Abstract
Differences in striatal dopamine (DA) function may be related to differences in the degree of social attachment to others. Using positron emission tomography (PET), socially detached persons demonstrate reduced DA D2/3 receptor (D2/3R) availability in the striatum. However, previous PET studies have only used antagonist radiotracers for D2/3R and have not specifically examined regions of interest (ROIs) such as the ventral striatum (VS). In 32 healthy persons, we investigated the relationship between self-reported attachment and DA D2/3R availability in striatal and extrastriatal ROIs as measured using the agonist radiotracer [11C]-(+)-PHNO. Surprisingly, more social attachment—as measured by the attachment subscale of the temperament and character inventory—was related to less [11C]-(+)-PHNO binding in the VS (r(30) =−.43, p = .01). This relationship held in a subsample who also completed the detachment subscale of the Karolinska Scales of Personality (r(10) = .62, p = .03). However, no relationships were observed with BPND in the dorsal striatum or D3R-specific ROIs. One potential explanation for these findings is that persons who are more socially detached have less endogenous DA occupying D2/3R in the VS. This interpretation warrants investigation by future research. These findings may help us better understand the neurochemical basis of attachment.
Keywords: TCI, KSP, dopamine, [11C]-(+)-PHNO, D2/3R, attachment
Introduction
Engaging in social relationships is fundamental to human well-being and is related to mental health (Bowlby, 1988). The neurochemical dopamine (DA) acting at D2/3 receptors (D2/3R) in the striatum is hypothesized to be critical for the formation and maintenance of social attachments and their rewarding value (Aragona et al., 2006; Gingrich, Liu, Cascio, Wang, & Insel, 2000). The relationship between striatal D2/3R availability and social detachment in healthy humans has been examined with positron emission tomography (PET) and the antagonist radiotracer [11C]-raclopride. Individuals with high social detachment demonstrate less D2/3R availability in the dorsal striatum (DS) (Breier et al., 1998; Farde, Gustavsson, & Jonsson, 1997). Detachment has also been associated with a D2R gene promoter variant related to reduced D2R expression (Jonsson et al., 2003). Consistent with previous work in monkeys (Morgan et al., 2002; Nader & Czoty, 2005), a positive correlation has been observed between social status/support and striatal D2/3R availability in humans measured with [11C]-raclopride (Martinez et al., 2010).
Previous PET studies examining detachment and D2/3R availability have only looked at the DS, not the ventral striatum (VS). This is pertinent given the VS’s dual role in social and drug reward (Insel, 2003; Lee et al., 2014; Tops, Koole, H, & Buisman-Pijlman, 2014) and the therapeutic potential of altering social neurocircuits to treat drug addictions (El Rawas et al., 2012; Fritz et al., 2011; Zernig, Kummer, & Prast, 2013). Finally, only antagonist radiotracers have been employed to investigate striatal D2/3R availability and sociability.
[11C]-(+)-PHNO is an agonist radiotracer for D2/3R which has preferential affinity for D3R over D2R (Narendran et al., 2006; Wilson et al., 2005). [11C]-(+)-PHNO is currently the only probe available for use in humans that can allow differentiation between D3R over D2R. This is relevant because these G-protein-coupled receptors show several differences that may have an impact on behavior. In contrast to D2R, the D3R has a unique “rigid” configuration (Vanhauwe, Josson, Luyten, Driessen, & Leysen, 2000) which contributes to its very high affinity for DA (>20-fold higher than D2) (Freedman et al., 1994). Unlike the D2R receptor, the D3R may be activated by tonic DA levels in the brain given its high affinity for DA (Levesque et al., 1992), attenuating any effects of DA fluctuation related to synaptic phasic DA release (Tsukada et al., 1999). Moreover, knock-out mice for the DA D3R have shown extracellular DA levels twice as high as their wild-type littermates (Joseph et al., 2002). While the D2R receptor is broadly distributed in the cerebral cortex and subcortical regions (Gurevich & Joyce, 1999), the D3R receptor has a more restricted distribution to the limbic system, amygdala, hippocampus, thalamus, hypothalamus, and midbrain (substantia nigra and ventral tegmental area), regions known to be involved in modulation of drive, affect, and memory (Nakajima et al., 2013).
[11C]-(+)-PHNO has ~20–40-fold selectivity of D3R over D2R (Freedman et al., 1994; Gallezot et al., 2012; Rabiner et al., 2009; G. Searle et al., 2010; Seeman, Ulpian, Larsen, & Anderson, 1993), resulting in a differential contribution of D2R and D3R to the [11C]-(+)-PHNO signal across different regions of interest (ROI). The estimated percent of the [11C]-(+)-PHNO signal in vivo in humans attributed to D3R across ROIs are: the substantia nigra (~100%), hypothalamus (~100%), ventral pallidum (~75%), globus pallidus (~65%), VS (~26%), and dorsal caudate-putamen (negligible) (Graff-Guerrero et al., 2010; Searle et al., 2013; Tziortzi et al., 2011).
Some studies, though few, have examined the relationship between social behavior and D3R function/expression, noting unique differences from D2R. For example, unlike antagonism of D2R, antagonizing D3R in the prefrontal cortex of rodents increases social recognition and social discrimination (see review: Nakajima et al., 2013). In both healthy persons and cocaine dependent subjects, less D3R availability in the midbrain has been correlated with greater self-reported social status (Matuskey et al., 2015). Thus, exploring the relationship between attachment and in vivo D3R availability in humans is novel, and may offer new insights as several investigations have noted potential differences in D2R versus D3R regulation in several conditions (Boileau, Nakajima, & Payer, 2015; Le Foll, Wilson, Graff, Boileau, & Di Ciano, 2014; Nakajima et al., 2013).
As an agonist [11C]-(+)-PHNO binding to D2/3R is also more sensitive to competition with endogenous DA in vivo in humans (Caravaggio, Borlido, Wilson, & Graff-Guerrero, 2015; Caravaggio et al., 2014). For example, it has been demonstrated in humans that [11C]-(+)-PHNO is more sensitive to displacement by amphetamine than [11C]-raclopride (Ginovart et al., 2006; Shotbolt et al., 2012; Willeit et al., 2008). For [11C]-raclopride, it has been reported that 16% of the variance in baseline binding in the striatum can be accounted for by endogenous DA (r(31) = −.40, p = .02) (Kegeles, Martinez, Slifstein, Laruelle, & Abi-Dargham, 2014). For [11C]-(+)-PHNO this is far greater, with 59% of the variance in baseline binding being explained by endogenous DA in the DS [caudate (r(8) = −.77, p = .01) and putamen (r (8) = −.77, p = .009)], and 42% in the VS (Caravaggio et al., 2014). Thus, using [11C]-(+)-PHNO offers a novel probe into how neurochemistry may be related to personality traits and social functioning (Suridjan et al., 2012).
Given the limited ROIs previously examined and the use of only [11C]-raclopride, we explored whether self-reported social attachment in healthy humans is related to DA D2/3R availability with [11C]-(+)-PHNO. Based on previous findings, we hypothesized that attachment would be positively correlated with D2/3R availability in the DS. Furthermore, we explored whether attachment is related to binding in the VS and in the extrastriatal D3R-specific ROIs.
Elucidating in vivo neurochemical correlates of attachment in healthy humans may help us better understand the biological basis of the innate desire for intersubjectivity and how this innate reward may go awry in neuropsychiatric disorders.
Materials and methods
Participants
PET data previously reported in healthy participants were reanalyzed using improved ROI delineation techniques for the purpose of the current investigation (Graff-Guerrero et al., 2009, 2008). Participants were right-handed adults free of any major medical or psychiatric disorders as determined by clinical interview, the Mini International Neuropsychiatric Interview, basic laboratory tests, and electrocardiography. At inclusion and before the PET scan, participants were required to have a negative urine screen for drugs of abuse and/or pregnancy. All participants provided written informed consent and were nonsmokers. Moreover, history of drug abuse was an exclusion criterion for being scanned. This study was approved by the Research Ethics Board of the Centre for Addiction and Mental Health, Toronto.
Self-reported attachment
Before undergoing a [11C]-(+)-PHNO PET scan, subjects completed the attachment subscale of the Temperament and Character Inventory (TCI) (Cloninger, 1987). This measure is thought to capture the subjective reward value of social relationships. Persons who score high on attachment are characterized as desiring social closeness, eager to help and please others, sympathetic, sentimental, and sensitive to praise and rejection (Cloninger, 1987). Conversely, persons who score low on attachment are characterized as socially and emotionally detached, content to be alone, practical, self-reliant, and independent. They are insensitive to social cues and pressures, and quick to discontinue relationships that are no longer gratifying (Cloninger, 1987). A subset of participants also completed another self-report measure of social attachment, the detachment scale of the Karolinska Scales of Personality (KSP) (Gustavsson et al., 2000). Persons with high detachment scores are described as showing indifference toward social relationships, being socially cold and aloof (Farde et al., 1997). The Cronbach’s alpha for the attachment scale of the TCI has been reported to be .71 (Cloninger, Przybeck, & Svrakic, 1994; Miettunen et al., 2004) and .58–.62 for the detachment scale of the KSP (Ortet, Ibáñez, Llerena, & Torrubia, 2002). Given the retrospective nature of our dataset, unfortunately other relevant measures of social cognition were not collected for these [11C]-(+)-PHNO scans.
PET imaging
The radiosynthesis of [11C]-(+)-PHNO and the acquisition of PET images have been described in detail elsewhere (Graff-Guerrero et al., 2008; Wilson et al., 2005). Briefly, [11C]-propionyl chloride was reacted with 9-hydroxynaph-thoxazine to generate a [11C]-amide which was subsequently reduced by lithium aluminum hydride. Purification by HPLC and formulation gave radiochemically pure [11C]-(+)-PHNO as a sterile, pyrogen-free solution suitable for human studies. PET images were acquired using a high-resolution head-dedicated PET camera system (CPS-HRRT; Siemens Molecular Imaging, USA), which measures radioactivity in 207 brain slices with a thickness of 1.2 mm each. The in-plane resolution was ~2.8 mm full-width at half-maximum. Transmission scans were acquired using a 137Cs (T1/2 = 30.2 years, E = 662 keV) single photon point source to provide attenuation correction, and the emission data were acquired in list mode. The raw data were reconstructed by filtered-back projection. After completion of the emission acquisition, both the transmission and emission data were transferred to an off-line data processing system for image reconstruction. The emission data were re-binned into a series of 3D sinograms. Scanning time was 90 min in length, wherein 30 frames were defined: 1–15 of 1-min duration and 16–30 of 5-min duration. For each 3D sinogram, gaps were filled and corrections for photon attenuation and detector normalization were applied. The gap-filled sinograms were scatter-corrected before applying Fourier rebinning to convert the 3D sinograms into 2D sinograms. The 2D sonograms were then reconstructed into image space using a 2D filtered-back projection algorithm, with a Hann filter at Nyquist cutoff frequency and the images calibrated to nCi/mL. A custom-fitted thermoplastic mask (Tru-Scan Imaging, Annapolis) was made for each subject and used with a head fixation system during PET scans to reduce any movement during the acquisition. [11C]-(+)-PHNO was injected as a bolus followed by a flush of 2 mL saline into an intravenous line placed in an antecubital vein. The average time of injection was 12:47 pm. The mean radioactivity dose was 9.63(±1.3) mCi, with a specific activity of 1143.32 (±326.5) mCi/μmol, and an injected mass of 2.1(±.5) μg. None of the participants included in this sample reported nausea given the [11C]-(+)-PHNO injection. The PET data were redefined into 30 frames (1–15 of 1-min duration and16–30 of 5-min duration).
Image analysis
The region of interest (ROI)-based analysis for [11C]-(+)-PHNO has been described in detail elsewhere (Graff-Guerrero et al., 2008; Tziortzi et al., 2011). Time activity curves (TACs) from ROIs were obtained from the dynamic PET images in native space with reference to each subjects co-registered MRI image. The co-registration of each subjects MRI to PET space was done using the normalized mutual information algorithm (Studholme, Hill, & Hawkes, 1997) as implemented in SPM2 (SPM2, Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm). The TACs were analyzed using the Simplified Reference Tissue Method (SRTM) (Lammertsma & Hume, 1996) which has been validated for use with [11C]-(+)-PHNO (Ginovart et al., 2007). The cerebellum was used as the reference region to derive a quantitative estimate of binding—binding potential relative to the non-displaceable compartment (BPND)—as defined by the consensus nomenclature for in vivo imaging of reversibly binding radioligands (Innis et al., 2007). The basic function implementation of the SRTM (Gunn, Lammertsma, Hume, & Cunningham, 1997) was applied to the dynamic PET images to generate parametric voxelwise BPND maps using PMOD (v2.7, PMOD Technologies, Zurich, Switzerland). These images were spatially normalized into Montréal Neurological Institute (MNI) brain space by nearest neighbor interpolation with a voxel size fixed in 2 × 2 × 2 mm3 using SPM2. Regional BPND estimates were then derived from ROIs defined in MNI space, except for the hypothalamus and ventral pallidum ROIs. The VS and DS (dorsal caudate, hereafter caudate and dorsal putamen, hereafter putamen) were defined according with the criteria of Mawlawi et al. (2001). The globus pallidus, ventral pallidum, and hypothalamus ROIs were defined according to the criteria of Tziortzi et al. (2011). Regional BPND estimates for the hypothalamus and ventral pallidum were derived using the SRTM from ROIs drawn by hand, according to the aforementioned criteria of Tziortzi and colleagues.
Statistical analysis
Our a-priori hypothesis was to examine the relationship between self-reported attachment and detachment with [11C]-(+)-PHNO BPND in the VS and DS in an attempt to extend and replicate previous literature. Notably, the subscale of the TCI measuring attachment is the only subscale of the TCI found to be related to D2/3R availability in previously published PET studies. For the a-priori TCI analyses in the full-sample of subjects, the p-value for significance was set at p = .02 (Bonferroni corrected α = .05/3 ROIs). Findings with the attachment scale of the TCI and [11C]-(+)-PHNO BPND were then corroborated within a subsample of subjects who also completed the detachment scale of the KSP. Since this is the first such investigation with an agonist radiotracer with preferential affinity for D3R over D2R, we conducted exploratory analyses examining the relationship between these self-report measures and BPND in the D3R specific regions: substantia nigra, globus pallidus, ventral pallidum, and hypothalamus. For these exploratory analyses, the p-value for significance was set at p = .01 (Bonferroni corrected α = .05/4 ROIs). Age and gender were controlled for using partial Pearson correlations. Statistical analyses were conducted using SPSS (v.12.0; SPSS, Chicago, IL, USA) and GraphPad (v.5.0; GraphPad Software, La Jolla, CA, USA). To our knowledge, this is to date the largest sample (n = 32) to examine how [11C]-(+)-PHNO BPND is related to any self-report measure, not just personality (Boileau et al., 2015; Caravaggio et al., 2014; Di Ciano et al., 2015; Matuskey et al., 2015; Payer et al., 2015; Suridjan et al., 2012). Moreover, this sample is larger than those studies examining the relationship between baseline [11C]-raclopride BPND and attachment/detachment. As such, our sample size (n = 32) was adequately powered (1 − β error probability = .80) to detect significant correlations with a medium to large Cohen’s d effect size (d > .46), given α = .05, two-tailed. The a-priori power analysis for a correlation was computed using the statistical software package G*Power (Faul, Erdfelder, Buchner, & Lang, 2009). Normality of variables was determined using the D’Agostino–Pearson test. The significance level for all tests was set at p < .05 (two-tailed).
Results
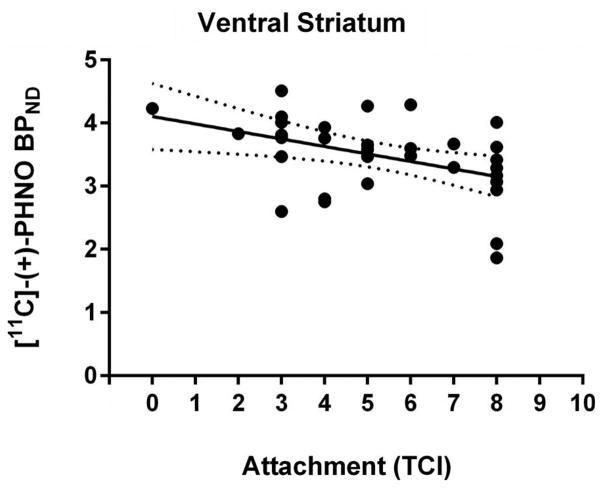
Thirty-two healthy participants (22 males, 10 females; age range: 18–49, mean = 32, SD = 8.9) participated in the study. All 32 participants completed the attachment sub-scale of the TCI, while 12 of these subjects also completed the detachment scale of the KSP (males; age range: 20–39, mean = 30, SD = 6.5). Within the full sample, self-reported attachment was not correlated with age (r(30) = −.04, p = .82). There was no significant difference in self-reported attachment between men and women (t (30) = −1.8, p = .09). Attachment was negatively correlated with [11C]-(+)-PHNO BPND in the VS (r(30) = −.43, p = .01) (Cohen’s d = −1.06) (see Figure 1), a relationship that remained statistically significant after controlling for age and gender (r(28) = −.41, p = .02) (Cohen’s d = −1.03). Attachment was not correlated with BPND in the DS: caudate (r(30) = −.22, p = .23) and putamen (r(30) = −.16, p = .40). Controlling for age and gender did not significantly affect these null results: caudate (r(28) = −.16, p = .39) and putamen (r(28) = −.07, p = .71). Removing an individual who had the lowest attachment score (a score of zero) did not change any of the results, indicating that the relationship between attachment scores and [11C]-(+)-PHNO BPND in the VS was not being driven by a potential outlier (r(29) = −.39, p = .03) (Cohen’s d = −.92).
Figure 1.
The relationship between [11C]-(+)-PHNO BPND in the ventral striatum and attachment measured with the temperament and character inventory. The dashed lines demarcate the 95% confidence interval.
In the D3R-specific regions, attachment was not correlated with [11C]-(+)-PHNO BPND in the substantia nigra (r(30) = −.29, p = .11), globus pallidus (r (30) = −.25, p = .17), ventral pallidum (r(30) = −.35, p = .05), or hypothalamus (r(29) = .32, p = .08) (note: for one subject [11C]-(+)-PHNO BPND could not be reliably estimated in the hypothalamus). Controlling for age and gender did not significantly affect any of these null results: substantia nigra (r(28) = −.23, p = .22), globus pallidus (r(28) = −.23, p = .22), ventral pallidum (r(28) = −.29, p = .13), and hypothalamus (r (27) = .19, p = .32).
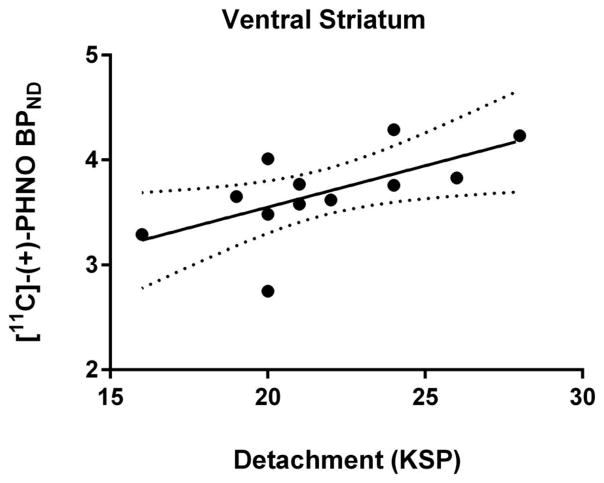
Within the subsample of participants who completed both scales, self-reported attachment and detachment were highly negatively correlated (r(10) = −.71, p = .009). Detachment was not correlated with age (r(10) = −.14, p = .66). Detachment was positively correlated with [11C]-(+)-PHNO BPND in the VS (r(10) = .62, p = .03) (Cohen’s d = 2.01) (see Figures 2 & 3). This survived statistically controlling for age (r(9) = .62, p = .04) (Cohen’s d = 2.01). Detachment was not correlated with BPND in the DS: caudate (r(10) = .16, p = .61) and putamen (r(10) = .17, p = .60). Controlling for age did not significantly affect these null results: caudate (r(9) = .15, p = .65) and putamen (r(9) = .13, p = .70).
Figure 2.
The relationship between [11C]-(+)-PHNO BPND in the ventral striatum and detachment measured with the Karolinska Scales of Personality. The dashed lines demarcate the 95% confidence interval.
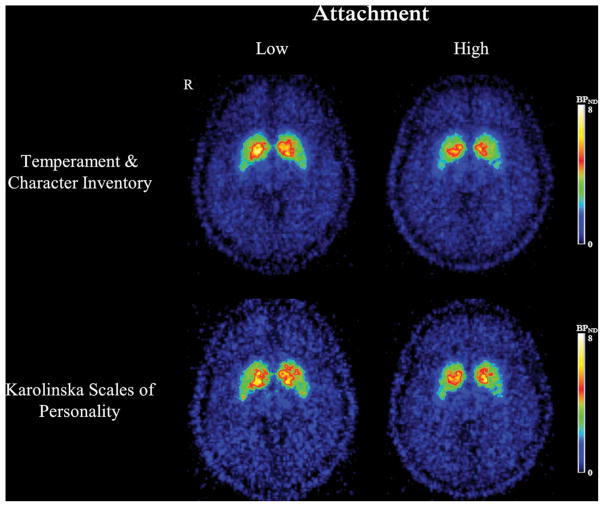
Figure 3.
Averaged [11C]-(+)-PHNO BPND maps of persons in the first versus fourth quartile of attachment scores measured with the temperament and character inventory (n = 9 per group) and the Karolinska Scales of Personality (n = 4 per group). Depicted is a transaxial slice of the ventral straitum.
Detachment was not correlated with [11C]-(+)-PHNO BPND in the D3R-specific regions: substantia nigra (r (10) = .19, p = .56), globus pallidus (r(10) = .35, p = .27), ventral pallidum (r(10) = .50, p = .10), and hypothalamus (r(10) = −.09, p = .79). Controlling for age did not significantly affect any of these null results: substantia nigra (r(9) = .17, p = .62), globus pallidus (r (9) = .38, p = .26), ventral pallidum (r(9) = .49, p = .13), and hypothalamus (r(9) = −.15, p = .65).
Discussion
We examined whether self-reported attachment in healthy humans was related to DA D2/3R availability measured with the agonist radiotracer [11C]-(+)-PHNO. This investigation examined in humans the relationship between attachment and D2/3R availability in several striatal and extrastriatal regions. Moreover, it was explored whether there is a relationship between attachment and receptor availability in regions where the majority of the [11C]-(+)-PHNO signal is due to binding to D3R: the substantia nigra, hypothalamus, ventral pallidum, and globus pallidus. We hypothesized, based on previous findings in humans and animals (Breier et al., 1998; Farde et al., 1997; Martinez et al., 2010; Morgan et al., 2002) that increased attachment would be positively related to [11C]-(+)-PHNO BPND in the striatum. Surprisingly, we observed using two different scales that attachment was negatively correlated with [11C]-(+)-PHNO BPND in the VS. While this finding is surprising, it is prima facie consistent with work examining the relationship between social status and [11C]-(+)-PHNO BPND in healthy persons and persons with cocaine-dependence (Matuskey et al., 2015). Notably, low social status has been associated with reduced social attachment through other indirect factors, such as substance abuse, violence, neglect, etc., which has been reviewed elsewhere (Green, Furrer, & McAllister, 2007; Sherry, Adelman, Farwell, & Linton, 2013). Using the Barratt Simplified Measure of Social Status, Matuskey and colleagues found a trend negative correlation between social status and [11C]-(+)-PHNO BPND in the VS of healthy persons (r(14) = −.44, p = .09). Notably, using this scale positive correlations have been observed between social status/support and striatal D2/3R availability measured with [11C]-raclopride (Martinez et al., 2010).
We did not observe a relationship between social attachment and [11C]-(+)-PHNO BPND in the DS. A previous PET study failed to find a significant relationship between [11C]-raclopride BPND in the DS and differences in attachment measured with the TCI, despite finding a significant negative correlation when measured with the KSP (Breier et al., 1998). A subsequent PET study also failed to find a relationship between [11C]-raclopride BPND in the DS and detachment-like traits measured with the NEO Personality Inventory-Revised (Kestler, Malhotra, Finch, Adler, & Breier, 2000). It has been suggested that reduced DS D2/3R availability may be related to some specific aspect captured by the KSP not shared by the other attachment scales, or to differences in psychometric properties (Kestler et al., 2000). Similarly, despite these measures being highly correlated with each other in the same persons, a D2R gene promoter linked to reduced D2R density was associated with attachment measured with the KSP but not the TCI (Jonsson et al., 2003). In the current investigation, we found converging results with multiple measures: using both the TCI and KSP, less attachment was associated with more [11C]-(+)-PHNO BPND in the VS. It is possible that we were underpowered due to our sample size to observe a relationship between D2/3R availability in the DS and detachment measured with the KSP. Another potential explanation for this discrepancy with previous literature may be the increased sensitivity of [11C]-(+)-PHNO to endogenous DA in the DS. That is, true differences in receptor number possibly captured by [11C]-raclopride may be masked by concurrent changes in endogenous DA levels.
Differences in radioligand binding in vivo are usually explained by changes in at least one of three parameters: the number of available receptors, endogenous DA levels, and receptor affinity for the ligand. While several interpretations exist which are non-mutually exclusive, we speculate that a plausible interpretation of our current findings is that persons who score high on attachment have more endogenous DA occupying D2/3R in the VS, resulting in reduced baseline [11C]-(+)-PHNO BPND. Subsequently, we outline our rationale.
One interpretation of our findings is that persons who score high on attachment have more endogenous DA occupying D2/3R in the VS, reducing baseline [11C]-(+)-PHNO BPND. Thus, perhaps persons who do not report receiving reward or pleasure from social relationships have less endogenous DA occupying D2/3R in the VS. Such an interpretation seems biologically plausible for several reasons. First, it is consistent with findings in animals suggesting that increased DA signaling at D2/3R in the VS facilitates social behaviors, while decreased signaling diminishes them (Aragona et al., 2006; Gingrich et al., 2000). Unfortunately, the PET studies, examining how attachment is related to DA synthesis capacity (Laakso et al., 2003) and DA transporter availability (Laakso et al., 2000), have not distinguished between the DS and VS. At least in persons with schizophrenia endogenous DA levels in the VS measured with [11C]-raclopride were found to be inversely correlated with an item on the positive and negative syndrome scale which captures passivity, apathy, and social withdrawal (Kegeles et al., 2010). Second, this interpretation is consistent with the observations that [11C]-(+)-PHNO is more sensitive to changes in endogenous DA than [11C]-raclopride. The high sensitivity of [11C]-(+)-PHNO BPND to endogenous DA may be capturing changes in endogenous DA levels in the VS at D2/3R across attachment scores which cannot be captured by [11C]-raclopride. Similarly, this sensitivity to endogenous DA may also explain the null result observed between attachment scores and [11C]-(+)-PHNO BPND in the DS. However, it is possible that we were underpowered to observe a relationship between D2/3R availability in the DS and detachment measured with the KSP. Future studies estimating endogenous DA levels at D2/3R in humans should collect measures of social attachment to validate this interpretation.
Another interpretation of our finding is that persons who score low on attachment have more D2R and/or D3R expression in the VS. Notably, while the VS is a mixed D2R and D3R region, the majority of the [11C]-(+)-PHNO signal in this ROI is from D2R (~74%) (Tziortzi et al., 2011). An increase in D2R expression in the VS with lower attachment would be inconsistent with the aforementioned human and animal literature. For D3R, it is less clear what would be expected. We did not observe a significant relationship between attachment and [11C]-(+)-PHNO BPND in the D3R-specific ROIs. Notably, the trend relationship observed in the ventral pallidum was in the opposite direction of the VS: a positive correlation. However, further human and animal research is required to elucidate the specific role of VS D3R in the rewarding aspects of affiliative behaviors. Finally, it is important to note that for the D3R-specific ROIs, the ventral pallidum and hypothalamus, the model fitting (% covariance) and test–retest values are worse than for the D2R specific ROIs (Gallezot et al., 2014; Searle et al., 2013). This may in part explain our null findings in these regions, and future studies should examine attachment with other D3R-specific radiotracers. However, [11C]-(+)-PHNO is currently the only in vivo probe available to quantify D3R in the living human brain. Future studies employing agonist radiotracers which are more specific to D2R—such as [11C]-NPA and [11C]-MNPA—would help clarify the relationship between attachment and availability of striatal D2R versus D3R (Finnema, Bang-Andersen, Wikstrom, & Halldin, 2010; Van Wieringen et al., 2014).
We are unaware of any evidence to suggest that reduced affinity at the agonist binding site of the D2/3R in the VS should be related to increased social attachment. In vitro studies have yielded inconsistent results regarding the relationship between social isolation in rodents and changes in D2R affinity (Del Arco, Zhu, Terasmaa, Mohammed, & Fuxe, 2004; King, Seeman, Marsden, & Fone, 2009); a neurocorrelate of increased sensitivity to psychostimulants (King et al., 2009). Therefore, we believe it is unlikely that our findings reflect changes in D2/3R affinity in the VS.
There are several limitations to the current investigation. It has been noted that the injected mass of [11C]-(+)-PHNO is not within ideal radiotracer conditions (i.e., <1.5 ng/kg) (Gallezot et al., 2012). The specific activity required to obtain tracer conditions is not possible with the available radiosynthesis method. While this limitation is currently unavoidable, it is perhaps worth noting that the relationship between attachment and [11C]-(+)-PHNO BPND in the VS survives controlling for the subjects’ injected mass (r(29) = −.49, p = .005), injected mass per kilogram (r(29) = −.41, p = .03), amount injected (r(29) = −.46, p = .009), and specific activity (r(29) = −.49, p = .005), respectively. It has been suggested that [11C]-(+)-PHNO BPND in D3R-rich regions is underestimated if SRTM quantification is used in conjunction with 90 min of data acquisition (Girgis et al., 2011). Thus, using arterial plasma-based kinetic models following 120 min of emission data is more ideal for quantifying [11C]-(+)-PHNO BPND in D3R-rich regions (Girgis et al., 2011). Moreover, use of arterial plasma-based kinetic models would circumvent limitations associated with using reference tissue methods, namely concerns about specific binding to D3R in cerebellar reference tissue (Searle et al., 2013). Future [11C]-(+)-PHNO studies should collect and analyze data accordingly. Moreover, this study was retrospective, reanalyzing previously collected PET data using improved methods to delineate striatal subregions. Unfortunately, other measures were not collected from these subjects such as social status or social support which would have added further clarification to our results. Further, we only examined the relationship between attachment and D2/3R availability in healthy humans. Future PET studies should examine this relationship in persons with neuropsychiatric illnesses. Several lines of evidence suggest that emotional processing/attachment is sexually dimorphic (Cosgrove, Mazure, & Staley, 2007; Del Giudice, 2011; DeWall et al., 2012). Unfortunately, we only had 10 females in our sample, precluding any meaningful analyses to examine the interactions between gender, attachment, and D2/3R availability. This is something that should be explored by future investigations, using for example, PET and fMRI in the same subjects. Moreover, we did not record the menstrual cycle of our female participants, nor if they were taking contraceptive medications. Given its increased sensitivity to DA over other radiotracers, future [11C]-(+)-PHNO studies should examine the effect of menstrual cycle and contraception use on D2/3R availability, as it has been done with other radiotracers (Kaasinen, Någren, Hietala, Farde, & Rinne, 2001; Nordström, Olsson, & Halldin, 1998; Patrizia Riccardi et al., 2006). We also did not record the relationship status of our participants—a variable which should be investigated by future PET studies. Thus, our findings with [11C]-(+)-PHNO warrant replication with these limitations taken into due consideration.
This is the first investigation to (1) examine whether self-reported attachment in humans is related to DA D2/3R availability in specific subregions of the striatum such as the VS, (2) explore such a relationship with an agonist radiotracer, and (3) explore potential relationships between attachment and receptor availability in D3R-rich ROIs. We observed a negative correlation between self-reported attachment scores and DA D2/3R availability in the VS of healthy humans as measured with [11C]-(+)-PHNO. Our data, in conjunction with previous research, suggests that persons who are less socially attached—perceiving relationships as less rewarding—have less endogenous DA occupying D2/3R in the VS. However, other interpretations cannot currently be ruled out, requiring investigation by future studies. Such knowledge will have important implications for better understanding the neurochemical basis of social affiliation and how this system may go awry in persons with neuropsychiatric disorders.
Acknowledgments
The authors would like to thank the PET Centre staff at the Centre for Addiction and Mental Health for technical assistance in data collection. They also thank Wanna Mar, Zhe Feng, Thushanthi Balakumar, Danielle Uy, and Sofia Raitsin for their ongoing support and assistance.
Funding
This study was funded by Canadian Institutes of Health Research [grant number MOP-114989]; US National Institute of Health [grant number RO1MH084886-01A2]. Dr. Nakajima reports having received grants from Japan Society for the Promotion of Science and Inokashira Hospital Research Fund and speaker’s honoraria from GlaxoSmith Kline, Janssen Pharmaceutical, Pfizer, and Yoshitomiyakuhin within the past 3 years. Dr. Graff-Guerrerro currently receives research support from the following external funding agencies: Canadian Institutes of Health Research, the US National Institute of Health, and the Mexico Instituto de Ciencia y Tecnologıa para la Capital del Conocimiento en el Distrito Federal (ICyTDF). He has also received professional services compensation from Abbott Laboratories, Gedeon-Richter Plc, and Lundbeck; grant support from Janssen; and speaker compensation from Eli Lilly.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ariel Graff-Guerrero, http://orcid.org/0000-0001-9301-2171
References
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience. 2006;9(1):133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Boileau I, Nakajima S, Payer D. Imaging the D3 dopamine receptor across behavioral and drug addictions: Positron emission tomography studies with [11C]-(+)-PHNO. European Neuropsychopharmacology. 2015;25(9):1410–1420. doi: 10.1016/j.euroneuro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Bowlby J. A secure base: Clinical applications of attachment theory. London: Routledge; 1988. [Google Scholar]
- Breier A, Kestler L, Adler C, Elman I, Wiesenfeld N, Malhotra A, Pickar D. Dopamine D2 receptor density and personal detachment in healthy subjects. The American Journal of Psychiatry. 1998;155(10):1440–1442. doi: 10.1176/ajp.155.10.1440. [DOI] [PubMed] [Google Scholar]
- Caravaggio F, Borlido C, Wilson A, Graff-Guerrero A. Examining endogenous dopamine in treated schizophrenia using [11C]-(+)-PHNO positron emission tomography: A pilot study. Clinica Chimica Acta. 2015;23(15):00152–00157. doi: 10.1016/j.cca.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaggio F, Nakajima S, Borlido C, Remington G, Gerretsen P, Wilson A, Graff-Guerrero A. Estimating endogenous dopamine levels at D2 and D3 receptors in humans using the agonist radiotracer [(11)C]-(+)-PHNO. Neuropsychopharmacology. 2014;39(12):2769–2776. doi: 10.1038/npp.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Archives of General Psychiatry. 1987;44(6):573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM. The Temperament and Character Inventory (TCI): A guide to its development and use. MO: Center for psychobiology of personality, Washington University St. Louis; 1994. [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biological Psychiatry. 2007;62(8):847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Arco A, Zhu S, Terasmaa A, Mohammed AH, Fuxe K. Hyperactivity to novelty induced by social isolation is not correlated with changes in D2 receptor function and binding in striatum. Psychopharmacology. 2004;171(2):148–155. doi: 10.1007/s00213-003-1578-8. [DOI] [PubMed] [Google Scholar]
- Del Giudice M. Sex differences in romantic attachment: A meta-analysis. Personality & Social Psychology Bulletin. 2011;37(2):193–214. doi: 10.1177/0146167210392789. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Masten CL, Powell C, Combs D, Schurtz DR, Eisenberger NI. Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience. 2012;7(2):184–192. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Guranda M, Lagzdins D, Tyndale RF, Gamaleddin I, Selby P, Le Foll B. Varenicline-induced elevation of dopamine in smokers: A preliminary [C]-(+)-PHNO PET Study. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Rawas R, Klement S, Salti A, Fritz M, Dechant G, Saria A, Zernig G. Preventive role of social interaction for cocaine conditioned place preference: Correlation with FosB/DeltaFosB and pCREB expression in rat mesocortico-limbic areas. Frontiers in Behavioral Neuroscience. 2012;6(8) doi: 10.3389/fnbeh.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farde L, Gustavsson JP, Jönsson E. D2 dopamine receptors and personality traits. Nature. 1997;385(6617):590. doi: 10.1038/385590a0. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Bang-Andersen B, Wikstrom HV, Halldin C. Current state of agonist radioligands for imaging of brain dopamine D2/D3 receptors in vivo with positron emission tomography. Current Topics in Medicinal Chemistry. 2010;10(15):1477–1498. doi: 10.2174/156802610793176837. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Patel S, Marwood R, Emms F, Seabrook GR, Knowles MR, McAllister G. Expression and pharmacological characterization of the human D3 dopamine receptor. The Journal of Pharmacology and Experimental Therapeutics. 1994;268(1):417–426. [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Zernig G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addiction Biology. 2011;16(2):273–284. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T, Rabiner EA. Affinity and selectivity of [(1)(1)C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse. 2012;66(6):489–500. doi: 10.1002/syn.21535. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Zheng MQ, Lim K, Lin SF, Labaree D, Matuskey D, Malison RT. Parametric imaging and test-retest variability of (1)(1)C-(+)-PHNO binding to D(2)/D(3) dopamine receptors in humans on the high-resolution research tomograph PET scanner. Journal of Nuclear Medicine. 2014;55(6):960–966. doi: 10.2967/jnumed.113.132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2000;114(1):173–183. doi: 10.1037/0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, Wilson AA. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. Journal of Neurochemistry. 2006;97(4):1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S, Wilson AA. Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. Journal of Cerebral Blood Flow and Metabolism. 2007;27(4):857–871. doi: 10.1038/sj.jcbfm.9600411. [DOI] [PubMed] [Google Scholar]
- Girgis RR, Xu X, Miyake N, Easwaramoorthy B, Gunn RN, Rabiner EA, Slifstein M. In vivo binding of antipsychotics to D3 and D2 receptors: A PET study in baboons with [11C]-(+)-PHNO. Neuropsychopharmacology. 2011;36(4):887–895. doi: 10.1038/npp.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mamo D, Shammi CM, Mizrahi R, Marcon H, Barsoum P, Kapur S. The effect of antipsychotics on the high-affinity state of D2 and D3 receptors: A positron emission tomography study With [11C]-(+)-PHNO. Archives of General Psychiatry. 2009;66(6):606–615. doi: 10.1001/archgenpsychiatry.2009.43. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Redden L, Abi-Saab W, Katz DA, Houle S, Barsoum P, Kapur S. Blockade of [11C] (+)-PHNO binding in human subjects by the dopamine D3 receptor antagonist ABT-925. The International Journal of Neuropsychopharmacology. 2010;13(3):273–287. doi: 10.1017/S1461145709990642. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Kapur S. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C] raclopride in healthy humans. Human Brain Mapping. 2008;29(4):400–410. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B, Furrer C, McAllister C. How do relationships support parenting? Effects of attachment style and social support on parenting behavior in an at-risk population. American Journal of Community Psychology. 2007;40(1–2):96–108. doi: 10.1007/s10464-007-9127-y. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6(4):279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: Comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20(1):60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Gustavsson JP, Bergman H, Edman G, Ekselius L, Von Knorring L, Linder J. Swedish universities scales of personality (SSP): Construction, internal consistency and normative data. Acta Psychiatrica Scandinavica. 2000;102(3):217–225. doi: 10.1034/j.1600-0447.2000.102003217.x. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Insel TR. Is social attachment an addictive disorder? Physiology & Behavior. 2003;79(3):351–357. doi: 10.1016/S0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Cichon S, Gustavsson JP, Grunhage F, Forslund K, Mattila-Evenden M, Nothen MM. Association between a promoter dopamine D2 receptor gene variant and the personality trait detachment. Biological Psychiatry. 2003;53(7):577–584. doi: 10.1016/S0006-3223(02)01732-8. [DOI] [PubMed] [Google Scholar]
- Joseph JD, Wang YM, Miles PR, Budygin EA, Picetti R, Gainetdinov RR, Wightman RM. Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D(3) receptors. Neuroscience. 2002;112(1):39–49. doi: 10.1016/s0306-4522(02)00067-2. S0306452202000672[pii] [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Någren K, Hietala J, Farde L, Rinne JO. Sex differences in extrastriatal dopamine D2-like receptors in the human brain. American Journal of Psychiatry. 2001;158(2):308–311. doi: 10.1176/appi.ajp.158.2.308. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, Laruelle M. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Archives of General Psychiatry. 2010;67(3):231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Martinez D, Slifstein M, Laruelle M, Abi-Dargham A. Baseline [11C]raclopride binding potential is inversely related to D2/3 receptor stimulation by endogenous dopamine. Neuropsychopharmacology. 2014;39:S112–S290. doi: 10.1038/npp.2014.280. [DOI] [Google Scholar]
- Kestler LP, Malhotra AK, Finch C, Adler C, Breier A. The relation between dopamine D2 receptor density and personality: Preliminary evidence from the NEO personality inventory-revised. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2000;13(1):48–52. [PubMed] [Google Scholar]
- King MV, Seeman P, Marsden CA, Fone KC. Increased dopamine D2High receptors in rats reared in social isolation. Synapse. 2009;63(6):476–483. doi: 10.1002/syn.20624. [DOI] [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Kajander J, Bergman J, Paranta M, Solin O, Hietala J. Prediction of detached personality in healthy subjects by low dopamine transporter binding. The American Journal of Psychiatry. 2000;157(2):290–292. doi: 10.1176/appi.ajp.157.2.290. [DOI] [PubMed] [Google Scholar]
- Laakso A, Wallius E, Kajander J, Bergman J, Eskola O, Solin O, Hietala J. Personality traits and striatal dopamine synthesis capacity in healthy subjects. The American Journal of Psychiatry. 2003;160(5):904–910. doi: 10.1176/appi.ajp.160.5.904. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3 Pt 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wilson AA, Graff A, Boileau I, Di Ciano P. Recent methods for measuring dopamine D3 receptor occupancy in vivo: Importance for drug development. Frontiers in Pharmacology. 2014;5:161. doi: 10.3389/fphar.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Glassman M, King-Casas B, Kelly DL, Stein EA, Schroeder J, Salmeron BJ. Complexity of oxytocins effects in a chronic cocaine dependent population. European Neuropsychopharmacology. 2014;24(9):1483–1491. doi: 10.1016/j.euroneuro.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(17):8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, Kleber HD. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biological Psychiatry. 2010;67(3):275–278. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Gaiser EC, Gallezot JD, Angarita GA, Pittman B, Nabulsi N, Malison RT. A preliminary study of dopamine D2/3 receptor availability and social status in healthy and cocaine dependent humans imaged with [11C](+)PHNO. Drug and Alcohol Dependence. 2015;154:167–173. doi: 10.1016/j.drugalcdep.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. Journal of Cerebral Blood Flow and Metabolism. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Miettunen J, Kantojärvi L, Ekelund J, Veijola J, Karvonen JT, Peltonen L, Joukamaa M. A large population cohort provides normative data for investigation of temperament. Acta Psychiatrica Scandinavica. 2004;110(2):150–157. doi: 10.1111/j.1600-0047.2004.00344.x. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader MA. Social dominance in monkeys: Dopamine D2 receptors and cocaine self-administration. Nature Neuroscience. 2002;5(2):169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: Genetic predisposition versus environmental modulation. The American Journal of Psychiatry. 2005;162(8):1473–1482. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, Graff-Guerrero A. The potential role of dopamine D3 receptor neurotransmission in cognition. European Neuropsychopharmacology. 2013;23(8):799–813. doi: 10.1016/j.euroneuro.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Laruelle M. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60(7):485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Nordström AL, Olsson H, Halldin C. A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Research: Neuroimaging. 1998;83(1):1–6. doi: 10.1016/S0925-4927(98)00021-3. [DOI] [PubMed] [Google Scholar]
- Ortet G, Ibáñez MI, Llerena A, Torrubia R. The underlying traits of the Karolinska Scales of Personality (KSP) European Journal of Psychological Assessment. 2002;18(2):139–148. doi: 10.1027//1015-5759.18.2.139. [DOI] [Google Scholar]
- Patrizia Riccardi MD, David Zald PD, Rui Li MS, Sohee Park PDM, Sib Ansari MS, Benoit Dawant PD, Robert Kessler MD. Sex differences in amphetamine-induced displacement of [18 F]fallypride in striatal and extrastriatal regions: A PET study. American Journal of Psychiatry. 2006;163(9):1639–1641. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- Payer DE, Guttman M, Kish SJ, Tong J, Strafella A, Zack M, Boileau I. [(1)(1)C]-(+)-PHNO PET imaging of dopamine D(2/3) receptors in Parkinson’s disease with impulse control disorders. Movement Disorders. 2015;30(2):160–166. doi: 10.1002/mds.26135. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, Laruelle MA. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse. 2009;63(9):782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, Laruelle M. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biological Psychiatry. 2010;68(4):392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Searle GE, Beaver JD, Tziortzi A, Comley RA, Bani M, Ghibellini G, Gunn RN. Mathematical modelling of [(1)(1)C]-(+)-PHNO human competition studies. NeuroImage. 2013;68:119–132. doi: 10.1016/j.neuroimage.2012.11.033. [DOI] [PubMed] [Google Scholar]
- Seeman P, Ulpian C, Larsen RD, Anderson PS. Dopamine receptors labelled by PHNO. Synapse. 1993;14(4):254–262. doi: 10.1002/(ISSN)1098-2396. [DOI] [PubMed] [Google Scholar]
- Sherry A, Adelman A, Farwell L, Linton B. 17 The impact of social class on parenting and attachment. The Oxford Handbook of Social Class in Counseling. 2013;1:275. [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, Van Der Aart J, Abanades S, Rabiner EA. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. Journal of Cerebral Blood Flow and Metabolism. 2012;32(1):127–136. doi: 10.1038/jcbfm.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme C, Hill DL, Hawkes DJ. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Medical Physics. 1997;24(1):25–35. doi: 10.1118/1.598130. [DOI] [PubMed] [Google Scholar]
- Suridjan I, Boileau I, Bagby M, Rusjan PM, Wilson AA, Houle S, Mizrahi R. Dopamine response to psychosocial stress in humans and its relationship to individual differences in personality traits. Journal of Psychiatric Research. 2012;46(7):890–897. doi: 10.1016/j.jpsychires.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Tops M, Koole SL, IJH, Buisman-Pijlman FT. Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacology, Biochemistry, and Behavior. 2014;119:39–48. doi: 10.1016/j.pbb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N. Is synaptic dopamine concentration the exclusive factor which alters the in vivo binding of [11C] raclopride?: PET studies combined with microdialysis in conscious monkeys. Brain Research. 1999;841(1–2):160–169. doi: 10.1016/s0006-8993(99)01834-x. S0006-8993(99)01834-X[pii] [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: Dissection of D3 signal and anatomy. NeuroImage. 2011;54(1):264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Van Wieringen JP, Shalgunov V, Janssen HM, Fransen PM, Janssen AG, Michel MC, Elsinga PH. Synthesis and characterization of a novel series of agonist compounds as potential radiopharmaceuticals for imaging dopamine D(2)/(3) receptors in their high-affinity state. Journal of Medicinal Chemistry. 2014;57(2):391–410. doi: 10.1021/jm401384w. [DOI] [PubMed] [Google Scholar]
- Vanhauwe JF, Josson K, Luyten WH, Driessen AJ, Leysen JE. G-protein sensitivity of ligand binding to human dopamine D(2) and D(3) receptors expressed in Escherichia coli: Clues for a constrained D(3) receptor structure. The Journal of Pharmacology and Experimental Therapeutics. 2000;295(1):274–283. [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, Kapur S. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33(2):279–289. doi: 10.1038/sj.npp.1301400. [DOI] [PubMed] [Google Scholar]
- Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, Ginovart N. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. Journal of Medicinal Chemistry. 2005;48(12):4153–4160. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- Zernig G, Kummer KK, Prast JM. Dyadic social interaction as an alternative reward to cocaine. Frontiers in Psychiatry. 2013;4(100):00100. doi: 10.3389/fpsyt.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]