ABSTRACT
Recent years have seen a proliferation of methods leading to successful organ decellularization. In this experiment we examine the feasibility of a decellularized liver construct to support growth of functional multilineage cells. Bio-chamber systems were used to perfuse adult rat livers with 0.1% SDS for 24 hours yielding decellularized liver scaffolds. Initially, we recellularized liver scaffolds using a human tumor cell line (HepG2, introduced via the bile duct). Subsequent studies were performed using either human tumor cells co-cultured with human umbilical vein endothelial cells (HUVECs, introduced via the portal vein) or rat neonatal cell slurry (introduced via the bile duct). Bio-chambers were used to circulate oxygenated growth medium via the portal vein at 37C for 5-7 days. Human HepG2 cells grew readily on the scaffold (n = 20). HepG2 cells co-cultured with HUVECs demonstrated viable human endothelial lining with concurrent hepatocyte growth (n = 10). In the series of neonatal cell slurry infusion (n = 10), distinct foci of neonatal hepatocytes were observed to repopulate the parenchyma of the scaffold. The presence of cholangiocytes was verified by CK-7 positivity. Quantitative albumin measurement from the grafts showed increasing albumin levels after seven days of perfusion. Graft albumin production was higher than that observed in traditional cell culture. This data shows that rat liver scaffolds support human cell ingrowth. The scaffold likewise supported the engraftment and survival of neonatal rat liver cell slurry. Recellularization of liver scaffolds thus presents a promising model for functional liver engineering.
KEYWORDS: liver, bioengineering, decellularization, recellularization, organ scaffold, rat
INTRODUCTION
Whole organ bioengineering has the potential to completely eliminate the donor organ shortage that currently cripples clinical liver transplantation. Perfusion decellularization, which was first demonstrated in a cardiac model,1 has since provided the field of regenerative medicine with a new platform to grow whole organs. Decellularized organ bio-scaffolds may preserve native organ architecture which has been shown to influence cell signaling, function, and differentiation. Furthermore, preservation of vasculature channels can permit rapid exchange of nutrients and oxygen to growing and functioning cells.2 Following the success of perfusion decellularization in the cardiac model, the principle has been applied to other whole organs such as the kidney,3-6 lungs,7-9 and liver.6,10-18
A number of effective strategies for the decellularization of rat livers have been described by Uygun et al.,10 Bao et al.19 and Baptista et al.11, via the portal vein using 1% SDS and 1% Triton X-100,or only 1% Triton X-100 respectively.20 However, the ability to reproducibly generate a robust recellularized construct has proven elusive.7,11,17,21 Early studies attempted to seed the scaffold with primary hepatocytes, and these efforts were followed by the use of stem and progenitor cells.10,11,17,21,22 More recent work has demonstrated the importance of cell-to-cell interactions, thus highlighting the importance of multiple cell lineages in recreating a functional native liver.21
Transplantation of the recellularized construct has resulted in rapid graft thrombosis, likely secondary to exposed collagen in the de-endothelialized vasculature.19 Progress in the field is limited by the inability to generate a fully endothelialized construct containing multiple cell lines comparable to a native organ.
Here we demonstrate a novel method of organ generation, resulting in partially re-endothelialized construct populated with multilineage cells able to exhibit metabolic function.
RESULTS
Decellularization is rapid and reproducible
Perfusion decellularization proceeded uneventfully, resulting in gross (Fig. 2B) and histologic evidence of decellularization (Fig. 3A, H&E) with preservation of the extracellular matrix (Fig. 3B, collagen IV).
FIGURE 2.
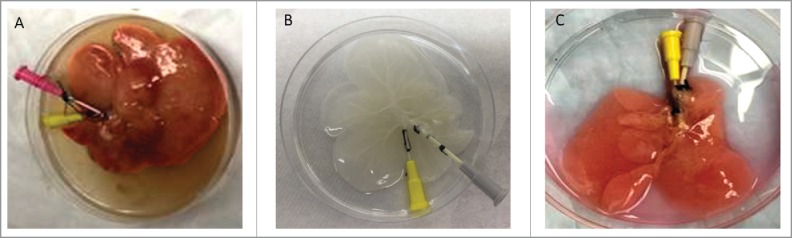
(A) Native liver after flushing shows slightly blanched liver parenchyma, (B) Decellularized liver shows a fully intact, translucent scaffold with a visible vascular tree, (C) Recellularized liver, after 5 days of perfusion, shows loss of translucency with an opaque pink color.
FIGURE 3.
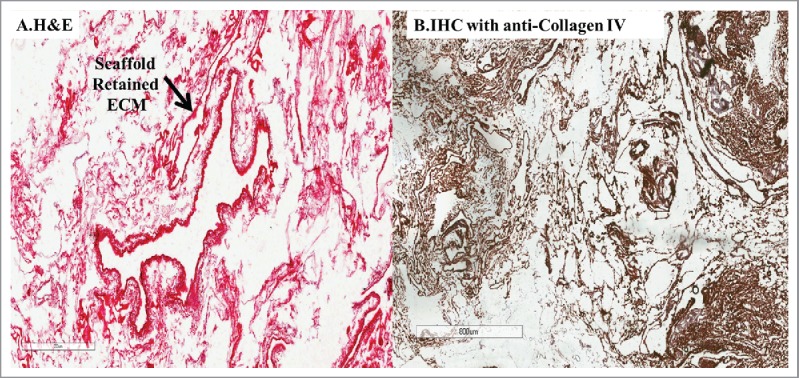
(A) H&E staining of decellularized liver scaffold, after perfusion with 0.1% SDS for 24 hours, showed absence of cells with only ECM visible, (B) IHC staining for type IV collagen on the decellularized scaffold, showed conservation of the ECM of the liver architecture.
Success of parenchymal repopulation depends on route of cell infusion
The introduction of HepG2 cells via the bile duct resulted in even cell distribution throughout the liver parenchyma (Figs. 4A-H&E, 4B-cell tracking, 4C-DAPI). Native liver DAPI staining is noted to be similar to that of decellularized livers scaffolds recellularized via the bile duct (Figs. 5A, 5C DAPI), while there is no evidence of nuclear staining in decellularized livers (Fig. 5B, DAPI).
FIGURE 4.
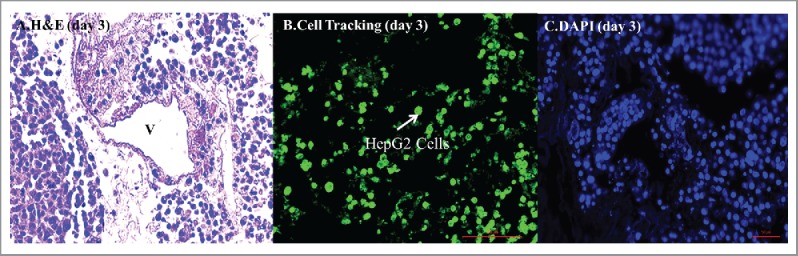
(A) Recellularization through the common bile duct using HepG2 cells 5 days after recellularization H&E staining of the scaffolds injected through the bile duct shows diffuse ubiquitous distribution of hepatocytes in the liver parenchyma while avoiding the vasculature, confirmed by (B) cell tracking and (C) DAPI staining.
FIGURE 5.
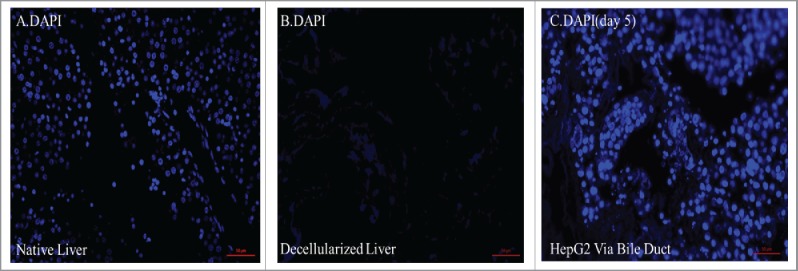
(A) DAPI nuclear staining showed the presence of cells in the native liver, (B) cells were not present within the decellularized construct. (C) DAPI nuclear staining returned following recellularization of livers through the bile duct with HepG2 at day 5 showing cells throughout the construct.
Construct supports multilineage cell growth in varying compartments
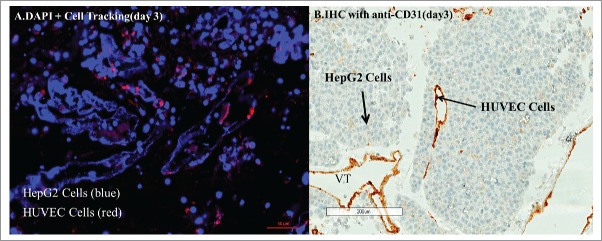
Concomitant introduction of HepG2 cells via the bile duct and HUVECs via the portal vein demonstrated HepG2 cell growth within the liver parenchyma with HUVEC cells lining the endothelium (Figs. 6A CD31, 6B cell tracking).
FIGURE 6.
(A) Cell tracking of HUVECs (red stain) after 3 days of recellularization of the liver scaffold with HUVECs via the portal vein and HepG2 via the common bile duct, counter stained with DAPI (blue), shows clusters of HUVECs separate from most clusters of HepG2 cells. (B) IHC-CD31 staining for HUVEC cells after injection through portal vein, with HepG2 cells through the bile duct at day 3 shows the vasculature is lined by HUVECs (brown staining).
Neonatal cells were isolated successfully, and the construct supported multilineage neonatal cell growth
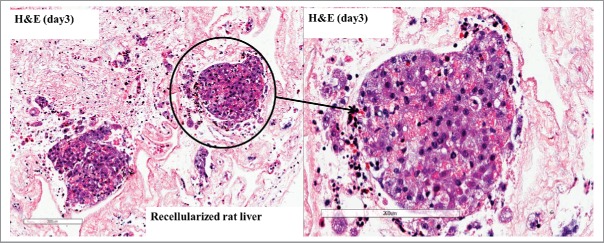
Neonatal cell viability following isolation was 90%, yielding 60–80 × 106 cells per neonatal liver. The liver scaffold supported growth of these cells (Fig. 7 H&E). Scaffolds recellularized with a neonatal liver cell slurry demonstrated the presence of CK-7 positive cells, in comparable distribution to native neonatal liver; indicating the presence of choalngiocytes seeded inside the scaffold. (Fig. 8B).
FIGURE 7.
H&E of the recellularized scaffold with a neonatal liver slurry perfused through the common bile duct at day 3 shows cells distributed through the scaffold.
FIGURE 8.
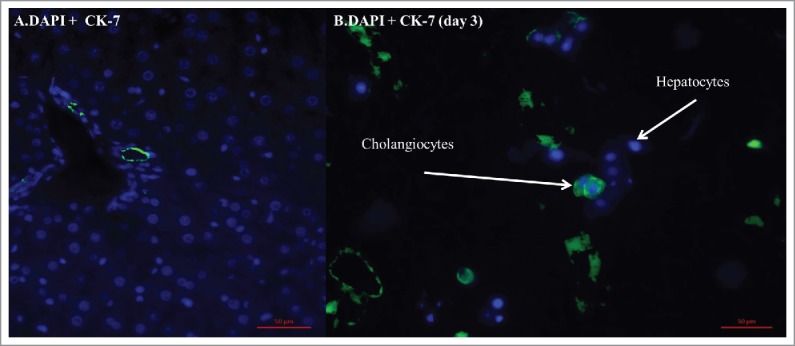
(A) IF-CK7 staining for cholangiocytes (green) within a native neonatal rat liver. (B) IF-CK7 staining for cholangiocytes demonstrates; cholangiocytes are present at day 3 within the rat liver scaffold recellularized with neonatal rat cell.
DNA quantification in decellularized and recellularized grafts
Decellularized scaffolds showed markedly decreased DNA content in the construct after the process was completed, with an average of 5.93 ng/ul +/− 1.37 (n = 10), this compared to native livers at a with an average of 412.9 ng/ul +/− 176.8 (n = 10) (Fig. 9). When scaffolds were recellularized with HepG2 cells through the bile duct and HUVECs through the portal vein increased levels of DNA with an average of 41.77 ng/ul +/−13.34 (n = 10) were noted, while recellularization with neonatal rat liver slurry resulted in slightly higher levels of DNA with an average of 52.3 ng/ul, +/− 34.7 (n = 10).
FIGURE 9.
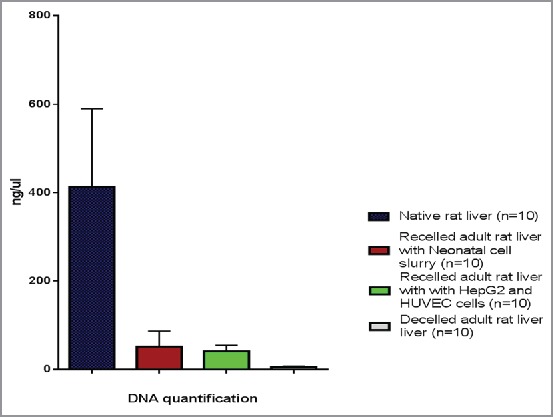
DNA quantification demonstrates that decellularized livers had markedly decreased DNA content and recellularized scaffolds showed a significant increase in DNA content at 5 days post recellularization, compared to native rat livers.
Albumin production
Neonatal liver cell slurry constructs (n = 3) demonstrated increase levels of albumin production during the 7 days of perfusion with an average of 75.9 mg/dl +/− 31.7. Conversely, neonatal liver cells cultured in culture flasks showed a decline in albumin production by day 7 (Fig. 10).
FIGURE 10.
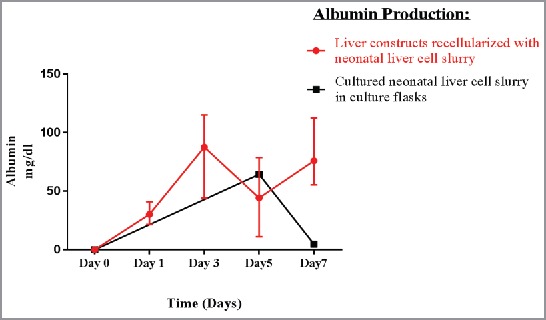
Albumin production increased in effluent media of the constructs throughout perfusion, while decreased in the cell culture media. This suggests that, the scaffold is important to cell function.
Construct Survival
Livers were perfused for 5–7 days; perfusion was terminated electively at day 7(n = 40). At the conclusion of perfusion, the majority of livers showed gross and histologic evidence of cell colonies.
DISCUSSION
There is currently no consensus regarding the optimal method of decellularization. A number of methods have been proposed using different combinations and varying concentrations of SDS and Triton X-100 on different animal models including murine, rabbit, porcine, ferret and rat.8,10,13,23-29 The goal of this process is to remove all cellular material while retaining the native ECM structure, and most established protocols have successfully demonstrated this to be quite possible. It remains to be seen whether the preservation of growth factors and essential proteins found on the ECM will play an important role in promoting the proliferation and function of new parenchymal and non-parenchymal cells.20
Several studies have demonstrated success with decellularization by using a lower concentration of SDS and several concentrations of Triton X-100.25,26 We simplified the decellularization process by only using 0.1% SDS. The effectiveness of this method in removing all cellular material from the ECM was verified by H&E and DNA extraction. The basement membrane remains intact with retained type IV collagen. This method of decellularization demonstrated a gentle and effective, yet less laborious, method of decellularization.
Although innumerable studies have met with success in decellularization, this same level of success has not been encountered with the recellularization process. Several methods of recellularization have been explored to repopulate the liver scaffold in order to facilitate robust cell growth throughout the ECM parenchymal space. These have included injecting cells directly into the parenchymal space, perfusion through the portal vein and perfusion through the hepatic vein.13,19,24,25,27 One method which has not yet been documented is perfusion through the bile duct. The unique architecture of the portal triad suggests that the biliary infusion will theoretically be able to deliver cells throughout the liver ECM.
Our first series of recellularizations was performed as a proof of concept using HepG2 cells, due to ease of cell culture and economic efficiency. In our experience, using the bile duct as a route for recellularization was feasible and showed uniform parenchymal distribution throughout the scaffold. This novel technique of perfusion through the common bile duct may allow future studies to improve the distribution of cells into the parenchymal space.
Two major obstacles to creating a transplantable bioengineered liver are complete re-endothelialization of the vascular tree17 and growing both parenchymal and non-parenchymal cells on the same ECM scaffolding with appropriate organization and cell compartment localization.10 Endothelialization of the vasculature is necessary to prevent thrombosis during transplantation as any exposed collagen will activate platelets and the extrinsic coagulation cascade making successful transplantation impossible. Engrafting both parenchymal and non-parenchymal cells is necessary to establish normal liver function.
We sought to overcome the challenges of re-endothelialization with simultaneous repopulation of the parenchyma on the same ECM by infusing HUVEC cells and HepG2 cells. HUVECs were able to successfully proliferate throughout the vasculature, lining the vessel lumen, while HepG2 cells proliferated in the parenchymal space. This demonstrated the feasibility of engrafting multiple cell types in the appropriate cellular compartment. The ability to concurrently engraft cells of different lineages has the added benefit of having the potential to facilitate cell-to-cell interactions, specifically those between endothelial cells and hepatocytes, which may play a prominent role in the pathways leading to liver regeneration.30
Finally, we used a neonatal rat liver slurry as a novel method to engraft both parenchymal and non-parenchymal cells onto the ECM. Current research has shown success in hepatocyte transplantation using neonatal hepatocytes to assist cellular proliferation of injured livers.31-33 A neonatal liver has the full contingent of pluripotent cells necessary to repopulate an ECM with parenchymal hepatocytes and non-parenchymal cells including sinusoidal endothelial cells, stellate cells and biliary epithelial cells. This method may have a distinct advantage over similar methods using pluripotent stem cells34 as the neonatal liver cells have already traversed to the hepatic lineage and should be primed for cell-to-matrix communication to enhance proliferation, localization, and functionality. We have demonstrated obvious foci of neonatal hepatocytes proliferating in the parenchymal space of the ECM using this strategy. These recellularized constructs demonstrated their ability to sustain metabolic functional activity, as measured by albumin production, when compared to cells cultured in flasks. This suggests that biological constructs are superior to other non-biological scaffolds, perhaps because they provide the adequate environment for seeded cells to differentiate and function.11 This experience forms the bases of future experiments investigating the ability of the multiple cell types present in the neonatal liver cell slurry to repopulate ubiquitously and differentiate in functionally appropriate compartments within the scaffold.
METHODS
Animals
Male Wistar (200–300 g) (Harlan Laboratories) were used for liver procurement. All animals were kept under constant environmental conditions with a day-night cycle and free access to food and water. The rats were euthanized by carbon dioxide inhalation, followed by cervical dislocation. All procedures were performed according to the Office of Animal Welfare Assurance and the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine.
Procurement technique
After euthanasia, rats were positioned in the supine position on a sterile field and the skin prepared with 70% EtOH. A vertical midline incision was made from the xiphoid to the pubis exposing both the abdominal and thoracic cavity. Blood was vented from the right ventricle. A butterfly catheter was inserted into the left ventricle and 60 mL of heparinized phosphate buffered saline (PBS) (10 units/ml) was injected to prevent thrombosis and evacuate any clotted blood. The common bile duct and portal vein were cannulated with 24 gauge and 16 gauge angiocatheters, respectively. The catheters were secured with 3–0 silk ties. The liver was then excised by dividing all remaining attachments. Following explant, an additional 60 mL of heparinized PBS was flushed through the liver via the portal vein until the effluent was clear.
Perfusion chamber
The same closed circuit bioreactor chamber (Fig. 1) was utilized for both decellularization and recellularization. A 1000 mL Nalgene (Thermofisher, CAT# 2116–1000) container with a screw top lid was utilized as the basis of the chamber. Two inlets and one outlet were constructed by drilling three separate holes into the lid. Two way connectors Nalgene™ HDPE Quick Disconnects (Thermofisher, CAT# 6150–0020) were fixed within each of these openings, allowing tubing to be connected at either end while maintaining a closed system. The first inlet was utilized to introduce perfusate into the portal vein, while the second inlet (closed during decellularization) was used to introduce 95% O2 and 5% CO2 directly into the media during recellularization. The outlet line returned perfusate to the pump in our closed loop system. Sterilized flexible silicon tubing (Masterflex, Cat# 96410–17) was used for all perfusion lines. A single Masterflex “Easy-load” pump (Masterflex, Cat# 7553–85) was used and set at a flow rate of 25 mL/min for continuous perfusion. During decellularization the chambers were kept inside a hood at room temperature, while during recellularization the chambers were kept in an incubator at 38°C with 5% CO2 and 95% O2.
FIGURE 1.

Schematic of bioreactor with pump setup. The bioreactor is filled with the medium of choice depending on the experiment. One inlet and one outlet on the top of the bioreactor allows for media to be removed from the reactor and recirculated to the organ scaffold with a roller pump, while the third inlet is used for gas exchange in the closed system during recellularization.
Decellularization
The liver explant was perfused for 60 minutes via the portal vein, vented through the unligated vena cava into the bioreactor, and suspended in 400 mL of 0.1% sodium dodecyl sulfate (SDS) (Bio-Rad, Cat# 1610302). Effluent from the liver was drained into the bioreactor, and then was returned to the pump via the outlet line, thus maintaining a continuous perfusion. After the initial 2 hours, the perfusate was exchanged for another 400 mL of 0.1% SDS and perfused for an additional 2 hours. The perfusate was then exchanged a final time with another 400 mL of 0.1% SDS and perfused overnight (approximately 14 hours). The following day the perfusate was replaced with 400 mL of sterile PBS and perfused for 2 hours followed by a second 400 mL sterile PBS perfusion for 2 hours.
Human hepatocellular carcinoma (HepG2) cell, human umbilical vein endothelial cell (HUVEC) and neonatal cell slurry cultures
In perfusions utilizing isolated HepG2 cells, cells were cultured and perfused utilizing Eagle's minimum essential medium (EMEM) with Earle's balanced salt solution (EBSS) w/L-Glutamine 500 ml (Lonza, Cat# 12–611F) with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Cat# S11150) and 1% penicillin/streptomycin (Lonza, Cat# 17-602E). HUVECs were cultured and passaged utilizing endothelial growth medium (EGM-2) Bulletkit (Lonza, Cat# CC-3162). When both HUVECs and HepG2 cells were seeded onto the same construct, Dulbecco's modified Eagle's medium (DMEM) (Corning, Cat# 10-014-CV) with EGM-2 SingleQuot Kit Supplement & Growth Factors (Lonza, Cat# CC-4176) and 1% penicillin (Lonza, Cat# 17-602E) was utilized. HUVECs and HepG2 cells were co-cultured in this media for 3 days prior to seeding to allow adaption to the media. All HUVECs and HepG2 cells were harvested once they reached a confluency of 80–90%. The neonatal liver cell slurry was cultured utilizing the Hepatocyte Culture Media BulletKit (Lonza, Cat# CC3198) with 1% penicillin/streptomycin (Lonza, Cat# 17-602E).
Neonatal liver cell slurry isolation
Three-day old neonatal Wistar rats (Harlan Laboratories) were used for liver procurement. A modified two-step collagenase digestion method was used to isolate the hepatocytes. Livers were placed in Hanks' Balanced Buffer Solution without calcium, magnesium and phenol red (GIBCO, Cat# 14175095) overnight in a petri dish at 4°C. This was followed by gentle mechanical and enzymatic dissociation utilizing 0.1% collagenase A (Sigma, Cat# 10103578001) while on a shaker. Isolated cells were filtered through a 100 μm cell strainer (Fisher Scientific, Cat# 352360). Viability was assessed using Trypan Blue (Sigma, Cat# T8154). This step was repeated three times at fifteen minute intervals which resulted in most of the slurry dissociating. Immediately following isolation, the neonatal cell slurry was utilized for recellularization as above.
Recellularization
The recellularization chamber was filled with 250 mL of media (specific for the particular cell line used) and provided with a 95% O2/5% CO2 gas mixture via the additional inlet line. The liver was perfused via the portal vein angiocatheter with media alone for 1 hour. After this initial flushing, 100–200 × 106 cells were eluted into 12 mL of media and subsequently injected in 3 mL aliquots every 15 minutes through either the portal vein, common bile duct or both depending on the experiment. After the final aliquot of cells was injected, the liver was placed in a media-filled Petri dish in an incubator at 37°C for 3 hours to facilitate cell adhesion. The liver was then returned to the bioreactor and perfused with media for 5–7 days with a continuous perfusion of media at 25 mL/min. The media was exchanged on day 3 and on day 5.
Cell tracking
HUVECs and HepG2 cells were labeled using CytoPainter cell tracking staining kits to identify their locations in the recellularized scaffold. HepG2 cells were labeled using the green fluorescent staining kit (Abcam, Cat# ab138891) and HUVECs were labeled with the red fluorescent staining kit (Abcam, Cat# ab138893). All specimens were then embedded in paraffin and stained for nuclear DNA with 4′, 6-diamidino-2-phenylindole (DAPI) (Biolegend, Cat# 422801). Slides were examined via fluorescent microscope (ZEISS, Observer.Z1).
Immunohistochemistry
4 μm paraffin-embedded sections were initially deparaffinized: slides were dipped in xylene for 30 minutes, followed by 100% ethanol for 20 minutes, 95% ethanol for 10 minutes, 70% ethanol for 5 minutes, distilled water for 5 minutes, and then phosphate buffered saline (PBS) for 15 minutes. Primary staining antibodies were mouse monoclonal anti-human CD31, endothelial cell clone JC70A (Dako, Cat# IS61030-2) for HUVECs, and anti-collagen IV antibody (Thermofisher Scientific, Cat# MA5-14100). Secondary antibodies used for anti-CD 31 and anti-collagen IV staining were EnVision FLEX Mini Kit, High pH HRP Mouse (Dako, Cat# K8023) and Biotinylated Horse anti-mouse IgG (Vector Laboratories, Cat# BA-2000), respectively.
Immunofluorescence
4um paraffin-embedded sections were initially deparrafainized. The primary staining antibody was rabbit monoclonal anti-CK 7(Abcam, Cat# ab181598). The secondary antibody for anti-CK 7 was goat anti-rabbit IgG H&L (Alexa Fluor® 488) (Abcam, Cat# ab150077). All specimens were stained for nuclear DNA with 4′, 6-diamidino-2-phenylindole (DAPI) (Biolegend, Cat # 422801). Slides were examined via fluorescent microscope (ZEISS, Observer.Z1).
Hematoxylin and eosin staining
H&E staining was processed by the Dermatopathology lab in the Center for Innovative Biomedical Resources at the University of Maryland School of Medicine utilizing standard techniques.
4′, 6-diamidino-2-phenylindole (DAPI) staining
Previously deparaffinized slides were obtained. A working solution of DAPI (Biolegend, Cat#422801) suspended in PBS was prepared. The solution was applied to the slides for 30 seconds and then washed in PBS. The slides were mounted using fluorescent mounting media (Dako, Cat#S3023). Slides were maintained in the dark for four hours and then examined via fluorescent microscope.
DNA quantification
DNA quantification of a 25 mg sample was performed on native liver, neonatal liver, and decellularized and recellularized liver constructs. Each recellularized liver scaffold was sampled three times from a different lobe of the liver. Samples were then processed using the DNeasy® Blood & Tissue Kit (Qiagen, Cat# 69504).
Albumin detection assay
Albumin levels were measured in the effluent media used for perfusing liver constructs (n = 3) repopulated with neonatal liver cell slurry on days 1, 3, 5 and 7. In parallel, cells isolated from the same neonatal livers were cultured in culture flasks and albumin levels were measured on the same time points. The media was partially changed for both the liver constructs and cultured cells on days 3 and 5 to maintain adequate levels of growth factors and nutrients. Albumin was detected in each sample using QuantiChrom™ BCG Albumin Assay Kit (BioAssay systems, Cat # DIAG-250) and the results were adjusted to the baseline albumin content used for perfusion and culture. Samples were analyzed via SpectraMAx M3.
ABBREVIATIONS
- ECM
Extracellular Matrix
- HepG2
Human Hepatocellular Carcinoma Cell
- HUVEC
Human Umbilical Vein Endothelial Cell
- SDS
Sodium Dodecyl Sulfate
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- [1].Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 2008; 14(2):213-21; PMID:18193059; http://dx.doi.org/ 10.1038/nm1684 [DOI] [PubMed] [Google Scholar]
- [2].Knight E, Przyborski S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J Anat 2015; 227(6):746-56; PMID:25411113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ross EA, Abrahamson DR, St John P, Clapp WL, Williams MJ, Terada N, Hamazaki T, Ellison GW, Batich CD. Mouse stem cells seeded into decellularized rat kidney scaffolds endothelialize and remodel basement membranes. Organogenesis 2012; 8(2):49-55; PMID:22692231; http://dx.doi.org/ 10.4161/org.20209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 2013; 19(5):646-51; PMID:23584091; http://dx.doi.org/ 10.1038/nm.3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Katari R, Peloso A, Zambon JP, Soker S, Stratta RJ, Atala A, Orlando G. Renal bioengineering with scaffolds generated from human kidneys. Nephron Exp Nephrol 2014; 126(2):119. PMID:24854653; http://dx.doi.org/ 10.1159/000360684 [DOI] [PubMed] [Google Scholar]
- [6].Wang Y, Bao J, Wu Q, Zhou Y, Li Y, Wu X, Shi Y, Li L, Bu H. Method for perfusion decellularization of porcine whole liver and kidney for use as a scaffold for clinical-scale bioengineering engrafts. Xenotransplantation 2015; 22(1):48-61; PMID:25291435; http://dx.doi.org/ 10.1111/xen.12141 [DOI] [PubMed] [Google Scholar]
- [7].Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, Mayeux JP, Gregory AN, Wang G, Townley IK, et al.. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A 2012; 18(23–24):2437-52; PMID:22764775; http://dx.doi.org/ 10.1089/ten.TEA.2011.0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 2010; 16(8):927-33; PMID:20628374; http://dx.doi.org/ 10.1038/nm.2193 [DOI] [PubMed] [Google Scholar]
- [9].O'Neill JD, Anfang R, Anandappa A, Costa J, Javidfar J, Wobma HM, Singh G, Freytes DO, Bacchetta MD, Sonett JR, et al.. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg 2013; 96(3):1046-55; discussion 1055–6. PMID:23870827; http://dx.doi.org/ 10.1016/j.athoracsur.2013.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, et al.. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 2010; 16(7):814-20; PMID:20543851; http://dx.doi.org/ 10.1038/nm.2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 2011; 53(2):604-17; PMID:21274881 [DOI] [PubMed] [Google Scholar]
- [12].Lang R, Stern MM, Smith L, Liu Y, Bharadwaj S, Liu G, Baptista PM, Bergman CR, Soker S, Yoo JJ, et al.. Three-dimensional culture of hepatocytes on porcine liver tissue-derived extracellular matrix. Biomaterials 2011; 32(29):7042-52; PMID:21723601; http://dx.doi.org/ 10.1016/j.biomaterials.2011.06.005 [DOI] [PubMed] [Google Scholar]
- [13].Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, Holley LS, Gauthier PK. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res 2012; 173(1):e11-25; PMID:22099595; http://dx.doi.org/ 10.1016/j.jss.2011.09.033 [DOI] [PubMed] [Google Scholar]
- [14].Park KM, Woo HM. Systemic decellularization for multi-organ scaffolds in rats. Transplant Proc 2012; 44(4):1151-4; PMID:22564650 [DOI] [PubMed] [Google Scholar]
- [15].Uygun BE, Price G, Saedi N, Izamis ML, Berendsen T, Yarmush M, Uygun K. Decellularization and recellularization of whole livers. J Vis Exp 2011; (48):2394; PMID:21339718; http://dx.doi.org/ 10.3791/2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ren H, Shi X, Tao L, Xiao J, Han B, Zhang Y, Yuan X, Ding Y. Evaluation of two decellularization methods in the development of a whole-organ decellularized rat liver scaffold. Liver Int 2013; 33(3):448-58; PMID:23301992; http://dx.doi.org/ 10.1111/liv.12088 [DOI] [PubMed] [Google Scholar]
- [17].Zhou P, Huang Y, Guo Y, Wang L, Ling C, Guo Q, Wang Y, Zhu S, Fan X, Zhu M, et al.. Decellularization and Recellularization of Rat Livers With Hepatocytes and Endothelial Progenitor Cells. Artif Organs 2016; 40(3):E25-38; PMID:26637111; http://dx.doi.org/ 10.1111/aor.12645 [DOI] [PubMed] [Google Scholar]
- [18].Yagi H, Fukumitsu K, Fukuda K, Kitago M, Shinoda M, Obara H, Itano O, Kawachi S, Tanabe M, Coudriet GM, et al.. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant 2013; 22(2):231-42; PMID:22943797; http://dx.doi.org/ 10.3727/096368912X654939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bao J, Shi Y, Sun H, Yin X, Yang R, Li L, Chen X, Bu H. Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell Transplant 2011; 20(5):753-66; PMID:21054928; http://dx.doi.org/ 10.3727/096368910X536572 [DOI] [PubMed] [Google Scholar]
- [20].Faulk DM, Wildemann JD, Badylak SF. Decellularization and cell seeding of whole liver biologic scaffolds composed of extracellular matrix. J Clin Exp Hepatol 2015; 5(1):69-80; PMID:25941434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kadota Y, Yagi H, Inomata K, Matsubara K, Hibi T, Abe Y, Kitago M, Shinoda M, Obara H, Itano O, et al.. Mesenchymal stem cells support hepatocyte function in engineered liver grafts. Organogenesis 2014; 10(2):268-77; PMID:24488046; http://dx.doi.org/ 10.4161/org.27879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Khan AA, Vishwakarma SK, Bardia A, Venkateshwarulu J. Repopulation of decellularized whole organ scaffold using stem cells: an emerging technology for the development of neo-organ. J Artif Organs 2014; 17(4):291-300; PMID:25030000; http://dx.doi.org/ 10.1007/s10047-014-0780-2 [DOI] [PubMed] [Google Scholar]
- [23].Zhou P, Lessa N, Estrada DC, Severson EB, Lingala S, Zern MA, Nolta JA, Wu J. Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transpl 2011; 17(4):418-27; PMID:21445925; http://dx.doi.org/ 10.1002/lt.22270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Soto-Gutierrez A, Zhang L, Medberry C, Fukumitsu K, Faulk D, Jiang H, Reing J, Gramignoli R, Komori J, Ross M, et al.. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods 2011; 17(6):677-86; PMID:21375407; http://dx.doi.org/ 10.1089/ten.TEC.2010.0698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shupe T, Williams M, Brown A, Willenberg B, Petersen BE. Method for the decellularization of intact rat liver. Organogenesis 2010; 6(2):134-6; PMID:20885860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mirmalek-Sani SH, Sullivan DC, Zimmerman C, Shupe TD, Petersen BE. Immunogenicity of decellularized porcine liver for bioengineered hepatic tissue. Am J Pathol 2013; 183(2):558-65; PMID:23747949; http://dx.doi.org/ 10.1016/j.ajpath.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, Ott HC. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg 2011; 92(3):998-1005; discussion 1005–6. PMID:21871290; http://dx.doi.org/ 10.1016/j.athoracsur.2011.05.018 [DOI] [PubMed] [Google Scholar]
- [28].Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al.. Tissue-engineered lungs for in vivo implantation. Science 2010; 329(5991):538-41; PMID:20576850; http://dx.doi.org/ 10.1126/science.1189345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, Winston S, Wang J, Walls S, Nichols JE, et al.. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A 2010; 16(8):2565-80; PMID:20408765; http://dx.doi.org/ 10.1089/ten.tea.2009.0730 [DOI] [PubMed] [Google Scholar]
- [30].Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 2001; 294(5542):559-63; PMID:11577199; http://dx.doi.org/ 10.1126/science.1063889 [DOI] [PubMed] [Google Scholar]
- [31].Suzuki A, Taniguchi H, Zheng YW, Takada Y, Fukunaga K, Seino K, Yazawa K, Otsuka M, Yoshiki A, Kusakabe M, et al.. Proliferative and functional ability of transplanted murine neonatal hepatocytes in adult livers. Transplant Proc 2000; 32(7):2370-1; PMID:11120204 [DOI] [PubMed] [Google Scholar]
- [32].Tolosa L, Pareja-Ibars E, Donato MT, Cortés M, López S, Jiménez N, Mir J, Castell JV, Gómez-Lechón MJ. Neonatal livers: a source for the isolation of good-performing hepatocytes for cell transplantation. Cell Transplant 2014; 23(10):1229-42; PMID:23803290 ;http://dx.doi.org/ 10.3727/096368913X669743 [DOI] [PubMed] [Google Scholar]
- [33].Ibars EP, Cortes M, Tolosa L, Gómez-Lechón MJ, López S, Castell JV, Mir J. Hepatocyte transplantation program: Lessons learned and future strategies. World J Gastroenterol 2016; 22(2):874-86; PMID:26811633; http://dx.doi.org/ 10.3748/wjg.v22.i2.874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhu S, Rezvani M, Harbell J, Mattis AN, Wolfe AR, Benet LZ, Willenbring H, Ding S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature 2014; 508(7494):93-7; PMID:24572354; http://dx.doi.org/ 10.1038/nature13020 [DOI] [PMC free article] [PubMed] [Google Scholar]