Abstract
Background
Vulnerability to the reduction in natural light associated with fall/winter is generally accepted as the main trigger of Seasonal Affective Disorder (SAD), while light therapy is a treatment of choice of the disorder. However, the relationship between exposure to light and mood regulation remains unclear. As compared to green light, blue light was shown to acutely modulate emotion brain processing in healthy individuals. Here, we investigated the impact of light on emotion brain processing in patients with SAD and healthy controls and its relationship with retinal light sensitivity.
Methods
Fourteen symptomatic untreated patients with SAD (34.5 ± 8.2 y.o.; 9F) and sixteen healthy controls (32.3 ± 7.7 y.o.; 11F) performed an auditory emotional task in functional Magnetic Resonance Imaging (fMRI) during the fall/winter season, while being exposed to alternating blue and green monochromatic light. Scotopic and photopic retinal light sensitivities were then evaluated using electroretinography.
Results
Blue light enhanced responses to auditory emotional stimuli in the posterior hypothalamus in patients with SAD, while green light decreased these responses. These effects of blue and green light were not observed in healthy controls despite similar retinal sensitivity in SAD and control subjects.
Conclusions;
These results point to the posterior hypothalamus as the neurobiological substrate involved in specific aspects of SAD, including a distinctive response to light and altered emotional responses.
Keywords: Seasonal Affective Disorder, Light, Hypothalamus, Emotion, Mood, melanopsin, fMRI
Introduction
Winter Seasonal Affective Disorder (SAD) is a recurrent major depressive disorder occurring in fall/winter with full remission in spring/summer (1–4). Patients with SAD tend to report typical depression complaints such as decreased mood and motivation, but also atypical symptoms such as hypersomnia and fatigue, and hyperphagia (particularly for carbohydrates) associated with weight gain, which implies alteration in sleep/wake regulation (5–6) and possibly in metabolism (7). Despite substantial research efforts, the mechanisms underlying the disorder are not established. Vulnerability to day length shortening associated with fall/winter is generally accepted as the main triggering factor of the disorder. Indeed, SAD prevalence varies with latitude [to reach up to 3% in Canada and possibly even up to 10% at higher latitude (2, 4)] and light therapy is a treatment of choice for the disorder with symptom improvements observed within a few weeks of daily (generally morning) light exposures (8). However, the mechanism linking exposure to light and mood regulation is still largely unknown.
Retinal light sensitivity was recently reported to be abnormal in patients with SAD during the depressive episode, with normalization of retinal function after 4 weeks of light therapy in winter (9). Light also regulates circadian rhythms (10) and acutely affects many processes other than vision such as melatonin secretion, alertness, sleep, performance and cognition (11–14). These effects of light are mediated by a photoreception system, which recruits intrinsically photosensitive retinal ganglion cells (ipRGCs) expressing the photopigment melanopsin (15–16), in addition to rods and cones (17). these melanopsin ipRGCs present a maximal sensitivity to blue light (460–480nm) and confer a shorter wavelength maximal sensitivity to non-visual responses to light as compared to the photopic visual system which is maximally sensitivity to green light (~550nm) (17).
Seasonal changes in the spectral composition of light occur, with relatively less blue light in winter (18) and recent data showed that blue light therapy is effective to treat SAD (19–22). In addition, blue light therapy requires light levels significantly lower than the recommended 10,000 lux of white light, suggesting that non-classic photoreception and melanopsin-expressing ipRGCs contribute to the therapeutic effects of light exposure. Functional magnetic resonance imaging (fMRI) studies in healthy individuals showed that, as compared with green monochromatic light, exposure to blue monochromatic light exerts an acute influence on cerebral activations associated to the processing of auditory emotional stimuli, notably in the hypothalamus and amygdala (23). Because these brain areas, involved in emotional processing, are also implicated in mood regulation and mood disorders (23), this acute effect of light could also be involved in the long term regulation of mood by light, possibly through melanopsin-based photoreception. Winter depression in SAD could thus be caused by some abnormal influence of light (or lack of light) on brain responses to emotionally-relevant signals.
Here, we studied the acute impact of light on auditory emotional processing in SAD and investigated the role of classical and non-classical photoreception in the disorder. We measured retinal light sensitivity and examined the effect of blue and green light exposures on the brain responses to neutral and emotional auditory stimuli in untreated symptomatic patients with SAD and healthy controls in fall/winter. We hypothesized that, during the symptomatic episode of SAD, the impact of light exposure on auditory emotional processing would be abnormal, in key brain areas for emotion regulation, such as the amygdala and hypothalamus. We also hypothesized that retinal dysfunction in SAD, which alters the light signal reaching the brain, would be related to the influence of light on these emotional responses.
Methods and Materials
More details can be found as online supplemental information.
Subjects
Patients with SAD and controls were recruited in the Montreal area (latitude ~45°30′N). They were aged between 18 and 45 (Table 1 for complete characteristics) and gave written informed consent. The study was approved by the institutional Regroupement Neuroimagerie/Québec Ethics Committee.
Table 1.
Subjects characteristics (mean +/− SD)
| SAD | CONTROLS | Pvalue | |
|---|---|---|---|
| NUMBER OF SUBJECTS | 14 | 16 | |
| AGE [≥18 ; ≤45 y.o] | 34.5 ± 8.17 | 32.25 ± 7.66 | 0.74 |
| BODY MASS INDEX [≤27] | 24.01 ± 3.28 | 23.02 ± 2.43 | 0.30 |
| SEX [M/F] | 5/9 | 5/11 | 0.8 # |
| SEASONALITY SCORE (24) | 14.07 ± 3.08 | 3 ± 2.69 | < 0.001 |
| DEPRESSION LEVEL SCORE (25) | 24 ± 10.54 | 1.38 ± 1.45 | < 0.001 |
| SIGHSAD TOTAL SCORE [≥25] (26) | 31.88 ± 5.91 | n/a | |
| SIGHSAD ATYPICAL ITEMS only [≥9] (26) | 15.64 ± 4.22 | n/a | |
| ANXIETY LEVEL (29) | 11.65 ± 9.6 | 1.81 ± 2.6 | < 0.001 |
| SLEEP DISTURBANCE (28) | 7.64 ± 3.15 | 2.56 ± 1.5 | < 0.001 |
| DAYTIME PROPENSITY TO FALL ASLEEP (63) | 14.15 ± 4.28 | 6.5 ± 4.78 | 0.001 |
| CHRONOTYPE (64) | 53.93 ± 11.69 | 50.44 ± 10.26 | 0.4 |
| LATERALITY [LEFT/RIGHT] | 1/13 | 1/15 | 0.92 # |
| YEARS OF EDUCATION | 15.63 ± 3.12 | 15.76 ± 2.34 | 0.81 # |
| Women using ORAL CONTRACEPTIVE | 2/9 | 4/11 | 0.49 # |
| Women in LUTEAL PHASE | 2/9 | 3/11 | 0.70 # |
| ETHINICITY [Afro-American/Caucasian] | 1/13 | 1/15 | 0.92 # |
| BORN OUTSIDE QUEBEC * | 4 | 5 | 0.87 # |
| BORN OUTSIDE QUEBEC IN A “SOUTHERN” COUNTRY (>5° south away from Montréal) | 1 | 1 | 0.92 # |
| SMOKING HABITS [smoking/non smoking] | 4/10 | 1/15 | 0.10 # |
| DATE OF EXPERIMENT [dd/mm/yy] (From 21/11/08 to 07/02/09) | 22/12/08 ± 27d | 22/12/08 ± 29d | 0.94 |
| SLEEP TIME prior to experiment | 22:54h ± 0:46h | 23:40h ± 1:09h | 0.073 |
| WAKE TIME prior to experiment | 07:30h ± 0:58h | 07:22h ± 1:00h | 0.74 |
| SLEEP DURATION prior to experiment | 8.6 ± 0.76 | 7.89 ± 0.54 | 0.006 |
| SUBJECTIVE SLEEPINESS immediately prior to fMRI experiment (65) | 5.36 ± 1.78 | 3.25 ± 1.15 | 0.006 |
| STIMULI VOLUME in fMRI [arbitrary units] | 675 ± 435.3 | 628.1 ± 401.2 | 0.76 |
| FIRST 40s LIGHT EXPOSURE in fMRI: Blue/Green | 7/7 | 8/8 | 1 # |
None of the subjects’ characteristics showing a significant difference between SAD and controls explained alone the reported differences in fMRI activations, as indicated by regression analyses in SPM5. Therefore the present result cannot be attributed to a single clinical symptom such as levels of depression or anxiety, seasonality, or sleep/wake disturbances.
p-values computed with Chi-squared test, otherwise with unpaired t-test
See supplemental results for more details on the place of birth aspect.
Patients
Candidates had to present a Global Seasonality Score (GSS) ≥9 on the Seasonal Pattern Assessment Questionnaire (SPAQ) (24), perceive seasonal changes as at least a “moderate” problem, and present a score ≥11 on the Beck Depression Inventory II (25). A psychologist then determined the presence of SAD symptoms based on the Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorder version (total score ≥25; atypical item score ≥9) (26). The psychologist also excluded bipolarity [using the Mood Disorder Questionnaire (27)] or other psychiatric or medical disorders (using a semi-structured interview). Sixteen patients completed the protocol but two were excluded for technical reasons. All patients participating to the experiment were studied in the absence of any treatment (light therapy or drugs).
Controls
Control subjects were matched with patients for sex, laterality and age (±2 years except for one female and one male pairs for which the difference was 4 and 6 years, respectively). Control subjects presented a GSS <8, with “no problem” with seasonal changes, and a semi-structured interview established the absence of a medical or psychiatric disorder. No sleep disturbances were reported as determined by the Pittsburgh Sleep Quality Index Questionnaire (28) (score ≤5). All showed normal scores on the 21-item Beck Anxiety Inventory (29) and Beck Depression Inventory II (25) (scores <11).
Participants
All participants were moderate alcohol consumers (<7 alcohol unit/week), and were not on medication. They were asked to refrain from alcohol containing beverages for at least 36h before the experiment. Smokers were included but smoking was not allowed for the duration of the laboratory experimentations. None had worked on night shifts during the preceding year or traveled across more than one time zone during the last 2 months. All subjects had been living in the province of Quebec for at least 3 years. Females were not pregnant or breast-feeding and were more than 1 year post-partum. Absence of ophthalmic disorder (e.g. glaucoma, color blindness) was assessed by an optometrist (standard examination). Participants completed additional questionnaires but the scores of these questionnaires were not used as inclusion criteria (Table 1).
At least one week before the experiment, participants were familiarized to the MR environment during a short MRI session during which a structural image of the brain was acquired. Volunteers were requested to follow a regular sleep schedule based on their preferred sleep times and durations during the 5 days preceding the experimentation. Compliance was verified using sleep logs and actigraphy (Actiwatch-L; MiniMitter/Respironics, OR, USA).
Experimental protocol
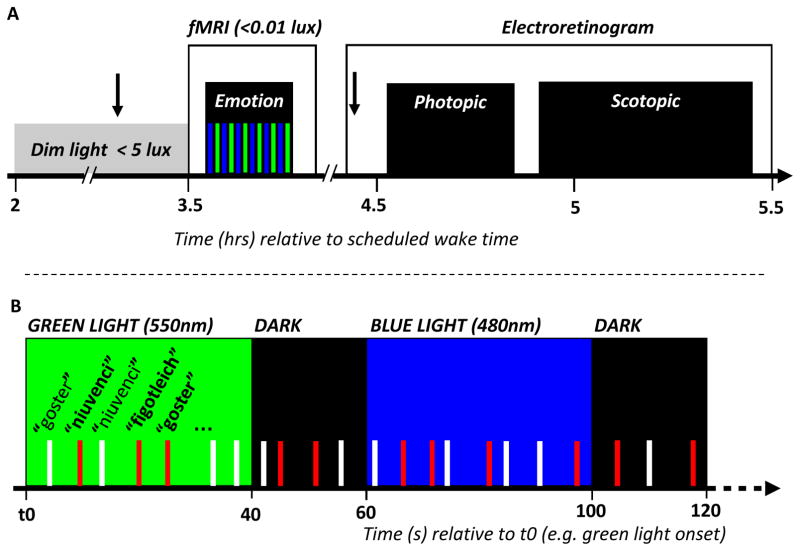
Participants arrived at the laboratory 2h after habitual wake time and were maintained in dim-light (<5 lux) for 1.5h (Figure 1a). One drop of tropicamide 1% was administered in each eye 20min before entering the scanner to inhibit pupillary constriction. During the fMRI session (12min), subjects performed an emotional auditory task while being exposed to alternating 40s periods of blue (480nm) and green (550nm) monochromatic lights [full width at half maximum (FWHM): 10nm], separated by 15-to-25s periods of darkness (30) (order blue and green light was counter-balanced across subject within each group). The fMRI session was followed by photopic and scotopic electroretinogram (ERG) recordings characterizing cone and rod photoreception, respectively.
Figure 1. experimental design.
a. General protocol.
Arrow: pupil dilator administration. Time relative to scheduled wake time (hr).
Subject performed an emotional task in fMRI (see b. for details) before photopic and scotopic ERGs were recorded.
b. Detailed fMRI procedures
Time (s) relative to t0, a time point arbitrary chosen as a green light onset of the session.
The task consisted in a gender discrimination of auditory vocalizations while exposed to alternating
 (480nm) and
(480nm) and
 (550nm) monochromatic light (counter balanced order). Light exposures lasted 40s and were separated by 15-to-25s periods of darkness. Anger (
(550nm) monochromatic light (counter balanced order). Light exposures lasted 40s and were separated by 15-to-25s periods of darkness. Anger (
 bars) and neutral (white bars) prosody vocalizations of the three pseudo-word type (“goster”, “niuvenci” or “figotleich”) were pseudo-randomly and evenly administered throughout each light conditions across the entire session (inter stimuli interval: 3 to 11s; mean: 4.8s).
bars) and neutral (white bars) prosody vocalizations of the three pseudo-word type (“goster”, “niuvenci” or “figotleich”) were pseudo-randomly and evenly administered throughout each light conditions across the entire session (inter stimuli interval: 3 to 11s; mean: 4.8s).
Technical issue
In accordance with our previous studies (31–32) and work of others [e.g. (12–13)], we set the photon densities of both monochromatic lights used in fMRI at an equal level, so that comparisons between blue and green exposures could reveal non-classic modulation of brain responses. The irradiance used (1013 photon/cm2/s) was intermediate between the two irradiances of our prior investigation of the impact of light on emotion processing (30), and had successfully been used in another study on the impact of light on auditory working memory (32). A technical problem, however, accidentally set blue and green light irradiance levels at 1.1×1013 and 0.9×1013 photons/cm2/s, respectively (which corresponds to 1.5 and 20 lux, respectively). This affected all data acquisitions and prevented direct comparisons between blue and green exposures, but did not compromise comparisons between patients and controls for blue and green light separately.
FMRI task
Acoustic stimuli consisted of three meaningless words (“goster”, “niuvenci”, “figotleich”) pronounced by professional actor (half females) with two different modalities, anger and neutral prosody, as validated by extensive behavioral assessments (33) and in previous experiments (30, 34–35). Note that negative and positive emotions are mediated through common (but not completely identical) pathways (36) but our experience is that negative emotion elicits more robust responses, less influenced by individual valence perception (37). Stimuli were presented to the subject via headphones from an audio player. The task of the subject was to press one of two buttons on a keypad (with their right hand) upon discriminating the gender of the speaker pronouncing the pseudo-word. The goal of the study to measure brain responses to emotional words was hidden from the subjects. Stimuli were matched in term of duration (750ms) and mean acoustic energy. Anger and neutral prosodies were evenly assigned to each light condition (blue, green, darkness).
FMRI acquisitions
FMRI data were acquired using a 3 Tesla MR scanner (TIM-TRIO, Siemens, Germany). Multislice T2*-weighted fMRI images were obtained with a gradient echo-planar sequence (32 axial slices; voxel size: 3.4×3.4×3mm3 with 30% of gap; matrix size 64×64×32; repetition time = 2180ms; echo time = 40ms; flip angle = 90°). Structural brain images consisted of a T1-weighted 3D MDEFT (38) (repetition time = 7.92ms, echo time = 2.4ms, time of inversion = 910ms, flip angle = 15°, field of view = 256×224mm2, matrix size = 256×224, voxel size = 1×1×1mm3).
Electroretinography acquisitions
ERG recordings were undertaken 4.5h after habitual wake time, following the fMRI session. One drop of tropicamide 1% was administered in each eye again 15min before the first ERG. Recordings were obtained with DTL electrodes (Shieldex 33/9 Thread, Germany) placed deep in the conjunctival sac, with reference electrodes placed on the canthi and ground on the forehead (39). Flash stimulations were administered using a ganzfeld dome (Color dome, Diagnosys LLC, Lowell, MA, USA) to achieve full field retinal stimulation. Participants were first adapted to a background light (25.5cd/m2) for 15min before being administered a series of white light flashes of increasing intensity (range: −1.12 to 1.375 log cd/m2/s; stimuli interval: 1 to 5s) to generate a photopic luminance response. Participants were then dark-adapted for 30min (0lux) before being presented with a series of light flashes (480nm broadband blue light to better stimulate rods, which present peak sensitivity at around 505nm) of increasing intensity (range: −4.25 to −1.00 log cd/m2/s; stimuli intervals: 1.5s -low intensity; 5s -high intensity), to generate a scotopic luminance response.
Data analysis
Behavior
Behavioral data were analyzed with Statistica 6.1 (StatSoft France, France). Mixed ANOVAs with group as the between-subjects factor (SAD, controls) and prosody (neutral, anger) as the within-subject factor were used to compare reaction times and accuracy on the fMRI task.
FMRI
Brain functional volumes were analyzed using Statistical Parametric Mapping software (SPM5 - http://www.fil.ion.ucl.ac.uk/spm). They were realigned, coregistered, spatially normalized (MNI space; standard SPM5 parameters) and smoothed (FWHM: 8mm). The analysis was conducted in two steps, accounting respectively for individual-level fixed effects and group-level random effects. Changes in regional brain responses were estimated using a general linear model in which emotional and neutral stimuli in each light condition, blue and green light onset and offset were modeled using stick functions (“events”) convolved with a canonical haemodynamic response function. A parametric modulation was added to each regressor to track any linear change of the amplitude of brain responses across time. Regressors derived from the realignment of functional volumes were considered as covariates of no interest. High-pass filtering was implemented in the matrix design using a cut-off period of 256s to remove low frequency drifts from the time series. Serial correlations in the fMRI signal were estimated using an autoregressive (order 1) plus white noise model and a restricted maximum likelihood algorithm.
The summary statistic images resulting from the contrasts of interest were further smoothed (FWHM: 6mm) and entered in the random effects analyses. This second level analyses consisted in 2 sample t-test on independent measures with unequal variance, which constituted maps of the t-statistics thresholded at puncorrected = 0.001. One sample t-tests were also computed to identify if the observed effect was significant in each population separately. Statistical inferences were performed after correction for multiple comparisons at a threshold of pcorrected = 0.05. Corrections for multiple comparisons were computed on the entire brain volume (Family Wise Error) or on small spherical volumes around a priori locations of activation (10mm radius), which were expected in structures involved in the processing of emotional auditory stimuli (34–35), in arousal regulation (40–41), in the impact of light on non-visual brain function (30–32, 42), or brain areas to which the melanopsin-expressing ipRGC project (43–44). Multiple regression analyses were carried out with questionnaire scores (Table 1) using standard SPM5 procedure.
ERG
One control subject did not complete the photopic and scotopic ERG assessment because of technical problems and photopic ERG data of another control subject were accidentally not recorded. Log K, which is the intensity necessary to reach half of the saturating amplitude of the ERG b-wave and constitutes a measure of retinal sensitivity (9, 39), was computed for scotopic and photopic data of all the other subjects using sigmoidal curve fitting (Prism 4, GraphPad, La Jolla, CA, USA). Two sample t-tests compared scotopic and photopic LogK.
Results
Demographics
As expected, patients with SAD presented high SIGH-SAD scores and were significantly more seasonal, anxious, and depressed than controls (Table 1). SAD patients reported feeling sleepier than controls during the day in general and presented significantly more sleep disturbances. Sleep duration and subjective sleepiness immediately prior to the experiment were also significantly higher in SAD patients. By contrast, possible confounds such as age, BMI, education level and chronotype did not differ significantly between groups. Wake time prior to the experiment and the date of the experiment were also similar in both groups.
Performance to the fMRI task
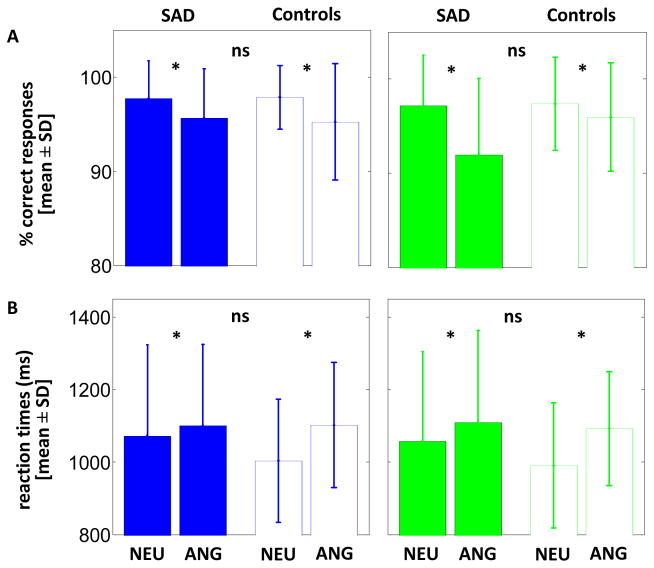
Accuracy to the gender discrimination task was high (>90%) in both light conditions, but tended to be higher for neutral than anger prosody [mean ± SD; blue: neutral (97.8 ± 3.6%) > anger (95.4 ± 5.7%), F=3.94, df=1,28, p=0.057; green: neutral (97.3 ± 5.1%) > anger (94.1 ± 7.1%), F=3.36; df=1,28; p=0.077] (Figure 2a). In both light conditions, reaction times were significantly slower for anger than neutral prosody [mean ± SD; blue: neutral (1034 ± 211ms) < anger (1101 ± 195ms), F=7.22, df=1,28, p=0.012; green: neutral (1022 ± 210ms) < anger (1101 ± 204ms), F=9.63, df=1,28, p=0.004] (Figure 2b). Critically, accuracy and reaction times did not differ between patients and controls (F<2.5, df=1,28, p>0.12) with no group-by-prosody interactions (F<2.2, df=1,28, p>0.14).
Figure 2. behavioral results of the fMRI task.
a. Accuracy (mean ± SD); b. Reaction times (mean ± SD).
SAD: Patients with SAD patient; CON: Control subjects; NEU: Neutral prosody; ANG: Anger prosody; * significant differences (p ≤ 0.05); ns: non-significant difference (p > 0.05).
These results indicate that the emotional content of the stimuli was equally well perceived by patients and controls, preventing behavior bias in the fMRI analyses comparing both groups.
FMRI results
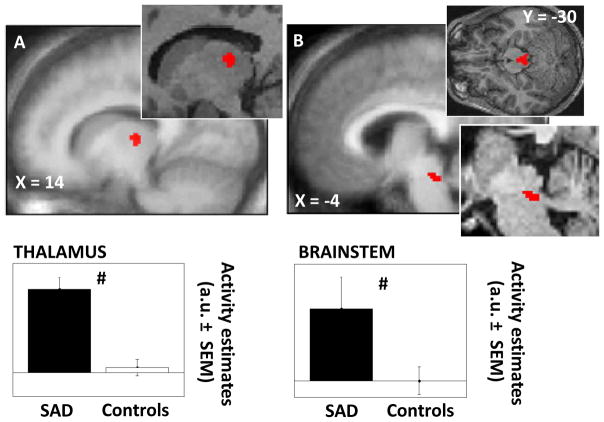
The clinical manifestation of a mood disorder, i.e. the depressive episode, alters normal brain function (23). Therefore, before investigating how blue and green light modulate brain responses to neutral or angry prosody stimuli, we assessed whether brain responsiveness to all stimuli differed between patients and controls, i.e. irrespective of the light and prosody conditions. We found that, as compared with controls, patients with SAD presented increased responses to all auditory stimuli in a dorso-posterior area of the thalamus compatible with the dorsal pulvinar, and a dorsal area of brainstem located next to superior cerebellar peduncle encompassing several nuclei of the ascending arousing system (Figure 3; Table 2a). Multiple regression analyses showed that the group differences in thalamic and brainstem responsiveness were not related to a subjects’ single characteristic that differed between SAD and control participants (cf. Table 1).
Figure 3. Significant differences between patients with SAD and healthy controls in the brain responses to all auditory stimulus types (irrespective of light and prosody condition).
a. Thalamus (dorsal and posterior); b. Brainstem (median-posterior, next to superior cerebellar peduncle).
Results are overlaid over the mean structural image of all subjects. Insets: enlargements in representative subjects. Graphs: activity estimates (arbitrary unit – a.u. ± SEM) of the brain responses to all auditory stimulus types. # pcorrected ≤ 0.05 (group difference).
Table 2.
Functional MRI results
| Brain areas | Side | X Y Z | Z score | P value |
|---|---|---|---|---|
| a. All stimuli types (irrespective of the light and prosody conditions) | ||||
| SAD > Controls | ||||
| Thalamus 1 | R | 14 −18 4 | 3.61 | 0.010 |
| Brainstem 1 | L | −2 −28 −22 | 3.58 | 0.011 |
| Controls > SAD | ||||
| No significant voxel | ||||
| b. Anger prosody stimuli | ||||
| [Blue > dark] × [SAD > Controls] | ||||
| Hypothalamus 2, 4 | L | −2 −2 −12 | 3.21 | 0.027 |
| [Blue > dark] × [Controls > SAD] | ||||
| No significant voxel | ||||
| [Green > dark] × [SAD > Controls] | ||||
| No significant voxel | ||||
| [Green > dark] × [Controls > SAD] | ||||
| Hypothalamus 3, 4 | L | −4 −2 −18 | 3.13 | 0.032 |
XYZ: relative coordinates (mm) in MNI space.
The same two significant clusters of voxel are obtained in the thalamus and brainstem if the analyses only included 1) all stimuli in darkness; 2) all stimuli under green light exposure; 3) all stimuli under blue light exposure; 4) emotional stimuli under blue or green light exposure ; 5) neutral stimuli in under blue or green light exposure (i.e. theses differences between groups are observed for every stimuli subgroups).
Clusters not affected by an exclusive mask (p=0.05) of the (Neutral × [Blue > dark] × [SAD > Controls]) contrast, indicating that the light condition effect was specific to the emotional (angry prosody) stimuli.
Clusters not affected by an exclusive mask (p=0.05) of the (Neutral × [Green > dark] × [SAD > Controls]) contrast, indicating that the light condition effect was specific to the emotional (angry prosody) stimuli.
Because of the difference in irradiance level between blue and green light (see technical issue in the methods section) the contrast computing the interaction between blue and green light conditions [(Blue > Green)] × [SAD > Controls] is not valid. However, if this contrast is nevertheless computed, it shows a single significant difference in the hypothalamus (−2 0 −18 mm; Z = 3.73; psvc = 0.006) which further strengthens the results obtained for each light condition separately.
Brain responses to auditory stimuli under blue or green light exposures were compared with brain responses in darkness to take into account the global difference in brain responsiveness and allow for group comparisons. Analyses revealed that, as compared to darkness, blue light exposure increased responses to angry prosody stimuli in the posterior hypothalamus, dorso-lateral to mammillary bodies, in patient with SAD (Figure 4; Table 2b). In contrast, under green light exposure, responses to these emotional stimuli were decreased in a slightly more ventral hypothalamic area in patients with SAD. Importantly, these effects of blue and green light were not observed in controls and were significantly different between patients and controls. Again, multiple regression analyses showed that these results were not significantly related to subjects’ single characteristics that differed between groups (cf. Table 1). Finally, no impact of light wavelength on the processing of neutral auditory stimuli was found in either group, demonstrating the specificity of the effects for emotional stimuli and for the patients with SAD.
Figure 4. Significant differences between patients with SAD and healthy controls in the impact of blue and green light exposure on the brain responses to auditory emotional stimuli.
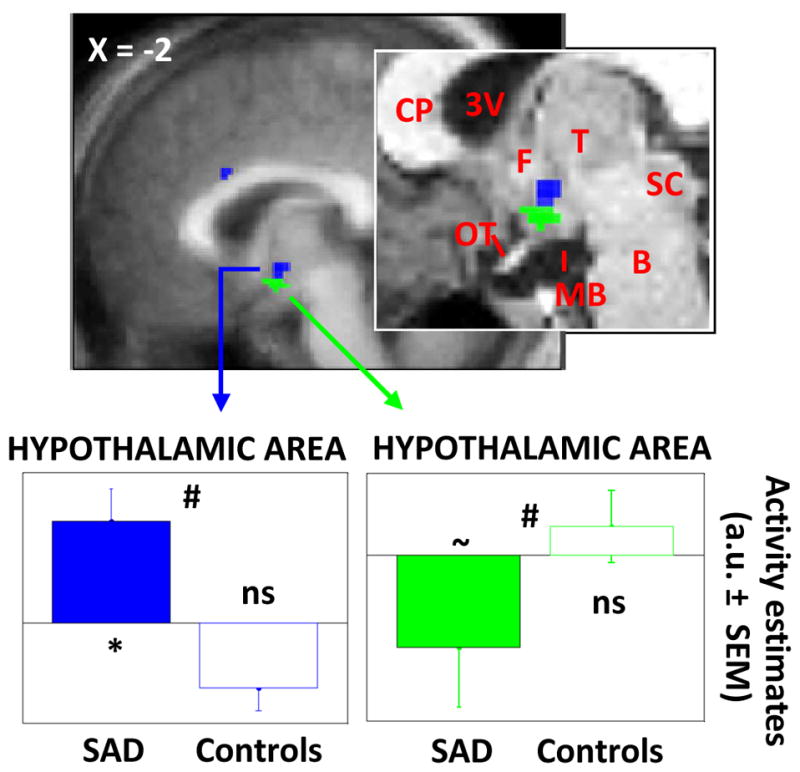
Results are overlaid over the mean structural image of all subjects. Inset: enlargement in a representative subject. 3V: third ventricle; B: brainstem; CP: corpus collusum; F: fornix; MB: mammillary bodies; OT: optic tract; SC: superior colliculus; T: thalamus. Graphs: change in activity estimates (arbitrary unit – a.u. ± SEM) between the light condition (blue, green) and the darkness condition for the processing of auditory emotional stimuli. * pcorrected ≤ 0.05 (in SAD patients taken in isolation); ~ pcorrected = 0.07 (in SAD patients taken in isolation); ns puncorrected > 0.1 (in controls taken in isolation); # pcorrected ≤ 0.05 (group difference).
ERG results
Scotopic and photopic light sensitivity, as indicated by LogK, did not differ between patients and controls [mean ± SD; Photopic LogK: patients (0.108 ± 0.093), controls (0.108 ± 0.102), T=0.008, df=26, p=0.99; Scotopic LogK: patients (−2.72 ± 0.12), controls (−2.75 ± 0.11), T=0.66, df=27, p=0.51].
Discussion
These results demonstrate that exposure to light has an acute impact on emotional brain processing in untreated symptomatic patients with SAD in fall/winter and that this impact depends on light spectral composition. As compared with healthy controls, blue light increased and green light decreased responses to auditory emotional stimuli in the posterior hypothalamus. This study also reveals that, in the context of our protocol, SAD patients presented increased thalamic and brainstem responsiveness to vocal stimuli regardless of their emotional content and of the light condition.
Compared to controls, patients showed higher thalamic activation to auditory stimuli in the dorsal pulvinar and in regions of the brainstem compatible with the locus coeruleus and dorsal raphe nucleus (although fMRI spatial resolution does not allow identification of specific brainstem nuclei). The locus coeruleus and dorsal raphe nucleus are implicated in reward regulation and depression (45) and constitute an important source of norepinephrin and serotonin, respectively. Interestingly, serotonin levels appear to be influenced by season and bright sunlight (46) and altered serotonin receptor functions have been described in SAD (2–3, 47). Animal data also showed that complete light deprivation reduced noradrenergic projections from the locus coeruleus to the prefrontal cortex (48), which is essential for cognition (49). In addition, metabolic and serotoninergic dysfunction in the pulvinar has been related to depression (50). Therefore, the differential responsiveness to vocal stimuli could constitute a marker of a general increased sensitivity in SAD during the fall/winter depressive episode, speculatively related to serotonin and norepinephrin functions.
Taking into account baseline differences between groups (i.e. responses under blue or green light were compared to darkness), emotional processing was affected by blue and green light in a single area of the brain, pointing to light-induced variation in hypothalamic reactivity specific to SAD, at least during the fall/winter symptomatic episode. These effects were not observed with neutral stimuli showing their specificity for the processing of emotional stimuli. In other words, they were not caused by an overall change in brain reactivity throughout the 40s light exposure. In healthy individuals and as compared with green light, we showed that blue light exposure increased the functional connectivity between the amygdala, temporal cortex voice-sensitive area and a hypothalamic area located in the vicinity of the present significant hypothalamic cluster (30). Dysfunction in hypothalamus-related functions is typically observed in SAD, as indicated by changes in sleep, feeding, metabolism and motivation (2–3, 5–7). One plausible implication of our findings is that exposure to light participates in the long-term normalization of these hypothalamic functions and lead to remission. However, based on our protocol, we cannot determine if it is the case or whether these abnormal hypothalamic responses to light constitute a trait-marker triggering the disorder when light availability declines, or a state-marker secondary to other phenomenon.
Importantly, the data showed no performance differences between groups, which ensures that our results are not due to behavioral differences during data acquisition (e.g. differences in task difficulty). Moreover, both populations did not differ for many other possible confounds such as age, sex, education level, wake time, dates of experiments, etc. As expected, however, SAD patients differed from controls for several aspects typically related to their pathology such as daytime sleepiness, anxiety and depression levels, sleep duration, and seasonality. While several of these factors are likely to have contributed to our results, none of them was identified by regression analyses as significantly contributing to the results on their own.
The spatial resolution of fMRI does not allow to determine which hypothalamic nucleus was specifically affected by light but a number of posterior hypothalamic nuclei receive retinal projections directly, or indirectly, through the suprachiasmatic nucleus (17, 51). Some of them, such as the hypocretin/MCH postero-lateral hypothalamus, are involved in the regulation of sleep, wakefulness, motivation and metabolism. Through their numerous projections, Hcrt-MCH neurons regulate activity in nuclei of the ascending arousal system of the brainstem, including the locus coeruleus and dorsal raphe nucleus, and in the thalamus (52). Likewise, light of various wavelengths could also affect the processing of emotional stimuli in the paraventricular nucleus of the hypothalamus, which is involved in emotional responses (53) and vegetative regulation (54).
Scotopic (rod-dependent) light sensitivity did not differ between patients and controls, which contrast with our predictions based on previous observations of lower rod sensitivity in SAD (9, 55–56). This discrepancy cannot be attributed to the sample of patients because SIGH-SAD scores, depression and seasonality levels were similar to those reported in previous studies on SAD [e.g. (19, 47)], including those investigating retinal sensitivity (9, 55). It should be noted, however, that it is the seasonal change in rod retinal sensitivity which has been most reported to be abnormal in SAD, while differences between patients and controls were not systematically detected in fall/winter (55–57). Regarding cone function, only one study so far reported decreased function in symptomatic patients with SAD in fall/winter (9), a result that was not observed in the current study. Only short term light history (preceding hours) was closely controlled in the present protocol. We cannot therefore exclude that longer-term light history influenced our ERG results (58). However, there seem to be no indication in the literature for difference in light history between SAD patients and healthy controls (59). In spite of this, our results suggest that abnormal rod or cone function cannot account for the altered hypothalamic responses observed in SAD under blue and green light exposures.
The irradiance level we used are compatible with the recruitment of melanopsin-expressing ipRGCs (60) and a polymorphism in the melanopsin gene has been linked to SAD (61). However all photoreceptors are likely to have contributed (17), especially given the results obtained with green light, and further research is warranted to identify how each photoreceptors participates to the influence of light on emotional brain processing in patients and healthy individuals. Nonetheless, our results support that the wavelength of light is an important factor for light therapy as well as for optimal indoor lighting, particularly for individuals more vulnerable to seasonal light variation, such as SAD patients, but also for the important part of the population, namely sub-syndromal SAD sufferers (up to 18% of the North American general population), who experience intermediate seasonal emotional, mood and vigilance problems that, although bothersome, do not reach clinical significance (62).
The acute impact of light on emotional brain responses may not be related to its long-term impact on mood regulation. However, emotions and mood are intimately related. Mood alteration in mood disorders modify emotional brain responses, while emotional responses can greatly influenced (subsequent) mood (23). Furthermore, although the impact of light on emotional processing might differ between negative and positive stimuli, common brain pathways respond to emotional stimuli regardless of emotional valence direction (36), supporting that similar effects of light are likely take place for positive emotions.
As a whole, the results provide experimental evidence for a central role of the hypothalamus in the seasonal-light-decline sensitivity present in SAD. Abnormal light responsiveness in the posterior hypothalamus constitutes a neurobiological substrate of SAD during the fall/winter depressive episode which could trigger the disorder or, conversely, lead to remission. Future studies should address these questions and compare symptomatic and asymptomatic states in the same individuals, in fall/winter, before and after light therapy, and spring/summer.
Supplementary Material
Acknowledgments
We thank N. Brault, O. Collignon, A. Cyr, M. Desrosiers, S. Frenette, C. Hurst, F. Lesage, P. Orban, N. Ouakli, J. Paquet, F. Peters, C. Phillips and G. Poirier for their help. This study was supported by a grant from the Institut de Recherche en Santé du Canada (IRSC-CHIR), and fellowships from the Fonds de la Recherche en Santé du Québec (FRSQ) and the Fonds Québecois de la Recherche sur la Nature et les Technologies (FQRNT).
Footnotes
Financial Disclosures
The authors declare no conflict of interest
References
- 1.Rosenthal N, Sack D, Gillin J, Lewy A, Goodwin F, Davenport Y, et al. Seasonal affective disorder : a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 2.Westrin A, Lam RW. Seasonal affective disorder: a clinical update. Ann Clin Psychiatry. 2007;19:239–246. doi: 10.1080/10401230701653476. [DOI] [PubMed] [Google Scholar]
- 3.Levitan RD. The chronobiology and neurobiology of winter seasonal affective disorder. Dialogues Clin Neurosci. 2007;9:315–324. doi: 10.31887/DCNS.2007.9.3/rlevitan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magnusson A, Partonen T. The diagnosis, symptomatology, and epidemiology of seasonal affective disorder. CNS Spectr. 2005;10:625–634. doi: 10.1017/s1092852900019593. [DOI] [PubMed] [Google Scholar]
- 5.Cajochen C, Brunner DP, Krauchi K, Graw P, Wirz-Justice A. EEG and subjective sleepiness during extended wakefulness in seasonal affective disorder: circadian and homeostatic influences. Biol Psychiatry. 2000;47:610–617. doi: 10.1016/s0006-3223(99)00242-5. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz PJ, Rosenthal NE, Wehr TA. Band-specific electroencephalogram and brain cooling abnormalities during NREM sleep in patients with winter depression. Biol Psychiatry. 2001;50:627–632. doi: 10.1016/s0006-3223(01)01097-6. [DOI] [PubMed] [Google Scholar]
- 7.Pinchasov BB, Shurgaja AM, Grischin OV, Putilov AA. Mood and energy regulation in seasonal and non-seasonal depression before and after midday treatment with physical exercise or bright light. Psychiatry Res. 2000;94:29–42. doi: 10.1016/s0165-1781(00)00138-4. [DOI] [PubMed] [Google Scholar]
- 8.Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–663. doi: 10.1017/s1092852900019611. [DOI] [PubMed] [Google Scholar]
- 9.Lavoie MP, Lam RW, Bouchard G, Sasseville A, Charron MC, Gagne AM, et al. Evidence of a biological effect of light therapy on the retina of patients with seasonal affective disorder. Biol Psychiatry. 2009;66:253–258. doi: 10.1016/j.biopsych.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Duffy JF, Czeisler CA. Effect of Light on Human Circadian Physiology. Sleep Med Clin. 2009;4:165–177. doi: 10.1016/j.jsmc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brainard GC, Hanifin JP. Photons, clocks, and consciousness. J Biol Rhythms. 2005;20:314–325. doi: 10.1177/0748730405278951. [DOI] [PubMed] [Google Scholar]
- 12.Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- 13.Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 14.Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13:429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 17.Hatori M, Panda S. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol Med. 2010;16:435–446. doi: 10.1016/j.molmed.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorne HC, Jones KH, Peters SP, Archer SN, Dijk DJ. Daily and seasonal variation in the spectral composition of light exposure in humans. Chronobiol Int. 2009;26:854–866. doi: 10.1080/07420520903044315. [DOI] [PubMed] [Google Scholar]
- 19.Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs) Biol Psychiatry. 2006;59:502–507. doi: 10.1016/j.biopsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JL, Glod CA, Dai J, Cao Y, Lockley SW. Lux vs. wavelength in light treatment of Seasonal Affective Disorder. Acta Psychiatr Scand. 2009;120:203–212. doi: 10.1111/j.1600-0447.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 21.Desan PH, Weinstein AJ, Michalak EE, Tam EM, Meesters Y, Ruiter MJ, et al. A controlled trial of the Litebook light-emitting diode (LED) light therapy device for treatment of Seasonal Affective Disorder (SAD) BMC Psychiatry. 2007;7:38. doi: 10.1186/1471-244X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strong RE, Marchant BK, Reimherr FW, Williams E, Soni P, Mestas R. Narrow-band blue-light treatment of seasonal affective disorder in adults and the influence of additional nonseasonal symptoms. Depress Anxiety. 2009;26:273–278. doi: 10.1002/da.20538. [DOI] [PubMed] [Google Scholar]
- 23.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenthal N, Bradt G, Wehr T. Seasonal Pattern Assessment Questionnaire (SPAQ) Bethesda MD: National Institute of Mental Health; 1984. [Google Scholar]
- 25.Steer RA, Ball R, Ranieri WF, Beck AT. Further evidence for the construct validity of the Beck depression Inventory-II with psychiatric outpatients. Psychol Rep. 1997;80:443–446. doi: 10.2466/pr0.1997.80.2.443. [DOI] [PubMed] [Google Scholar]
- 26.Williams J, Link M, Rosenthal N, Amira L, Terman M. Structured Interview Guide for the Hamilton Depression rating scale-seasonal affective disorder version (SIGH-SAD) (revised edn) New York State: Psychiatric Institute, New York; 1994. [Google Scholar]
- 27.Hirschfeld RM, Williams JB, Spitzer RL, Calabrese JR, Flynn L, Keck PE, Jr, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873–1875. doi: 10.1176/appi.ajp.157.11.1873. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 30.Vandewalle G, Schwartz S, Grandjean D, Wuillaume C, Balteau E, Degueldre C, et al. Spectral quality of light modulates emotional brain responses in humans. Proc Natl Acad Sci U S A. 2010;107:19549–19554. doi: 10.1073/pnas.1010180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandewalle G, Gais S, Schabus M, Balteau E, Carrier J, Darsaud A, et al. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb Cortex. 2007;17:2788–2795. doi: 10.1093/cercor/bhm007. [DOI] [PubMed] [Google Scholar]
- 32.Vandewalle G, Schmidt C, Albouy G, Sterpenich V, Darsaud A, Rauchs G, et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS One. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banse R, Scherer KR. Acoustic profiles in vocal emotion expression. J Pers Soc Psychol. 1996;70:614–636. doi: 10.1037//0022-3514.70.3.614. [DOI] [PubMed] [Google Scholar]
- 34.Grandjean D, Sander D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, et al. The voices of wrath: brain responses to angry prosody in meaningless speech. Nat Neurosci. 2005;8:145–146. doi: 10.1038/nn1392. [DOI] [PubMed] [Google Scholar]
- 35.Sander D, Grandjean D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, et al. Emotion and attention interactions in social cognition: brain regions involved in processing anger prosody. Neuroimage. 2005;28:848–858. doi: 10.1016/j.neuroimage.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Sterpenich V, Albouy G, Boly M, Vandewalle G, Darsaud A, Balteau E, et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007;5:e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- 39.Hébert M, Lachapelle P, Dumont M. Reproducibility of electroretinograms recorded with DTL electrodes. Doc Ophthalmol. 1996;91:333–342. doi: 10.1007/BF01214651. [DOI] [PubMed] [Google Scholar]
- 40.Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998;18:8979–8989. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt C, Collette F, Leclercq Y, Sterpenich V, Vandewalle G, Berthomier P, et al. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 2009;324:516–519. doi: 10.1126/science.1167337. [DOI] [PubMed] [Google Scholar]
- 42.Vandewalle G, Balteau E, Phillips C, Degueldre C, Moreau V, Sterpenich V, et al. Daytime light exposure dynamically enhances brain responses. Curr Biol. 2006;16:1616–1621. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 43.Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, et al. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8:e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 46.Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD. Effect of sunlight and season on serotonin turnover in the brain. Lancet. 2002;360:1840–1842. doi: 10.1016/s0140-6736(02)11737-5. [DOI] [PubMed] [Google Scholar]
- 47.Willeit M, Sitte HH, Thierry N, Michalek K, Praschak-Rieder N, Zill P, et al. Enhanced serotonin transporter function during depression in seasonal affective disorder. Neuropsychopharmacology. 2008;33:1503–1513. doi: 10.1038/sj.npp.1301560. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez MM, Aston-Jones G. Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc Natl Acad Sci U S A. 2008;105:4898–4903. doi: 10.1073/pnas.0703615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- 50.Young KA, Holcomb LA, Bonkale WL, Hicks PB, Yazdani U, German DC. 5HTTLPR polymorphism and enlargement of the pulvinar: unlocking the backdoor to the limbic system. Biol Psychiatry. 2007;61:813–818. doi: 10.1016/j.biopsych.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 51.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 52.Adamantidis A, de Lecea L. Sleep and metabolism: shared circuits, new connections. Trends Endocrinol Metab. 2008;19:362–370. doi: 10.1016/j.tem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 54.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, et al. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 55.Hebert M, Beattie CW, Tam EM, Yatham LN, Lam RW. Electroretinography in patients with winter seasonal affective disorder. Psychiatry Res. 2004;127:27–34. doi: 10.1016/j.psychres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Hebert M, Dumont M, Lachapelle P. Electrophysiological evidence suggesting a seasonal modulation of retinal sensitivity in subsyndromal winter depression. J Affect Disord. 2002;68:191–202. doi: 10.1016/s0165-0327(00)00192-0. [DOI] [PubMed] [Google Scholar]
- 57.Gagne AM, Hebert M. Atypical pattern of rod electroretinogram modulation by recent light history: A possible biomarker of seasonal affective disorder. Psychiatry Res. 2010 doi: 10.1016/j.psychres.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Beaulieu C, Rufiange M, Dumont M, Lachapelle P. Modulation of ERG retinal sensitivity parameters with light environment and photoperiod. Doc Ophthalmol. 2009;118:89–99. doi: 10.1007/s10633-008-9137-6. [DOI] [PubMed] [Google Scholar]
- 59.Dumont M, Beaulieu C. Light exposure in the natural environment: Relevance to mood and sleep disorders. Sleep Med. 2007 doi: 10.1016/j.sleep.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Guler AD, et al. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roecklein KA, Rohan KJ, Duncan WC, Rollag MD, Rosenthal NE, Lipsky RH, et al. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2009;114:279–285. doi: 10.1016/j.jad.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kasper S, Wehr T, Bartko J, Gaist P, Rosenthal N. Epidemiological findings of seasonal changes in mood and behavior. Arch Gen Psychiatry. 1989;46:823–833. doi: 10.1001/archpsyc.1989.01810090065010. [DOI] [PubMed] [Google Scholar]
- 63.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 64.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 65.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.