Abstract
Background and Objectives
New-onset diabetes and concomitant weight loss occurring several months before the clinical presentation of pancreatic cancer (PC) appear to be paraneoplastic phenomena caused by tumor-secreted products. Our recent findings have shown exosomal adrenomedullin (AM) is important in development of diabetes in PC. Adipose tissue lipolysis might explain early onset weight loss in PC. We hypothesize that lipolysis-inducing cargo is carried in exosomes shed by PC and is responsible for the paraneoplastic effects. Therefore, in this study we investigate if exosomes secreted by PC induce lipolysis in adipocytes and explore the role of AM in PC exosomes as the mediator of this lipolysis.
Design
Exosomes from patient derived cell lines and from plasma of PC patients and non-PC controls were isolated and characterized. Differentiated murine (3T3-L1) and human adipocytes were exposed to these exosomes to study lipolysis. Glycerol assay and western blotting were used to study lipolysis. Duolink assay was used to study AM and AM receptor (ADMR) interaction in adipocytes treated with exosomes.
Results
In murine and human adipocytes we found that both AM and PC-exosomes promoted lipolysis, which was abrogated by AM receptor blockade. AM interacted with its receptor on the adipocytes, activated p38 and ERK1/2 MAPKs and promoted lipolysis by phosphorylating hormone sensitive lipase. PKH67 labeled PC-exosomes were readily internalized into adipocytes and involved both caveolin and macropinocytosis as possible mechanisms for endocytosis.
Conclusions
Pancreatic cancer secreted exosomes induce lipolysis in subcutaneous adipose tissue; exosomal adrenomedullin is a candidate mediator of this effect.
Keywords: Adrenomedullin, pancreatic cancer, exosome, paraneoplastic syndrome, lipolysis
INTRODUCTION
Most pancreatic cancer (PC) patients die within 6 months of cancer diagnosis.1 Though multifactorial in etiology, a major contributor to early mortality in PC is the profound and rapid loss of adipose tissue and skeletal muscle mass. In one study, stabilization of weight improved survival in PC2 and pharmacological blockade of soft tissue loss dramatically prolonged survival in animal models.3, 4
Our goal is to understand the molecular mechanisms of adipose tissue loss in PC in order to develop novel strategies for prevention, which could lead to significantly improved survival rates for PC. Early weight loss in PC begins many months prior to the onset of cachexia.5 This weight loss is paradoxically associated with development of new-onset diabetes5 and is likely due to loss of adipose tissue, as it is not associated with any symptoms of muscle loss, a hallmark of cachexia. We proposed that new-onset diabetes and the associated weight loss in PC are paraneoplastic phenomena caused by tumor-secreted products.5, 6. A number of candidate “lipid mobilizing factors” in PC have been proposed. However, blood levels of these factors, such as TNF-α7, 8, and IL-69 are not consistently elevated in all PC patients. We therefore sought to determine how PC causes fat loss. Our study is based on the novel premise that the effects of PC on remote tissues, including adipose tissue, are mediated by exosomes shed by PC.
Extracellular vesicles (EVs) are membranous vesicles that are secreted from a variety of different cell types. There are three classes of EVs: microvesicles, apoptotic blebs, and exosomes. Exosomes (30–150 nm), the smallest of the three are shed in large numbers by tumor cells into conditioned media and plasma.10, 11 We have identified exosomes in conditioned media from PC cell lines, primary PC cell lines and in the plasma of PC patients. Reports, including ours,12, 13 suggest that tumor-derived exosomes can effectively target cells through the transfer of their contents (e.g., bioactive lipids, receptors, mRNAs, miRNA). Cancer exosomes by virtue of their cargo can have local and systemic effects. Based on our data, we propose that PC-exosomes contain lipolysis-inducing factors that lead to loss of adipose tissue in PC.
The exosomal lipolysis-inducing factor that we studied is adrenomedullin (AM), a 52 amino acid peptide ubiquitously expressed in many cells including adipocytes14 serves as a modulator of various physiological functions.15 We proposed that tumor-derived AM is a candidate mediator of β-cell dysfunction leading to new-onset diabetes in PC.6 Since diabetes and weight loss in PC are frequent and often occur concurrently, we tested for exosomal AM as a mediator of adipose tissue loss in PC.
AM is known to stimulate adipose tissue lipolysis in an autocrine manner as AM receptors are found on the surface of normal adipocytes.16. Binding of AM to its receptor (ADMR) on the adipocyte surface activates extracellular signal regulated (ERK1 and ERK2) and p38 MAPK pathways in addition to the commonly known cAMP/PKA pathway.17, 18
The role of exosomes in lipolysis and its effectors is unknown. In the present study we observed that lipolysis in 3T3-L1 cells and in human adipocytes increased upon exposure to PC-exosomes. Increase in lipolysis is attributed to AM contained within PC-exosomes, as AM receptor blockade led to abrogation of the effect of exosomes and activation of ERK1/2 and p38 MAPKs in both murine and human adipocytes. Our data supports the notion that PC-induced lipolysis is mediated by exosomal AM.
MATERIAL AND METHODS
All research protocols involving human subjects were approved by Mayo Clinic IRB. Blood collection, tissue sample procurement, handling, processing and storage of samples and informed consents were per Mayo Clinic IRB policies. Subcutaneous adipose tissue was obtained from non-cancer subjects undergoing abdominal surgeries. Normal pancreas sections were obtained from autopsy specimens. Serum samples for exosome isolation and pancreatic resection specimen for cell line generation and immunohistochemistry were obtained under Mayo Clinic Pancreatic Cancer SPORE.
Reagents
AM peptide (1–52 AA, Cat# AS-60447) and AM inhibitor (22–52 AA AM peptide, Cat# AS-60449 receptor antagonist) were from Anaspec. TNF alpha (Cat# T0157, Sigma), p38 MAPK inhibitor (SB203580, Cat# BML-E1286-001, Enzo Life Sciences) or MEK1/2 inhibitor (U0126, Cat# 9903S, Cell Signaling).
PC patient-derived cell lines
PC patient-derived cell lines were developed from xenografts of pancreatic cancer tissue from resection specimen. The following cell lines have been developed and characterized in our laboratory as reported previously19: 6741-1 (MCPAN014), 6413-1 (MCPAN013), 5822-1 (MCPAN008), 7135-1, 7426-1, 7291-1. The numbers correspond to unique patient de-identifiers. These were cultured in DMEM/F12 media. Characterization of the cell lines: 6741-1 (MCPAN014), 6413-1 (MCPAN013), 5822-1 (MCPAN008)) includes sequencing and DNA fingerprinting.
Isolation of exosomes
Cells were grown to 70–80% confluence, washed with PBS and cultured in respective media with microvesicle-free fetal bovine serum for 72 hours. Conditioned media was collected, centrifuged twice at 3,000 rpm for 10 minutes at 4°C to remove debris. The supernatant was centrifuged at 100,000 × g for 60 minutes to pellet exosomes. Exosomes were washed in PBS and centrifuged at 100,000 × g for 60 minutes. The pellet was resuspended in PBS. Exosomes from 6 ml of heparinized blood from human subjects were isolated by the same method. Protein concentrations of exosomes were taken using the Bicinchoninic acid assay (Pierce).
Isolation and differentiation of human preadipocytes from human adipose tissue
Preadipocytes were isolated from subcutaneous adipose tissue as per protocol20, 21 and differentiated for 15 days in differentiation media21 with addition of fetuin (1mg/ml).
Culture and differentiation of 3T3-L1 preadipocytes
Murine 3T3-L1 preadipocytes were obtained from the American Type Tissue Culture Repository and cultured in DMEM containing 25 mM glucose and 10% fetal calf serum. Cells were differentiated as per Zen-Bio’s recommendation.22 Experiments were conducted with differentiated adipocytes (10–14 days).
Oil Red O staining of adipocytes
Adipocytes were stained with Oil Red O solution (Sigma).17 Images were taken on Axiovert microscope with a Prog Res C3 camera.
NanoTracker analysis
Exosome fractions were analyzed on the NanoSight NS300. At least 1000 particles were counted (5 videos, each of 60 second duration) and analyzed with Nanoparticle Tracking Analysis (NTA) 2.3 software.
Internalization of labeled exosomes
3T3-L1 and human adipocytes were seeded in a 12 well plate (~30,000/well) and differentiated. Cells were treated with 50 µg PKH67 (Sigma)-labeled PANC1 exosomes for 3 hours at 4°C and 37°C and imaged using Zeiss LSM 780 confocal microscope.
Western blot analysis
Cell lysates of adipocytes were prepared in NP40 buffer and quantified using the bicinchoninic acid assay (Pierce). Exosomes were solubilized in NP40 buffer. Each well was loaded with 15 µg protein unless otherwise specified and probed overnight at 4°C with primary antibodies. The following antibodies were used: human adrenomedullin (Phoenix Pharmaceuticals), p44/42 MAPK (Cell Signaling Technology), phospho-p44/42 (Cell Signaling Technology), phospho-p38 (Cell Signaling Technology), p38 (Cell Signaling Technology), HSL (Cell Signaling Technology), phospho-HSL (Cell Signaling Technology), TNF-α (Cell Signaling Technology), β-actin as the loading control (Santa Cruz Biotechnology, Inc.), p16 (M-156) sc-1207 rabbit polyclonal (Santa Cruz Biotechnology, Inc.), CA 19-9 (Abcam), TSG101 (Novus Biologicals), Alix (Thermo scientific) and Anti-CD63 antibody (MEM-259) mouse monoclonal (Abcam).
Immunoprecipitation
IP for AM was performed as per recommendations of catch and release V2.0 millipore kit. The exosomes (amount as indicated) were solubilized in NP40 buffer. IP was carried overnight at 4°C and precipitated proteins were eluted under denaturing conditions per manufacturer’s protocols. The eluted proteins were run in two parallel sets in the same 4–15% gel- one set was probed with AM antibody (1:1000) and the other set with AM antibody and blocking peptide (AM peptide) pre-incubated at final concentration of 10 µg/ml with AM antibody for 2 hours at room temperature.
Duolink™ Assay System
Duolink™ fluorescence method was employed as per manufacturer’s recommendations (O link Biosciences). 3T3-L1 adipocytes (10,000 cells) were seeded and differentiated in chamber slides. Adipocytes were serum-starved overnight (DMEM and 2% BSA) and treated in microvesicle-free DMEM with 0.5% BSA for 24 hours. Human adrenomedullin antibody (Phoenix Pharmaceuticals) and CRLR antibody (Santa Cruz Technology, Inc) were used to assess AM/ADMR interactions. Images were taken with Zeiss LSM 780 confocal microscope.
Glycerol estimation assay
Differentiated adipocytes were serum-starved overnight in DMEM and 2% BSA (fatty acid free) and treated in microvesicle-free DMEM (phenol red free) with 0.5% BSA for 24 hours. Supernatants were assayed for glycerol levels with free glycerol reagent (Sigma) as per manufacturer’s recommendations.
Immunohistochemical staining
Immunohistochemistry for AM expression was performed on PC tissues (n= 56) and histologically normal pancreas (n=2). Phospho-perilipin expression was done on pancreatic tissues of cancer patients (n=5) and on histologically normal pancreas (n=3). Human adrenomedullin antibody (Phoenix Pharmaceuticals) and anti-phospho-perilipin1 serine 522 antibody (Vala Sciences) were used. Slides were counterstained with hematoxylin and images were taken with axioplan light microscope (Zeiss).
Mechanism for exosomes endocytosis
Differentiated human adipocytes, serum-starved overnight in DMEM with 2% BSA were treated with inhibitors of different endocytic pathways. Cells were then washed with PBS and treated with PKH67-labelled exosomes overnight. Chlorpromazine was used at 5, 10 and 20 µM concentration. 5-(N-Ethyl-N-isopropyl) amiloride was used at 10, 50 and 100 µM concentration. Nystatin was used at three different concentrations of 10, 27 and 54 µM. Nocodazole was used at concentrations of 1, 5 and 10 µM.
Statistical analysis
GraphPad Prism software was used for all analysis. P values less than 0.05 were considered statistically significant. Mean±SEM are reported. Student t-test or ANOVA with Tukey’s post-hoc adjustment were used unless otherwise specified.
RESULTS
Characterization of PC-derived extracellular vesicles
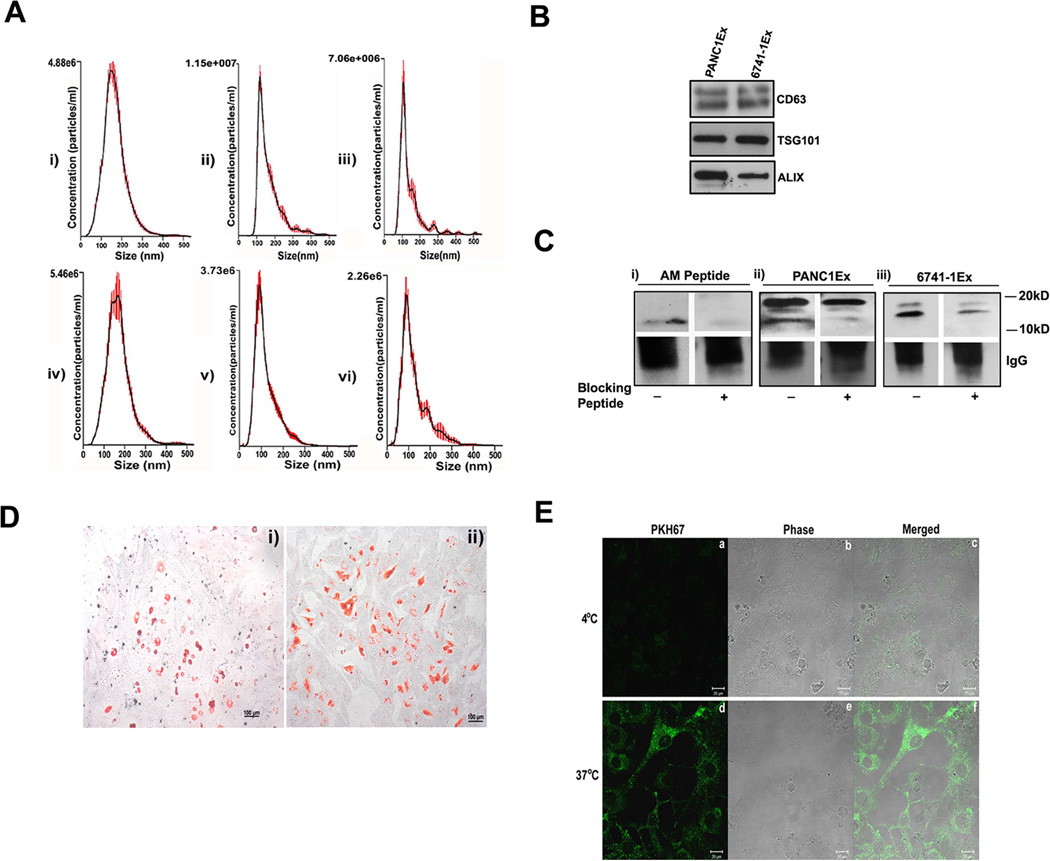
NanoTracker analysis of microvesicle fraction (isolated as detailed in methods) confirmed size distribution consistent with exosomes (shown in Figure 1A: i–iii for exosomes from PC cell lines; Figure 1A: iv–vi and Supp. Figure 1A: i for exosomes from PC patient plasma and Supp. Figure 1A: ii from non-PC control subject plasma). Further, exosomal identity markers CD63, TSG101 and ALIX were confirmed by western blot analysis (shown in Figure 1B for exosomes from PC cell lines and Supp. Figure 1B for exosomes from patient plasma).
Figure 1. Characterization of PC-derived extracellular vesicles.
(A, i–iii) The size distribution of microvesicle fraction of pancreatic cancer cell lines (n=3) and (A, iv–vi) of plasma from PC patients (n=3). (B) Western blot showing expression of exosomal markers: CD63, TSG101 and Alix in PC cell line derived exosomes (PANC1Ex & 6741-1Ex). (C) Western blot showing immuno-precipitated forms of AM; with i) AM peptide (6ngs) ii) PANC1Ex (100µg) and iii) 6741-1Ex (200µg). AM peptide used as a positive control. (+) refers to Blocking of AM antibody with (+) and without AM blocking peptide (−). (D) Oil red O staining of differentiated: i) 3T3-L1 and ii) human adipocytes. (E) Confocal microscopy showing fluorescence (a,d), phase contrast (b,e) and merged images (c,f) of uptake of labeled exosomes in 3T3-L1 cells at 4 and 37°C.
PC-derived exosomes contain AM
Immunoprecipitation of solubilized exosomes with AM antibody showed bands migrating to 20kD and 10kD in PANC-1 (Figure 1C: ii) and to 18kD and 14kD in 6741-1 (Figure 1C: iii). Each of these bands (Figure 1C) were blocked by pre-incubation of AM antibody with AM peptide prior to exposure including blocking of the 10kD band of AM peptide itself (Figure 1C: i) confirming the specificity of the bands. In exosomes from PC patient plasma (PC), only mature AM band (10kD) migrating at the same level as mature AM peptide run as parallel control was detected by immunoprecipitation (Supp. Figure 1C: i). Similarly, AM band at 10kD was also detected in exosomes from non-PC control subjects (Control) with levels varying from very low compared to that in PC (Supp. Figure 1C: i) to comparable level as that in PC patient exosomes (Supp. Figure 1C: ii). Quantification for AM N≥6 in each group is shown in (Supp. Figure 1C: iii) demonstrating increased levels in exosome from PC patients.
Cell line PC-exosomes enter adipocytes
Oil Red O staining of differentiated murine (3T3-L1) and human adipocytes revealed dye-stained oil droplets confirming differentiation of adipocytes (Figure 1D: i–ii). Internalization of PKH67 labeled PC-cell line exosomes was observed into 3T3-L1 (Figure 1E) and human adipocytes (Supp. Figure 2) when imaged at 3 hours after treatment with exosomes. No uptake occurred at 4°C. Further, we observed onset of exosome internalization in human adipocytes within 15 minutes of treatment by fluorescent microscopy (data not shown).
AM and PC-exosomes induce lipolysis in subcutaneous adipocytes
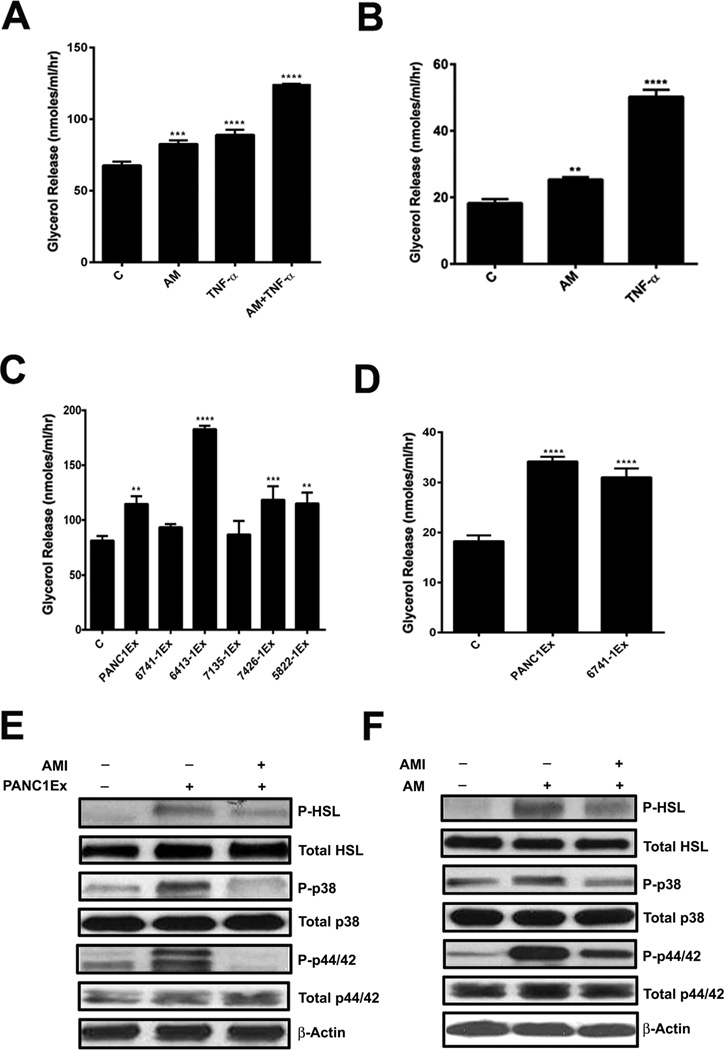
Lypolysis was assessed by glycerol release and western blot of markers of activated lipolysis (phosphorylation of Hormone Sensitive Lipase- HSL, p38 and p44/42 MAP Kinase/ERK). Glycerol assay in the supernatant demonstrated lipolysis in response to AM peptide in 3T3-L1 cells (Figure 2A) human adipocytes (Figure 2B) to PC-exosomes from various cell lines in 3T3-L1 cells (Figure 2C) and human adipocytes (Figure 2D). Treatment with TNF-α, a known lipolytic agent, was used as a positive control to validate the assay.23 Further, western blotting of treated human adipocytes demonstrated activation of lipolysis by PANC-1 exosomes (Figure 2E) and AM peptide (Figure 2F). These findings were further confirmed by an increase in free fatty acid levels measured in supernatant after treatment with AM peptide and PC cell line exosomes (Supp. Figure 3A). No increase in cell death was observed in human adipocytes after treatment with exosomes as confirmed by MTT assay (Supp. Figure 3B).
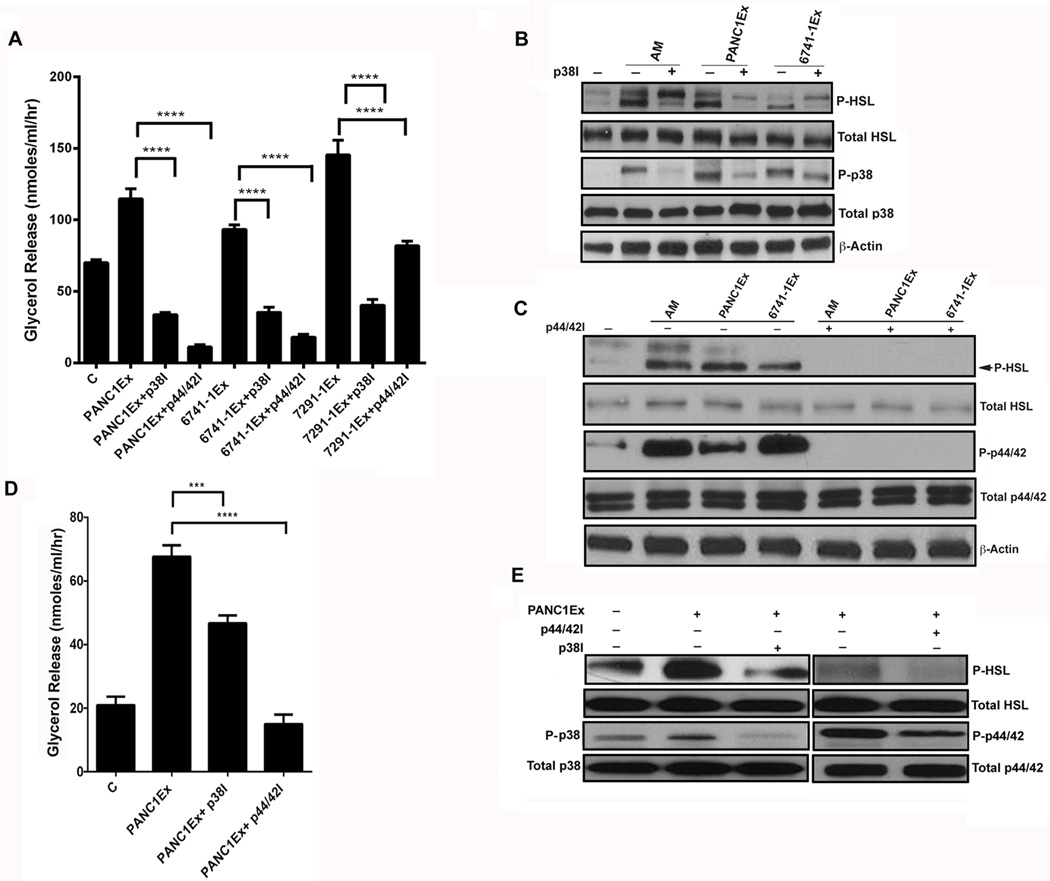
Figure 2. AM and PC-exosomes induce lipolysis in adipocyte.
(A) Glycerol levels in 3T3-L1 cells treated with AM (10nM), TNF-α (50ng/ml), or AM (10nM) & TNF-α (50ng/ml) and compared to untreated cells, C. (B) Glycerol levels in human adipocytes treated with AM (10nM) or TNF-α (50ng/ml) and compared to untreated cells, C. (C) Glycerol levels in 3T3-L1 cells treated with exosomes from a commercial PC cell line (PANC1Ex) or PC xenograft cell lines (6741-1Ex, 6413-1Ex, 7135-1Ex, 7426-1Ex, & 5822-1Ex) and compared to untreated cells, C. (D) Glycerol levels in human adipocytes treated with PANC1 and 6741-1 exosomes. (E) Western blot showing expression of P-HSL upon treatment with PANC1Ex concomitant with activation of MAPKs (p38 &p44/42) in human adipocytes. (F) Western blot showing expression of phospho-HSL upon treatment with AM peptide concomitant with activation of MAPKs (p38 & p44/42). All cells were treated for 24 hours with either the lipolytic agent(s) or exosomes. **, P<0.01; ***, P<0.001; ****, P<0.0001.
Exosomes from PC patients activate lipolysis in subcutaneous adipocytes
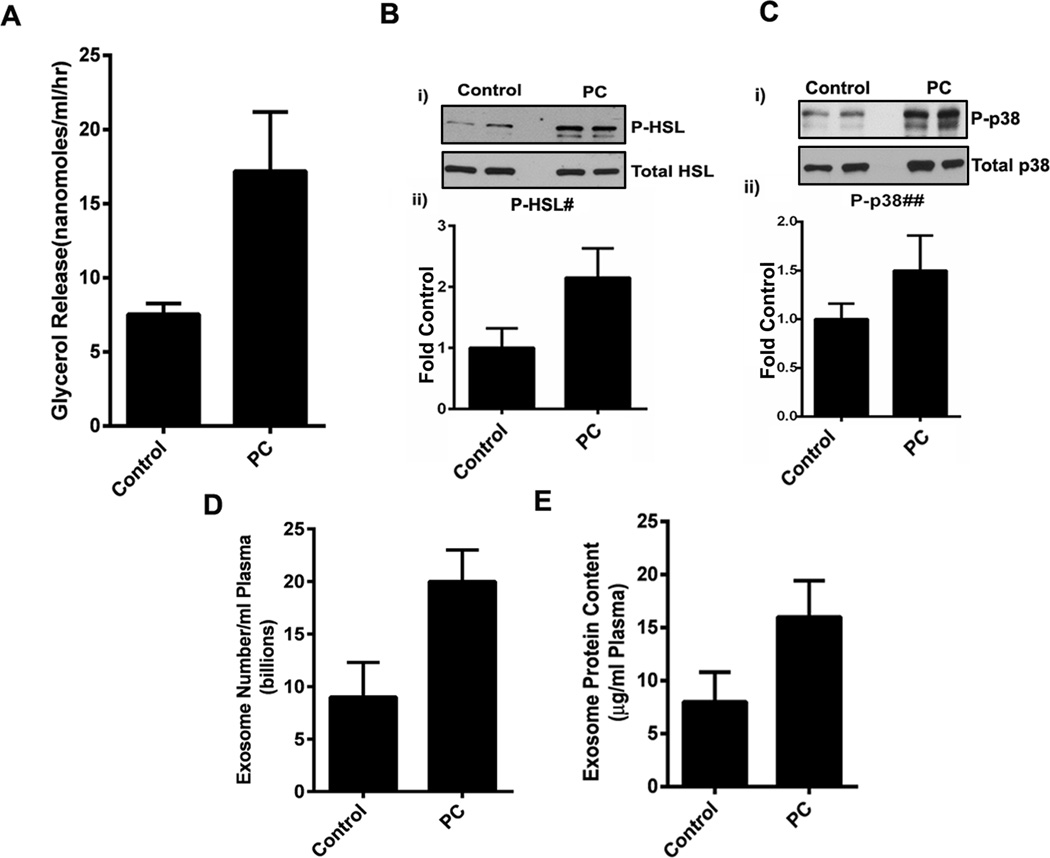
Exosomes from PC patients activated lipolysis in human subcutaneous adipocytes compared to exosomes from non-PC control subjects as shown by glycerol assay in the supernatants (Figure 3A and Supp. Figure 4A) and phosphorylation of HSL and p38 in the treated adipocytes (Figure 3B, 3C and Supp. Figure 4B). As previously shown, AM levels were significantly elevated (1.7±.29 fold) in exosomes from PC patients compared to those from non-PC control subjects although some degree of variability was observed between subjects (Supp. Figure 1C: iii). Further, we observed that PC patients had a mean excess of 11 billion exosomes per ml of plasma (Figure 3D) and a mean excess of 8µg protein in the exosomal fraction per ml of plasma (Figure 3E).
Figure 3. Exosomes from PC patients activate lipolysis in subcutaneous adipocytes.
(A) Comparison of mean glycerol release in human adipocytes treated with exosomes (25µg) from PC patients (PC; n=3) and non-PC control subjects (Control; n=3). (B, i) Western blot showing expression of phospho HSL in adipocytes treated with exosomes from PC patients (PC; n=2) in comparison to non-PC control subjects (Control; n=2). (B, ii) Densitometry analysis of mean phospho-HSL/total HSL band intensity in non-PC control subjects (Control) vs PC patients (PC) as fold control. #, p=0.08. (C, i) Western blot showing expression of phospho p38 in human adipocytes treated with PC exosomes (PC; n=4) in comparison to exosomes from non-PC control subjects (Control; n=4). (C, ii) Densitometry analysis representing mean phospho-p38/total p38 expression in non-PC control subjects (Control: n=4) vs PC patients (PC; n=4) represented as fold control. ##, p=0.38. (D) Comparison of mean exosome number in non-PC control subjects (Control; n=5) with PC patients per ml of plasma (PC: n=7), p=0.03. (E) Comparison of protein content (µg) per ml of plasma in non-PC control subjects (Control: n=5) with PC patients per ml of plasma (PC; n=7), p=0.09.
PC-exosomal AM interacts with AM receptor (ADMR) in adipocytes
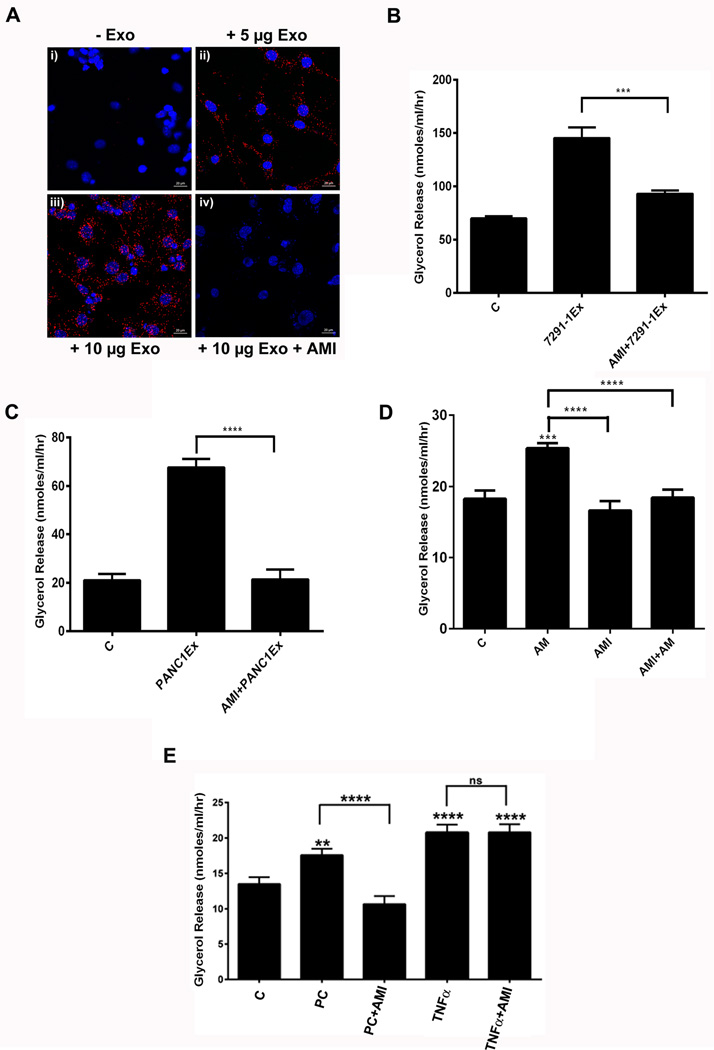
The Duolink Assay showed increasing AM/ADMR interactions, characterized by red punctate dots, in the presence of increasing amounts of PC-exosomes (Figure 4A: ii–iii). PC-exosomes treatment with AM inhibitor abolished the receptor/ligand interactions, as indicated by less red punctate dots (Figure 4A: iv). In addition, treatment with AM and exosomes did not change Crlr and Ramp2 (known components of functional ADMR) expression in 3T3-L1 adipocytes in comparison to control, confirming that the lipolytic effect is due to an enhanced interaction of exosomal AM and ADMR on the adipocytes and not merely due to increase in AM receptors (Supp. Figure 5A–B). Further, no change in expression of perilipin 1 (PLIN1) was observed on treatment with AM suggesting that lack of effects at the transcription level (Supp. Figure 5C). Phosphorylation of perilipin is a known initial event in lipolysis pathway.
Figure 4. Lipolysis induced by PC exosomes is dependent on exosomal AM.
(A) AM/ADMR interactions assessed by Duolink assay in 3T3-L1 cells: i) untreated group, ii) PC exosomes (5µg), iii) PC-exosomes (10µg), or iv) pretreatment with AMI (1nM) for 3 hours followed by addition of PC-exosomes (10µg) for 24 hours. (B) Glycerol release in 3T3-L1 adipocytes treated with exosomes isolated from a PC cell line (7291-1Ex) with and without AMI (1nM). (C) Human adipocytes pre-treated with AMI (10nM) for 3 hours followed by treatment with PC-exosomes (PANC1Ex) or treated with PANC1Ex alone for 24 hours. (D) Glycerol release in human adipocytes treated with AM (10nM), AMI (1nM), and AMI (1nM) and AM (10nM) for 24 hours. (E) Glycerol release in human adipocytes treated with PC patient exosomes (PC), PC exosomes (PC) with AMI (10nM), TNF-α (50ng/ml) and TNF-α (50ng/ml) with AMI (10nM) for 24 hrs. C refers to untreated human adipocytes. **, P<0.01; ****, p<0.0001.
Lypolysis induced by PC exosomes is dependent on exosomal AM
AM receptor antagonist abrogated lipolysis in 3T3-TL cells (Figure 4B) and human subcutaneous adipocytes (Figure 4C, 2E) induced by PC cell line exosomes. In parallel experiments, AM receptor antagonist also abrogated lipolysis induced by AM peptide (Figure 4D, 2F) confirming that AM receptor antagonist was functional in the experimental setting but did not change the lipolytic effect of TNF-α (Figure 4E) thus excluding non-specific effects of the antagonist. Similarly, lipolysis induced by PC patient exosome was also abrogated by AM receptor antagonist (Figure 4E).
Lipolytic effect of AM is mediated through MAP kinases p38 and ERK 1/2 in subcutaneous adipocytes
The western blot data (Figure 2 E–F, 3 B–C, Supp. Figure 4B) suggest that lipolysis induced by AM/ADMR interaction involves phosphorylation of HSL. Phosphorylation of HSL is dependent on activation of p38 and p44/42 MAP Kinase/ERK pathways, the known parallel lipolytic pathways in adipocytes. We further tested this possibility by using p38 MAPK inhibitor (SB203580) or MEK1/2 (p44/42) inhibitor (U0126). We observed partial inhibition of lipolysis induced by AM and PC-exosomes in the presence of p38 MAPK inhibitor (SB203580) in 3T3-L1 (Figure 5 A, B). Near complete abrogation of lipolysis induced by AM and PC-exosomes was observed in the presence of MEK1/2 inhibitor (U0126) in 3T3-L1 (Figure 5 A, C). Similar results were seen in human adipocytes (Figure 5 D, E). Additional, cytotoxic effects of these inhibitors were excluded by MTT assay (Supp. Figure 3B).
Figure 5. Lipolytic effect of AM is mediated through MAP kinases (p38 and ERK1/2) in subcutaneous adipocytes.
(A) Glycerol release in 3T3-L1 cells treated with PC-exosomes (PANC1Ex, 6741-1Ex & 7291-1Ex., 50 µg) and inhibitors of the MAPK pathway: p38 inhibitor (p38I; 10 µM), p44/42 inhibitor (p44/42I; 10 µM) and compared to exosomes only treatment group, C refers to untreated cells. ****, P<0.0001. B) Western blot showing phosphorylation of p38 and HSL in cells treated with p38I and AM (10 nM) or p38I and PC-exosomes (PANC1Ex & 6741-1Ex) for 24 hours. (C) Western blot showing phosphorylation of p44/42 and HSL in cells treated with p44/42I (10 µM) with AM peptide (10 nM), p44/42I with PC-exosomes (PANC1Ex & 6741-1Ex) and compared to AM, PANC1Ex and 6741-1Ex treated groups respectively. + and − refers to treatment with and without inhibitor. (D) Glycerol release in human adipocytes treated with p38 inhibitor (p38I; 20 µM) and p44/42 inhibitor (p44/42I; 10 µM) for 6 hours followed by incubation with PANC1Ex (50 µg) or treatment with PANC1Ex (50 µg) alone for 24 hours. (B) Western blot depicting phosphorylated levels of p38, p44/42, and HSL in human adipocytes treated with inhibitor (+) and without inhibitor in the presence (+) and absence (−) of PANC1 exosomes.
PC-exosomes do not contain TNFα at functionally significant levels for lipolysis
We measured TNFα by ELISA in PC-cell line exosomes and PC exosomes from patient plasma to investigate possible confounding of the lipolysis results by TNFα. As shown in Supp. Figure 6A, in 50 µg exosomes (same amount used for treatment in the lipolysis experiments), TNFα level was < 10 pg. However, no glycerol release was seen at 10 pg or even 100 pg of TNFα (experiment carried out in 1 ml, same volume as in the lipolysis experiments) but lipolysis was seen at 50 ng of TNFα (Supp. Figure 6B). This confirms lack of functionality with the levels of TNFα in PC-exosomes in regard to lipolysis.
Confirmation of AM upregulation in PC resection specimen
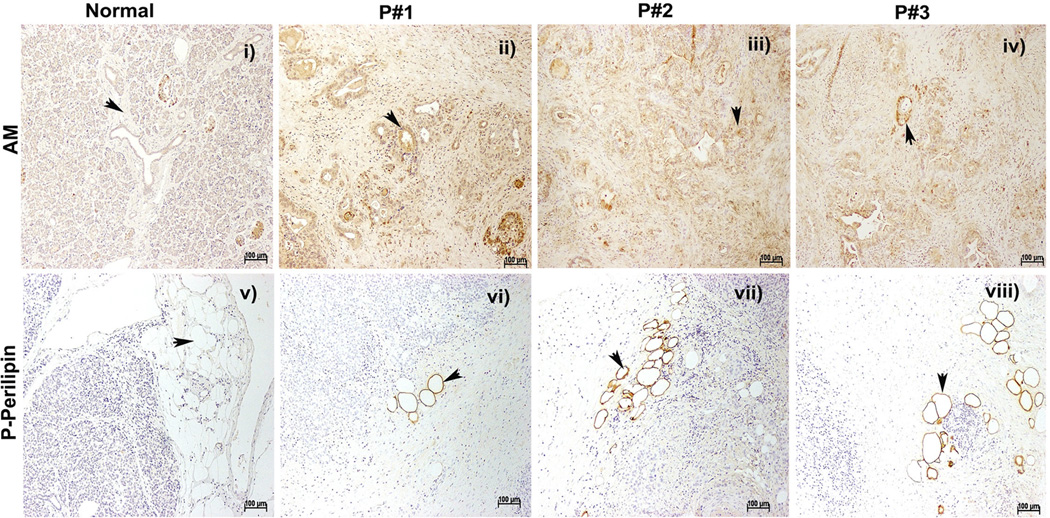
Immunohistochemical staining for AM revealed increased expression of AM in PC in the resection specimens of pancreas from PC patients (Figure 6: ii–iv) in comparison to normal pancreas from autopsy specimens (Figure 6: i). Interestingly, phosphorylation of perilipin, an early event in lipolysis, was observed in the peri-pancreatic adipose tissue in the PC resections (Figure 6: vi–viii) but not in the normal pancreas (Figure 6: v).
Figure 6. Confirmation of AM up-regulation in PC resection specimen.
Immunohistochemical staining for AM in tumor affected pancreatic ducts and cancerous regions in PC patients (P#1–3, ii–iv) in comparison to normal subjects (i).
Immunohistochemical staining for phospho-perilipin 1 in lipid droplets of adipocytes of PC patients (P#1–3, vi–viii) in comparison to pancreatic tissues from normal autopsies (v), positive staining represented by arrowheads.
Mechanism for entry of PC-exosomes into adipocytes
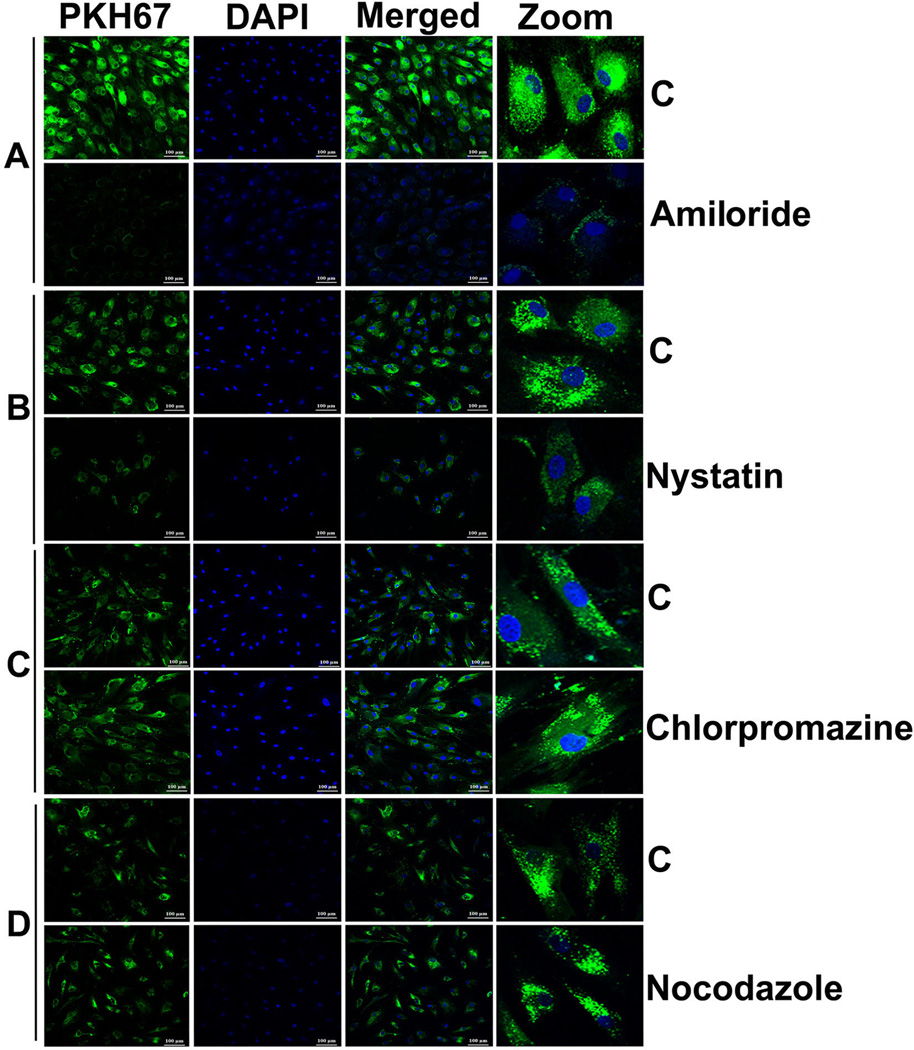
Treatment of human adipocytes with inhibitors of various endocytic pathways revealed the involvement of macropinocytotic and caveolin-dependent mechanisms for internalization of PKH67 labeled exosomes. Treatment with an inhibitor of macropinocytosis (amiloride, 50 uM) for 30 minutes inhibited uptake of PC-exosomes (Figure 7A). Treatment with an inhibitor of caveolin/lipid raft mediated endocytosis (nystatin, 54 uM) for 30 minutes also inhibited the uptake of PKH67 labeled exosomes in comparison to control (Figure 7B). In contrast, treatment with an inhibitor of clathrin-mediated endocytic pathway (chlorpromazine, 20 uM) for 30 minutes and a microtubule destabilizer (nocodazole, 1uM) for 10 minutes did not significantly affect the internalization of labeled exosomes compared to the control untreated group (Figure 7, C–D). Thus, macropinocytosis and caveolin/lipid raft-mediated endocytosis are most likely the mechanisms for internalization of exosomes into adipocytes.
Figure 7. Mechanism for entry of PC-exosomes into adipocytes.
Confocal images (20×) of differentiated human adipocytes pre-treated with various inhibitors of endocytic pathways and incubated with labeled PANC1Ex for overnight at 37°C. (A) Amiloride (50 µM; 30 minutes) (B) Nystatin (54 µM; 30 minutes) (C) chlorpromazine (20 µM; 30 minutes) and nocodazole (1 µM; 10 minutes). C refers to treatment with labeled exosomes only.
DISCUSSION
Based on our clinical observations, we postulated that in PC patients, tumor-secreted products mediate a paraneoplastic phenomenon that results in early, pronounced, unintentional weight loss, primarily the loss of adipose tissue.5 Tumor-derived exosomes have been shown to play a role in tumor progression and transport cargo that can profoundly influence remote tissues.24 Therefore, we examined the role of PC-exosomes and their content as potential mediators of lipolysis. In this report, we show that exosomes released by PC contain adrenomedullin that induces lipolysis in subcutaneous adipose tissue.
Due to its known involvement in new-onset diabetes, a paraneoplastic syndrome in pancreatic cancer,5, 6, 19 we examined the role of adrenomedullin (AM) in cancer-associated lipolysis. AM has been found to have varied effects on lipolysis depending upon the cellular context. In the murine cell line 3T3-F442A, AM failed to promote lipolysis25; however, in rat adipocytes, AM promoted lipolysis by increasing phosphorylation of hormone sensitive lipase (HSL).17 In our study involving murine and human adipocytes, we observed that both recombinant and exosomal AM promoted lipolysis through activation of p38 and p44/42; this effect was abrogated with AM inhibitor treatment.
Our data convincingly demonstrates that PC-exosomes induce lipolysis and this effect is abrogated by AM receptor blockade suggesting that PC-exosome induced lipolysis is dependent on exosomal AM. Further, we show that PC-exosomes do not contain functionally significant TNFα, a well-known lipolytic factor. We acknowledge that additional lipolytic mechanisms such as IL6 and other known factors might be operational in PC induced lipolysis either independently or in conjunction with PC-exosomal AM. Exploring all such mechanisms is beyond the scope of this manuscript and future studies will be needed. However, in this study, we describe a novel mechanism of PC-induced lipolysis that is mediated by PC exosomes and is exosomal-AM dependent.
Our data show that AM levels in PC exosomes from patient plasma is elevated compared to exosomes from non-PC control subjects although we observed some degree of variability in the levels between subjects. In addition, we found that the total number of exosomes is much higher in PC plasma compared to non-PC control subjects. We believe that taken together, these might explain the significantly increased lipolysis in PC patients but not in controls. In addition, possible mechanisms targeting exosomes from PC to adipocytes might exist such that PC exosomes have higher potency and efficacy in the pharmacologic sense for lipolysis compared to control exosomes. Exosomal tissue targeting and selective uptake mechanisms are poorly understood and future studies will be required to explore such possibilities.
Western blot analysis of PC-cell line derived exosomes showed multiple bands including bands at ~22kD seen consistently and variably at 18kD, ~14kD and 10kD (data not shown) suggesting possibility of multiple processed forms of AM (based on predicted molecular weights- 185 AA PreproAM (22kD), 164 AA ProAM (18kD), Intermediate form of AM ~14kD and 52 AA mature AM (~10kD). We were able to eliminate non-specific bands observed in traditional western blot by resorting to immunoprecipitation. Detection of variable precursor forms of AM in lysates from various cancer cell lines26 and variability based on the processing of the samples have been reported previously. Whether detection of various forms of AM suggest that packaging of AM in different forms in the exosomes is perhaps dependent on the cellular conditions and/or existence of possible mechanisms of post-translational modifications that could process precursor forms to mature forms of AM is only speculative at this time. Interestingly, in exosomes from human subjects, we consistently observed only the mature AM band at 10kD.
We observed that recombinant AM and exosomal AM mediate their effects through interaction with AM receptors on adipocytes. A functional AM receptor (AMR or ADMR) contains a member of the seven transmembrane domain G-protein coupled receptor superfamily named calcitonin receptor-like receptor (CRLR) and one of the three single transmembrane domain bearing proteins RAMPs (RAMP1/2/3) as coreceptors. RAMPs associate with CRLR in the endoplasmic reticulum and are transported to the plasma membrane.27 In pancreatic adenocarcinoma, there is a strong colocalization of CRLR with RAMP1/RAMP2 but not with RAMP3 suggesting that RAMP1/2 but not RAMP3 are the main coreceptors for CRLR in pancreatic adenocarcinoma.28 The Duolink™ Assay System29 not only revealed the interaction of exosomal AM with the AM receptor (ADMR) but, also validated the role of exosomal AM in lipolysis. Since the Duolink™ Assay indicated that AM/ADMR interactions occur inside the cell, we also examined the mechanism of exosome internalization in adipocytes. Exosomes can be internalized via different mechanisms including macropinocytosis, clathrin- or caveolin-mediated endocytosis, or phagocytosis.30–32 The method of exosome internalization can vary depending on their content, size, their fate after internalization, and the signaling status of the cells.33 We tested inhibitors of various endocytic pathways and found caveolin-mediated endocytosis and macropinocytosis as possible mechanisms for exosome internalization in adipocytes (Figure 7). Our data suggest that there are several means of exosome internalization and their association with various endosomal compartments.
In summary, our data demonstrate that PC-exosomes induce lipolysis in murine and human subcutaneous adipocytes. Further, PC-exosomes contain AM that interacts with AM receptors on adipocytes. The lipolytic effect of exosomes was abrogated by AM receptor blockade. Together, these data shows that PC exosome induced lipolysis is mediated by exosomal AM. Our results strongly support the notion that early onset weight loss observed in PC patients preceding onset of PC related symptoms are due to PC-induced lipolysis mediated by exosomal AM. We believe this work provides insight into early onset paraneoplastic effects of PC; further research in this area will be crucial in identifying targets and developing strategies for early detection of PC.
Supplementary Material
Significance of this study.
What is already known on this subject?
-
➢
Unintentional weight loss is observed in pancreatic cancer patients beginning several months before the onset of cancer related symptoms.
-
➢
Weight loss occurs in conjunction with progression of pancreatic cancer induced diabetes, another recently characterized paraneoplastic effect of pancreatic cancer.
-
➢
Adrenomedullin, a pluripotent hormone secreted by pancreatic cancer has been identified to induce β-cell dysfunction and is associated with pancreatic cancer induced diabetes.
What are the new findings?
-
➢
Pancreatic cancer secreted exosomes induce lipolysis in adipose tissue.
-
➢
Adrenomedullin is contained in pancreatic cancer secreted exosomes.
-
➢
Adrenomedullin containing pancreatic cancer exosomes promote lipolysis in both murine and human adipocytes and involve activation of MAPKs.
How might it impact on clinical practice in the foreseeable future?
-
➢
Identification of specific factors contained in pancreatic exosomes and their role in lipolysis will aid in early detection of pancreatic cancer and improve quality of life of pancreatic cancer patients.
Acknowledgments
The authors would like to thank James Tarara and Kristin Mantz of Mayo Clinic Microscopy Core for assistance with microscopy work, Dr. Ying Wang for assistance with immunohistochemistry, and Dr. Krishnendu Pal for assistance in experimental design.
Financial Support: Study was supported by CA78383, CA150190 and Florida Cancer Research Chair Fund (Florida #3J) to DM, CA100685 to STC, NIH training grant 5T32CA148073-05 to GS and NJ.
Footnotes
Disclosure of Potential Conflict of Interests: No potential conflict of interests.
REFERENCES
- 1.Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut. 2013;62:317–326. doi: 10.1136/gutjnl-2012-303588. [DOI] [PubMed] [Google Scholar]
- 2.Davidson W, Ash S, Capra S, et al. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin Nutr. 2004;23:239–247. doi: 10.1016/j.clnu.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Glass DJ. Treating cancer cachexia to treat cancer. Skelet Muscle. 2011;1:2. doi: 10.1186/2044-5040-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou X, Wang JL, Lu J, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Sah RP, Nagpal SJ, Mukhopadhyay D, et al. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10:423–433. doi: 10.1038/nrgastro.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal G, Ramachandran V, Javeed N, et al. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in beta cells and mice. Gastroenterology. 2012;143:1510–1517. e1. doi: 10.1053/j.gastro.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karayiannakis AJ, Syrigos KN, Polychronidis A, et al. Serum levels of tumor necrosis factor-alpha and nutritional status in pancreatic cancer patients. Anticancer Res. 2001;21:1355–1358. [PubMed] [Google Scholar]
- 8.Socher SH, Martinez D, Craig JB, et al. Tumor necrosis factor not detectable in patients with clinical cancer cachexia. J Natl Cancer Inst. 1988;80:595–598. doi: 10.1093/jnci/80.8.595. [DOI] [PubMed] [Google Scholar]
- 9.Okada S, Okusaka T, Ishii H, et al. Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin Oncol. 1998;28:12–15. doi: 10.1093/jjco/28.1.12. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino D, Kirkbride KC, Costello K, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inal JM, Kosgodage U, Azam S, et al. Blood/plasma secretome and microvesicles. Biochim Biophys Acta. 2013;1834:2317–2325. doi: 10.1016/j.bbapap.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 12.D'Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–1299. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh AK, Secreto CR, Knox TR, et al. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010;115:1755–1764. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Jiang C, Wang X, et al. Adrenomedullin is a novel adipokine: adrenomedullin in adipocytes and adipose tissues. Peptides. 2007;28:1129–1143. doi: 10.1016/j.peptides.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 16.Fukai N, Yoshimoto T, Sugiyama T, et al. Concomitant expression of adrenomedullin and its receptor components in rat adipose tissues. Am J Physiol Endocrinol Metab. 2005;288:E56–E62. doi: 10.1152/ajpendo.00586.2003. [DOI] [PubMed] [Google Scholar]
- 17.Iemura-Inaba C, Nishikimi T, Akimoto K, et al. Role of adrenomedullin system in lipid metabolism and its signaling mechanism in cultured adipocytes. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1376–R1384. doi: 10.1152/ajpregu.90467.2008. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 19.Javeed N, Sagar G, Dutta SK, et al. Pancreatic Cancer-derived Exosomes Causes Paraneoplastic beta-cell Dysfunction. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. American journal of physiology. Regulatory, integrative and comparative physiology. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 21.Hauner H, Petruschke T, Russ M, et al. Effects of tumour necrosis factor alpha (TNF alpha) on glucose transport and lipid metabolism of newly-differentiated human fat cells in cell culture. Diabetologia. 1995;38:764–771. doi: 10.1007/s001250050350. [DOI] [PubMed] [Google Scholar]
- 22.Rayalam S, Della-Fera MA, Yang JY, et al. Resveratrol potentiates genistein's antiadipogenic and proapoptotic effects in 3T3-L1 adipocytes. J Nutr. 2007;137:2668–2673. doi: 10.1093/jn/137.12.2668. [DOI] [PubMed] [Google Scholar]
- 23.Green A, Rumberger JM, Stuart CA, et al. Stimulation of lipolysis by tumor necrosis factor-alpha in 3T3-L1 adipocytes is glucose dependent: implications for long-term regulation of lipolysis. Diabetes. 2004;53:74–81. doi: 10.2337/diabetes.53.1.74. [DOI] [PubMed] [Google Scholar]
- 24.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33:441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 25.Harmancey R, Senard JM, Pathak A, et al. The vasoactive peptide adrenomedullin is secreted by adipocytes and inhibits lipolysis through NO-mediated beta-adrenergic agonist oxidation. FASEB J. 2005;19:1045–1047. doi: 10.1096/fj.04-2868fje. [DOI] [PubMed] [Google Scholar]
- 26.Miller MJ, Martinez A, Unsworth EJ, et al. Adrenomedullin expression in human tumor cell lines. Its potential role as an autocrine growth factor. J Biol Chem. 1996;271:23345–23351. doi: 10.1074/jbc.271.38.23345. [DOI] [PubMed] [Google Scholar]
- 27.Poyner DR, Sexton PM, Marshall I, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 28.Keleg S, Kayed H, Jiang X, et al. Adrenomedullin is induced by hypoxia and enhances pancreatic cancer cell invasion. Int J Cancer. 2007;121:21–32. doi: 10.1002/ijc.22596. [DOI] [PubMed] [Google Scholar]
- 29.Leuchowius KJ, Weibrecht I, Soderberg O. In situ proximity ligation assay for microscopy and flow cytometry. Curr Protoc Cytom. 2011;Chapter 9(Unit 9):36. doi: 10.1002/0471142956.cy0936s56. [DOI] [PubMed] [Google Scholar]
- 30.Escrevente C, Keller S, Altevogt P, et al. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng D, Zhao WL, Ye YY, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 32.Fitzner D, Schnaars M, van Rossum D, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 33.Svensson KJ, Christianson HC, Wittrup A, et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288:17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.