Abstract
We performed transcriptome profiling of human immortalized myoblasts (MB) transiently expressing double homeobox transcription factor 4 (DUX4) and double homeobox transcription factor 4 centromeric (DUX4c) and identified 114 and 70 genes differentially expressed in DUX4- and DUX4c-transfected myoblasts, respectively. A significant number of differentially expressed genes were involved in inflammation, cellular migration and chemotaxis suggesting a role for DUX4 and DUX4c in these processes. DUX4 but not DUX4c overexpression resulted in upregulation of the CXCR4 (C-X-C motif Receptor 4) and CXCL12 (C-X-C motif ligand 12 also known as SDF1) expression in human immortalized myoblasts. In a Transwell cell migration assay, human bone marrow-derived mesenchymal stem cells (BMSCs) were migrating more efficiently towards human immortalized myoblasts overexpressing DUX4 as compared to controls; the migration efficiency of DUX4-transfected BMSCs was also increased. DUX4c overexpression in myoblasts or in BMSCs had no impact on the rate of BMSC migration. Antibodies against SDF1 and CXCR4 blocked the positive effect of DUX4 overexpression on BMSC migration. We propose that DUX4 controls the cellular migration of mesenchymal stem cells through the CXCR4 receptor.
Keywords: DUX4, CXCR4, SDF1, signalling, migration
INTRODUCTION
Human genome harbors 333 genes and pseudogenes with homeobox sequences encoding homeodomain DNA binding motif. Most of homeobox genes are transcription factors of which many are known to play key roles in animal development (for review see [1]). Among all homeobox genes, the double homeobox (DUX) family is one of the most enigmatic. DUX family members numbered from 1 to 5 [2–4] are encoded within tandem repeats of macrosatellite DNA and their multiple polymorphic copies are spread over human genome. Two other members of the DUX family, DUXA [5] and DUXB [6] are single-copy genes encoded on chromosome 19 and 16, respectively.
Double homeobox protein 4 (DUX4) and its nearly identical homologue DUX4 centromeric (DUX4c) are the best known of all DUX proteins (for review see [7]). Multiple copies of DUX4 ORFs reside within 3.3 kb-long macrosatellite repeats on chromosomes 4q35 and 10q26 also called D4Z4 [8, 3]. The copy number of DUX4 ORF may vary from several units to several hundred making it the highest copy number ORF in human genome [9]. The single-copy of DUX4c gene is located 42 kb proximally to D4Z4 array on chromosome 4q35 [10].
The alternative splicing of DUX4 pre-mRNA results in the production of either a full-length 424 amino acid-long or truncated 160-aa protein lacking the C-terminal transactivation domain (DUX4-s) [11–12]. High level of full-length DUX4 overexpression was shown to be toxic for mouse and human cultured cells [13–16]. In vivo, ubiquitous DUX4 overexpression is detrimental for zebrafish [17] and Xenopus [18] development. Muscle-specific DUX4 overexpression resulted in tissue deterioration [19, 18, 20] specific overexpression in other tissues types was not tested. DUX4 toxicity has been linked to p53-dependent apoptosis induction [19, 13, 14, 19] and has been shown to require the C terminus [15] and the integrity of DNA binding domains [19, 15] of DUX4. Other biological effects of DUX4 overexpression in vitro include an increased sensitivity to oxidative stress and an inhibition of myogenic differentiation of human and mouse myogenic progenitor cells [14, 21].
In contrast to DUX4, high level of DUX4c [22, 23] or DUX4-s [24] expression is not toxic for the cells in culture. DUX4c overexpression induced human myoblast proliferation [22] and inhibited myogenic differentiation [23]; phenotypic effects of DUX4-s overexpression were not described, however it has been shown to inhibit DUX4 target genes when overexpressed together with DUX4 [12]. Neither ubiquitous nor muscle-specific DUX4c expression in vivo interfered with embryogenesis or muscle tissue integrity in Xenopus [18]. Similarly, DUX4-s injection did not affect normal development of zebrafish embryos [20].
To better understand the mechanism of DUX4 impact on the cell, we performed transcriptome profiling of human primary myoblasts overexpressing full-length DUX4 and DUX4c. One of the most striking findings of this analysis was an apparent upregulation of a significant number of chemokine genes. Chemokines is a superfamily of vertebrate-specific protein ligands interacting with rhodopsin-like G-protein-coupled receptors (GPCRs) [25]. The primary role of chemokines is the control of leukocyte traffic and recruitment to inflamed tissues (for review see [26–30]). Some of constitutively produced chemokines are known to play a role in processes unrelated to innate or acquired immunity (for review see [31–32]). Of these, CXCL12 (C-X-C motif ligand 12), also known as Stromal-derived factor 1 (SDF1) [33]) and its primary receptor CXCR4 (C-X-C motif Receptor 4) [34] clearly stand apart due to an important role in development regulation [35], hematopoiesis, angiogenesis [36–37], stem cell migration [38] and wound healing (reviewed in [39–40]). In addition, CXCR4 is one of the major GPCR receptors controlling the migration of various cell types including leukocytes, HSPC, MSC (reviewed in [41].
MSC (mesenchymal stem or mesenchymal stromal cells) are multipotent stem cells capable of differentiation into chondrocytes, osteocytes, adipocytes and, more controversially, to other cell types (for review see [42]). The primary site of MSC localization is bone marrow, although cells with similar properties were also found in other tissues (for review see [43]). In adult organism, MSC are thought to contribute to tissue homeostasis. Tissue damage results in MSC mobilization from bone marrow and their homing to damaged tissue where they either differentiate to replenish damaged cells, stimulate differentiation of the tissue-resident stem cells, stimulate vascularization and control inflammation thus restoring the damage (reviewed in [44]). CXCR4-SDF1 signaling is known to be required for MSC homing and retention to their niche in bone marrow (for review see [43]) and has also been shown to stimulate MSC cell migration in vitro [45, 46]. We have shown that DUX4 overexpression results in upregulation of CXCR4 and SDF1 genes in several cell types and stimulates the migration of BMSC in a CXCR4- and SDF1-dependent manner. Our results thus establish DUX4 as a novel regulator of cell mobility.
RESULTS
Transcriptome profiling of DUX4- and DUX4c-overexpressing human myoblasts
Several transcriptomic studies have been performed on cells overexpressing DUX4, at the same time its homologue DUX4c is much less studied. We argued that the difference in transcriptome profiles of DUX4 and DUX4c might help us to better understand the functional differences between these two proteins. We thus performed transcriptome profiling of DUX4- and DUX4c- transfected immortalized human myoblasts (MB) at two time points (12 and 24 h) following transfection. Overall, we have identified 130 differentially expressed genes of which 60 genes were differentially expressed only in DUX4-transfected cells, 16 genes only in DUX4c transfected cells and 54 genes were differentially expressed both in DUX4- and DUX4c-transfected cells (Figure 1). With rare exceptions, all significantly overexpressed DUX4 target genes found in our study were already described previously [12] (Tables 1–4 and Supplementary Table S1). DUX4 is known to induce apoptosis in various cell types including human myoblasts. Indeed, we observed a significant induction of apoptosis and cell mortality in DUX4-transfected MB 72 h after the transfection (data not shown). However, 24 h after the transfection, apoptosis and cell mortality were significantly increased neither in DUX4- nor in DUX4c-transfected MB indicating that our transcriptomic data obtained at earlier time points may contain information about other functional activities of DUX4 (Supplementary Figure S1).
Figure 1. Genes differentially expressed 12- and 24 h after the transfection of human immortalized myoblasts (MB) with DUX4 or DUX4c plasmids as compared to empty vector; yellow: no differential expression (-1.5 < FC < 1.5); red: upregulated (FC > 1.5); green: downregulated (FC < −1.5).
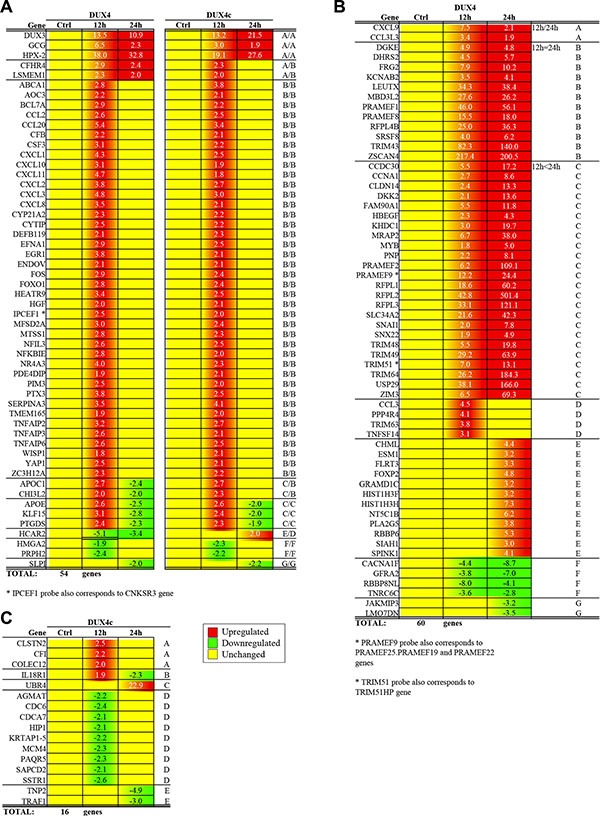
(A) Genes differentially expressed in both DUX4- and DUX4c-transfected MB; genes upregulated at both 12 h and 24 h, only at 12 h or 24 h time-points are labeled with (A, B and D) respectively; genes upregulated at 12 h but downregulated at 24 h time-point are labeled with C; genes downregulated at both 12 h and 24 h, only at 12 h or 24 h time-points are labeled with (E, F and G) respectively. (B) Genes differentially expressed only in DUX4-transfected MB; genes upregulated at both 12 h and 24 h time-points with the expression level at 12 h higher, equal or lower than at 24 h are labeled with (A, B or C) respectively; genes upregulated only at 12 h or 24 h time-points are labeled with D and E respectively; genes downregulated at both time-points or only at 24 h time-point are labeled with F and G respectively. (C) Genes differentially expressed only in DUX4c-transfected MB; genes upregulated at 12 h or 24 h or downregulated at 12 h or 24 h time-points are labeled with A, C, D and E respectively; genes upregulated at 12 h but downregulated at 24 h time-points are labeled with B.
Table 1. Genes differentially expressed in hIMB cells 12 h after DUX4-plasmid transfection.
| DUX4 12 h | ||||
|---|---|---|---|---|
| FC | Gene | Description | ref | |
| 217.4 | ZSCAN4 | zinc finger and SCAN domain containing 4 | ‡ | |
| 82.3 | TRIM43 | tripartite motif containing 43 | ‡ | |
| 46.0 | PRAMEF1 | PRAME family member 1 | ‡ | |
| 42.8 | RFPL2 | ret finger protein-like 2 | ‡ | |
| 38.1 | USP29 | ubiquitin specific peptidase 29 | ‡ | |
| * | 38.0 | HPX-2 | homeobox HPX-2 | |
| 34.3 | LEUTX | leucine twenty homeobox | ||
| 33.1 | RFPL3 | ret finger protein-like 3 | ‡ | |
| 29.2 | TRIM49 | tripartite motif containing 49 | ‡ | |
| 27.6 | MBD3L2 | methyl-CpG binding domain protein 3-like 2 | ‡ | |
| 26.2 | TRIM64 | tripartite motif containing 64 | ‡ | |
| 25.0 | RFPL4B | ret finger protein-like 4B | ‡ | |
| 21.6 | SLC34A2 | solute carrier family 34 (type II sodium/phosphate contransporter). member 2 | ‡ | |
| 18.6 | RFPL1 | ret finger protein-like 1 | ‡ | |
| 15.5 | PRAMEF8 | PRAME family member 8 | ‡ | |
| * | 13.5 | DUX3 | double homeobox 3 | |
| 12.2 | PRAMEF9; PRAMEF25; PRAMEF19; PRAMEF22 | PRAME family member 9;PRAME family member 25;PRAME family member 19;PRAME family member 22 | ‡ | |
| 7.9 | FRG2 | FSHD region gene 2 | ||
| 7.5 | CXCL9 | chemokine (C-X-C motif) ligand 9 | ||
| 7.0 | TRIM51. TRIM51HP | tripartite motif-containing 51;tripartite motif-containing 51H. pseudogene | ||
| 6.7 | MRAP2 | melanocortin 2 receptor accessory protein 2 | ||
| * | 6.5 | GCG | glucagon | |
| 6.5 | ZIM3 | zinc finger. imprinted 3 | ||
| 6.2 | PRAMEF2 | PRAME family member 2 | ‡ | |
| 5.5 | FAM90A1 | family with sequence similarity 90. member A1 | ‡ | |
| 5.5 | TRIM48 | tripartite motif containing 48 | ‡ | |
| 5.5 | CCDC30 | coiled-coil domain containing 30 | ||
| * | 5.4 | CCL20 | chemokine (C-C motif) ligand 20 | |
| 4.9 | DGKE | diacylglycerol kinase. epsilon 64kDa | ||
| * | 4.8 | CXCL3 | chemokine (C-X-C motif) ligand 3 | |
| * | 4.7 | CXCL11 | chemokine (C-X-C motif) ligand 11 | |
| 4.5 | CCL3 | chemokine (C-C motif) ligand 3 | ||
| 4.5 | DHRS2 | dehydrogenase/reductase (SDR family) member 2 | ||
| * | 4.3 | CXCL1 | chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity. alpha) | |
| 4.1 | PPP4R4 | protein phosphatase 4. regulatory subunit 4 | ||
| * | 4.0 | NR4A3 | nuclear receptor subfamily 4. group A. member 3 | |
| 4.0 | SRSF8 | serine/arginine-rich splicing factor 8 | ||
| 3.8 | TRIM63 | tripartite motif containing 63. E3 ubiquitin protein ligase | ||
| * | 3.8 | CXCL2 | chemokine (C-X-C motif) ligand 2 | |
| * | 3.8 | PTX3 | pentraxin 3. long | |
| * | 3.8 | EGR1 | early growth response 1 | |
| * | 3.5 | CXCL8 | chemokine (C-X-C motif) ligand 8 | |
| * | 3.5 | SERPINA3 | serpin peptidase inhibitor. clade A (alpha-1 antiproteinase. antitrypsin). member 3 | |
| 3.5 | KCNAB2 | potassium voltage-gated channel. shaker-related subfamily. beta member 2 | ||
| 3.4 | CCL3L3 | chemokine (C-C motif) ligand 3-like 3 | ||
| * | 3.4 | HEATR9 | HEAT repeat containing 9 | |
| * | 3.2 | TNFAIP2 | tumor necrosis factor. alpha-induced protein 2 | |
| 3.1 | TNFSF14 | tumor necrosis factor (ligand) superfamily. member 14 | ||
| * | 3.1 | KLF15 | Kruppel-like factor 15 | |
| * | 3.1 | CXCL10 | chemokine (C-X-C motif) ligand 10 | |
| * | 3.1 | CSF3 | colony stimulating factor 3 (granulocyte) | |
| ... | ... | ... | ||
| –3.6 | TNRC6C | trinucleotide repeat containing 6C | ||
| –3.8 | GFRA2 | GDNF family receptor alpha 2 | ||
| –4.4 | CACNA1F | calcium channel. voltage-dependent. L type. alpha 1F subunit | ||
| * | –5.1 | HCAR2 | hydroxycarboxylic acid receptor 2 | |
| –8.0 | RBBP8NL | RBBP8 N-terminal like | ||
Asterisk labels genes that are also differentially expressed in DUX4c-transfected IMBs.
labels genes previously descried DUX4 target genes [12].
Table 4. Genes differentially expressed in hIMB cells 20 h after DUX4c-plasmid transfection.
| DUX4c 24 h | |||
|---|---|---|---|
| FC | Gene | Description | |
| * | 27.6067 | HPX-2 | homeobox HPX-2 |
| 22.8739 | UBR4 | ubiquitin protein ligase E3 component n-recognin 4 | |
| * | 21.4539 | DUX3 | double homeobox 3 |
| * | –2.0324 | KLF15 | Kruppel-like factor 15 |
| * | –2.1708 | SLPI | secretory leukocyte peptidase inhibitor |
| –2.2869 | IL18R1 | interleukin 18 receptor 1 | |
| –2.9995 | TRAF1 | TNF receptor-associated factor 1 | |
| –4.9447 | TNP2 | transition protein 2 (during histone to protamine replacement) | |
Asterisk labels genes that are also differentially expressed in DUX4-transfected IMBs.
Table 2. Genes differentially expressed in hIMB cells 24 h after DUX4-plasmid transfection.
| DUX4 24 h | ||||
|---|---|---|---|---|
| FC | Gene | Description | ref | |
| 501.4 | RFPL2 | ret finger protein-like 2 | ‡ | |
| 200.5 | ZSCAN4 | zinc finger and SCAN domain containing 4 | ‡ | |
| 184.3 | TRIM64 | tripartite motif containing 64 | ‡ | |
| 166.0 | USP29 | ubiquitin specific peptidase 29 | ‡ | |
| 140.0 | TRIM43 | tripartite motif containing 43 | ‡ | |
| 121.1 | RFPL3 | ret finger protein-like 3 | ‡ | |
| 109.1 | PRAMEF2 | PRAME family member 2 | ‡ | |
| 69.3 | ZIM3 | zinc finger. imprinted 3 | ||
| 63.9 | TRIM49 | tripartite motif containing 49 | ‡ | |
| 60.2 | RFPL1 | ret finger protein-like 1 | ‡ | |
| 56.1 | PRAMEF1 | PRAME family member 1 | ‡ | |
| 42.3 | SLC34A2 | solute carrier family 34 (type II sodium/phosphate contransporter). member 2 | ‡ | |
| 38.4 | LEUTX | leucine twenty homeobox | ||
| 38.0 | MRAP2 | melanocortin 2 receptor accessory protein 2 | ||
| 36.3 | RFPL4B | ret finger protein-like 4B | ‡ | |
| * | 32.8 | HPX-2 | homeobox HPX-2 | |
| 26.2 | MBD3L2 | methyl-CpG binding domain protein 3-like 2 | ‡ | |
| 24.4 | PRAMEF9. PRAMEF25. PRAMEF19. PRAMEF22 | PRAME family member 9;PRAME family member 25;PRAME family member 19;PRAME family member 22 | ‡ | |
| 19.8 | TRIM48 | tripartite motif containing 48 | ‡ | |
| 19.7 | KHDC1 | KH homology domain containing 1 | ‡ | |
| 18.0 | PRAMEF8 | PRAME family member 8 | ‡ | |
| 17.2 | CCDC30 | coiled-coil domain containing 30 | ||
| 13.6 | DKK2 | dickkopf WNT signaling pathway inhibitor 2 | ||
| 13.3 | CLDN14 | claudin 14 | ||
| 13.1 | TRIM51. TRIM51HP | tripartite motif-containing 51;tripartite motif-containing 51H. pseudogene | ||
| 11.8 | FAM90A1 | family with sequence similarity 90. member A1 | ‡ | |
| * | 10.9 | DUX3 | double homeobox 3 | |
| 10.24 | FRG2 | FSHD region gene 2 | ||
| 8.61 | CCNA1 | cyclin A1 | ||
| 8.14 | PNP | purine nucleoside phosphorylase | ||
| 7.75 | SNAI1 | snail family zinc finger 1 | ||
| 7.34 | HIST1H3H | histone cluster 1. H3h | ||
| 6.19 | NT5C1B | 5′-nucleotidase. cytosolic IB | ||
| 6.17 | SRSF8 | serine/arginine-rich splicing factor 8 | ||
| 5.69 | DHRS2 | dehydrogenase/reductase (SDR family) member 2 | ||
| 5.33 | RBBP6 | retinoblastoma binding protein 6 | ||
| 4.96 | MYB | v-myb avian myeloblastosis viral oncogene homolog | ||
| 4.92 | SNX22 | sorting nexin 22 | ||
| 4.79 | FOXP2 | forkhead box P2 | ||
| 4.78 | DGKE | diacylglycerol kinase. epsilon 64kDa | ||
| 4.37 | CHML | choroideremia-like (Rab escort protein 2) | ||
| 4.32 | HBEGF | heparin-binding EGF-like growth factor | ||
| 4.14 | KCNAB2 | potassium voltage-gated channel. shaker-related subfamily. beta member 2 | ||
| 4.10 | SPINK1 | serine peptidase inhibitor. Kazal type 1 | ||
| 3.84 | PLA2G5 | phospholipase A2. group V | ||
| 3.30 | FLRT3 | fibronectin leucine rich transmembrane protein 3 | ||
| 3.22 | HIST1H3F | histone cluster 1. H3f | ||
| 3.20 | ESM1 | endothelial cell-specific molecule 1 | ||
| 3.17 | GRAMD1C | GRAM domain containing 1C | ||
| 3.01 | SIAH1 | siah E3 ubiquitin protein ligase 1 | ‡ | |
| ... | ... | ... | ||
| –3.19 | JAKMIP3 | Janus kinase and microtubule interacting protein 3 | ||
| * | –3.36 | HCAR2 | hydroxycarboxylic acid receptor 2 | |
| –3.51 | LMO7DN | LMO7 downstream neighbor | ||
| –4.10 | RBBP8NL | RBBP8 N-terminal like | ||
| –7.00 | GFRA2 | GDNF family receptor alpha 2 | ||
| –8.72 | CACNA1F | calcium channel. voltage-dependent. L type. alpha 1F subunit | ||
Asterisk labels genes that are also differentially expressed in DUX4c-transfected IMBs.
Table 3. Genes differentially expressed in hIMB cells 12 h after DUX4c-plasmid transfection.
| DUX4c 12 h | |||
|---|---|---|---|
| FC | Gene | Description | |
| * | 19.1 | HPX-2 | homeobox HPX-2 |
| * | 13.2 | DUX3 | double homeobox 3 |
| * | 4.1 | SERPINA3 | serpin peptidase inhibitor. clade A (alpha-1 antiproteinase. antitrypsin). member 3 |
| * | 3.8 | ABCA1 | ATP-binding cassette. sub-family A (ABC1). member 1 |
| * | 3.4 | CCL20 | chemokine (C-C motif) ligand 20 |
| * | 3.0 | GCG | glucagon |
| * | 3.0 | CXCL3 | chemokine (C-X-C motif) ligand 3 |
| * | 2.7 | CXCL2 | chemokine (C-X-C motif) ligand 2 |
| * | 2.7 | TNFAIP2 | tumor necrosis factor. alpha-induced protein 2 |
| * | 2.7 | APOC1 | apolipoprotein C-I |
| * | 2.6 | APOE | apolipoprotein E |
| * | 2.6 | MFSD2A | major facilitator superfamily domain containing 2A |
| 2.5 | CLSTN2 | calsyntenin 2 | |
| * | 2.5 | HEATR9 | HEAT repeat containing 9 |
| * | 2.5 | EFNA1 | ephrin-A1 |
| * | 2.5 | CCL2 | chemokine (C-C motif) ligand 2 |
| * | 2.5 | CXCL1 | chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity. alpha) |
| * | 2.5 | TNFAIP6 | tumor necrosis factor. alpha-induced protein 6 |
| * | 2.5 | NFIL3 | nuclear factor. interleukin 3 regulated |
| * | 2.5 | PTX3 | pentraxin 3. long |
| * | 2.4 | FOXO1 | forkhead box O1 |
| * | 2.4 | KLF15 | Kruppel-like factor 15 |
| * | 2.4 | FOS | FBJ murine osteosarcoma viral oncogene homolog |
| * | 2.3 | NR4A3 | nuclear receptor subfamily 4. group A. member 3 |
| * | 2.3 | CHI3L2 | chitinase 3-like 2 |
| * | 2.3 | MTSS1 | metastasis suppressor 1 |
| * | 2.3 | DEFB119 | defensin. beta 119 |
| * | 2.3 | CFHR4 | complement factor H-related 4 |
| * | 2.3 | PTGDS | prostaglandin D2 synthase 21kDa (brain) |
| * | 2.2 | CSF3 | colony stimulating factor 3 (granulocyte) |
| * | 2.2 | CYTIP | cytohesin 1 interacting protein |
| * | 2.2 | BCL7A | B-cell CLL/lymphoma 7A |
| 2.2 | CFI | complement factor I | |
| * | 2.2 | CYP21A2 | cytochrome P450. family 21. subfamily A. polypeptide 2 |
| * | 2.2 | ZC3H12A | zinc finger CCCH-type containing 12A |
| * | 2.1 | CXCL8 | chemokine (C-X-C motif) ligand 8 |
| * | 2.1 | PDE4DIP | phosphodiesterase 4D interacting protein |
| * | 2.1 | CFB | complement factor B |
| * | 2.1 | HGF | hepatocyte growth factor (hepapoietin A; scatter factor) |
| * | 2.1 | WISP1 | WNT1 inducible signaling pathway protein 1 |
| * | 2.1 | EGR1 | early growth response 1 |
| * | 2.1 | YAP1 | Yes-associated protein 1 |
| 2.1 | AOC3 | amine oxidase. copper containing 3 | |
| * | 2.1 | TNFAIP3 | tumor necrosis factor. alpha-induced protein 3 |
| * | 2.1 | ENDOV | endonuclease V |
| * | 2.0 | PIM3 | Pim-3 proto-oncogene. serine/threonine kinase |
| * | 2.0 | NFKBIE | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor. epsilon |
| * | 2.0 | IPCEF1; CNKSR3 | interaction protein for cytohesin exchange factors 1; CNKSR family member 3 |
| * | 2.0 | LSMEM1 | leucine-rich single-pass membrane protein 1 |
| * | 2.0 | TMEM165 | transmembrane protein 165 |
| 2.0 | COLEC12 | collectin sub-family member 12 | |
| ... | ... | ... | |
| – 2.1 | HIP1 | huntingtin interacting protein 1 | |
| – 2.1 | SAPCD2 | suppressor APC domain containing 2 | |
| – 2.1 | CDCA7 | cell division cycle associated 7 | |
| * | – 2.2 | PRPH2 | peripherin 2 (retinal degeneration. slow) |
| – 2.2 | AGMAT | agmatine ureohydrolase (agmatinase) | |
| – 2.2 | KRTAP1-5 | keratin associated protein 1-5 | |
| * | – 2.3 | HMGA2 | high mobility group AT-hook 2 |
| – 2.3 | PAQR5 | progestin and adipoQ receptor family member V | |
| – 2.3 | MCM4 | minichromosome maintenance complex component 4 | |
| – 2.4 | CDC6 | cell division cycle 6 | |
| – 2.6 | SSTR1 | somatostatin receptor 1 | |
Asterisk labels genes that are also differentially expressed in DUX4-transfected IMBs.
According to GeneOntology analysis, significant known functions of the majority of genes differentially expressed in DUX4 and DUX4c-overexpressing myoblasts included inflammation, chemotaxis, metabolism and apoptosis (Figure 2 and Supplementary Table S2). These functional categories have already been attributed to DUX4 target genes in previous transcriptomic studies of genes differentially expressed in DUX4-overexpressing murine C2C12 myoblasts [14], human primary myoblasts [12] and rhabdomyosarcoma cells [47]; furthermore, functional involvement of ectopically expressed DUX4 in certain aspects of cellular metabolism and regulation of apoptosis has been previously demonstrated [13, 14].
Figure 2. Functional classification of genes differentially expressed both in DUX4- and DUX4c- transfected human immortalized myoblasts (MB) (A) or only in DUX4- or DUX4c-transfected MB (B) at two time points (12- and 24 h) after the transfection.
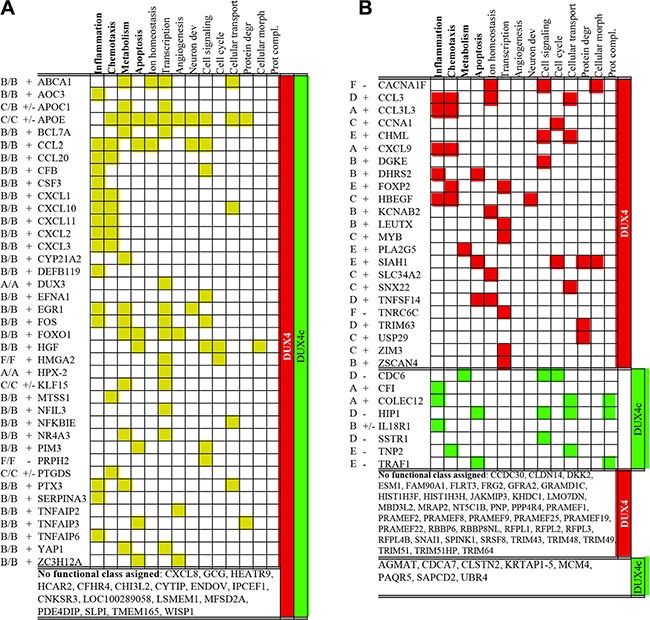
Names of superclusters composed of several gene ontology functional categories (see supplementary Table S2 for the composition of superclusters) of which at least one was statistically significant (p-value < 0.05, FDR < 20) are in bold. Gene expression level at various time-points is indicated with letters (A–E or F) as in Figure 1; “+” and “−” indicate whether a gene was consistently up- or downregulated at all time points tested; “+/−” means that a gene was upregulated and one time-point but downregulated at the other.
Several functional categories including, transcription, angiogenesis, neuron development, cell signaling, cellular transport, protein degradation, and protein complex assembly that have not reach statistical significance in our study, were attributed to DUX4 target genes previously [14, 12, 47]. The validity of some of these functional categories has been experimentally proven: DUX4 overexpression in human myoblasts has been shown to induce genes involved in protein degradation [16] and interfere with protein ubiquitination [48]. DUX4 overexpression in mouse embryonic stem cells (ES) has been shown to induce neuroectoderm program [49]; the involvement of DUX4 in transcription regulation has also been addressed [14]. Finally, DUX4 target genes found in this study also include FRG2 and KLF15 - genes shown previously to be overexpressed in FSHD; this is in agreement with an observation that DUX4 target genes are differentially expressed in FSHD [50].
As compared to DUX4c, DUX4 overexpression resulted in a differential expression of more genes involved in chemotaxis, ion transport, and protein degradation. Conversely, all differentially expressed genes related to protein complex assembly were exclusively controlled by DUX4c (Figure 2).
DUX4 overexpression results in upregulation of CXCR4 and SDF1 gene expression in various cell types
DUX4 and DUX4c transcriptome signatures contained genes encoding chemokines. Of 18 chemokine genes reliably detected by the microarray (intensity > 50) in MB, 10 and 7 were at least 1.5-fold upregulated in DUX4- and DUX4c-overexpressing MB, respectively (Figure 3A, 3B). A relatively weakly expressed CXCR4 chemokine receptor gene was upregulated 2 to 5-fold in DUX4- but not DUX4c-overexpressing MB as compared to the controls. Other chemokine receptor genes were either undetectable or not differentially expressed. qPCR and immunofluorescence microscopy analysis of CXCR4 expression in MB and mesenchymal stromal cells isolated from human bone marrow cells (BMSC) transfected with the DUX4 plasmid confirmed the results of transcriptome profiling (Figures 4, 5 and Supplementary Figure S3). Furthermore, we observed a significant upregulation of CXCR4 expression in several other cell types transfected with DUX4 including human keratinocyte cell line HaCat, dermal fibroblasts Dfb and mesenchymal stromal cells isolated from adipose tissue (ADAS) (data not shown).
Figure 3. Chemokine (A, B) or chemokine receptor (C, D) gene mRNA expression (microarray) in human immortalized myoblasts (MB) 12 h or 24 h after transfection with DUX4 (A, C) or DUX4c (B, D) plasmids.
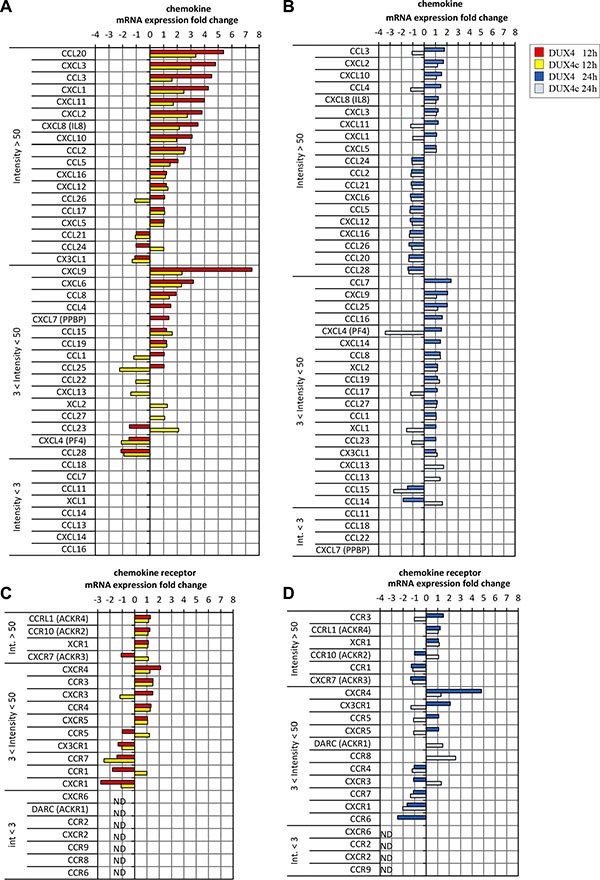
Gene names are divided in 3 classes: highly expressed (int > 50), low expression (3 < int < 50) and non-detectable (in < 3) sorted according to fold change.
Figure 4. DUX4-transfected cells overexpress SDF1 and CXCR4 genes.
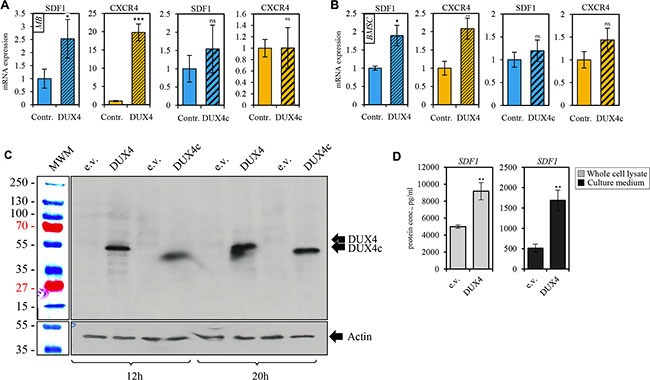
qRT-PCR analysis of the expression of CXCR4 and SDF1 mRNA in human immortalized myoblasts (MB) (A) and bone marrow mesenchymal stem cells (BSMC) (B) transiently transfected with DUX4- or, DUX4c-expressing plasmids or an empty vector (pCI-Neo). Average of three independent experiments is shown, error bar represent standard deviation (SD), t-test p-value < 0.05 (*). Gene expression level of the control sample normalized to GAPDH was set to 1. (C) Western blot analysis of protein lysates of human immortalized myoblasts (MB) transfected with DUX4 and DUX4c plasmids for 12 and 20 h. DUX4 (52-kDa) and DUX4c (47-kDa) proteins were stained with 9A12 antibody. (D) SDF1 protein concentration was measured using ELISA in the whole cell lysate (diluted 10-fold) or cell culture medium (concentrated 20 times) of human immortalized myoblasts (MB) 24 h after transfection with pCI-Neo-DUX4, or pCI-Neo (e.v.) plasmids. Average and standard deviation of 3 independent experiments are shown, t-test p-value < 0.05 (*).
Figure 5. DUX4-transfected cells overexpress SDF1 and CXCR4.
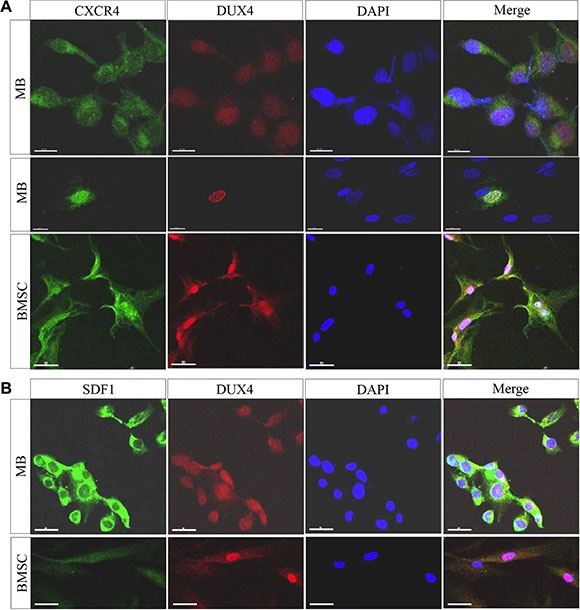
Immunofluorescence analysis of CXCR4 and DUX4 expression in human immortalized myoblasts (MB) (A) and bone marrow-derived mesenchymal stromal cells (BMSCs) (B) 24 h after the transfection with pCI-NeoDUX4 plasmid. Formaldehyde-fixed cells were stained with CXCR4, SDF1 and DUX4 antibodies, representative images are shown; scale bar 50 μm.
CXCR4 receptor is activated by the interaction with its only known ligand SDF1. Our microarray analysis did not detect a differential expression of SDF1 gene (Figure 3C, 3D); however, we observed a moderate but statistically significant upregulation of SDF1 mRNA and protein in DUX4- but not DUX4c-transfected MB (Figure 4A, 4B). DUX4-transfected myoblasts also secreted more SDF1 protein into extracellular medium (Figure 4D). SDF1 is also known to bind to a scavenger receptor CXCR7, however, no change in CXCR7 expression level was observed upon DUX4 or DUX4c overexpression (Figure 3C, 3D). It is also unlikely that DUX4 expression is essential for CXCR4 or SDF1 expression as these genes are known to be expressed in various cells types, and DUX4 expression is confined to embryonic and germinal cells [17, 11].
DUX4 overexpression stimulates migration of mesenchymal stem cells
The efficiency of CXCR4 signaling depends on the expression level of both SDF1 and CXCR4 genes (reviewed in [41]. Our results thus suggested that DUX4 might regulate cell mobility and migration. To test the migration and chemoattractive properties of cells overexpressing DUX4 and DUX4c, we used a Transwell assay. Migrating BMSC were plated on an upper layer of a permeable membrane placed in a Transwell insert and chemoattractant-producing human myoblasts were plated to the lower chamber section of the Transwell system (Figure 6H). To test the effect of DUX4- and DUX4c overexpression on chemoattractive properties of MB, we transfected them with DUX4 and DUX4c plasmids and counted the number of untransfected stromal cells derived from human bone marrow cells (BMSC) that crossed the membrane (Figure 6A). We observed a more efficient migration of BMSC towards DUX4- but not DUX4c overexpressing MB. The effect was completely blocked if antibodies against SDF1 were added to the culture medium (Figure 6F). We have also tested whether DUX4 overexpression could increase chemoattractive properties of other cell types. We overexpressed DUX4 in TE671 rhabdomyosarcoma cell line, known to be resistant to high levels of DUX4 expression [13, 51], human immortalized myoblasts (MB) and immortalized keratinocyte cells line HaCat and found that DUX4 overexpression also increases the chemoattractivity of these cells to BMSC (data not shown).
Figure 6. DUX4 and DUX4c expression increases migration rate of human bone marrow stem cells (BMSC) towards human immortalized myoblasts (MB) in a transwell assay.
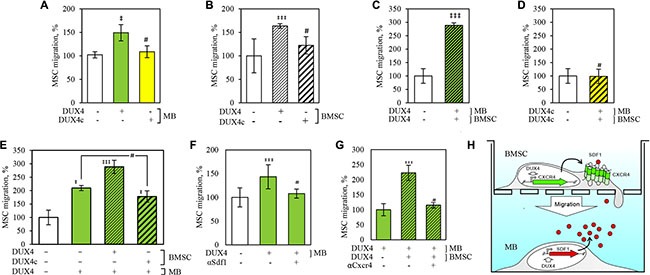
BMSC and MB cells were plated to the upper- and the lower wells of a Transwell chamber respectively and the number of BMSC that crossed the membrane and adhered to its lower surface was quantified. (A) Migration of non-transfected BMSCs towards MB transfected with DUX4-, DUX4c-plasmid or an empty-vector control (e.v.); (B) Migration of BMSCs transfected with DUX4-, DUX4c-plasmid or e.v. towards non-transfected MB; (C) Migration of BMSCs transfected with DUX4- or e.v. towards DUX4- or e.v.-transfected MB; (D) Migration of BMSCs transfected with DUX4c- or e.v. towards DUX4c- or e.v.-transfected MB; (E) Migration of BMSCs transfected with DUX4-, DUX4c- or e.v. towards DUX4- or e.v.-transfected MB; (F) Effect of SDF1 antibody on BMSC migration towards MB transfected with DUX4-plasmid or e.v. (G) Effect of CXCR4 antibody on migration of BMSC transfected with DUX4-plasmid or e.v. towards DUX4-plasmid or e.v.-transfected MB; (H) Schematic representation of DUX4 overexpression impact on BMSC cell migration towards MB. (*), p-value < 0.05 as compared to control; #, non-significant; migration to e.v.-transfected cells was set to 100%, error bars correspond to SD.
Next, we tested whether DUX4 or DUX4c overexpression could modify migration properties of BMSC, and observed an increase of migration rate of DUX4- but not DUX4c-trasfected BMSC towards untransfected MB (Figure 6B). This effect was further increased if DUX4 but not DUX4c was simultaneously overexpressed in both BMSC and MB (Figure 6C, 6D). Adding CXCR4 antibodies to the culture medium completely abolished the effect of DUX4 overexpression on cell migration (Figure 6G). Cell migration experiments have been carried out 24 h after the transfection as apoptosis and necrosis rate of the transfected cells at this time point was not significantly different from non-transfected controls (Supplementary Figure S1) arguing against the possibility that cytotoxic effect of DUX4 overexpression could introduce a bias in our assay.
To rule out the possibility that DUX4 overexpression could affect BMSC and ADAS multipotency, we then induced adipogenic differentiation of DUX4-transfected BMSC. We found the number of resulting adipocytes unchanged as compared to a control plasmid-transfected BMSCs, thus arguing against the possibility that DUX4 altered adipogenic differentiation potential of BMSC and ADAS (Supplementary Figure S2).
We conclude that DUX4 but not DUX4c overexpression increases chemoattractivity of several cell types for MSC migration and also increased migration rate of BMSC when overexpressed in them. The difference in transcriptome signatures of DUX4 and DUX4c-overexpressing myoblasts is thus clearly translated into different biological roles of these transcription factors.
DISCUSSION
DUX4 and SDF1-CXCR4 axis in the normal organism
DUX4 is a powerful transcriptional regulator with an unknown physiological role in normal cells. In vitro, full-length DUX4 is expressed in human embryonic and mesenchymal stem cells [17] and induced pluripotent stem cells [11]; its expression is downregulated in the process of differentiation of these cells. DUX4 expression cannot be detected in fully-differentiated cells such as primary myoblasts or fibroblasts where only DUX4-s, a shorter non-toxic form of DUX4 resulting from an alternative splicing of DUX4 gene could be detected.
In normal adult human tissues, full-length DUX4 is expressed only in testis; in somatic tissues including skeletal muscles, liver and heart only DUX4-s transcript was detectable [11]. The pattern of full-length DUX4 expression is indicative of a role at early stages of human development. However, besides the observation that DUX4 overexpression induced neuroectodermal program in murine embryonic stem cells, developmental functions of DUX4 remain unknown. Linking DUX4 with cellular migration described here may thus contribute to the understanding of a physiological role of DUX4 in development.
In the course of vertebrate development, SDF1 and CXCR4 are essential for colonization of the bone marrow by hematopoietic stem and progenitor cells (HSPCs) (reviewed in [52, 53, 39, 38]), colonization of gonads by primordial germ cells (PGCs) [54] (reviewed in [38]); neurogenesis (reviewed in [38, 55]); cardiogenesis and vascular formation [56]; limb myogenesis [57–61]. It is tempting to speculate that as an activator of CXCR4 and SDF1 expression, DUX4 might be also involved in these processes.
The role of DUX4 in development could, in principle, be addressed using loss of function mouse models. Mouse genome contains DUX4 homologs Duxbl (also named Duxl) [62, 63] and Dux. The degree of amino acid identity of Duxbl and DUX4 reaches 65% in the most conserved region (second homeobox DNA binding domain) [62]. Expression pattern of Duxbl (ovary, eyes, testes and brain of adult mice and developing muscles of mouse embryos [64]) demonstrates a certain similarity to the DUX4 expression pattern. Functional analysis of Duxbl demonstrated that Duxbl overexpression increased myoblast proliferation but repressed their myogenic differentiation [65] which to certain extent recapitulated the effect of DUX4overexpression on myogenic differentiation.
Another mouse homolog, Dux is only 60% identical to DUX4 in its most conserved region; its expression pattern is also less similar to that of DUX4. Dux expression was demonstrated in adult mouse brain, heart liver and lungs and was almost undetectable in skeletal muscle [62]; functional analysis of this gene was not conducted. Duxbl or Dux loss-of-function mouse models are not yet available, and their relevance for understanding the physiological role is not clear.
DUX4 and SDF1-CXCR4 axis in pathology
While in normal adult organism the role of DUX4 is unknown and might be limited to germline, the role of DUX4 in pathology (i.e. in Facioscapulohumeral dystrophy or FSHD) has been extensively explored. In this disease, DUX4 expression could be detected in muscle cells and tissues [11] and is thought to contribute to the pathological phenotype by inducing apoptosis, provoking the sensitivity to oxidative stress and inhibiting myogenic differentiation. Inhibition of myogenic differentiation is one of the best understood functions of DUX4 in FSHD. Murine muscle satellite cells expressing human DUX4 are unable to properly differentiate in vitro [21] or regenerate skeletal muscle tissue in vivo [66].
Inhibition of the myogenic program by DUX4 could be explained by a similarity of recognition sites of DUX4 and Pax3/7 transcription factors essential for the commitment of myogenic progenitors resulting in an interference with the early myogenic differentiation steps [14]. It has also been demonstrated that DUX4 induced the expression of muscular atrophy-related genes [67, 16] myogenic microRNAs [68] and transcription factors [14, 47] suggesting that DUX4 might also interfere with later stages of myogenic differentiation.
Interestingly, CXCR4 is also involved in the regulation of myogenic differentiation. CXCR4 is expressed on the surface of both proliferating and differentiated C2C12 cells [58, 61, 69]. SDF1 expression is increased while CXCR4 decreased during myogenic differentiation of rat myoblasts [70]; however, the role of CXCR4-SDF1 axis in myogenic differentiation remains controversial. Some reports have shown inhibition of myogenic differentiation by SDF1 of CXCR4 signaling [69] or no effect [70]. Other reports have shown that SDF1 induced myotube formation while CXCR4 inhibition via siRNA blocked C2C12 differentiation [71]. The mechanism of CXCR4-dependent regulation of myogenic differentiation is thought to involve MAPK signaling. Intriguingly, MAPK signaling-related genes were identified in DUX4 transcriptomic signature [47]. CXCR4 as one of DUX4 target genes might thus also contribute to FSHD etiology.
Besides an abnormal myogenic differentiation, an accumulation of leukocytes has been also documented in FSHD muscles [72]; the mechanism of this phenomenon is currently unknown. It has been demonstrated that CXCR4 receptor plays a key role in chronic inflammatory conditions such as inflammatory bowel disease (reviewed in [73]), chronic allergic lung inflammation, idiopathic pulmonary fibrosis, liver fibrosis [74], rheumatoid arthritis (reviewed in [75]).
Our results allow us to put forward a hypothesis that DUX4 overexpression resulting in SDF1 expression in FSHD muscles might result in an increased migration of leukocytes to FSHD muscles. It is plausible therefore that DUX4 might be involved in these processes as its aberrant expression in FSHD muscles might activate SDF1 and thus attract circulating CXCR4+ leukocytes which would result in their infiltration to FSHD muscles. Again, this possibility could, in principle, be tested using mouse models of ectopic expression of human DUX4 that have recently been described. Mice expressing human DUX4 under the control of its natural promoter did not show visible phenotypic anomalies except for the eye keratitis of unknown etiology [21]. At the same time, mice expressing DUX4 under the control of a leaky doxycycline-inducible promoter in retina, testis, skin, brain, kidney, and lung, had scaly skin, muscle weakening, increased retina neovascularization, males demonstrated a defect in gametogenesis [66]. Although the latter mouse model had some phenotypical features common to FSHD, its relevance as a model of FSHD is thus unclear.
DUX4 in cancer
Finally, DUX4 is also known to be expressed in many cancer cell lines including rhabdomyosarcoma RD (CCL-136) and RMS13 (CRL-2061) [13, 76], cervix carcinoma HeLa, lung adenocarcinoma A549 cell lines [76] and in non-small cell lung cancer (NSCLC) cells [76]; DUX4 or a homologous transcript was detected in cervical carcinoma cell line C33A [77]. Recurrent DUX4 fusions have been detected in B cell acute lymphoblastic anemia [78], embryonal rhabdomyosarcoma [79], Ewing-like sarcomas [80] and pediatric primitive round cell sarcomas [81]. The mechanism of DUX4 upregulation in cancer cells remains unknown, although it has been proposed that it is linked to demethylation of the 3.3 kb repeats harboring DUX4 ORF [82, 77, 83].
Tumor cells are known to secrete SDF, inflammatory cytokines and growth factors which together promote MSC homing to the tumor site [84]. MSC infiltration has been proven beneficial for some tumor types [85]. One possibility that is suggested by our results but has not been tested in this study is that DUX4 might contribute to the MSC infiltration into tumors. Another possibility that was not addressed in the present study is that DUX4 expression might influence metastatic potential of cancer cells via CXCR4-SDF1 axis. SDF1-CXCR4 signaling is also involved in migration of cancer cells to the sites of metastasis (SDF1-expressing tissues, such as bone marrow and lung) including breast, lung, ovarian, thyroid, rhabdomyosarcoma and others (over 20 human tumor types (reviewed in [86]) [53, 87, 86–89]. Our study thus prompts to test whether DUX4 overexpressing tumors demonstrate an increased metastatic potential as compared to DUX4-negative tumors.
MATERIALS AND METHODS
Cell culture conditions, plasmids and transfection
Human immortalized myoblasts (MB) generated from a healthy subject (a kind gift of Dr. V. Mouly, Institute of Myology, Paris) were cultured in the media composed of 4 parts of high-glucose DMEM, 1 part of Medium 199 (Sigma #M4530) and supplemented with 20% FBS 4 mM L-glutamine 50 mkg/ml gentamicin, 1 mkg/ml Amphotericin B, 2.5 ng/ml human recombinant HGF (Sigma #H1404) and 1 mkM dexamethasone (Sigma #D4902) as described previously [90].
Human primary myoblasts were isolated from skeletal muscle biopsies of healthy subjects and purified using CD56/NCAM magnetic beads (Miltenyi Biotec) as previously described [91], preserved in liquid nitrogen and transported for future use. After unfreezing, the myoblasts were cultured in proliferation medium (high-glucose DMEM (here and below #D6546, Sigma), 20% FBS FBS (Millerium #BWSTS1810/500), 4 mM L-glutamine (Sigma #68540–25 G), 50 μg/ml gentamicin (G1397 Sigma), 1 mkg/ml Amphotericin B (Fungizone, Gibco #15290–018)), passaged at a cell confluence not exceeding 30 % and used for transfection and tests up to passage 5 or earlier to avoid cellular senescence and spontaneous differentiation.
Both primary and immortalized myoblasts were cultured in cell culture dishes coated with collagen using sterile 0.1% solution of collagen powder (#C7661 Sigma) in 0.2% acetic acid.
Human bone marrow-derived mesenchymal cells (BMSC) were isolated as previously described [92]. Briefly, mononuclear cells were isolated from bone marrow aspirates from healthy donors using centrifugation in Histopaque-1077 density gradient (Sigma) at 400 g for 30 min at room temperature; the resulting cells were washed 3 times with DMEM, plated at 10^6 cells/cm2 in cell culture flasks (Greiner Bio-One) and cultured the growth medium (low-glucose DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS) (HyClone), 2 mM GlutaMAXTM (Gibco), and 50 U/ml of penicillin and 50 ug/ml of streptomycin (Gibco); medium was changed every 3 days.
Human adult adipose tissue-derived stem cells (ADAS) were isolated as previously described [92]. Briefly, the adipose tissue aspirates was washed with phosphate-buffered saline (PBS), containing 50 U/ml of penicillin and 50 ug/ml of streptomycin (Gibco), then digested at 37°C for 60 min with 0.075% collagenase type I (Worthington). The cell suspension was washed with DMEM, containing 10% FBS and centrifuged at 300 g for 10 min. The pellet was resuspended in 160 mM NH4Cl and incubated at room temperature for 10 min to lyse contaminating red blood cells. The cell suspension was centrifuged as detailed above, the pellet was resuspended in DMEM supplemented with 20% FCS filtered through a 100-um nylon mesh to remove cellular debris, plated at culture flasks and incubated overnight at 37°C, 5% CO2. Following incubation, the flasks were washed extensively with PBS to remove residual nonadherent blood cells. ADAS were cultured in growth medium DMEM/F12 (Gibco), supplemented with 10% FBS, 2 mM GlutaMAXTM, 50 U/ml of penicillin and 50 ug/ml of streptomycin (Gibco). The medium was changed every 3 days, cells were maintained at subconfluent levels.
TE-671 and HaCat cell lines were cultured in DMEM medium supplemented with 4.5 g/L glucose (Gibco), 2 mM L-glutamine, 10% fetal bovine serum and 100 u/ml penicillin and 100 ug/ml of streptomycin.
Adipogenic differentiation
ADAS and BMSC were plated in 12-well plates at a density of 5 × 103 cells cm-2 and transfected with DUX4-, DUX4c- or control vector when indicated. After 24 h the growth media was replaced with adipogenic induction medium: DMEM, 10% FBS, 0.5 mM isobutyl-methylxantine (Sigma), 1 uM dexamethasone (Sigma), 10 uM insulin (Sigma), 0.2 mM indomethacin (Sigma); the medium was changed every three days. After 21 days cells were fixed with 4% paraformaldehyde in PBS and lipid droplets were revealed by histochemical staining with Oil Red O dye (Sigma) for neutral fats. For quantification, Oil Red O stain was extracted with 100% isopropanol for 5 min, absorbance was measured at 492 nm and normalized to the number of cells; 100% isopropanol was used as a background control; the experiments were performed in quadruplicates.
Transfection. 8 × 104 of human MB cells were resuspended in 400 mkl of corresponding culturing medium, mixed with 80 mkl of OptiMEM (Gibco #31985062) containing 240 ng of plasmid DNA and 0.24 mkl Lipofectamine 2000 (Invitrogen, #11668–019) and plated into 1 well of a 12-well plate (2.1 × 104 cells/cm2); medium was changed 6 h after the transfection. The transfection efficiency was around 40%. 2 × 104 BMSC or ADAS cells were plated in a well of a12-well plate (5 × 103 cells/cm2) and transfected using 1 μg of plasmid DNA and 1 mkl of Lipofectamine 2000™ (Invitrogen) according to the supplier's instructions; medium was changed 6 h after the transfection. The transfection efficiency was 80–90%.
To overexpress DUX4 and DUX4c, we used pCI-Neo-DUX4 [3] and pCI-Neo-DUX4c [22] plasmids containing human DUX4 and DUX4c ORFs genes under control of the CMV promoter (a kind gift of Alexandra Belayew and Frederique Coppée, University of Mons, Belgium); Following control plasmids were used: pCI-Neo (Promega), phrGFP (Stratagene) and pCI-Neo-DUX1 [2].
Immunofluorescence staining
24 h after the transfection MB or BMSC cells were fixed with 2% PFA (Euromedex) in PBS for 5 min, permeabilized with 0.5% triton X-100 (Sigma-Aldrich) in PBS for 5 min, blocked with 5% BSA (Euromedex) in PBS for 1 h, incubated with mouse monoclonal antibodies against DUX4 clone 9A12 [67] (kindly donated by Alexandra Belayew, University of Mons, Belgium) diluted 1:50; rabbit polyclonal antibody against CXCR4 (Abcam #ab 2074) diluted 1:100 or rabbit polyclonal antibody against SDF1 (CXCL12) (Abcam #ab 9797) diluted 1:200 in 2.5% PBS in BSA, stained with Alexa Fluor 488-conjugated anti-rabbit IgG (Life Technologies #A-21441, 1:100) for 2 h or Alexa Fluor 488 anti-mouse IgG (Life Technologies #A-21200, 1:100) for 1 h 2.5% PBS in BSA and mounted with a mounting medium containing DAPI (Vector laboratories), observed under a fluorescent microscope (Microvision instruments, excitation/emission: 488/519 nm, green fluorescence);
Western blot
Whole cell protein extracts were prepared from frozen immortalized or primary myoblast cell pellets using TENT buffer (150 mM NaCl, 1 mM EDTA, 50 mM Tris-HCl pH7.5, 0.5% NP-40) [93] supplemented with anti-protease and anti-phosphatase inhibitor cocktails (#04693159001, #04906845001, Roche), separated on 8% PAAG, transferred to a nitrocellulose membrane (Amersham), blocked with 5% milk/PyTBST, hybridized with primary antibodies 9A12 [67] recognizing DUX4 and DUX4c diluted 1:2000, washed with 1 × PyTBST (10 mM Tris-HCl pH7.4, 75 mM NaCl, 1 mM EDTA, 0.1% Tween 20) [94] to reduce background, hybridized with secondary antibodies conjugated to HRP (SantaCruz #sc2030, #sc2768, #sc2005) diluted 1.2000, revealed with ECL+ (GE Healthcare), exposed to X-ray film (Amersham) and developed in a chemical film processor (FujiFilm). Quantification of scanned X-ray images was performed using ImageJ. After exposition the membranes were stripped in a solution containing 2%SDS and 100 mM beta-mercaptoethanol buffered with 62.5 mM TrisHCl, reblocked in 5% BSA/PBS overnight and rehybridized with mouse monoclonal antibodies against actin (Millipore #mab1501) diluted 1:10 000.
Flow cytometry apoptosis assay was performed using a CF™488A Annexin V and 7-AAD Apoptosis Kit (Biotium, cat # 30060). 24 or 72 h after transfection, MB or BMSC cells were trypsinized, centrifuged, resuspended in PBS. To avoid the loss dead cells, the media with detached cells was also collected and centrifuged at 300 g for 5 min, the cells pellet was resuspended in PBS. The cells were stained according manufacture protocol. Briefly, cells were resuspended at 5 × 106 cells/ml in 1X Annexin Binding Buffer, 5 ul of Annexin V and 2 ul of 7-AAD working solutions were added into the cell suspension and incubated at 4°C for 30 min in a dark. Then 400 ul of 1X Annexin Binding Buffer was added and probes were analyzed by flow cytometry on the Cell Lab Quanta SC (Beckman Coulter, Brea, CA, USA)
Transcriptome profiling
RNA from human MB cells 12- and 20 h after the transfection with pCI-Neo-DUX4, pCI-Neo-DUX4c or pCI-Neo plasmids was extracted using Trizol (Invitrogen) as instructed by the producer and further purified with silica column cleanup using Nucleospin RNA Extraction kit (Macherey Nagel). 500 ng of RNA were used to synthesize Cy3- (control) and Cy5- (DUX4- and DUX4c-transfected MB) labeled probes in a two-step procedure using Agilent Fluorescent Low Input Linear Amplification kit. Mix. Labeled probes were then hybridized to Gene Expression microarrays (4 × 44 k #G4112F, Agilent) and scanned using Agilent G2505C DNA Microarray scanner as instructed by the manufacturer. Scanned images were then analysed using the Feature Extraction software (Agilent, version 10.5.1.1) and gene expression fold change was calculated using Rosetta Resolver (version 7.2.2.0). In the case of DUX4-transfected cells, genes with FC < -3 and Int1 (Cy3) > 50 were considered downregulated; genes with FC > 3 and Int2 (Cy5) > 50 were considered upregulated; in the case of DUX4c-transfected cells, genes with FC < -2 and Int1 (Cy3) > 50 were considered downregulated; genes with FC > 2 and Int2 (Cy5) > 50 were considered upregulated. Gene symbols and descriptions corresponding to significant Agilent IDs were retrieved from the Agilent microarray annotation file and db2db and DAVID gene ID conversion tools. In case of disagreement between the sources, HGNC database (http://www.genenames.org/) was consulted. Probes corresponding to non-annotated genes were not analyzed. Protein-coding genes differentially expressed in DUX4 and DUX4c transfected MB were used for functional annotation with DAVID (http://david.abcc.ncifcrf.gov/) using GOTERM_BP_FAT list and the default background. The stringency of functional annotation clustering was set to “medium”. Clusters with similar biological functions containing at least one significant GO term (acceptable significance: p-value < 0.05, FDR < 20) were manually combined using Microsoft Excel with Ablebits add-ons “Cell merge” (https://www.ablebits.com/) resulting is superclusters “Cell cycle”, “Apoptosis” etc.; The significance of a supercluster was considered to be equal to p-value and FDR corresponding to the most significant GO term within this supercluster.
RT-PCR
Total RNA was extracted using Trizol (Invitrogen) as instructed by the producer; the traces of phenol and salts were eliminated with 2 supplementary chlorophorm extractions, ethanol precipitation and two additional washes with 70% ethanol. 100 ng of purified RNA was then reverse transcribed using Revertaid H minus Reverse transcriptase (Fermentas #EP0451) as instructed by the producer, random hexamers and other RT components were also from Fermentas. cDNA was then diluted 10 times and 2 mkl was mixed with primers (300 nM final) FastStart Universal SYBR Green Master mix (ROX) (Roche #04913850001) in a final volume of 20 mkl and analyzed on Step One plus instrument (Applied Biosystems). The sequence of primers (Invitrogen) used: SDF1-F2 5′-GAACGCCAAGGTCGTGGTCGT; SDF1-R2 5′-TCTGTAGCTCAGGCTGACGGGC; CXCR4-F1 5′-A AAGTACCAGTTTGCCACGGC; CXCR4-R1 5′-GCATG ACGGACAAGTACAGGCT; GAPDH-F2 5′-TCATTTC CTGGTATGACAACGA; GAPDH-R2 5′-TACATGGCA ACTGTGAGGAG; Reactions were performed in triplicates, DDct method was used to analyze data [95].
Migration assays
Migration assays were carried out in a 24-well transwell system equipped with porous (8 μm) polycarbonate membranes. 2 × 105 MB cells were resuspended in 600 μL of growth medium supplemented with 1% FBS and plated into the lower chamber of the transwell system; 5.0 × 104 BMSC cells were resuspended in 200 mkl of growth medium supplemented with 1% FBS and plated into Transwell inserts which were then placed into another transwell system with the lower chamber filled with 600 μl of serum-free medium. After 24 hours the cells on the inserts or in lower chambers were transfected as indicated, 6 hours after the transfection the media was changed and the inserts with BMSC, were placed into the wells with MB cells and incubated at 37 °C for 24 h. When indicated, CXCR4 (Abcam, #ab10403) or SDF1 (CXCL12) (Abcam, #ab9797) antibodies were added to the medium at 10 mkg/ml and 4 mkg/ml concentrations respectively. The inserts were then discarded, and upper sides of the filters were swabbed to remove the cells that did not cross the membrane. The cells present on the lower side of the filters were then fixed in 4 % paraformaldehyde, stained with DAPI and the counted under the microscope. All the experiments were performed in duplicate. The following control antibodies were used: rabbit IgG control (AB-105-C, R&D systems) and mouse IgG2b isotype control (MAB004, R&D systems).
SDF-1α ELISA assay
3 × 106 MB cells were plated on 10 cm dish and after 24 h were transfected with 8 μg of plasmid DNA using JetPEI as instructed by the producer (Polyplus transfection), ratio plasmid:JetPEI was 1:2; medium was changed 24 h after the transfection. To quantify secreted CXCL12, the medium was collected 48 h after the transfection, centrifuged at 10 000 rpm for 10 min and concentrated 20 times using a 15 ml spin concentrators (5 KDa MWCO) (Agilent Technologies). To quantify intracellular CXCL12, whole cell lysates were prepared in 500 μl of RIPA buffer and diluted 10 times prior to analysis. Samples were analyzed in triplicates using human SDF 1α ELISA Kit (Abcam #ab100637).
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
The research has been supported by the MEGAFSHD grant from the Association Française contre les Myopathies (AFM) to YSV. PD was supported by Association de Recherche Contre le Cancer (ARC), and the Amis FSH association. The work of EK was partly conducted in the frame of the IDB RAS government program of basic research № 0108-2014-0004.
Footnotes
CONFLICTS OF INTEREST
None.
REFERENCES
- 1.Zhong YF, Holland PW. The dynamics of vertebrate homeobox gene evolution: gain and loss of genes in mouse and human lineages. BMC Evol Biol. 2011;11:169. doi: 10.1186/1471-2148-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding H, Beckers MC, Plaisance S, Marynen P, Collen D, Belayew A. Characterization of a double homeodomain protein (DUX1) encoded by a cDNA homologous to 3. 3 kb dispersed repeated elements. Hum Mol Genet. 1998;7:1681–1694. doi: 10.1093/hmg/7.11.1681. [DOI] [PubMed] [Google Scholar]
- 3.Gabriels J, Beckers MC, Ding H, De Vriese A, Plaisance S, van der Maarel SM, Padberg GW, Frants RR, Hewitt JE, Collen D, Belayew A. Nucleotide sequence of the partially deleted D4Z4 locus in a patient with FSHD identifies a putative gene within each 3. 3 kb element. Gene. 1999;236:25–32. doi: 10.1016/s0378-1119(99)00267-x. [DOI] [PubMed] [Google Scholar]
- 4.Beckers M, Gabriels J, van der Maarel S, De Vriese A, Frants RR, Collen D, Belayew A. Active genes in junk DNA? Characterization of DUX genes embedded within 3. 3 kb repeated elements. Gene. 2001;264:51–57. doi: 10.1016/s0378-1119(00)00602-8. [DOI] [PubMed] [Google Scholar]
- 5.Booth HA, Holland PW. Annotation, nomenclature and evolution of four novel homeobox genes expressed in the human germ line. Gene. 2007;387:7–14. doi: 10.1016/j.gene.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Holland PW, Booth HA, Bruford EA. Classification and nomenclature of all human homeobox genes. BMC Biol. 2007;5:47. doi: 10.1186/1741-7007-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewitt JE. Loss of epigenetic silencing of the DUX4 transcription factor gene in facioscapulohumeral muscular dystrophy. Hum Mol Genet. 2015 doi: 10.1093/hmg/ddv237. [DOI] [PubMed] [Google Scholar]
- 8.Hewitt JE, Lyle R, Clark LN, Valleley EM, Wright TJ, Wijmenga C, van Deutekom JC, Francis F, Sharpe PT, Hofker M. Analysis of the tandem repeat locus D4Z4 associated with facioscapulohumeral muscular dystrophy. Hum Mol Genet. 1994;3:1287–1295. doi: 10.1093/hmg/3.8.1287. [DOI] [PubMed] [Google Scholar]
- 9.Alkan C, Kidd JM, Marques-Bonet T, Aksay G, Antonacci F, Hormozdiari F, Kitzman JO, Baker C, Malig M, Mutlu O, Sahinalp SC, Gibbs RA, Eichler EE. Personalized copy number and segmental duplication maps using next-generation sequencing. Nat Genet. 2009;41:1061–1067. doi: 10.1038/ng.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright TJ, Wijmenga C, Clark LN, Frants RR, Williamson R, Hewitt JE. Fine mapping of the FSHD gene region orientates the rearranged fragment detected by the probe p13E-11. Hum Mol Genet. 1993;2:1673–1678. doi: 10.1093/hmg/2.10.1673. [DOI] [PubMed] [Google Scholar]
- 11.Snider L, Geng LN, Lemmers RJ, Kyba M, Ware CB, Nelson AM, Tawil R, Filippova GN, van der Maarel SM, Tapscott SJ, Miller DG. Facioscapulohumeral dystrophy: incomplete suppression of a retrotransposed gene. PLoS Genet. 2010 doi: 10.1371/journal.pgen.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng LN, Yao Z, Snider L, Fong AP, Cech JN, Young JM, van der Maarel SM, Ruzzo WL, Gentleman RC, Tawil R, Tapscott SJ. DUX4 activates germline genes, retroelements, and immune mediators: implications for facioscapulohumeral dystrophy. Dev Cell. 2012;22:38–51. doi: 10.1016/j.devcel.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowaljow V, Marcowycz A, Ansseau E, Conde CB, Sauvage S, Matteotti C, Arias C, Corona ED, Nunez NG, Leo O, Wattiez R, Figlewicz D, Laoudj-Chenivesse D, et al. The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein. Neuromuscul Disord. 2007;17:611–623. doi: 10.1016/j.nmd.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Bosnakovski D, Xu Z, Gang EJ, Galindo CL, Liu M, Simsek T, Garner HR, Agha-Mohammadi S, Tassin A, Coppee F, Belayew A, Perlingeiro RR, Kyba M. An isogenetic myoblast expression screen identifies DUX4-mediated FSHD-associated molecular pathologies. The EMBO J. 2008;27:2766–2779. doi: 10.1038/emboj.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corona ED, Jacquelin D, Gatica L, Rosa AL. Multiple protein domains contribute to nuclear import and cell toxicity of DUX4, a candidate pathogenic protein for facioscapulohumeral muscular dystrophy. PLoS ONE. 2013;8:e75614. doi: 10.1371/journal.pone.0075614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanderplanck C, Ansseau E, Charron S, Stricwant N, Tassin A, Laoudj-Chenivesse D, Wilton SD, Coppee F, Belayew A. The FSHD Atrophic Myotube Phenotype Is Caused by DUX4 Expression. PLoS ONE. 2011 doi: 10.1371/journal.pone.0026820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snider L, Asawachaicharn A, Tyler AE, Geng LN, Petek LM, Maves L, Miller DG, Lemmers RJ, Winokur ST, Tawil R, van der Maarel SM, Filippova GN, Tapscott SJ. RNA transcripts, miRNA-sized fragments and proteins produced from D4Z4 units: new candidates for the pathophysiology of facioscapulohumeral dystrophy. Hum Mol Genet. 2009;18:2414–2430. doi: 10.1093/hmg/ddp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wuebbles RD, Long SW, Hanel ML, Jones PL. Testing the effects of FSHD candidate gene expression in vertebrate muscle development. Int J Clin Exp Pathol. 2010;3:386–400. [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace LM, Garwick SE, Mei W, Belayew A, Coppee F, Ladner KJ, Guttridge D, Yang J, Harper SQ. DUX4, a candidate gene for facioscapulohumeral muscular dystrophy, causes p53-dependent myopathy in vivo. Ann Neurol. 2010;69:540–552. doi: 10.1002/ana.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsuhashi H, Mitsuhashi S, Lynn-Jones T, Kawahara G, Kunkel LM. Expression of DUX4 in zebrafish development recapitulates facioscapulohumeral muscular dystrophy. Hum Mol Genet. 2013;22:568–577. doi: 10.1093/hmg/dds467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krom YD, Thijssen PE, Young JM, den Hamer B, Balog J, Yao Z, Maves L, Snider L, Knopp P, Zammit PS, Rijkers T, van Engelen BG, Padberg GW, et al. Intrinsic epigenetic regulation of the D4Z4 macrosatellite repeat in a transgenic mouse model for FSHD. PLoS Genet. 2013 doi: 10.1371/journal.pgen.1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ansseau E, Laoudj-Chenivesse D, Marcowycz A, Tassin A, Vanderplanck C, Sauvage S, Barro M, Mahieu I, Leroy A, Leclercq I, Mainfroid V, Figlewicz D, Mouly V, et al. DUX4c is up-regulated in FSHD. It induces the MYF5 protein and human myoblast proliferation. PLoS ONE. 2009 doi: 10.1371/journal.pone.0007482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosnakovski D, Lamb S, Simsek T, Xu Z, Belayew A, Perlingeiro R, Kyba M. DUX4c, an FSHD candidate gene, interferes with myogenic regulators and abolishes myoblast differentiation. Exp Neurol. 2008;214:87–96. doi: 10.1016/j.expneurol.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Geng LN, Tyler AE, Tapscott SJ. Immunodetection of human double homeobox 4. Hybridoma (Larchmt) 2011;30:125–130. doi: 10.1089/hyb.2010.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeVries ME, Kelvin AA, Xu L, Ran L, Robinson J, Kelvin DJ. Defining the origins and evolution of the chemokine/chemokine receptor system. J Immunol. 2006;176:401–415. doi: 10.4049/jimmunol.176.1.401. [DOI] [PubMed] [Google Scholar]
- 26.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 27.Baggiolini M. Chemokines, leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 28.Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol. 2015:7. doi: 10.1101/cshperspect.a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esche C, Stellato C, Beck LA. Chemokines: key players in innate and adaptive immunity. J Invest Dermatiol. 2005;125:615–628. doi: 10.1111/j.0022-202X.2005.23841.x. [DOI] [PubMed] [Google Scholar]
- 30.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 31.Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- 32.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 33.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 35.Raz E, Mahabaleshwar H. Chemokine signaling in embryonic cell migration: a fisheye view. Development. 2009;136:1223–1229. doi: 10.1242/dev.022418. [DOI] [PubMed] [Google Scholar]
- 36.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leuk Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 37.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Lewellis SW, Knaut H. Attractive guidance: how the chemokine SDF1/CXCL12 guides different cells to different locations. Semin. Cell Dev Biol. 2012;23:333–340. doi: 10.1016/j.semcdb.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagasawa T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J Mol Med. 2014;92:433–439. doi: 10.1007/s00109-014-1123-8. [DOI] [PubMed] [Google Scholar]
- 40.Martins-Green M, Petreaca M, Wang L. Chemokines and Their Receptors Are Key Players in the Orchestra That Regulates Wound Healing. Adv Wound Care. 2013;2:327–347. doi: 10.1089/wound.2012.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma M, Afrin F, Satija N, Tripathi RP, Gangenahalli GU. Stromal-derived factor-1/CXCR4 signaling: indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow. Stem Cell Dev. 2011;20:933–946. doi: 10.1089/scd.2010.0263. [DOI] [PubMed] [Google Scholar]
- 42.Marquez-Curtis LA, Janowska-Wieczorek A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed Res Int. 2013;2013:561098. doi: 10.1155/2013/561098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984–991. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 44.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7:19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem cells (Dayton, Ohio) 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 47.Sharma V, Harafuji N, Belayew A, Chen YW. DUX4 Differentially Regulates Transcriptomes of Human Rhabdomyosarcoma and Mouse C2C12 Cells. PLoS ONE. 2013 doi: 10.1371/journal.pone.0064691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Homma S, Beermann ML, Boyce FM, Miller JB. Expression of FSHD-related DUX4-FL alters proteostasis and induces TDP-43 aggregation. Ann Clin Transl Neurol. 2015;2:151–166. doi: 10.1002/acn3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dandapat A, Hartweck LM, Bosnakovski D, Kyba M. Expression of the human FSHD-linked DUX4 gene induces neurogenesis during differentiation of murine embryonic stem cells. Stem Cells Dev. 2013;22:2440–2448. doi: 10.1089/scd.2012.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao Z, Snider L, Balog J, Lemmers RJ, Van Der Maarel SM, Tawil R, Tapscott SJ. DUX4-induced gene expression is the major molecular signature in FSHD skeletal muscle. Hum Mol Genet. 2014;23:5342–5352. doi: 10.1093/hmg/ddu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu H, Wang Z, Jin S, Hao H, Zheng L, Zhou B, Zhang W, Lv H, Yuan Y. Dux4 induces cell cycle arrest at G1 phase through upregulation of p21 expression. Biochem Biophys Res Commun. 2014;446:235–240. doi: 10.1016/j.bbrc.2014.02.105. [DOI] [PubMed] [Google Scholar]
- 52.Broxmeyer HE. Regulation of hematopoiesis by chemokine family members. Int J Hematol. 2001;74:9–17. doi: 10.1007/BF02982544. [DOI] [PubMed] [Google Scholar]
- 53.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 54.Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O'Brien W, Raz E, Littman D, Wylie C, Lehmann R. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development. 2003;130:4279–4286. doi: 10.1242/dev.00640. [DOI] [PubMed] [Google Scholar]
- 55.Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- 56.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 57.Odemis V, Lamp E, Pezeshki G, Moepps B, Schilling K, Gierschik P, Littman DR, Engele J. Mice deficient in the chemokine receptor CXCR4 exhibit impaired limb innervation and myogenesis. Mol Cell Neurosci. 2005;30:494–505. doi: 10.1016/j.mcn.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 58.Vasyutina E, Stebler J, Brand-Saberi B, Schulz S, Raz E, Birchmeier C. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Dev. 2005;19:2187–2198. doi: 10.1101/gad.346205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yusuf F, Rehimi R, Morosan-Puopolo G, Dai F, Zhang X, Brand-Saberi B. Inhibitors of CXCR4 affect the migration and fate of CXCR4+ progenitors in the developing limb of chick embryos. Dev Dyn. 2006;235:3007–3015. doi: 10.1002/dvdy.20951. [DOI] [PubMed] [Google Scholar]
- 60.Masyuk M, Abduelmula A, Morosan-Puopolo G, Odemis V, Rehimi R, Khalida N, Yusuf F, Engele J, Tamamura H, Theiss C, Brand-Saberi B. Retrograde migration of pectoral girdle muscle precursors depends on CXCR4/SDF-1 signaling. Histochem Cell Biol. 2014 doi: 10.1007/s00418-014-1237-7. [DOI] [PubMed] [Google Scholar]
- 61.Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, Janowska-Wieczorek A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem cells (Dayton, Ohio) 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- 62.Clapp J, Mitchell LM, Bolland DJ, Fantes J, Corcoran AE, Scotting PJ, Armour JA, Hewitt JE. Evolutionary conservation of a coding function for D4Z4, the tandem DNA repeat mutated in facioscapulohumeral muscular dystrophy. Am. J Hum Genet. 2007;81:264–279. doi: 10.1086/519311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawazu M, Yamamoto G, Yoshimi M, Yamamoto K, Asai T, Ichikawa M, Seo S, Nakagawa M, Chiba S, Kurokawa M, Ogawa S. Expression profiling of immature thymocytes revealed a novel homeobox gene that regulates double-negative thymocyte development. J Immunol. 2007;179:5335–5345. doi: 10.4049/jimmunol.179.8.5335. [DOI] [PubMed] [Google Scholar]
- 64.Wu SL, Tsai MS, Wong SH, Hsieh-Li HM, Tsai TS, Chang WT, Huang SL, Chiu CC, Wang SH. Characterization of genomic structures and expression profiles of three tandem repeats of a mouse double homeobox gene: Duxbl. Dev Dyn. 2010;239:927–940. doi: 10.1002/dvdy.22210. [DOI] [PubMed] [Google Scholar]
- 65.Wu SL, Li GZ, Chou CY, Tsai MS, Chen YP, Li CJ, Liou GG, Chang WW, Chen SL, Wang SH. Double homeobox gene, Duxbl, promotes myoblast proliferation and abolishes myoblast differentiation by blocking MyoD transactivation. Cell Tissue Res. 2014;358:551–566. doi: 10.1007/s00441-014-1974-x. [DOI] [PubMed] [Google Scholar]
- 66.Dandapat A, Bosnakovski D, Hartweck LM, Arpke RW, Baltgalvis KA, Vang D, Baik J, Darabi R, Perlingeiro RC, Hamra FK, Gupta K, Lowe DA, Kyba M. Dominant lethal pathologies in male mice engineered to contain an X-linked DUX4 transgene. Cell Rep. 2014;8:1484–1496. doi: 10.1016/j.celrep.2014.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dixit M, Ansseau E, Tassin A, Winokur S, Shi R, Qian H, Sauvage S, Matteotti C, van Acker AM, Leo O, Figlewicz D, Barro M, Laoudj-Chenivesse D, et al. DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1. Proc Natl Acad Sci USA. 2007;104:18157–18162. doi: 10.1073/pnas.0708659104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dmitriev P, Stankevicins L, Ansseau E, Petrov A, Barat A, Dessen P, Robert T, Turki A, Lazar V, Labourer E, Belayew A, Carnac G, Laoudj-Chenivesse D, et al. Defective regulation of microRNA target genes in myoblasts from facioscapulohumeral dystrophy patients. J Biol Chem. 2013;288:34989–35002. doi: 10.1074/jbc.M113.504522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Odemis V, Boosmann K, Dieterlen MT, Engele J. The chemokine SDF1 controls multiple steps of myogenesis through atypical PKCzeta. J Cell Sci. 2007;120:4050–4059. doi: 10.1242/jcs.010009. [DOI] [PubMed] [Google Scholar]
- 70.Brzoska E, Kowalewska M, Markowska-Zagrajek A, Kowalski K, Archacka K, Zimowska M, Grabowska I, Czerwinska AM, Czarnecka-Gora M, Streminska W, Janczyk-Ilach K, Ciemerych MA. Sdf-1 (CXCL12) improves skeletal muscle regeneration via the mobilisation of Cxcr4 and CD34 expressing cells. Biol Cell. 2012;104:722–737. doi: 10.1111/boc.201200022. [DOI] [PubMed] [Google Scholar]
- 71.Melchionna R, Di Carlo A, De Mori R, Cappuzzello C, Barberi L, Musaro A, Cencioni C, Fujii N, Tamamura H, Crescenzi M, Capogrossi MC, Napolitano M, Germani A. Induction of myogenic differentiation by SDF-1 via CXCR4 and CXCR7 receptors. Muscle Nerve. 2010;41:828–835. doi: 10.1002/mus.21611. [DOI] [PubMed] [Google Scholar]
- 72.Arahata K, Ishihara T, Fukunaga H, Orimo S, Lee JH, Goto K, Nonaka I. Inflammatory response in facioscapulohumeral muscular dystrophy (FSHD): immunocytochemical and genetic analyses. Muscle Nerve. 1995;2:S56–66. [PubMed] [Google Scholar]
- 73.Werner L, Guzner-Gur H, Dotan I. Involvement of CXCR4/CXCR7/CXCL12 Interactions in Inflammatory bowel disease. Theranostics. 2013;3:40–46. doi: 10.7150/thno.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wald O, Pappo O, Safadi R, Dagan-Berger M, Beider K, Wald H, Franitza S, Weiss I, Avniel S, Boaz P, Hanna J, Zamir G, Eid A, et al. Involvement of the CXCL12/CXCR4 pathway in the advanced liver disease that is associated with hepatitis C virus or hepatitis B virus. Eur J Immunol. 2004;34:1164–1174. doi: 10.1002/eji.200324441. [DOI] [PubMed] [Google Scholar]
- 75.Hummel S, Van Aken H, Zarbock A. Inhibitors of CXC chemokine receptor type 4: putative therapeutic approaches in inflammatory diseases. Curr Opin Immunology. 2014;21:29–36. doi: 10.1097/MOH.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 76.Lang DS, Marwitz S, Kugler C, Reinmuth N, Vollmer E, Zabel P, Reck M, Goldmann T. Double Homeobox Protein DUX4 in the Human Lung: Expression under Normal and Pathological Conditions. GJMR-C. 2014;2:1–9. [Google Scholar]
- 77.Katargin AN, Pavlova LS, Kisseljov FL, Kisseljova NP. Hypermethylation of genomic 3. 3-kb repeats is frequent event in HPV-positive cervical cancer. BMC Med Genomics. 2009;2:30. doi: 10.1186/1755-8794-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yasuda T, Tsuzuki S, Kawazu M, Hayakawa F, Kojima S, Ueno T, Imoto N, Kohsaka S, Kunita A, Doi K, Sakura T, Yujiri T, Kondo E, et al. Recurrent DUX4 fusions in B cell acute lymphoblastic leukemia of adolescents and young adults. Nat Genet. 2016;48:569–74. doi: 10.1038/ng.3535. [DOI] [PubMed] [Google Scholar]
- 79.Sirvent N, Trassard M, Ebran N, Attias R, Pedeutour F. Fusion of EWSR1 with the DUX4 facioscapulohumeral muscular dystrophy region resulting from t(4;22)(q35;q12) in a case of embryonal rhabdomyosarcoma. Cancer Genet Cytogenet. 2009;195:12–18. doi: 10.1016/j.cancergencyto.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 80.Kawamura-Saito M, Yamazaki Y, Kaneko K, Kawaguchi N, Kanda H, Mukai H, Gotoh T, Motoi T, Fukayama M, Aburatani H, Takizawa T, Nakamura T. Fusion between CIC and DUX4 up-regulates PEA3 family genes in Ewing-like sarcomas with t(4;19)(q35;q13) translocation. Hum Mol Genet. 2006;15:2125–2137. doi: 10.1093/hmg/ddl136. [DOI] [PubMed] [Google Scholar]
- 81.Graham C, Chilton-MacNeill S, Zielenska M, Somers GR. The CIC-DUX4 fusion transcript is present in a subgroup of pediatric primitive round cell sarcomas. Hum Pathol. 2012;43:180–189. doi: 10.1016/j.humpath.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 82.Cadieux B, Ching TT, VandenBerg SR, Costello JF. Genome-wide hypomethylation in human glioblastomas associated with specific copy number alteration, methylenetetrahydrofolate reductase allele status, and increased proliferation. Cancer Res. 2006;66:8469–8476. doi: 10.1158/0008-5472.CAN-06-1547. [DOI] [PubMed] [Google Scholar]
- 83.Paz MF, Wei S, Cigudosa JC, Rodriguez-Perales S, Peinado MA, Huang TH, Esteller M. Genetic unmasking of epigenetically silenced tumor suppressor genes in colon cancer cells deficient in DNA methyltransferases. Hum Mol Genet. 2003;12:2209–2219. doi: 10.1093/hmg/ddg226. [DOI] [PubMed] [Google Scholar]
- 84.Sun Z, Wang S, Zhao RC. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J Hematol Oncol. 2014;7:14. doi: 10.1186/1756-8722-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yagi H, Kitagawa Y. The role of mesenchymal stem cells in cancer development. Front Genet. 2013;4:261. doi: 10.3389/fgene.2013.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, Walenkamp AM. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013;49:219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 87.Santoni M, Bracarda S, Nabissi M, Massari F, Conti A, Bria E, Tortora G, Santoni G, Cascinu S. CXC and CC chemokines as angiogenic modulators in nonhaematological tumors. Biomed Res Int. 2014;2014:768758. doi: 10.1155/2014/768758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lourenco S, Teixeira VH, Kalber T, Jose RJ, Floto RA, Janes SM. Macrophage migration inhibitory factor-CXCR4 is the dominant chemotactic axis in human mesenchymal stem cell recruitment to tumors. J Immunol. 2015;194:3463–3474. doi: 10.4049/jimmunol.1402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murphy PM. Chemokines and the molecular basis of cancer metastasis. New Engl J Med. 2001;345:833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 90.Zhu CH, Mouly V, Cooper RN, Mamchaoui K, Bigot A, Shay JW, Di Santo JP, Butler-Browne GS, Wright WE. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging cell. 2007;6:515–523. doi: 10.1111/j.1474-9726.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 91.Barro M, Carnac G, Flavier S, Mercier J, Vassetzky Y, Laoudj-Chenivesse D. Myoblasts from affected and non-affected FSHD muscles exhibit morphological differentiation defects. J Cel Mol Med. 2010;14:275–289. doi: 10.1111/j.1582-4934.2008.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kiseleva EV, Chermnykh ES, Vorotelyak EA, Volozhinb AI, Vasiliev AV, Terskikh VV. Differentiation capacity of stromal fibroblast-like cells from human bone marrow, adipose tissue, hair follicle dermal papilla and derma. Cell Tissue Biol. 2009;3:42–49. [Google Scholar]
- 93.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 94.Malathi K, Xiao Y, Mitchell AP. Catalytic roles of yeast GSK3beta/shaggy homolog Rim11p in meiotic activation. Genetics. 1999;153:1145–1152. doi: 10.1093/genetics/153.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. BioTechniques. 2002;32:1372–1374. 1376, 1378–1379. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


