed proliferation and apoptosis. To analyze cell proliferation status in the tumors, we assayed for the proliferative marker Ki-67 by using immunohistochemistry. The IOD value of Ki-67 of the mice treated with metuzumab combined with GP was significantly decreased from 4191.12 ± 680.92 to 1281.69 ± 417.99 in A549 cells (p < 0.001, Fig. 2C), from 22713.76 ± 2217.17 to 11098.13 ± 1973.96 in NCI-460 cells (p < 0.001, Fig. S1A, B) and from 12873.21 ± 1978.95 to 6604.58 ± 971.51 in NCI-H520 cells (p < 0.001, Fig. S2A, B), comparing to the mice treated with GP alone, indicating metuzumab combined with GP could remarkable inhibit the tumor cell proliferation compared with those treated with GP alone. Apoptosis was analyzed by an immunohistochemistry-based TUNEL assay. The percentage of apoptotic cells were increased in the metuzumab combined with GP group from 34.32 ± 13.11% to 49.71 ± 16.09% in A549 cells (Fig. 2C), from 23.65 ± 9.45% to 36.28 ± 7.59% in NCI-H460 cells (Fig. S1A, C), and from 23.05 ± 5.06% to 34.52 ± 6.26% in NCI-H520 cells (Fig. S2A, C). Furthermore, the upregulation of the apoptotic marker, Bax and downregulation of the survival marker, Bcl-2 were founded in metuzumab combined with GP group in A549 (Fig. 2C), NCI-H460 (Fig. S1A, D and E) and NCI-H520 (Fig. S2A, D and E) cells compared with those in control, metuzumab and GP group.
Metuzumab enhanced gemcitabine induced cell proliferation, apoptosis and cell cycle in vitro
Our previously study demonstrated that metuzumab is a nonfucosylate antibody, and promote antibody-dependent cellular cytotoxicity (ADCC) efficacy in vivo,22 however, in this study, we found metuzumab apparently enhanced the antitumor efficacy of GP, but not satisfied to that of NP or TP. We speculated metuzumab enhanced chemosensitivity of NSCLC cells to GP is not simply due to ADCC effect, nor cisplatin. To test this hypothesis, we evaluated the antitumor efficacy of metuzumab combined with gemcitabine in vitro without effect cells. MTT assay was performed and the results were analyzed to establish the dose-inhibition efficiency curves and calculate the IC50 of metuzumab, GEM alone or combination to different NSCLC cells. As shown in Fig. 1B, metuzumab alone treatment cannot induce the cell death in NSCLC cell lines. The inhibition efficiencies of GEM, and metuzumab combined with GEM to A549, NCI-H460, and NCI-H520 cells were significantly higher than those metuzumab treated cells (p < 0.05), respectively. The IC50 values were significantly decreased in the metuzumab combined with GEM group, from 1.266 µM to 0.262 µM in A549 cells, from 1.371 µM to 0.310 µM in NCI-H460 cells, and from 1.251 µM to 0.307 µM in NCI-H520 cells, respectively, indicating that metuzumab could obviously enhance the chemosensitivity of NSCLC cells to gemcitabine. In addition, metuzumab alone did not inhibit PCNA expression, a cell proliferation marker, in A549, NCI-H460 and NCI-H520 cells, however, PCNA expression level significantly inhibited the cells treated with metuzumab combined with GEM, even compared with GEM treated cells (Fig. 3E).
Figure 3.
Combined effect of metuzumab and gemcitabine on cell cycle in A549, NCI-H460 and NCI-H520 cells. (A) Representative cell cycle profiles obtained by FACS analysis from propidium iodide stained A549, NCI-H460 or NCI-H520 cells treated with human IgG1, metuzumab, gemcitabine or metuzumab combined with gemcitabine. X-axis values correspond to DNA content; Y-axis values correspond to the number of events detected. Graph of the percentage of cells within each phase of the cell cycle in A549 (B), NCI-H460 (C) or NCI-H520 (D) cells obtained by FACS analysis. And data are presented as mean ± SD. (E) Western blot analysis on the expression levels of PCNA, cyclin D1 in A549, NCI-H460 or NCI-H520 cells treated with metuzumab or cHAb18, gemcitabine, and metuzumab or cHAb18 combined with gemcitabine for 24 hour. β-actin was used as the internal control. Gray-scale value was analyzed the same as above. The experiments were performed triplicate. The error bars indicate mean ± SD.
To investigate the possible mechanisms of metuzumab in synergism of GEM, we treated A549, NCI-H460 and NCI-H520 cells with saline, human IgG1 (hIgG1), metuzumab, GEM or metuzumab combined with GEM and evaluated cell cycle using PI staining and flow cytometry. As shown in Fig. 3A-D, the percentages of A549, NCI-H460 and NCI-H520 cells treated with metuzumab and GEM at S phase were decreased moderately from 15.36% to 5.45% (p < 0.001), 13.64% to 8.42% (p = 0.0002) or 15.37% to 8.77% (p < 0.001) comparing with the cells treated with GEM, and the percentages at G1 phase were increased significantly from 82.07% to 94.55% (p < 0.001), 86.37% to 91.58% (p = 0.005) or 82.11% to 91.23% (p < 0.001), whereas, hIgG1 had no apparent effect on cell cycles. Furthermore, cyclin D1 (CCND1), a cell-cycle regulating gene and a G1/S transition checkpoint marker was found to be downregulated expression in metuzumab combined with GEM treatment groups than that in GEM treatment alone in 3 cell lines (Fig. 3E). All these results suggested that metuzumab combined with GEM lead to a moderate G1-phase arrest of NSCLC cells.
For metuzumab combined with GEM caused a significant arrest of G1 phase in NSCLC cells, we further examined the apoptosis rate of A549, NCI-H460 and NCI-H520 cells treated with saline, hIgG1, metuzumab, GEM and metuzumab combined with GEM for 24 hour by flow cytometry. As shown in Fig. 4A-D, the apoptosis rate of A549, NCI-H460 and NCI-H520 cells treated with metuzumab combined with GEM were significantly increased from 43.65% to 74.13%, from 41.09% to 69.24% and from 40.98% to 70.69%, respectively when comparing with A549, NCI-H460 and NCI-H520 cells treated with GEM alone, however, the hIgG1 had no inhibition of apoptosis to the 3 cell lines compare with the cells treated with saline. Furthermore, the western blot analysis showed a significant upregulation of Bax in NSCLC cells treated with metuzumab combined with GEM comparing with the cells treated with GEM alone (Fig. 4E). On the other hand, expression of Bcl-2 and full-length caspases-3 were downregulated in metuzumab combined with GEM treated cells in all the cell lines tested indicating apoptosis (Fig. 4E). All these results suggested metuzumab combined with GEM could significant improve the apoptosis rate of NSCLC cells. In additionally, although the apoptosis rate of A549, NCI-H460 and NCI-H520 cells treated with metuzumab were slightly increased from 2.35% to 11.12%, from 1.857% to 10.49%, and from 1.107% to 10.61% when comparing with the cells treated with saline (Fig. 4A-D), western blot analysis did not demonstrate metuzumab induces cell apoptosis (Fig. 4E). On the other hand, we assessed the effect of cHAb18 on cell cycle and apoptosis by western blot analysis. Consistent with metuzumab, cHAb18 combined with GEM regressed PCNA, cyclin D1 (Fig. 3E), Bcl-2 and full-length caspase-3 express level (Fig. 4E), and upregulation of Bax (Fig. 4E), comparing with the cells treated with GEM alone.
Figure 4.
The effects of metuzumab combined with gemcitabine on apoptosis in A549, NCI-H460 and NCI-H520 cells. (A) Representative apoptosis profiles obtained by FACS analysis from Annexin V and propidium iodide stained in A549, NCI-H460 or NCI-H520 cells treated with human IgG1, metuzumab, gemcitabine or metuzumab combined with gemcitabine. Graphic presentation of data obtained from cell apoptosis analysis of A549 (B), NCI-H460 (C) or NCI-H520 (D) cells. Values are expressed as means (Q2+ Q3) ± SD of at least 3 independent experiments. (E) Western blot analysis on the expression levels of Bax, Bcl-2 and full length caspase-3 in A549, NCI-H460 or NCI-H520 cells treated with metuzumab or cHAb18, gemcitabine and metuzumab or cHAb18 combined with gemcitabine for 24 hour. β-actin was used as the internal control. Gray-scale value was analyzed the same as above. The numbers indicate relative protein expression normalized to β-actin. The bars represent each sample performed in triplicate, and the error bars represent mean ± SD. ***p < 0.001.
Augmentation of the cytotoxic effect of chemicals in NSCLC cells by metuzumab in vitro and in vivo
Deoxycytidine kinase (dCK) is a key rate-limiting enzyme response for conversion of GEM to active antimetabolite, the nucleotides gemcitabine diphosphate (dFdCDP) and triphosphate (dFdCTP). dFdCTP competes with endogenous deoxycytidine triphosphate (dCTP) for incorporation into newly synthesized DNA and inhibit DNA chain extends.23,24 As metuzumab increased induction of apoptosis in response to GEM, we hypothesized that CD147 inhibition could promote conversion of gemcitabine to active metabolite by dCK. The A549, NCI-H460 and NCI-H520 cells were treated with 10 μM hIgG1, cHAb18 or metuzumab. The expression level of dCK was determined by western blot analysis. An apparently increase in dCK was observed in metuzumab and cHAb18 treatment cells, the level of dCK increased about 3.15-fold in A549 cells, 2.11-fold in NCI-H460 cells and 2.96-fold in NCI-H520 cells treated with metuzumab, and increased about 3.11-fold, 2.09-fold and 2.88-fold in A549, NCI-H460 and NCI-H520 cells treated with cHAb18 antibody, respectively (Fig. 5A). In addition, the level of dCK in tumor obtained from metuzumab treated mice were determined by immunohistochemistry staining increased relative to the control group (Fig. 5B). Compared to the mouse in control group, the IOD of dCK value increased about 2.1-fold, 1.4-fold and 1.7-fold in A549, NCI-H460 and NCI-H520 cells xenograft mouse treated with metuzumab, respectively. These results provided evidences that metuzumab promoted the expression of dCK might be partly responsible to the enhanced cytotoxicity of gemcitabine in CD147 overexpression NSCLC cells. However, the mechanism need to be further study on CD147 regulates dCK expression.
Figure 5.
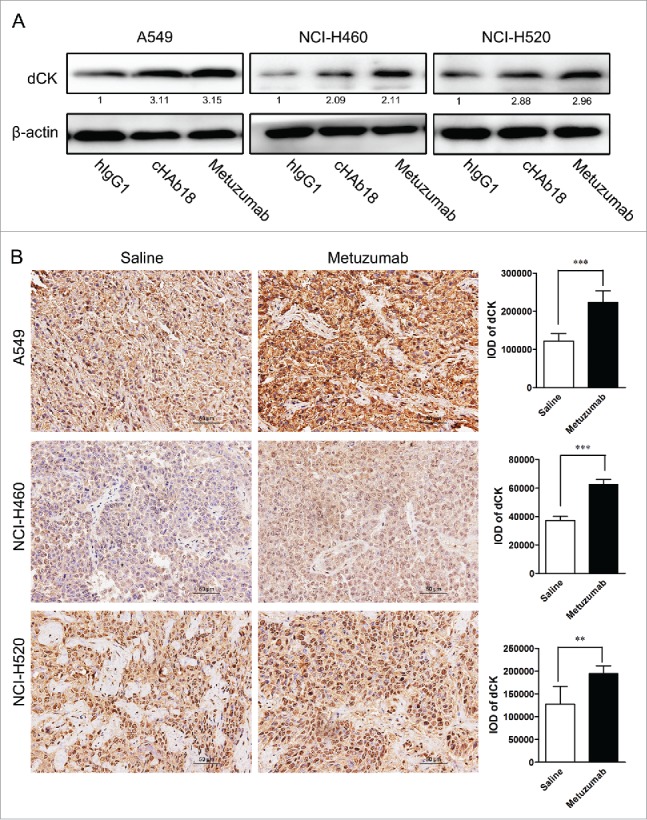
Metuzumab induces dCK expression in vitro and in vivo. (A) Western blot analysis on the level of dCK expression in A549, NCI-H460 or NCI-H520 cells treated with human IgG1, metuzumab or cHAb18 for 24 hour. β-actin was used as the internal control. Gray-scale value was analyzed the same as above. The numbers indicate relative protein expression normalized to β-actin. (B) Immunohistochemical staining for dCK expression in nude mice bearing A549 cell xenograft received saline or metuzumab treatment. Scale bar represents 50 μm. Histogram represents the integrated optical density (IOD) of dCK expression. The bars represent each sample performed in triplicate, and the error bars indicate mean ± SD. **p < 0.01. ***p < 0.001.
Discussion
Although non-small cell lung cancer (NSCLC) in humans remains incurable, available of alternative treatment regimens have contributed to inhibition of tumor growth and prolongation of survival of patients.25 For example, gemcitabine combined with cisplatin (GP), paclitaxel combined with cisplatin (TP) and vinorelbine combined with cisplatin (NP) have been accept being first-line of treatment of NSCLC. However, the effect was not satisfactory, the further development for targeted drug therapy based on antibody is still needed.
Metuzumab is an afucosylated human/mouse chimeric IgG1 isotype monoclonal antibody that targets the CD147, which induced ADCC activation and enhanced anti-tumor effect. We have previously reported that metuzumab can inhibit NSCLC cells growth in vitro and in vivo.22 Therapeutic mAbs are typically used in combination with one or more chemical drugs in the clinic.26 To study the effectiveness of metuzumab combination chemotherapy in treating NSCLC and provide some evidences for clinical application, we evaluated the antitumor effect of metuzumab combination with common chemotherapeutic drugs including GP, TP or NP in human NSCLC A549, NCI-H460 and NCI-H520 cells xenograft mouse. Our results showed that metuzumab is effective in reducing the tumor growth in 3 NSCLC xenograft mouse. The tumor volumes were 39.4%, 36.39% and 35.18% in A549, NCI-H460 and NCI-H520 xenograft mouse administrated with metuzumab compared with the volumes of xenograft mouse treated with saline, respectively, which is accordance with our previously study.22 Our results also indicated that metuzumab combined with GP could remarkable increase tumor inhibition rate in 3 NSCLC cells respectively, comparing with the cells treated with GP. However, metuzumab combined with NP or TP regimens did not enhanced tumor toxicity of NP or TP in A549 and NCI-H460 xenografts nude mice model, but a slight inhibit tumor growth of metuzumab combined with NP or TP were observed compared with that in NCI-H520 xenografts nude mice model treated with NP or TP (Fig. 1A). TUNEL results showed an elevated apoptosis rate of tumors generated from metuzumab combined with GP treated mice compared with that in metuzumab or GP group (Fig. 2C). These results suggestion metuzumab and GP interact synergistically to regress tumor growth in vivo.
To get a better understanding the mechanism by which metuzumab enhances the GP induced inhibition of tumor proliferation and apoptosis, we investigated the marker of key proteins known to regulate cell proliferation and apoptosis. We demonstrated that the level of Ki-67 and Bcl-2 protein were decreased and the level of Bax protein was increased in A549, NCI-H460 and NCI-H520 cells treated with either metuzumab or GP alone compared with control group. In addition, these changes in combination treatment group with metuzumab and GP were significantly greater than that in single treating group (Fig. 2C).
Furthermore, MTT analysis was performed when metuzumab or GEM was used individually and when those used in combination. Our results showed that compared with single agent GEM or metuzumab treatment, the cell death induced by metuzumab and GEM combination was significantly increased (Fig. 1B). This result indicated that the increase of anti-tumor effect of metuzumab combined with GP than that of GP possibly due to metuzumab promote GEM activity. Furthermore, cell cycle analysis and cell apoptosis detection were performed when metuzumab or GEM was used individually and when used in combination in vitro, but without effector cells, such as NK cell or peripheral blood mononuclear cell (PBMC). Our results showed that compared with single agent metuzumab or GEM treatment, the G1/S phase accumulation and cell apoptosis induced by their combination was significantly increased, indicating that metuzumab enhances the GEM-induced cell cycle arrest and apoptosis on A549, NCI-460 and NCI-H520 cells (Fig. 3A-D and Fig. 4A-D).
Previous studies demonstrated that GEM inhibits the expression of cyclin D and Bcl-2, inducing G1 phase arrest in many types of human cancer cells including NSCLC.27-29 Our results are consistent with these reports. The levels of cyclin D1 and Bcl-2 proteins were decreased in cells treated with GEM alone. In addition, these changes in combination treatment group with metuzumab and GEM were significantly greater than that in single treating group (Fig. 3E and Fig. 4E). On the other hand, it is known that Bax is an important protein that regulates the activation of caspase and leads cell to apoptosis.30 Caspase-3 is regarded as one of the main executors of apoptosis,31 and reduction of full-length caspase-3 is correlated with apoptosis. We observed significant increase of Bax and inhibition of full length caspase-3 expression by metuzumab plus GEM treatment than that in single treating group (Fig. 4E). These in vitro and in vivo results can partly explain the enhanced effects of GEM-induced cell cycle arrest and apoptosis on cells by metuzumab.
Gemcitabine (or 2′, 2′-difluorodeoxycytidine) is a pro-drug and a cytarabine analog that has been used widely as a chemotherapeutic agent for the treatment of multi-cancers.32 It exerts cellular toxicity through the phosphorylated to its mononucleotide by dCK, and subsequently by nucleotide kinases to its active metabolites, such as dFdCDP and dFdCTP.33 Accumulating evidences indicate that increase of dCK protein level34,35 or activation of dCK36,37 promote the antitumor effect of GEM. Our results showed for the first time that treatment of NSCLC cells with metuzumab can increase the expression of dCK in vitro and in vivo. As shown in Fig. 5, metuzumab treatment induced a 3.15-fold increase in A549 cells, 2.11-fold increase in NCI-H460 cells and 2.96-fold increase in NCI-H520 cells in dCK expression (Fig. 5A). And dCK level increased about 2.1-fold, 1.4-fold and 1.7-fold in A549, NCI-H460 and NCI-H520 cells xenograft mouse treated with metuzumab compared with that in control group (Fig. 5B), respectively. cHAb18 mAb, a fucosylated metuzumab, also enhanced dCK protein level (Fig. 5). These results suggested that binding to CD147 by metuzumab or cHAb18 is the main reason response of increase of dCK. However, the mechanism by which metuzumab induces expression of dCK remains elusive and needs to be further studied.
The potential off-target effect of mAb is attached to the red blood cells and cause hemolysis, and these effects exhibit species-specific properties. 38 In our preclinical study, metuzumab does not cause hemolysis in incubation with rabbit red blood cells (data not shown). No hemolysis and other several adverse reactions related to metuzumab were found in repeat-dose toxicity study in rats and cynomolgus monkeys, the no observed adverse effect level (NOAEL) were estimated to be 200 mg/kg in rats and in monkeys.22 However, the safety studies and off-target effect of metuzumab should be considered carefully in clinical trial.
In summary, we had shown that metuzumab enhanced the efficacy of gemcitabine through upregulation of dCK protein level by binding to CD147, which lead to downregulated the expression of Bcl-2, cyclin D1 and full length caspase-3, and upregulated of Bax, thereby increasing cell apoptosis and G1 phase arrested. This investigation suggests a potential clinical application of the combination regimen for the treatment of NSCLC cancer patients.
Materials and methods
Cell culture
Human NSCLC cell lines A549, NCI-H520 and NCI-H460 were purchased from the American Type Culture Collection. Cells were cultured in RPMI-1640 medium (Sigma, St Louis, MO, USA) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), 2 mmol/L L-glutamine, and 0.1mmol/L nonessential amino acids (Life Technologies), and maintained at 37°C humidified atmosphere containing 5% CO2. Cell lines authentication were assessed using short tandem repeat (STR) DNA profiling method.
Reagents
Metuzumab was generously provided by Pacific Meinuoke Biopharmaceutical Company (Changzhou, Jiangsu province, China) and diluted to certain concentration with sterile saline. Human IgG1 antibody was obtained from R&D Systems. Cisplatin was obtained from Qilu Pharmaceutical Co., Ltd. (China) and dissolved in sterile saline to a final concentration of 2 mg/mL. Gemcitabine was purchased from Eli Lilly and Company and dissolved in sterile saline to a final concentration of 200 mg/ml. Paclitaxel was obtained from Beijing SL Pharmaceutical Co., Ltd. (China) at a concentration of 6 mg/ml. Navelbine was provided from Pierre Fabre, Co., Ltd. (China) at a concentration of 10 mg/ml. All the chemicals were diluted in normal saline and dosed based on the weight of the mice receiving either treatment before each dosing regimen.
Antitumor efficacy studies in Mice
Athymic nude mice (female, 5–6 weeks old) were purchased from Vital River Laboratories of China. Animals were housed (5 per cage) in specific pathogen-free (SPF) condition, supplied with food and water ad libitum, and kept on a 12 h light/dark cycle. All studies were conducted in accordance with the Institutional Animal Care and Use Committee approved protocols. Human NSCLC cancer cells (5 × 106) were suspended in Hank's balanced salt solution and implanted in the right dorsal flank of the mice. When tumors reached a mean volume of 100 mm3, mice were randomized into experimental groups (n = 10 or 6). Mice in different treatment received a single dose of metuzumab (10mg/kg), GP (100 mg/kg gemcitabine and 1 mg/kg cisplatin), TP (6.6 mg/kg paclitaxel and 1 mg/kg cisplatin), NP (4 mg/kg navelbine and cisplatin 1 mg/kg, NP), metuzumab combined GP (metuzumab 10 mg/kg, gemcitabine 100 mg/kg and cisplatin 1 mg/kg), metuzumab combined TP (metuzumab 10 mg/kg, paclitaxel 6.6 mg/kg and cisplatin 1 mg/kg), metuzumab combined NP (metuzumab 10 mg/kg, navelbine 4 mg/kg and cisplatin 1 mg/kg), and saline, respectively. Antibody injections were administered twice weekly and chemical drug injections one weekly. Mice treated with saline were represented as control group.
Tumors size were measured twice a week with a digital caliper using the following formula: (width2×length)/226. Antitumor activity was assessed by calculating inhibition ratio of tumor volume (IRTV) based on medians by using following formula: [1-average RTVtreatment (day x)/average RTVcontrol (day x)] × 100%.
Methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay
The effect of metuzumab, gemcitabine or the metuzumab/gemcitabine combination on cell viability were assessed by MTT assay as described previously.39,40 Briefly, 5 × 103 NSCLC cells (A549, NCI-H460 and NCI-H520) were seeded in 96-well plate and cultured in 24 h, metuzumab, gemcitabine, the metuzumab/gemcitabine combination and saline were added to the cells. After additional 24h of respective treatments, 20 μL of MTT (5 mg/ml) was added into the wells and cells were incubated for 4 hours. Media was removed and replaced with 100 μL DMSO. Plate were read on a plate reader (BioRad Laboratories, Hercules, CA) at 490 nm, the reference wavelength was 690 nm. Inhibition of cell growth was measured as the percentage of viable cells relative to the control and calculated as follows: percent viable cells rate = 100%×ODT/ODC, where ODT is the average OD value of the treated samples, and ODC is the average OD value of the control samples. The assay was repeated 3 times. The mean optical density (OD ± SD) was calculated for each group.
Cell cycle and apoptosis analysis
Cell cycle and apoptosis were determined by flow cytometry. Cell cycle was determined using the Propidium Iodide (PtdIns) solution (Biolegend, San Diego, CA) according to the manufacturer's. 4 × 105 cells were seeded in 6-well plates and treated with saline, hIgG1, metuzumab, gemcitabine and metuzumab plus gemcitabine, respectively. The cells were harvested at 24 hours after drug treatment and fixed with 70% ethanol. The cells were then resuspended with PBS containing 100 μg/mL RNase and 50 μg/mL propidium iodide (PtdIns). Cell cycle profiles of 2 × 105 cells were analyzed using a FACScalibur flow cytometer (BD Biosciences).
Cell apoptosis was detected using the FITC-Annexin-V Apoptosis Detection Kit with PtdIns (Biolegend, San Diego, CA) according to manufacturer's. A total of 4 × 105 cells were planted in 6-wells culture plates and treated with saline, hIgG1, metuzumab and GEM alone, or metuzumab combined with GEM, respectively, for 24 hours. Then the cells were collected, resuspended in 500μl binding buffer, and stained with 5 μl Annexin V-FITC Conjugate and 10 μl of PtdIns Solution. The stained cells (1 × 105) were then analyzed using a FACSCalibur flow cytometer (BD Biosciences).
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) analysis
NSCLC tissues were fixed in 4% paraformaldehyde in PBS embedded in paraffin and cut into ∼5mm sections through the center of the tissue. The sections were stained with terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) analysis using the in situ cell-death detection kit (Keygen Bio-Technology Development Co., Ltd. Nanjing, China) following the manufacturer's protocol. The percentage of apoptotic cells were determined by counting at least 300 cells in 5 randomly selected fields41 and calculated as the number of apoptotic cells per number of total cells × 100%.42 The imaging of TUNEL staining were captured with an Olympus CX71 microscope (Olympus) and analysis using Image Pro Plus 6 software (Media Cybernetics, USA).
Western Blotting
Cells were harvested and lysed with RIPA lysis buffer (Beyotime Biotechnology, China). The total proteins were quantified by Bradford protein assay kit (PIERCE). The proteins were separated by SDS-PAGE and transferred into PVDF membrane. Membranes were blocked in blocking buffer (5% non-fat dry milk/0.1% Tween 20 in TBS) for 1h at room temperature, before being incubated at 4°C with the appropriate antibody in blocking buffer. The membranes were washed and incubated with the appropriated peroxidase conjugated secondary antibody. After washing, the proteins level was detected using ECL reagents (GE Healthcare). The following primary antibodies were used: anti-PCNA (Millipore, 1:250 dilution), anti-Bax (HangZhou HuaAn Biotechnology, China, 1:100 dilution), anti-Bcl-2 (Antibody Revolution, 1:250 dilution), anti-full length caspase-3 (Antibody Revolution, 1:100 dilution), and anti-dCK (Biorbyt, 1:500 dilution). Anti-rabbit (Pierce, 1:6000 dilution) or anti-mouse HRP-conjugated antibodies (Pierce, 1:3000 dilution) were used for secondary antibody reactions.
Immunohistochemistry staining
Immunohistochemistry staining were performed using Biotin-Streptavidin HRP Detection kit (Zhongshan Bio, Beijing, China) according to the manufacturer's procedure. 43 in brief, tumor tissues obtained from A549, NCI-H460 and NCI-H520 nude mice xenografts were formalin-fixed, paraffin embedded, and then cut into 5-μm sections. After antigen retrieval with autoclaving in citric acid, and inactivating endogenous peroxidase with 3% H2O2, the slides were incubated with the rabbit anti-mouse Ki67 monoclonal antibody (Abcam, 1:100 dilution), anti-Bax (HangZhou HuaAn Biotechnology, China, 1:100 dilution), anti-Bcl-2 (Antibody Revolution, 1:100 dilution) and anti-dCK (Biorbyt, 1:100 dilution) antibody overnight at 4°C. Second antibody conjugated with biotin was applied for 20 min at room temperature. To determine the distribution of metuzumab in tumor tissues, the slides were incubated with the biotin labeled goat anti-human antibody (ZSGB-BIO, China. 1: 150). The sections were developed in 3,3-diaminobenzidine(DAB) and counterstained with hematoxylin. The expression level was determined based on the integrated optical density (IOD) using Image Pro Plus 6 Software (Media Cybernetics, USA), according to the method described previously.44
Flow cytometry assay
Levels of CD147 expression on the lung cancer cells (A549, NCI-H460 and NCI-H520) were determined by flow cytometry using a FITC mouse anti-CD147 antibody (BD Biosciences, 1:500 dilution) and a FITC mouse anti-human IgG1 antibody (abcam, 1:500 dilution) as an isotype control. Relative antigen expression is reported as median fluorescence intensity (MFI).22
Statistical Analysis
For all cell line experiments, data were compiled from at least 3 independent experiments. All values are expressed as mean ± SD., One-way ANOVA analysis followed by t test was used to evaluate differences between the individual groups. Graphpad Prism 5 (San Diego, CA) was used for statistical analyses with p value < 0.05 were considered significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thanked Dr. Hao Tang and Dr. Qiang Feng (Pacific Meinuoke Biopharmaceutical Company, Changzhou, Jiangsu province, China) for the provided of metuzumab. Ms. Xiying Yao and Ms. Liqing Xu assisted with cell culture.
Funding
This work was supported in part by grants from National Basic Research Program (973 Program: 2015CB553701) and China National Science and Technology Major Project (2013ZX09301301).
Author contributions
ZZ and YZ conceived and designed the study. FF, BW, XXS, YMZ and HT performed the in vitro experiments and analyzed the data. GN, LJW, BW, MRHH, SSL, TYD and RH performed animal studies. FF wrote the paper. ZZ and ZY revised the manuscript and accepted the final version. All authors read and approved the final manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer J Clinicians 2013; 63(1):11-30; PMID:23335087; http://dx.doi.org/ 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, Shepherd FA. Non-small-cell lung cancer. Lancet 2011; 378(9804):1727-1740; http://dx.doi.org/ 10.1016/s0140-6736(10)62101-0 [DOI] [PubMed] [Google Scholar]
- 3.Spaks A, Svirina D, Spaka I, Jaunalksne I, Breiva D, Tracums I, Krievins D. CXC chemokine ligand 4 (CXCL4) is predictor of tumour angiogenic activity and prognostic biomarker in non-small cell lung cancer (NSCLC) patients undergoing surgical treatment. Biomarkers 2016; 21(5):474-8:1-4; PMID:27098116; http://dx.doi.org/ 10.3109/1354750X.2016.1172111 [DOI] [PubMed] [Google Scholar]
- 4.Stewart RL, Carpenter BL, West DS, Knifley T, Liu L, Wang C, Weiss HL, Gal TS, Durbin EB, Arnold SM, et al.. S100A4 drives non-small cell lung cancer invasion, associates with poor prognosis, and is effectively targeted by the FDA-approved anti-helminthic agent niclosamide. Oncotarget 2016; 7(23):34630-42; PMID:27127879; http://dx.doi.org/ 10.18632/oncotarget.8969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao HQ, Tian RH, Zhang ZH, Du KQ, Ni YM. Efficacy of pemetrexed plus platinum doublet chemotherapy as first-line treatment for advanced nonsquamous non-small-cell-lung cancer: a systematic review and meta-analysis. OncoTargets therapy 2016; 9:1471-1476; PMID:27042115; http://dx.doi.org/ 10.2147/OTT.S96160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Pereira K, Mohan P, Narayanan G, Wangpaichitr M, Savaraj N. Radiofrequency ablation in primary non-small cell lung cancer: What a radiologist needs to know. Indian J Radiol Imaging 2016; 26(1):81-91; PMID:27081229; http://dx.doi.org/ 10.4103/0971-3026.178347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinh TK, Fendler W, Chalubinska-Fendler J, Acharya SS, O'Leary C, Deraska PV, D'Andrea AD, Chowdhury D, Kozono D. Circulating miR-29a and miR-150 correlate with delivered dose during thoracic radiation therapy for non-small cell lung cancer. Radiat Oncol 2016; 11(1):61; PMID:27117590; http://dx.doi.org/ 10.1186/s13014-016-0636-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun XJ, Deng QH, Yu XM, Ji YL, Zheng YD, Jiang H, Xu YP, Ma SL. A phase II study of Endostatin in combination with paclitaxel, carboplatin, and radiotherapy in patients with unresectable locally advanced non-small cell lung cancer. BMC Cancer 2016; 16(1):266; PMID:27067521; http://dx.doi.org/ 10.1186/s12885-016-2234-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HK, Cho JH, Choi YS, Zo JI, Shim YM, Park K, Ahn MJ, Ahn YC, Kim K, Kim J. Outcomes of neoadjuvant concurrent chemoradiotherapy followed by surgery for non-small-cell lung cancer with N2 disease. Lung Cancer 2016; 96:56-62; PMID:27133751; http://dx.doi.org/ 10.1016/j.lungcan.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 10.Berardi R, Rinaldi S, Santoni M, Newsom-Davis T, Tiberi M, Morgese F, Caramanti M, Savini A, Ferrini C, Torniai M, et al.. Prognostic models to predict survival in patients with advanced non-small cell lung cancer treated with first-line chemo- or targeted therapy. Oncotarget 2016; 7(18):26916-24; PMID:27029035; http://dx.doi.org/ 10.18632/oncotarget.8309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franks SE, Jones RA, Briah R, Murray P, Moorehead RA. BMS-754807 is cytotoxic to non-small cell lung cancer cells and enhances the effects of platinum chemotherapeutics in the human lung cancer cell line A549. BMC Res Notes 2016; 9(1):134; PMID:26928578; http://dx.doi.org/ 10.1186/s13104-016-1919-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng L, Yao HP, Zhou YQ, Zhou J, Zhang R, Wang MH. Biological evaluation of antibody-maytansinoid conjugates as a strategy of RON targeted drug delivery for treatment of non-small cell lung cancer. J Exp Clin Cancer Res 2016; 35(1):70; PMID:27102688; http://dx.doi.org/ 10.1186/s13046-016-0347-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou BO, Nie J, Yang W, Huang C, Huang YE, Zhao H. Effect of hydrothorax EGFR gene mutation and EGFR-TKI targeted therapy on advanced non-small cell lung cancer patients. Oncology letters 2016; 11(2):1413-1417; PMID:26893752; http://dx.doi.org/ 10.3892/ol.2015.4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pallis AG, Serfass L, Dziadziusko R, van Meerbeeck JP, Fennell D, Lacombe D, Welch J, Gridelli C. Targeted therapies in the treatment of advanced/metastatic NSCLC. Eur J Cancer 2009; 45(14):2473-2487; PMID:19596191; http://dx.doi.org/ 10.1016/j.ejca.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Xu J, Chen L, Zhong WD, Zhang Z, Mi L, Zhang Y, Liao CG, Bian HJ, Jiang JL, et al.. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology 2009; 54(6):677-687; PMID:19438743; http://dx.doi.org/ 10.1111/j.1365-2559.2009.03280.x [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Liu S, Lei B, Li W, Lin N, Sheng W, Huang A, Shen H. Expression of HAb18G in non-small lung cancer and characterization of activation, migration, proliferation, and apoptosis in A549 cells following siRNA-induced downregulation of HAb18G. Mol Cell Biochem 2013; 383(1–2):1-11; PMID:24013786; http://dx.doi.org/ 10.1007/s11010-013-1722-7 [DOI] [PubMed] [Google Scholar]
- 17.Caudroy S, Polette M, Tournier JM, Burlet H, Toole B, Zucker S, Birembaut P. Expression of the extracellular matrix metalloproteinase inducer (EMMPRIN) and the matrix metalloproteinase-2 in bronchopulmonary and breast lesions. J Histochem Cytochem 1999; 47(12):1575-1580; PMID:10567441 [DOI] [PubMed] [Google Scholar]
- 18.Sidhu SS, Nawroth R, Retz M, Lemjabbar-Alaoui H, Dasari V, Basbaum C. EMMPRIN regulates the canonical Wnt/beta-catenin signaling pathway, a potential role in accelerating lung tumorigenesis. Oncogene 2010; 29(29):4145-4156; PMID:20514014; http://dx.doi.org/ 10.1038/onc.2010.166 [DOI] [PubMed] [Google Scholar]
- 19.Zhong X, Li M, Nie B, Wu F, Zhang L, Wang E, Han Y. Overexpressions of RACK1 and CD147 associated with poor prognosis in stage T1 pulmonary adenocarcinoma. Annals Surg Oncol 2013; 20(3):1044-1052; PMID:22592183; http://dx.doi.org/ 10.1245/s10434-012-2377-4 [DOI] [PubMed] [Google Scholar]
- 20.Fei F, Li X, Xu L, Li D, Zhang Z, Guo X, Yang H, Chen Z, Xing J. CD147-CD98hc complex contributes to poor prognosis of non-small cell lung cancer patients through promoting cell proliferation via the PI3K/Akt signaling pathway. Annals Surg Oncol 2014; 21(13):4359-4368; PMID:25084765; http://dx.doi.org/ 10.1245/s10434-014-3816-1 [DOI] [PubMed] [Google Scholar]
- 21.Zeng H, Qiu Y, Qu Y, Liang A, Deng A, Zhang W, Xiu B, Wang H, Wang H. Expression of CD147 in advanced non-small cell lung cancer correlated with cisplatin-based chemotherapy resistance. Neoplasma 2011; 58(5):449-454; PMID:21745000; http://dx.doi.org/25376611 10.4149/neo_2011_05_449 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Zhang Y, Sun Q, Feng F, Huhe M, Mi L, Chen Z. Preclinical pharmacokinetics, tolerability, and pharmacodynamics of metuzumab, a novel CD147 human-mouse chimeric and glycoengineered antibody. Mol Cancer Therapeutics 2015; 14(1):162-173; PMID:25376611; http://dx.doi.org/ 10.1158/1535-7163.MCT-14-0104 [DOI] [PubMed] [Google Scholar]
- 23.Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Annals Oncol 2006; 17(Suppl 5):v7-12; PMID:16807468; http://dx.doi.org/ 10.1093/annonc/mdj941 [DOI] [PubMed] [Google Scholar]
- 24.Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res 1991; 51(22):6110-6117; PMID:1718594 [PubMed] [Google Scholar]
- 25.de Carpeno JC, Baron MG, Aguiar J, Chacon JI, Feliu J, Garcia MJ, Madronal C, Colmenarejo A, Sanchez JJ, Ordonez A, et al.. Biweekly regimen of cisplatin, gemcitabine and vinorelbine for advanced non-small-cell lung cancer. Cancer Chemotherapy Pharmacol 2006; 58(2):266-271; PMID:16308698; http://dx.doi.org/ 10.1007/s00280-005-0143-z [DOI] [PubMed] [Google Scholar]
- 26.He Q, Gao J, Ge S, Wang T, Li Y, Peng Z, Li Y, Shen L. Axitinib alone or in combination with chemotherapeutic drugs exerts potent antitumor activity against human gastric cancer cells in vitro and in vivo. J Cancer Res Clin Oncol 2014; 140(9):1575-1583; PMID:24804814; http://dx.doi.org/ 10.1007/s00432-014-1693-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres C, Linares A, Alejandre MJ, Palomino-Morales RJ, Delgado JR, Perales S. Interplay between gemcitabine and erlotinib over pancreatic adenocarcinoma cells. Pancreas 2016; 45(2):269-280; PMID:26495790; http://dx.doi.org/ 10.1097/mpa.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 28.Toyota Y, Iwama H, Kato K, Tani J, Katsura A, Miyata M, Fujiwara S, Fujita K, Sakamoto T, Fujimori T, et al.. Mechanism of gemcitabine-induced suppression of human cholangiocellular carcinoma cell growth. Int J Oncol 2015; 47(4):1293-1302; PMID:26252371; http://dx.doi.org/ 10.3892/ijo.2015.3118 [DOI] [PubMed] [Google Scholar]
- 29.Maseki S, Ijichi K, Nakanishi H, Hasegawa Y, Ogawa T, Murakami S. Efficacy of gemcitabine and cetuximab combination treatment in head and neck squamous cell carcinoma. Mol Clin Oncol 2013; 1(5):918-924; PMID:24649271; http://dx.doi.org/ 10.3892/mco.2013.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem 1998; 273(13):7770-7775; PMID:9516487; http://dx.doi.org/ 10.1074/jbc.273.13.7770 [DOI] [PubMed] [Google Scholar]
- 31.Persad R, Liu C, Wu TT, Houlihan PS, Hamilton SR, Diehl AM, Rashid A. Overexpression of caspase-3 in hepatocellular carcinomas. Modern Pathol 2004; 17(7):861-867; PMID:15098015; http://dx.doi.org/ 10.1038/modpathol.3800146 [DOI] [PubMed] [Google Scholar]
- 32.Wong A, Soo RA, Yong WP, Innocenti F. Clinical pharmacology and pharmacogenetics of gemcitabine. Drug Metab Rev 2009; 41(2):77-88; PMID:19514966; http://dx.doi.org/ 10.1080/03602530902741828 [DOI] [PubMed] [Google Scholar]
- 33.Plunkett W, Huang P, Gandhi V. Preclinical characteristics of gemcitabine. Anti-Cancer Drugs 1995; 6(Suppl 6):7-13; PMID:8718419 [DOI] [PubMed] [Google Scholar]
- 34.Kerr M, Scott HE, Groselj B, Stratford MR, Karaszi K, Sharma NL, Kiltie AE. Deoxycytidine kinase expression underpins response to gemcitabine in bladder cancer. Clin Cancer Res 2014; 20(21):5435-5445; PMID:25224279; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galmarini CM, Clarke ML, Jordheim L, Santos CL, Cros E, Mackey JR, Dumontet C. Resistance to gemcitabine in a human follicular lymphoma cell line is due to partial deletion of the deoxycytidine kinase gene. BMC PHARMACOL 2004; 4:8; PMID:15157282; http://dx.doi.org/ 10.1186/1471-2210-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregoire V, Rosier JF, De Bast M, Bruniaux M, De Coster B, Octave-Prignot M, Scalliet P. Role of deoxycytidine kinase (dCK) activity in gemcitabine's radioenhancement in mice and human cell lines in vitro. Radiotherapy Oncol 2002; 63(3):329-338; PMID:12142097; http://dx.doi.org/ 10.1016/S0167-8140(02)00106-8 [DOI] [PubMed] [Google Scholar]
- 37.Al-Katib AM, Aboukameel A, Ebrahim A, Beck FW, Tekyi-Mensah SE, Raufi A, Ahmed Y, Mandziara M, Kafri Z. Modulation of deoxycytidine kinase (dCK) and glycogen synthase kinase (GSK-3beta) by anti-CD20 (rituximab) and 2-chlorodeoxyadenosine (2-CdA) in human lymphoid malignancies. Exp Hematol Oncol 2014; 3:31; PMID:25937997; http://dx.doi.org/ 10.1186/2162-3619-3-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nancy E, Nianyu L, Bailey K, Fort M, Stevenson R, Jawando R, Salyers K, Jawa V, Narayanan P, Stevens E, et al.. Unexpected thrombocytopenia and anemia in cynomolgus monkeys induced by a therapeutic human monoclonal antibody. Toxicol Pathol 2013; 41(7):951-969; PMID:23475561; http://dx.doi.org/27123085 10.1177/0192623312474727 [DOI] [PubMed] [Google Scholar]
- 39.Aras B, Yerlikaya A. Bortezomib and etoposide combinations exert synergistic effects on the human prostate cancer cell line PC-3. Oncol Letters 2016; 11(5):3179-3184; PMID:27123085; http://dx.doi.org/ 10.3892/ol.2016.4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohseni M, Samadi N, Ghanbari P, Yousefi B, Tabasinezhad M, Sharifi S, Nazemiyeh H. Co-treatment by docetaxel and vinblastine breaks down P-glycoprotein mediated chemo-resistance. Iranian J Basic Medical Sci 2016; 19(3):300-309; PMID:27114800 [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao TT, Wang YY, Zhang Y, Bai CH, Shen XC. Similar to spironolactone, oxymatrine is protective in aldosterone-induced cardiomyocyte injury via inhibition of calpain and apoptosis-inducing factor signaling. PloS One 2014; 9(2):e88856; PMID:24551180; http://dx.doi.org/ 10.1371/journal.pone.0088856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bian HB, Pan X, Yang JS, Wang ZX, De W. Upregulation of microRNA-451 increases cisplatin sensitivity of non-small cell lung cancer cell line (A549). J Exp Clin Cancer Res 2011; 30:20; PMID:21329503; http://dx.doi.org/ 10.1186/1756-9966-30-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Z, Lv G, Li Y, Li E, Li H, Zhou Q, Yang B, Cao W. Enhancement of anti-tumor effects of 5-fluorouracil on hepatocellular carcinoma by low-intensity ultrasound. J Exp Clin Cancer Res 2016; 35(1):71; PMID:27102814; http://dx.doi.org/ 10.1186/s13046-016-0349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elfayomy AK, Almasry SM, Attia GM, Habib FA. Enhanced expression of vascular endothelial growth factor and increased microvascular density in women with endometrial hyperplasia: a possible relationship with uterine natural killer cells. Romanian J Morphol Embryol 2015; 56(2 Suppl):725-734; PMID:26429165 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.