Abstract
Appropriate tissue morphogenesis strictly requires the developmental regulation of different types of nuclear movements. LINC (linker of nucleoskeleton and cytoskeleton) complexes are macromolecular scaffolds that span the nuclear envelope and physically connect the nuclear interior to different cytoskeletal elements and molecular motors, thereby playing essential roles in nucleokinesis. Recent studies dedicated to the in vivo disruption of LINC complexes not only confirmed their widespread role in nuclear dynamics, but also led to a vigorous regain of interest in the physiological relevance of nuclear positioning within cells and syncitia. In the present paper, we review the results of LINC complex disruption in vivo across different organisms and the potential implications of observed phenotypes in human diseases.
Keywords: interkinetic nuclear migration, linker of nucleoskeleton and cytoskeleton complex (LINC complex), nesprin, neuronal migration, nuclear positioning, SUN protein
LINC complexes
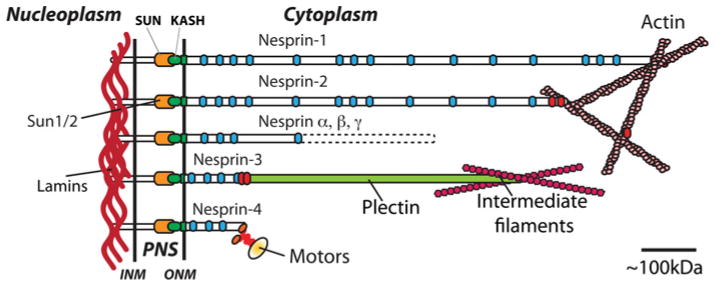
It has now been more than a decade since studies in Drosophila [1,2] and Caenorhabditis elegans [3] identified proteins belonging to what are now generally called LINC (linker of nucleoskeleton and cytoskeleton) complexes, a diverse assortment of macromolecular scaffolds that span the NE (nuclear envelope) and connect the nuclear interior to the cytoskeleton (Figure 1). In vertebrates, LINC complexes form through the interaction between two families of transmembrane proteins within the perinuclear space that separates the INM (inner nuclear membrane) from the ONM (outer nuclear membrane) of the NE (see [4,5] for recent reviews). As shown in Figure 1, one family corresponds to integral transmembrane SUN proteins (SUN1 and SUN2) that populate the INM. SUN protein nucleoplasmic regions interact directly with nuclear lamins [6,7]. A hallmark of SUN proteins is the evolutionarily conserved C-terminal SUN (Sad1/Unc84) domain made of ~150 C-terminal amino acids that protrudes into the perinuclear space. There, the SUN domain interacts with the KASH (Klarsicht/ANC-1/SYNE homology) domain, the evolutionarily conserved molecular signature of nesprins, the other family of LINC complex proteins that populate the ONM. In mammals, four genes encoding nesprin-1, -2, -3 and -4 have currently been identified [8]. They lead to the tissue- and development-specific synthesis of a plethora of KASH domain-containing proteins whose nucleoplasmic regions vary greatly in size (~40 kDa–1 MDa) and harbour a variable number of spectrin repeats known to provide interacting interfaces with the cytoskeleton [9,10]. Interactions between specific mammalian nesprins with various cytoskeletal networks and molecular motors have been delineated (Figure 1). The giant isoforms of nesprin-1 and -2 possess an N-terminal calponin homology region that binds directly to actin [11,12]. Nesprin-3 interacts with plectin, thereby connecting the nucleus to intermediate filaments [13,14]. Finally, connection of nesprins to molecular motors is illustrated by the interaction of nesprin-4, whose expression is restricted to secretory epithelia, with kinesin-1 [15] and of nesprin-1 and -2 with dynein complex components [16,17]. Early studies pointed to a central role for SUN proteins and nesprins in nuclear positioning. Indeed, mutations of either Klarsicht (KASH) or Klaroid (SUN) affect apical nuclear migration in developing Drosophila eye disc and mutations of Unc84 (SUN), Unc83 (KASH) or Anc1 (KASH) in C. elegans alter nuclear migration and/or anchorage during hypodermal syncytium development [18]. In vertebrates, recent studies not only confirmed the essential role of LINC complexes in different aspects of nuclear positioning in vivo, but also emphasized the physiological importance of nuclear positioning at various developmental stages.
Figure 1. Interactions of SUN proteins and nesprins.
Depiction of SUN proteins and nesprins whose interactions through evolutionarily conserved SUN and KASH domains provide macromolecular scaffolds that span the NE and mediate physical interactions between cytoplasmic and nucleoplasmic components (see the text for more details). Blue ovals are spectrin repeats; red ovals are actin-binding domains. PNS, perinuclear space.
Nuclear positioning in skeletal muscle
Mouse models of LINC complex disruption have clearly shown that SUN proteins and nesprins govern nuclear anchorage in skeletal muscle [19–22]. Myonuclei are regularly spaced along muscle fibres, whereas groups of four to five closely juxtaposed nuclei, called synaptic nuclei, are anchored just beneath arrays of acetylcholine receptors at the neuromuscular junction. Genetic ablation of Syne1, which encodes nesprin-1 in mice, results in mispositioning of both synaptic and extrasynaptic nuclei in skeletal muscle fibres [19,21]. However, loss of function of Syne2, which encodes nesprin-2, does not affect myonuclei anchorage [19]. This is rather surprising, because nesprin-1 and -2 are both expressed in skeletal muscle [23] and display similar architectural organization. Because the nesprin-1-encoding gene encodes a wide array of isoforms originating from the combined use of multiple internal promoters and alternative splicing [10], the identity of nesprin-1 isoforms that are involved in synaptic nuclei anchorage remains to be established. The giant isoform of nesprin-1, because of its ability to directly connect the NE to the actin network through its N-terminal actin-binding domain, is an ideal candidate for such a task. However, shorter nesprin isoforms are predominantly expressed in adult skeletal muscle in comparison with their giant counterparts [23,24]. These results therefore suggest that shorter nesprin-1 isoforms may potentially play a significant role in myonuclear anchorage as well. Because desmin is also required for myonuclei positioning [25] and nesprins have the potential to interact with and recruit intermediate filaments at the nuclear periphery [13,14], one can envisage a mechanism where smaller isoforms of nesprins may interact with desmin to mediate myonuclear anchorage. Taken together, these data clearly indicate that LINC complexes govern myonuclei anchorage in skeletal muscle fibres. However, whether abnormal positioning of synaptic nuclei is associated with the molecular aetiology of muscular dystrophies can be questioned, because there is a lack of correlation between myonuclei mispositioning and the development of muscular pathologies in different mouse models of nesprin-1 gene inactivation [19,21,26,27]. Furthermore, mutations of nesprin-1 associated with autosomal recessive cerebellar ataxia alter synaptic nuclei positioning [28], even though affected patients do not show any overt clinical manifestation of muscular dystrophy. Hence, it seems that new hypotheses need to be invoked in order to account for the reported mutations of nesprin-1 and -2 in muscle and cardiac pathologies [29,30]. In that respect, several lines of evidence point to KASH-less isoforms of nesprins. First, the use of KASH-less isoforms is not uncommon in C. elegans and Drosophila [18]. For example, the alternative splicing of the KASH domain of Klarsicht in Drosophila embryos redirects KASH-less isoform to lipid droplets rather than the NE [31]. Secondly, bioinformatic analyses strongly suggest the evolutionary conservation of the skipping of nesprin-1 penultimate exon that leads to KASH-less isoform synthesis in vertebrates and nesprin-1 and -2 transcripts that putatively encode KASH-less isoforms have been reported [10,21,24]. Thirdly, nesprin-2 immunoreactivity has been reported in Z-lines and sarcoplasmic reticulum of skeletal muscle [24] as well as at filipodia and microspikes [32]. These results, in addition to the property of spectrins to bind multiple cytoskeletal elements, therefore suggest that KASH-less nesprins may play important structural roles away from the NE that may also be impaired by mutations associated with cardiac or skeletal muscle pathologies. Examining these hypotheses will require both the identification of additional pedigrees to clearly establish the causality of reported nesprin mutations in skeletal muscle and cardiac pathologies and the sustained development of appropriate antibodies to unequivocally identify the complex pattern of nesprin isoforms synthesis in different tissues and cell types.
Nuclear positioning in the CNS (central nervous system)
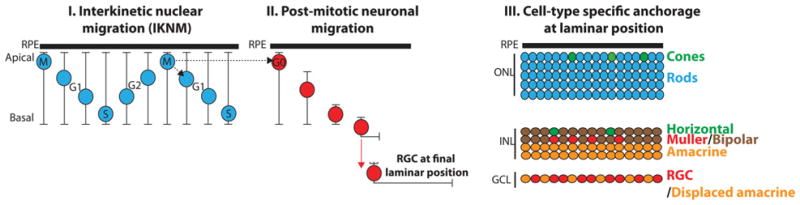
CNS development is accompanied by a spectacular array of nuclear movements that are illustrated throughout the development of the retina, an accessible extension of the CNS (Figure 2).
Figure 2. Depiction of nuclear movements associated with retinal development, an integral part of the CNS.
See the text for details. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RGC, retinal ganglion cells, the first cells to exit the cell cycle in the developing retina; RPE, retinal pigment epithelium.
The first of these movements, called IKNM (interkinetic nuclear migration), consists of oscillations of neuroblast nuclei between the apical and basal side of pseudostratified neuroepithelia (Figure 2, I). Remarkably, these nuclear movements are coupled to the cell cycle in that mitoses take place on the apical side while S-phase takes place at more basal locations. By opposition to neuronal migration (see below), nuclear movements during IKNM are not associated with any movement of the centrosome, which remains at the apical side of neuroepithelial cells. IKNM requires kinesins, dynein–dynactin complexes and actomyosin [33–35]. Importantly, interfering with cell-cycle progression blocks IKNM, whereas interfering with IKNM does not prevent cell-cycle progression. In the latter case, however, nuclear mispositioning during IKNM compromises the timing of neurogenesis [36]. For example, zebrafish strains carrying dynactin mutations display defective IKNM that result in larger and smaller populations of early-born and later-born neurons respectively [34]. Interestingly, down-regulation of the zebrafish nesprin-2 homologue also leads to an increased number of early-born neurons and a significant decrease in later-born neurons [34]. In agreement with a role of LINC complexes during IKNM, both nesprin-2−/− and Sun1/Sun2 DKO (double-knockout) mouse brain and retina tissues display ectopic mitotic and S-phase nuclei, indicating IKNM defects [16,17]. Furthermore, the early depletion of neural progenitors observed in nesprin-2−/− and Sun1/Sun2 DKO mouse brain also suggest an imbalance between differentiation and proliferation of neural precursors. These results, in conjunction with the evolutionarily conserved interaction between nesprins and molecular motors [15–17,37,38], strongly suggest that LINC complexes are indispensable to recruit molecular motors at the NE to sustain nuclear translocation during IKNM.
Upon exit from the cell cycle (Figure 2, II), post-mitotic neurons migrate from the apical side of the neuroblast layer towards their final laminar position [39]. It is now well established that neuronal migration is tightly coupled to nuclear translocation. Nuclear translocation consists of the forward saltatory movement of the nucleus behind the centrosome that moves at a relatively constant velocity. The physical coupling of the centrosome to the nucleus during nuclear translocation is absolutely essential [39–43]. A variety of human syndromes associated with the failure of radial neuronal migration and the ensuing abnormal lamination of the cerebral cortex is associated with the mutation of LIS1 and DCX [44–46]. These mutations severely impair centrosome coupling to the nucleus during nuclear translocation. Similarly, Sun1/Sun2 DKO and nesprin-2−/− mice display major disconnections between nuclei and centrosomes that result in failure of radial neuronal migration in the cerebral cortex [16]. Curiously, nesprin-2 alone is strictly required for radial neuronal migration within developing cortex and hippocampus, whereas it acts redundantly with nesprin-1 in cerebellum, midbrain and hindbrain [16]. Whether these observations reflect tissue-specific expression or tissue-specific function of either nesprin-1 or -2 isoforms remains to be established. The same question also arises for Klaroid (SUN) and Klarsicht (KASH) whose mutations solely affect eye development in Drosophila [38]. Alteration of the nuclear lamina also affects neuronal migration. Mutations of Lam Dm(0), which encodes a B-type lamin in Drosophila, phenocopy the same migration defect than Klaroid (SUN) and Klarsicht (KASH) mutants, suggesting that the nuclear lamina also plays essential role in neuronal migration [47]. Accordingly, Coffinier et al. [48,49] recently demonstrated that lamin B2−/− mice display profound lamination failures of the cerebral cortex and cerebellum owing to impaired neuronal migration. The lamin–SUN–nesprin ‘axis’ is therefore essential to neuronal migration.
Finally, within the adult retina (and the CNS in general), the positioning of nuclei from different cell types at very precise spatial locations is a striking, but poorly studied, phenomenon (Figure 2, III). For example, cone nuclei are strictly localized at the apical side of the ONL (outer nuclear layer) and Müller cells, whose cell bodies extend across the whole retina, invariably position their nuclei in the middle of the INL (inner nuclear layer). One can wonder how and why these cells position their nuclei at such specific locations. In zebrafish, either the forced expression of dominant-negative polypeptides or morpholino approaches targeting Lis1, the dynactin complex or LINC complexes in fully differentiated retina displace photoreceptor nuclei to basal locations [50]. A similar phenotype consisting of a more basal location of cone photoreceptor nuclei is also observed in retinas from Sun1−/− mice [17]. These results suggest that nuclear positioning in fully differentiated neurons is an active process that involves the same families of proteins that translocate the nucleus during neurogenesis. Finally, the impaired viability of zebrafish photoreceptors with mispositioned nuclei emphasizes the physiological requirement of LINC complexes in fully differentiated neurons. To that respect, it is interesting to note that the perinatal lethality of Sun1/Sun2 DKO mice could be partially rescued by the transgenic expression of Sun1 driven by a neuron-specific enolase promoter [22].
Concluding remarks
Taken together, these data clearly show that nuclear lamina connections to molecular motors via LINC complexes provide for evolutionarily conserved ‘nuts and bolts’ that are essential to neurogenesis, neuronal migration and CNS morphogenesis. To our knowledge, only one study directly associates mutations of LINC complex components with neurological disorders. Indeed, mutations of nesprin-1 have been reported in ARCA1 (autosomal recessive cerebellar ataxia 1) that involves the progressive development of locomotor abnormalities developing at early to mid-adulthood [28]. Given the recent progress in our understanding of the physiological functions of LINC complexes during CNS development and maintenance, ARCA1 mutations probably represent the tip of the iceberg regarding the involvement of LINC complex components in human neurological pathologies.
Acknowledgments
Funding
This work is supported by the National Institutes of Health [grant number GM084204], a research grant from the Muscular Dystrophy Association, a National Institutes of Health Training Grant [grant number T32 EY013360 (to D.R.)] and by awards to the Department of Ophthalmology and Visual Sciences at Washington University from a Research to Prevent Blindness, Inc. Unrestricted Grant, and the National Institutes of Health Vision Core Grant [grant number P30 EY002687].
Abbreviations used
- ARCA1
autosomal recessive cerebellar ataxia 1
- CNS
central nervous system
- DKO
double-knockout
- IKNM
interkinetic nuclear migration
- INM
inner nuclear membrane
- KASH
Klarsicht/ANC-1/SYNE homology
- LINC
linker of nucleoskeleton and cytoskeleton
- NE
nuclear envelope
- ONM
outer nuclear membrane
References
- 1.Mosley-Bishop KL, Li Q, Patterson L, Fischer JA. Molecular analysis of the klarsicht gene and its role in nuclear migration within differentiating cells of the Drosophila eye. Curr Biol. 1999;9:1211–1220. doi: 10.1016/s0960-9822(99)80501-6. [DOI] [PubMed] [Google Scholar]
- 2.Fischer-Vize JA, Mosley KL. Marbles mutants: uncoupling cell determination and nuclear migration in the developing Drosophila eye. Development. 1994;120:2609–2618. doi: 10.1242/dev.120.9.2609. [DOI] [PubMed] [Google Scholar]
- 3.Malone CJ, Fixsen WD, Horvitz HR, Han M. UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development. 1999;126:3171–3181. doi: 10.1242/dev.126.14.3171. [DOI] [PubMed] [Google Scholar]
- 4.Razafsky D, Hodzic D. Bringing KASH under the SUN: the many faces of nucleocytoskeletal connections. J Cell Biol. 2009;186:461–472. doi: 10.1083/jcb.200906068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN–KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol. 2010;26:421–444. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque F, Mazzeo D, Patel JT, Smallwood DT, Ellis JA, Shanahan CM, Shackleton S. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J Biol Chem. 2009;285:3487–3498. doi: 10.1074/jbc.M109.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellad JA, Warren DT, Shanahan CM. Nesprins LINC the nucleus and cytoskeleton. Curr Opin Cell Biol. 2011;23:47–54. doi: 10.1016/j.ceb.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Djinovic-Carugo K, Gautel M, Ylanne J, Young P. The spectrin repeat: a structural platform for cytoskeletal protein assemblies. FEBS Lett. 2002;513:119–123. doi: 10.1016/s0014-5793(01)03304-x. [DOI] [PubMed] [Google Scholar]
- 10.Simpson JG, Roberts RG. Patterns of evolutionary conservation in the nesprin genes highlight probable functionally important protein domains and isoforms. Biochem Soc Trans. 2008;36:1359–1367. doi: 10.1042/BST0361359. [DOI] [PubMed] [Google Scholar]
- 11.Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, Karakesisoglou I, Korenbaum E. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp Cell Res. 2004;295:330–339. doi: 10.1016/j.yexcr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Zhen YY, Libotte T, Munck M, Noegel AA, Korenbaum E. NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J Cell Sci. 2002;115:3207–3222. doi: 10.1242/jcs.115.15.3207. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postel R, Ketema M, Kuikman I, de Pereda JM, Sonnenberg A. Nesprin-3 augments peripheral nuclear localization of intermediate filaments in zebrafish. J Cell Sci. 2011;124:755–764. doi: 10.1242/jcs.081174. [DOI] [PubMed] [Google Scholar]
- 15.Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, Burke B. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci USA. 2009;106:2194–2199. doi: 10.1073/pnas.0808602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009;64:173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J, Lei K, Zhou M, Craft CM, Xu G, Xu T, Zhuang Y, Xu R, Han M. KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum Mol Genet. 2011;20:1061–1073. doi: 10.1093/hmg/ddq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starr DA, Fischer JA. KASH ‘n Karry: the KASH domain family of cargo-specific cytoskeletal adaptor proteins. BioEssays. 2005;27:1136–1146. doi: 10.1002/bies.20312. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Xu R, Zhu B, Yang X, Ding X, Duan S, Xu T, Zhuang Y, Han M. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development. 2007;134:901–908. doi: 10.1242/dev.02783. [DOI] [PubMed] [Google Scholar]
- 20.Apel ED, Lewis RM, Grady RM, Sanes JR. Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J Biol Chem. 2000;275:31986–31995. doi: 10.1074/jbc.M004775200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Felder A, Liu Y, Guo LT, Lange S, Dalton ND, Gu Y, Peterson KL, Mizisin AP, Shelton GD, et al. Nesprin 1 is critical for nuclear positioning and anchorage. Hum Mol Genet. 2009;19:329–341. doi: 10.1093/hmg/ddp499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei K, Zhang X, Ding X, Guo X, Chen M, Zhu B, Xu T, Zhuang Y, Xu R, Han M. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc Natl Acad Sci USA. 2009;106:10207–10212. doi: 10.1073/pnas.0812037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randles KN, Lam LT, Sewry CA, Puckelwartz M, Furling D, Wehnert M, McNally EM, Morris GE. Nesprins, but not Sun proteins, switch isoforms at the nuclear envelope during muscle development. Dev Dyn. 2010;239:998–1009. doi: 10.1002/dvdy.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Ragnauth CD, Skepper JN, Worth NF, Warren DT, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J Cell Sci. 2005;118:673–687. doi: 10.1242/jcs.01642. [DOI] [PubMed] [Google Scholar]
- 25.Ralston E, Lu Z, Biscocho N, Soumaka E, Mavroidis M, Prats C, Lomo T, Capetanaki Y, Ploug T. Blood vessels and desmin control the positioning of nuclei in skeletal muscle fibers. J Cell Physiol. 2006;209:874–882. doi: 10.1002/jcp.20780. [DOI] [PubMed] [Google Scholar]
- 26.Grady RM, Starr DA, Ackerman GL, Sanes JR, Han M. Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc Natl Acad Sci USA. 2005;102:4359–4364. doi: 10.1073/pnas.0500711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puckelwartz MJ, Kessler E, Zhang Y, Hodzic D, Randles KN, Morris G, Earley JU, Hadhazy M, Holaska JM, Mewborn SK, et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum Mol Genet. 2009;18:607–620. doi: 10.1093/hmg/ddn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gros-Louis F, Dupre N, Dion P, Fox MA, Laurent S, Verreault S, Sanes JR, Bouchard JP, Rouleau GA. Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat Genet. 2007;39:80–85. doi: 10.1038/ng1927. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 30.Puckelwartz MJ, Kessler EJ, Kim G, Dewitt MM, Zhang Y, Earley JU, Depreux FF, Holaska J, Mewborn SK, Pytel P, McNally EM. Nesprin-1 mutations in human and murine cardiomyopathy. J Mol Cell Cardiol. 2010;48:600–608. doi: 10.1016/j.yjmcc.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y, Jangi S, Welte MA. Organelle-specific control of intracellular transport: distinctly targeted isoforms of the regulator Klar. Mol Biol Cell. 2005;16:1406–1416. doi: 10.1091/mbc.E04-10-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawe HR, Adams M, Wheway G, Szymanska K, Logan CV, Noegel AA, Gull K, Johnson CA. Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J Cell Sci. 2009;122:2716–2726. doi: 10.1242/jcs.043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai JW, Lian WN, Kemal S, Kriegstein AR, Vallee RB. Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. Nat Neurosci. 2010;13:1463–1471. doi: 10.1038/nn.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Bene F, Wehman AM, Link BA, Baier H. Regulation of neurogenesis by interkinetic nuclear migration through an apical–basal notch gradient. Cell. 2008;134:1055–1065. doi: 10.1016/j.cell.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenk J, Wilsch-Brauninger M, Calegari F, Huttner WB. Myosin II is required for interkinetic nuclear migration of neural progenitors. Proc Natl Acad Sci USA. 2009;106:16487–16492. doi: 10.1073/pnas.0908928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baye LM, Link BA. Interkinetic nuclear migration and the selection of neurogenic cell divisions during vertebrate retinogenesis. J Neurosci. 2007;27:10143–10152. doi: 10.1523/JNEUROSCI.2754-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fridolfsson HN, Starr DA. Kinesin-1 and dynein at the nuclear envelope mediate the bidirectional migrations of nuclei. J Cell Biol. 2010;191:115–128. doi: 10.1083/jcb.201004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kracklauer MP, Banks SM, Xie X, Wu Y, Fischer JA. Drosophila klaroid encodes a SUN domain protein required for Klarsicht localization to the nuclear envelope and nuclear migration in the eye. Fly. 2007;1:75–85. doi: 10.4161/fly.4254. [DOI] [PubMed] [Google Scholar]
- 39.Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 40.Baye LM, Link BA. Nuclear migration during retinal development. Brain Res. 2008;1192:29–36. doi: 10.1016/j.brainres.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivas RJ, Hatten ME. Motility and cytoskeletal organization of migrating cerebellar granule neurons. J Neurosci. 1995;15:981–989. doi: 10.1523/JNEUROSCI.15-02-00981.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solecki DJ, Trivedi N, Govek EE, Kerekes RA, Gleason SS, Hatten ME. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron. 2009;63:63–80. doi: 10.1016/j.neuron.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6α signaling controls glial-guided neuronal migration. Nat Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- 44.Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci. 2007;10:970–979. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, Gleeson JG. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol. 2004;165:709–721. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wynshaw-Boris A, Gambello MJ. LIS1 and dynein motor function in neuronal migration and development. Genes Dev. 2001;15:639–651. doi: 10.1101/gad.886801. [DOI] [PubMed] [Google Scholar]
- 47.Patterson K, Molofsky AB, Robinson C, Acosta S, Cater C, Fischer JA. The functions of Klarsicht and nuclear lamin in developmentally regulated nuclear migrations of photoreceptor cells in the Drosophila eye. Mol Biol Cell. 2004;15:600–610. doi: 10.1091/mbc.E03-06-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coffinier C, Fong LG, Young SG. LINCing lamin B2 to neuronal migration: growing evidence for cell-specific roles of B-type lamins. Nucleus. 2010;1:407–411. doi: 10.4161/nucl.1.5.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, Toth JI, Fong LG, Young SG. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc Natl Acad Sci USA. 2010;107:5076–5081. doi: 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsujikawa M, Omori Y, Biyanwila J, Malicki J. Mechanism of positioning the cell nucleus in vertebrate photoreceptors. Proc Natl Acad Sci USA. 2007;104:14819–14824. doi: 10.1073/pnas.0700178104. [DOI] [PMC free article] [PubMed] [Google Scholar]