Abstract
The main characteristics of sleep-disordered breathing (SDB) are airflow limitation, chronic intermittent hypoxia, or apnea; which may lead to tissue hypoperfusion and recurrent arousal from sleep. These episodes of hypoxia or apnea can lead to tissue inflammation, and are causal factors of disturbed sleep in both men and women. Several lines of evidence suggest that sleep patterns differ along the lifespan in both male and female subjects, and this may result from the influence of female gonadotropic hormones on sleep. Compared to men, women have more sleep complaints, as women’s sleep is not only influenced by gonadotropins, but also by conditions related to these hormones, such as pregnancy. It is therefore not surprising that sleep disturbances are seen during menopause, too. Factors that may play a role in this type of SDB in women include vasomotor symptoms, changing reproductive hormone levels, circadian rhythm abnormalities, mood disorders, coexistent medical conditions, and lifestyle factors.
Keywords: CPAP, CVD, OSA, SDB, Cardiovascular, Estrogen, Gender, Hormones, Melatonin, Menopause, Perimenopause, Postmenopause, Premenopause, Progesterone, Sleep-disordered breathing, Obstructive sleep apnea, Women
INTRODUCTION
Women are continuously under the influence of hormonal changes from menarche to menopause, and pregnancy also causes hormonal fluctuations. These hormonal changes place women at an increased risk of sleep disturbance. Before menopause the risk of obstructive sleep apnea (OSA) is less than men, but women still experience more sleep problems than men. OSA is one of the major causative factors of disturbed sleep in the general population [1]. Women with OSA are considered a separate group from men with OSA; and women with OSA have polysomnographic differences from men.
OSA is also one of the major risk factors for cardiovascular disease (CVDs) in both men and women [2, 3]. Women with OSA have a higher risk of adverse pregnancy outcomes than women without OSA. Women with OSA also experience more depressive symptoms than men [4] (Figure 1).
Figure 1.
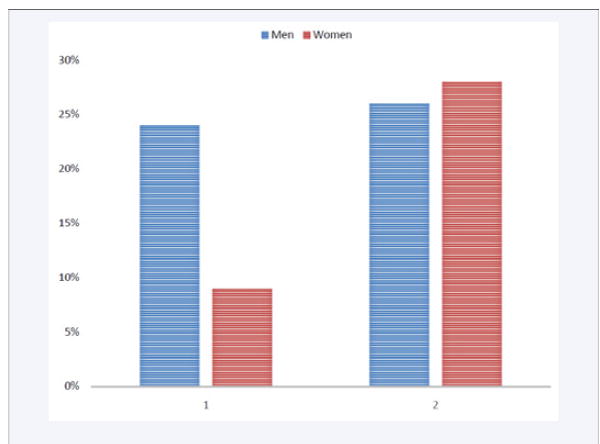
9% to 28% of women and 24% to 26% of males having apneic events in general population.
Although exercise, weight loss, and continuous positive airway pressure (CPAP) therapy have beneficial effects on sleep apnea, CPAP therapy also decreases cardiovascular morbidity and mortality [2,5].
Obstructive Sleep Apnea. Prevalence and Severity
One of the more common sleep disorders is OSA, a sleep-related breathing disorder (SDB) characterized either by intermittent episodes of breathing cessations (hypopnea) or complete collapse of the airway (apnea) [6] (Table 1). There are three categories of sleep apnea:
Table 1.
Categories of sleep apnea [6].
| Apnea/hypopnea index (AHI) /hour | ||
|---|---|---|
| Mild sleep apnea | Moderate sleep apnea | Severe sleep apnea |
| ≥5-15episodes/hour | ≥15-30 episodes/hour | ≥30 episodes/hour |
OSA can lead to oxidative stress, inflammatory processes, endothelial damage, sympathetic activation and metabolic dysregulation that predispose to atherosclerosis, and so OSA is a common cause of systemic hypertension [5] (Table 2).
Table 2.
OSA and increased comorbidities worldwide.
| OSA | Associated Comorbidities | Study Designed | N | Study Title | Future Intervention | Ref |
|---|---|---|---|---|---|---|
| Positive | Depression | Stepwise linear regression analysis. | 1,327 | Prevalence and Predisposing Factors for Depressive Status in Chinese Patients with Obstructive Sleep Apnea: A Large- Sample Survey. | CPAP | (Dai et al, 2016) |
| Positive | COPD | Cross-sectional | 404 | Predictive Factors Warrant Screening for Obstructive Sleep Apnea in COPD: a Taiwan National Survey | CPAP | (Hang et al, 2016) |
| Positive | Type 2 D.M HTN Ischemic Hrt Diseases Stroke Arrhythmias Depression | Cross-sectional | 1,704,905 | The Effect of Sex and Age on the Comorbidity Burden of OSA: An Observational Analysis from a Large Nationwide US Health Claims Database. | CPAP | (Mokhlesi et al, 2016) |
| Positive | HTN (39%) Obesity (34%) Depression (19%) GERD (18%) DM (15%) Hypercholesterolemia (10%) Asthma (4%) | Retrospective | 100 | Comorbidities Associated with Obstructive Sleep Apnea: A Retrospective Study | CPAP | (Pinto et al, 2016) |
| Positive | Nocturnal Heart block | Cross-sectional | 72 | Screening and Managing Obstructive Sleep Apnea in Nocturnal Heart Block Patients: An Observational Study | CPAP | WU et al, 2016) |
In the USA, twenty-five percent of women are at high risk for OSA [2]. In these women, the common symptoms of OSA were habitual snoring (61%), sleep onset insomnia (32%) or maintenance insomnia symptoms (19%),daytime sleepiness (24%), observed apnea (7%), body movement (60%) or restless legs syndrome (RLS) symptoms (33%). Women with obstructive sleep apnea also frequently reported chronic medical disorders [2]. Snoring was the most frequent symptom, where as observed pauses in breath accounted for a minority of the results in this study. Clinically, it indicates that in absence of observed pauses, screening for OSA should not be withheld. Some other symptoms e.g., nocturnal enuresis, are frequent among women with OSA, and clinicians should ask about them during the clinical examination of postmenopausal women [7] (Table 3).
Table 3.
Prevalence of high risk for OSA by (1) BMI or (2) Age [2].
| (1) BMI in women | OSA prevalence % | (2) Age of women | OSA Prevalence % |
|---|---|---|---|
| <25 | 9 | 18-29 | 19 |
| 25-30 | 21 | 30-49 | 25 |
| 31-35 | 57 | 50-64 | 32 |
| 36-40 | 64 | ||
| >40 | 74 |
Studies show a higher risk for OSA when age and BMI both increase together. Women who were older and had a higher BMI showed a higher risk for OSA [2] (Table 4).
Table 4.
Prevalence of high risk for OSA by Age and BMI [2].
| BMI | Age 18-29 yrs, OSA prevalence | Age 30-49 yrs, OSA prevalence | Age 50-64 yrs, OSA prevalence |
|---|---|---|---|
| <25 | 0-5% | 0-8% | 0-12% |
| 25-30 | 0-15% | 0-19% | 0-28% |
| >30 | 0-55% | 0-65% | 0-60% |
During OSA, episodes of hypoxemia can drop the oxyhemoglobin saturation from 95% to 80%, depending on the length of the period of apnea [8]. OSA is an independent risk factor for cardiovascular and cerebrovascular diseases. Due to hypoxia, the oxidative stress leads to overproduction of reactive oxygen species which can cause endothelial dysfunction, resulting in atherosclerosis [9]. The inflammatory marker C-reactive protein (CRP), tumor necrosis factor α (TNFα), and interleukin-6 (IL-6) were increased in patients with obstructive sleep apnea, and they were significantly higher in cases of severe obstructive sleep apnea, where the AHI was 15 or greater [10].
Sleep Disordered Breathing in Women and Cardiovascular Risks
As respiratory events during OSA can trigger an inflammatory process and endothelial damage, this metabolic dysregulation ultimately leads to atherosclerosis in the blood vessels, and is one of the major etiological factors in hypertension and ischemic heart diseases. OSA could be an independent risk factor for left ventricular hypertrophy in women, and is associated with incident heart failure and death among females, but not males. High levels of troponin T (TNT) were also noted in women during the study [11]. Patients with obstructive sleep apnea have worse diastolic dysfunction compared to those without OSA [12]. Obstructive sleep apnea is related to an increased risk of heart failure in women, after adjustment for previous myocardial infarction, menopausal status, age, waist circumference, alcohol or tobacco use, and hormone replacement therapy [3]. One major risk factor for cardiovascular diseases is visceral fat accumulation, and it is closely related to OSA. As increased fat deposition is more noted in men than women, it’s more of a major risk factor for men than women [13].
OSA is also associated with atrial fibrillation and heart failure, independent of obesity [14]. Even in participants with mild to moderate sleep disorders, the odds of hypertension were significantly increased. OSA is a major risk factor in hypertension and cardiovascular morbidity in the general population [15,16], but among OSA patients, treatment of the disease through CPAP can cause a significant reduction in blood pressure [5] (Figure 2).
Figure 2.
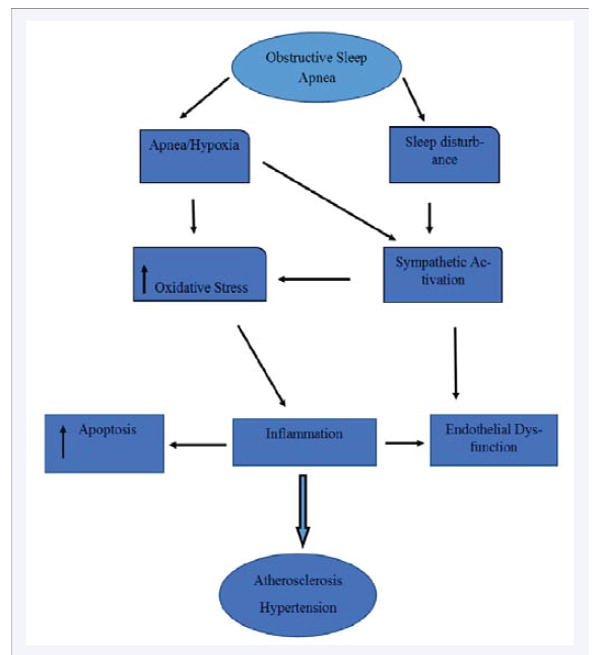
Consequences of sleep apnea/hypoxia in endothelial damage.
Sleep apnea is an independent risk factor for coronary artery disease, regardless of obesity. Coronary artery calcium (CAC), a marker of subclinical coronary artery disease, plays a major role in the formation of coronary artery atherosclerotic plaque. Buildup of CAC increases with the severity of sleep apnea [17]. The early finding of coronary artery atherosclerosis is intima media thickening, which is associated with OSA [18]. Patients with sleep apnea have a high risk of strokes, and women with OSA aged 35 or younger have a greater risk of strokes [19].
Placental Hypoxia
OSA in pregnant women may cause hypoxia in fetoplactenal circulation, as it is shown as normoblastemia and elevated placental carbonic anhydrase IX immunoreactivity, but the causative mechanism should be further investigated [20]. OSA does not have any adverse effects on neonatal and infant neuromotor development; but it may interfere with social development at age one [21].
OSA is also associated with increased pregnancy-related morbidity, preeclampsia and eclampsia, pulmonary embolism, and cardiomyopathy [22]. Further, pregnant women with OSA have a higher risk of gestational hypertension and are more likely to undergo a cesarean section than women without OSA [23]. During pregnancy, women with hypertension and diabetes are at a higher risk of sleep apnea and should regularly be screened for OSA [24]. An inflammatory marker IL-18 is found to be increased in pre-eclampsia patients with OSA, which might increase the risk of pre-eclampsia in OSA [25].
The adverse effects of obstructive sleep apnea are exacerbated by obesity, and there is a fivefold increase in hospital mortality as well. Treating OSA may improve the outcomes of pregnancy [26]. Another study shows that OSA is a risk factor for CVDs and cardiovascular mortality, and increased perinatal morbidity among pregnancies, such as gestational diabetes, gestational hypertension, and pre-eclampsia and eclampsia [27]. Patients with pre-eclampsia had more severe symptoms of OSA [28]. OSA increases during pregnancy, and 10% of women with OSA can develop sleep apnea [29]. There is an increased risk of low birth weight, preterm labor, small infants, caesarean section and preeclampsia, compared to pregnant women without OSA [4, 30-36] (Figure 3).
Figure 3.
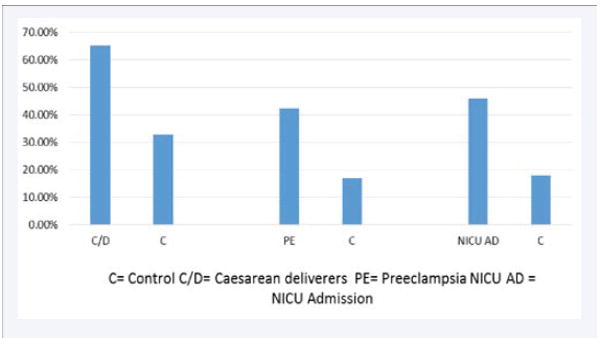
Sleep disordered breathing and adverse pregnancy outcomes.
Sleep Disordered Breathing and Menopause
Almost 50% women experience insomnia in mid-life, whether in initiating or maintaining sleep (or both). 20% of women develop OSA during menopause [37]. OSA in menopause is more significant compared to pre- and perimenopausal women, because in this age group the female physiological system is changed, and due to hormonal fluctuations during this age group (estrogen and progesterone decline is a major contributing factor in disturbed sleep), the decline of hormones pushes women more toward OSA. The incidence of OSA increases in the perimenopausal period and the menopausal transition [1]. Sleep disturbances during menopause have a significant negative impact on women’s social life, physical and psychological health, and workplace productivity [38].
The prevalence of OSA in females rises markedly after menopause. 47% to 67% of post-menopausal women have been found to have OSA. Women tend to gain weight after menopause, and this result in a higher BMI, larger neck circumference, and higher waist-hip ratio, but whether menopause also increases the chances of central obesity is still controversial. In this manner, the upper airway becomes anatomically different after menopause and results in compromised breathing during sleep. Thus, post-menopausal women have a higher prevalence of OSA as compared to pre-menopausal women. However, body weight does not appear to be the only factor responsible for this condition. Despite a comparable body mass index, post-menopausal women had more severe OSA, and spent a larger amount of sleep time with OSA when compared to pre-menopausal females [1] (Figure 4).
Figure 4.
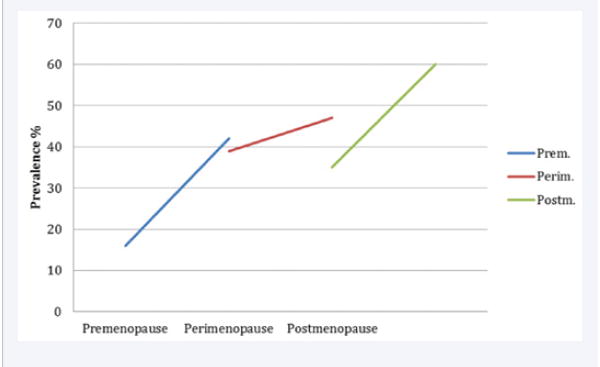
Sleep in Premenopause, Perimenopause, and Postmenopause.
Effect of OSA on Mood and Cognition
Lal et al (2016) reported that women who were at high risk of OSA had more frequent depressive symptoms and had worse cognition compared to women without OSA [39]. However, the prevalence of depressive symptoms was comparable between men and women with OSA [40].
Effect of OSA on Social Life and Work Disability
OSA symptoms can cause a worker to be absent from work for a long period. Daytime sleepiness, apnea/hypopnea episodes, and snoring can make a person unable to work. OSA can also be a cause of permanent disability. OSA is a sleep disorder, and is an independent risk factor in sleep disturbances, disabilities, social life dysfunctions, and driving problems [41, 42].
Night work, Daytime sleepiness, Economic Crises and Overall Health of a Worker
Night shift working can disturb the circadian rhythm and could also be a cause of daytime sleepiness and driving accidents. Poor health is related to poor sleep in night shift workers [43]. Working night shifts affect the psychosocial life, and can also cause increased sleepiness during the daytime. Night shift work impairs attention and performance [44]. In night shift workers, the disruption of the natural circadian rhythm leads to impaired cognitive functioning and excessive daytime sleepiness [45].
Economic crises in any nation put a negative impact on the psychosocial health of workers. Studies showed that economic crises in Italy not only increase the unemployment rate but also compromised the general health of a worker. The economic crises in the year 2007 and 2008, especially in the USA, globally impacted the overall health of workers and became a significant source of stress in the workplace. This global recession had negative effects on workers’ mental and physical health, and the suicide rate increased in this period. This recession involved many countries, and still continues in many of them. People are still feeling the effects of these crises in many parts of the world [46-48].
Treatment of Sleep Disordered breathing in women
Weight Loss and Exercise
Obesity and low levels of physical activity are associated with moderate to severe OSA. Exercise helps in decreasing weight, blood pressure, depression or anxiety, and fatigue [49].
Hormone Replacement Therapy
Hormone replacement therapy can help with other sleep disorders, such as vasomotor symptoms, but are not a helpful strategy in patients with obstructive sleep apnea [50]. Perimenopausal and postmenopausal women are treated with different pharmacological and non-pharmacological measures. The non-pharmacological approach that is widely used is cognitive behavioral therapy for insomnia. Therapeutic techniques may be beneficial for other menopausal symptoms, such as vasomotor symptoms and anxiety or depression [51-53]. Treatment of sleep disorders other than OSA during menopause will improve women’s mental and physical health and increase lifespan [54].
Studies shows that OSA is associated with sexual dysfunction in pre and post-menopausal women, but progesterone can play an important role in reducing sleep disordered breathing and can improve sexual function in pre and post-menopausal women [55].
CPAP
The conventional treatment for OSA is a continuous positive airway pressure (CPAP). This therapy uses a machine to deliver a constant airflow to a patient’s airway via a nasal, facial, or oral interface to maintain airway patency during sleep [56]. CPAP treatment significantly relieved OSA symptoms and improved functional status in both male and females, although women’s pathophysiological conditions and clinical presentations of OSA are different from men, and should be addressed further [54].
Early signs of atherosclerosis are improved if OSA is treated when it is an independent risk factor for atherosclerosis [57]. Untreated OSA in women causes serious cardiovascular outcomes and risk of stroke, but CPAP treatment reduces this [58].Severe OSA is a risk factor for CVD-associated death in women, and adequate treatment may reduce this risk [59]. In the US, among the older adult population, OSA is highly prevalent. Prevention and treatment of OSA can improve the quality of life and reduce disability-related healthcare expenses in this age group [60]. Further, CPAP treatment has been found to bring down the cortisol level among women with OSA [61].
OSA is one major risk factor for cardiovascular diseases. Hypoxia, which is the result of the upper airway obstruction, leads to inflammation in the blood vessels. Continuous CPAP therapy decreases inflammation in the blood vessels. Study shows that continuous CPAP therapy for more than three months improved the endothelial function compared to untreated patients [62].
DISCUSSION
In this article, we discuss the impact of OSA on women’s health and sleep. Good sleep hygiene, regular exercise, weight loss, and a balanced diet can help alleviate OSA in women. In addition, an adherence to CPAP therapy can provide a definite treatment to this breathing disorder, and can help maintain better sleep in women of childbearing age and menopausal women. OSA should be diagnosed early to get a definite diagnosis, to avoid confusing it with other medical disorders such as depression or anxiety due to similarities in the presentation of the symptoms. Early diagnosis and treatment of this condition can relieve sleep problems and the associated anxiety or depression disorders as well.
During pregnancy, OSA increases the risk of pre-eclampsia, eclampsia, caesarian deliveries, and neonatal death; as well as exaggerating hypertension and CVDs in the mother. In menopausal women, sleep apnea disturbs women’s sleep almost in the same ratio as men’s due to hormonal fluctuations (estrogen and progesterone decline in a menopausal woman). Post-menopausal women are at increased risk for vasomotor symptoms and other medical conditions, and have a greater likelihood to develop sleep disturbances. These sleep problems could be the causative factors of anxiety and depression, which need a proper diagnosis and treatment. Good sleep hygiene and appropriate treatment can provide a better night of sleep. CPAP therapy can improve the health in sleep apnea patients if it is used properly and regularly. Counseling regarding weight loss, diet, and exercise should be given at every office visit. There is a need to do more research for sleep disordered breathing in women so that women’s health could be improved without compromising quality of life.
CONCLUSION
There is a great amount of research being conducted on OSA in men and women in order to provide better treatment for patients, especially in primary care settings, not only in developed countries but in underdeveloped regions as well. OSA could be a major risk factor for significant morbidity and mortality, and is a known risk factor for hypertension. OSA prevalence is the same in Pakistan as in western countries (9%= women= 24% men) [63].
OSA not only disturbs sleep, but is also a major risk factor of CVDs. There is more anxiety and depression in women than men, depending on the severity of sleep apnea [64]. Exercise and weight loss is helpful in reducing the risk of sleep apnea. A lack of exercise and obesity can place patients at greater risk [65, 66].
Good sleep is essential for living a healthy life, and is an important determinant of life expectancy for women in their reproductive years, and during menopause and post-menopause. But, in menopause, women’s sleep is disturbed due to a lack of estrogen and progesterone [67]. Also, in menopause, sleep disorders are also associated with vasomotor symptoms, which could also be a causative factor of disturbed sleep in postmenopausal women [68-73].
Acknowledgments
FUNDING
This work is supported by the following funding agencies: R25-HL105444 and R25-HL116378 (NHLBI); R01-MD007716 (NIMHD) to GJL. However, the funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
DISCLOSURE STATEMENT
The authors have read the journal’s policy and have the following potential conflicts: This study was not an industry-supported study. S.R. Pandi-Perumal is a stockholder and the President and Chief Executive Officer of Somnogen Canada Inc., a Canadian Corporation. This does not alter his adherence to all of the journal policies. He declares that he has no competing interests that might be perceived to influence the content of this article. All remaining authors declare that they have no proprietary, financial, professional, nor any other personal interest of any nature or kind in any product or services and/or company that could be construed or considered to be a potential conflict of interest that might have influenced the views expressed in this manuscript.
References
- 1.Jehan S, Masters-Isarilov A, Salifu I, Zizi F, Jean-Louis G, Pandi-Perumal SR, et al. Sleep Disorders in Postmenopausal Women. J Sleep Disord Ther. 2015;4 [PMC free article] [PubMed] [Google Scholar]
- 2.Kapsimalis F, Kryger M. Sleep breathing disorders in the U.S. female population. J Womens Health (Larchmt) 2009;18:1211–1219. doi: 10.1089/jwh.2008.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ljunggren M, Byberg L, Theorell-Haglow J, Lindahl B, Michaelsson K, Lindberg E, et al. Increased risk of heart failure in women with symptoms of sleep-disordered breathing. Sleep Med. 2016;17:32–37. doi: 10.1016/j.sleep.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Louis J, Auckley D, Miladinovic B, Shepherd A, Mencin P, Kumar D, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120:1085–1092. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sánchez-de-la-Torre M, Campos-Rodriguez F, Barbé F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1:61–72. doi: 10.1016/S2213-2600(12)70051-6. [DOI] [PubMed] [Google Scholar]
- 6.Jenner R, Lorenzi-Filho G, Drager LF. Cardiovascular impact of obstructive sleep apnea: does gender matter. Expert Rev Cardiovasc Ther. 2014;12:281–283. doi: 10.1586/14779072.2014.884460. [DOI] [PubMed] [Google Scholar]
- 7.Koo P, McCool FD, Hale L, Stone K, Eaton CB. Association of obstructive sleep apnea risk factors with nocturnal enuresis in postmenopausal women. Menopause. 2016;23:175–182. doi: 10.1097/GME.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golbidi S, Badran M, Ayas N, Laher I. Cardiovascular consequences of sleep apnea. Lung. 2012;190:113–132. doi: 10.1007/s00408-011-9340-1. [DOI] [PubMed] [Google Scholar]
- 9.Badran M, Ayas N, Laher I. Cardiovascular complications of sleep apnea: role of oxidative stress. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/985258. 985258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svensson M, Venge P, Janson C, Lindberg E. Relationship between sleep-disordered breathing and markers of systemic inflammation in women from the general population. J Sleep Res. 2012;21:147–154. doi: 10.1111/j.1365-2869.2011.00946.x. [DOI] [PubMed] [Google Scholar]
- 11.Roca GQ, Redline S, Claggett B, Bello N, Ballantyne CM, Solomon SD, et al. Sex-Specific Association of Sleep Apnea Severity With Subclinical Myocardial Injury, Ventricular Hypertrophy, and Heart Failure Risk in a Community-Dwelling Cohort The Atherosclerosis Risk in Communities-Sleep Heart Health Study. Circulation. 2015;132:1329–1337. doi: 10.1161/CIRCULATIONAHA.115.016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glantz H, Thunström E, Johansson MC, Wallentin Guron C, Uzel H, Ejdebäck J et al. Obstructive sleep apnea is independently associated with worse diastolic function in coronary artery disease. Sleep Med. 2015;16:160–167. doi: 10.1016/j.sleep.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Harada Y, Oga T, Chihara Y, Azuma M, Murase K, Toyama Y et al. Differences in associations between visceral fat accumulation and obstructive sleep apnea by sex. Ann Am Thorac Soc. 2014;11:383–391. doi: 10.1513/AnnalsATS.201306-182OC. [DOI] [PubMed] [Google Scholar]
- 14.Otto ME, Belohlavek M, Romero-Corral A, Gami AS, Gilman G, Svatikova A et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298–1302. doi: 10.1016/j.amjcard.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 15.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 16.Xu HJ, Lan XF, Li QY, Zhou LN, Zhang XJ, Guo Q, et al. Factors affecting blood pressure profile in pre and postmenopausal women with obstructive sleep apnea hypopnea syndrome. Sleep Breath. 2015;19:169–174. doi: 10.1007/s11325-014-0983-z. [DOI] [PubMed] [Google Scholar]
- 17.Luyster FS, Kip KE, Aiyer AN, Reis SE, Strollo PJ., Jr Relation of obstructive sleep apnea to coronary artery calcium in non-obese versus obese men and women aged 45-75 years. Am J Cardiol. 2014;114:1690–1694. doi: 10.1016/j.amjcard.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salepci B, Fidan A, Ketenci SC, Parmaksiz ET, Comert SS, Kiral N, et al. The effect of obstructive sleep apnea syndrome and snoring severity to intima-media thickening of carotid artery. Sleep Breath. 2015;19:239–246. doi: 10.1007/s11325-014-1002-0. [DOI] [PubMed] [Google Scholar]
- 19.Chang CC, Chuang HC, Lin CL, Sung FC, Chang YJ, Hsu CY, et al. High incidence of stroke in young women with sleep apnea syndrome. Sleep Med. 2014;15:410–414. doi: 10.1016/j.sleep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Ravishankar S, Bourjeily G, Lambert-Messerlian G, He M, De Paepe ME, Gundogan F. Evidence of Placental Hypoxia in Maternal Sleep Disordered Breathing. Pediatr Dev Pathol. 2015;18:380–386. doi: 10.2350/15-06-1647-OA.1. [DOI] [PubMed] [Google Scholar]
- 21.Tauman R, Zuk L, Uliel-Sibony S, Ascher-Landsberg J, Katsav S, Farber M, et al. The effect of maternal sleep-disordered breathing on the infant’s neurodevelopment. Am J Obstet Gynecol. 2015;212:656. doi: 10.1016/j.ajog.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Yinon D, Lowenstein L, Suraya S, Beloosesky R, Zmora O, Malhotra A, et al. Pre-eclampsia is associated with sleep-disordered breathing and endothelial dysfunction. Eur Respir J. 2006;27:328–333. doi: 10.1183/09031936.06.00010905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma SK, Nehra A, Sinha S, Soneja M, Sunesh K, Sreenivas V, et al. Sleep disorders in pregnancy and their association with pregnancy outcomes: a prospective observational study. Sleep Breath. 2016;20:87–93. doi: 10.1007/s11325-015-1188-9. [DOI] [PubMed] [Google Scholar]
- 24.Karaduman M, Sari O, Aydoğan U, Akpak YK, Semiz A, Yilanlioğlu NC, et al. Evaluation of obstructive sleep apnea symptoms in pregnant women with chronic disease. J Matern Fetal Neonatal Med. 2016;29:3379–3385. doi: 10.3109/14767058.2015.1127346. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Zhang XL, Yuan P, Wang ZG, Chui XC, Peng JJ, et al. Clinical significance and intervention study of serum interleukin 18 in preeclampsia patients with coexisting obstructive sleep apnea. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:503–506. [PubMed] [Google Scholar]
- 26.Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998-2009. Sleep. 2014;37:843–849. doi: 10.5665/sleep.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cain MA, Ricciuti J, Louis JM. Sleep-disordered breathing and future cardiovascular disease risk. Semin Perinatol. 2015;39:304–309. doi: 10.1053/j.semperi.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Facco FL, Lappen J, Lim C, Zee PC, Grobman WA. Preeclampsia and Sleep-Disordered Breathing: A Case-Control Study. Pregnancy Hypertens. 2013;3:133–139. doi: 10.1016/j.preghy.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28:1299–1305. doi: 10.1093/sleep/28.10.1299. [DOI] [PubMed] [Google Scholar]
- 30.Blyton DM, Skilton MR, Edwards N, Hennessy A, Celermajer DS, Sullivan CE, et al. Treatment of sleep disordered breathing reverses low fetal activity levels in preeclampsia. Sleep. 2013;36:15–21. doi: 10.5665/sleep.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36:849–855. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 32.Champagne KA, Kimoff RJ, Barriga PC, Schwartzman K. Sleep disordered breathing in women of childbearing age & during pregnancy. Indian J Med Res. 2010;131:285–301. [PubMed] [Google Scholar]
- 33.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC, et al. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206:136. doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Fung AM, Wilson DL, Lappas M, Howard M, Barnes M, O’Donoghue F, et al. Effects of maternal obstructive sleep apnoea on fetal growth: a prospective cohort study. PLoS One. 2013;8:68057. doi: 10.1371/journal.pone.0068057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izci-Balserak B, Pien GW. Sleep-disordered breathing and pregnancy: potential mechanisms and evidence for maternal and fetal morbidity. Curr Opin Pulm Med. 2010;16:574–582. doi: 10.1097/MCP.0b013e32833f0d55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202:261. doi: 10.1016/j.ajog.2009.10.867. [DOI] [PubMed] [Google Scholar]
- 37.Hall MH, Kline CE, Nowakowski S. Insomnia and sleep apnea in midlife women: prevalence and consequences to health and functioning. F1000Prime Rep. 2015;7:63. doi: 10.12703/P7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolge SC, Balkrishnan R, Kannan H, Seal B, Drake CL. Burden associated with chronic sleep maintenance insomnia characterized by nighttime awakenings among women with menopausal symptoms. Menopause. 2010;17:80–86. doi: 10.1097/gme.0b013e3181b4c286. [DOI] [PubMed] [Google Scholar]
- 39.Lal C, DiBartolo MM, Kumbhare S, Strange C, Joseph JE. Impact of obstructive sleep apnea syndrome on cognition in early postmenopausal women. Sleep Breath. 2016;20:621–626. doi: 10.1007/s11325-015-1261-4. [DOI] [PubMed] [Google Scholar]
- 40.Edwards C, Mukherjee S, Simpson L, Palmer LJ, Almeida OP, Hillman DR, et al. Depressive Symptoms before and after Treatment of Obstructive Sleep Apnea in Men and Women. J Clin Sleep Med. 2015;11:1029–1038. doi: 10.5664/jcsm.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garbarino S, Guglielmi O, Sanna A, Mancardi GL, Magnavita N. Risk of Occupational Accidents in Workers with Obstructive Sleep Apnea: Systematic Review and Meta-analysis. Sleep. 2016;39:1211–1218. doi: 10.5665/sleep.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sivertsen B, Overland S, Glozier N, Bjorvatn B, Maeland JG, Mykletun A, et al. The effect of OSAS on sick leave and work disability. Eur Respir J. 2008;32:1497–1503. doi: 10.1183/09031936.00044908. [DOI] [PubMed] [Google Scholar]
- 43.Mucci N, Montalti M, Bini C, Cupelli V, Arcangeli G. Evaluation of the impact of night-work on health in a population of workers in Tuscany. G Ital Med Lav Ergon. 2012;34:381–384. [PubMed] [Google Scholar]
- 44.Narciso FV, Barela JA, Aguiar SA, Carvalho AN, Tufik S, de Mello MT. Effects of Shift Work on the Postural and Psychomotor Performance of Night Workers. PLoS One. 2016;11:0151609. doi: 10.1371/journal.pone.0151609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kazemi R, Haidarimoghadam R, Motamedzadeh M, Golmohamadi R, Soltanian A, Zoghipaydar MR, et al. Effects of Shift Work on Cognitive Performance, Sleep Quality, and Sleepiness among Petrochemical Control Room Operators. J Circadian Rhythms. 2016;14:1. doi: 10.5334/jcr.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S, Guo WJ, Tsang A, Mak AD, Wu J, Ng KL, et al. Evidence for the 2008 economic crisis exacerbating depression in Hong Kong. J Affect Disord. 2010;126:125–133. doi: 10.1016/j.jad.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Mucci N, Giorgi G, Cupelli V, Gioffrè PA, Rosati MV, Tomei F, et al. Work-related stress assessment in a population of Italian workers. The Stress Questionnaire. Sci Total Environ. 2015;502:673–679. doi: 10.1016/j.scitotenv.2014.09.069. [DOI] [PubMed] [Google Scholar]
- 48.Mucci N, Giorgi G, Roncaioli M, Fiz Perez J, Arcangeli G. The correlation between stress and economic crisis: a systematic review. Neuropsychiatr Dis Treat. 2016;12:983–993. doi: 10.2147/NDT.S98525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simpson L, McArdle N, Eastwood PR, Ward KL, Cooper MN, Wilson AC, et al. Physical Inactivity Is Associated with Moderate-Severe Obstructive Sleep Apnea. J Clin Sleep Med. 2015;11:1091–1099. doi: 10.5664/jcsm.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirer AG, Peppard PE, Palta M, Benca RM, Rasmuson A, Young T, et al. Menopausal hormone therapy and sleep-disordered breathing: evidence for a healthy user bias. Ann Epidemiol. 2015;25:779–784. doi: 10.1016/j.annepidem.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carpenter JS, Neal JG, Payne J, Kimmick G, Storniolo AM. Cognitive- behavioral intervention for hot flashes. Oncol Nurs Forum. 2007;34:37. doi: 10.1188/07.ONF.E1-E8. [DOI] [PubMed] [Google Scholar]
- 52.Keefer L, Blanchard EB. A behavioral group treatment program for menopausal hot flashes: results of a pilot study. Appl Psychophysiol Biofeedback. 2005;30:21–30. doi: 10.1007/s10484-005-2171-1. [DOI] [PubMed] [Google Scholar]
- 53.Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, et al. Sleep disturbance during the menopausal transition in a multiethnic community sample of women. Sleep. 2008;31:979–990. [PMC free article] [PubMed] [Google Scholar]
- 54.Ye L, Pien GW, Ratcliffe SJ, Weaver TE. Gender differences in obstructive sleep apnea and treatment response to continuous positive airway pressure. J Clin Sleep Med. 2009;5:512–518. [PMC free article] [PubMed] [Google Scholar]
- 55.Stavaras C, Pastaka C, Papala M, Gravas S, Tzortzis V, Melekos M, et al. Sexual function in pre- and post-menopausal women with obstructive sleep apnea syndrome. Int J Impot Res. 2012;24:228–233. doi: 10.1038/ijir.2012.20. [DOI] [PubMed] [Google Scholar]
- 56.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 58.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nuñez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV, et al. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189:1544–1550. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- 59.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM, et al. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156:115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 60.Spira AP, Stone KL, Rebok GW, Punjabi NM, Redline S, Ancoli-Israel S, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62:2040–2046. doi: 10.1111/jgs.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kritikou I, Basta M, Vgontzas AN, Pejovic S, Fernandez-Mendoza J, Liao D, et al. Sleep apnoea and the hypothalamic-pituitary-adrenal axis in men and women: effects of continuous positive airway pressure. Eur Respir J. 2016;47:531–540. doi: 10.1183/13993003.00319-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ciccone MM, Favale S, Scicchitano P, Mangini F, Mitacchione G, Gadaleta F, et al. Reversibility of the endothelial dysfunction after CPAP therapy in OSAS patients. Int J Cardiol. 2012;158:383–386. doi: 10.1016/j.ijcard.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 63.Haqqee R, Hussain SF, Mujib M, Ahmad HR. A hospital based preliminary report on sleep disordered breathing in Pakistani population. J Ayub Med Coll Abbottabad. 2002;14:2–4. [PubMed] [Google Scholar]
- 64.Aloia MS, Arnedt JT, Smith L, Skrekas J, Stanchina M, Millman RP, et al. Examining the construct of depression in obstructive sleep apnea syndrome. Sleep Med. 2005;6:115–121. doi: 10.1016/j.sleep.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Awad KM, Malhotra A, Barnet JH, Quan SF, Peppard PE. Exercise is associated with a reduced incidence of sleep-disordered breathing. Am J Med. 2012;125:485–490. doi: 10.1016/j.amjmed.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27:480–484. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 67.Landis CA, Moe KE. Sleep and menopause. Nurs Clin North Am. 2004;39:97–115. doi: 10.1016/j.cnur.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Moline ML, Broch L, Zak R, Gross V. Sleep in women across the life cycle from adulthood through menopause. Sleep Med Rev. 2003;7:155–177. doi: 10.1053/smrv.2001.0228. [DOI] [PubMed] [Google Scholar]
- 69.Dai Y, Li X, Zhang X, Wang S, Sang J, Tian X, et al. Prevalence and Predisposing Factors for Depressive Status in Chinese Patients with Obstructive Sleep Apnoea: A Large-Sample Survey. PLoS One. 2016;11:0149939. doi: 10.1371/journal.pone.0149939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hang LW, Hsu JY, Chang CJ, Wang HC, Cheng SL, Lin C, et al. Predictive factors warrant screening for obstructive sleep apnea in COPD: a Taiwan National Survey. Int J Chron Obstruct Pulmon Dis. 2016;11:665–673. doi: 10.2147/COPD.S96504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J. 2016;47:1162–1169. doi: 10.1183/13993003.01618-2015. [DOI] [PubMed] [Google Scholar]
- 72.Pinto JA, Ribeiro DK, Cavallini AF, Duarte C, Freitas GS. Comorbidities Associated with Obstructive Sleep Apnea: a Retrospective Study. Int Arch Otorhinolaryngol. 2016;20:145–150. doi: 10.1055/s-0036-1579546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu X, Liu Z, Chang SC, Fu C. Screening and managing obstructive sleep apnoea in nocturnal heart block patients: an observational study. Respir Res. 2016;17:16. doi: 10.1186/s12931-016-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


