Abstract
Purpose
Comorbid medical conditions are common among breast cancer survivors, contribute to poorer long-term survival and increased overall mortality, and may be ameliorated by weight loss. This secondary analysis evaluated the impact of a weight loss intervention on comorbid medical conditions immediately following an intervention (12-months) and one-year post-intervention (24-month) using data from the Exercise and Nutrition to Enhance Recovery and Good health for You (ENERGY) trial – a phase III trial which was aimed at and successfully promoted weight loss.
Methods
ENERGY randomized 692 overweight/obese women who had completed treatment for early stage breast cancer to either a one-year group-based behavioral intervention designed to achieve and maintain weight loss or to a less intensive control intervention. Minimal support was provided post-intervention. New medical conditions, medical conditions in which non-cancer medications were prescribed, hospitalizations, and emergency room visits were compared at baseline, year 1 and 2. Changes over time were analyzed using chi-squared tests, Kaplan-Meier and logistic regression analyses.
Results
At 12 months, women randomized to the intervention had fewer new medical conditions compared to the control group (19.6% vs. 32.2%, p<0.001); however, by 24 months, there was no longer a significant difference. No difference was observed in each of four conditions for which non-cancer medications were prescribed, hospital visits, or emergency visits at either 12 or 24 months.
Conclusions
These results support a short-term benefit of modest weight loss on the likelihood of comorbid conditions; however, recidivism and weight regain likely explain no benefit at one-year post-intervention follow-up.
Keywords: weight loss, breast cancer survivorship, comorbid conditions
INTRODUCTION
Early detection and effective initial treatments have led to a growing population of breast cancer survivors, with ~3.7 million women in the U.S. living with a history of breast cancer [1]. In addition to their cancer diagnosis, more than 40% of breast cancer survivors present with another chronic comorbid health condition at the time of their cancer diagnosis or shortly after diagnosis [2–4], and the development of new conditions post -diagnosis is common [5,6]. These comorbid medical conditions contribute to poorer long-term survival and increased overall mortality [2–4,7,8]. Among breast cancer survivors, there is great concern about recurrence of their cancer, but women over age 65 years are more likely to die of cardiovascular disease than from their breast cancer diagnosis [9]. Moreover, an increasing number of comorbid conditions are associated with poorer physical and mental quality of life among women with a previous diagnosis of breast cancer [10]. Determining effective ways to prevent the development of new chronic conditions among breast cancer survivors is imperative.
Overweight and obesity, as well as physical inactivity, are significant predictors for developing comorbidities among cancer survivors [5], as excess adiposity contributes to the development of medical conditions such as type 2 diabetes, hypertension, dyslipidemia, coronary heart disease, heart failure, obstructive sleep apnea, gastroesophageal reflux, and osteoarthritis [11]. In the U.S., approximately 70% of women diagnosed with breast cancer are overweight or obese at the time of their diagnosis [12]. This high prevalence is due in part to obesity being a major risk factor for postmenopausal estrogen-dependent breast cancer [13]. Once diagnosed, obesity increases the risk of overall mortality and breast cancer-specific mortality, which includes deaths caused by recurrence or a second primary breast cancer, in both pre- and postmenopausal women [14]. Weight gain following breast cancer diagnosis is common [15] and contributes to increased cancer-related symptoms and worse health-related quality of life [16–19].
Evidence in non-cancer patients suggests that weight loss and physical activity can prevent diabetes [20,21], improve diabetes management, blood pressure, and cardiovascular disease risk profiles [22,23] and lead to fewer hospitalizations, less medication use, and lower costs [24,25]. No studies to date, however, have evaluated the effect of weight loss on comorbid health conditions among breast cancer survivors.
The Exercise and Nutrition to Enhance Recovery and Good health for You (ENERGY) trial was a multicenter phase III randomized controlled trial of a one-year behavioral weight loss intervention followed by an observational year post-intervention follow up that previously reported a significant reduction in weight associated with the intervention at 12 (−6% versus −1.5%, p<0.001) and 24 months (−3.7% versus −1.3%, p<0.001), as well as significant decreases in both systolic and diastolic blood pressure at these time points [26]. The purpose of this secondary analysis was to evaluate the effect of the weight loss intervention on self-reported comorbidities as indicated by hospitalizations, emergency room (ER) visits, new medical conditions, and medical conditions for which medication was prescribed at the end of the intervention period and then follow-up at one year post-intervention.
METHODS
Study Design
The ENERGY trial was a multi-centered phase III randomized controlled trial of a one-year behavioral group-based weight loss program with telephone counseling and tailored newsletters with the primary aim to achieve and maintain at least a 7% initial body weight loss after two years among overweight/obese breast cancer survivors. The overall focus of the intervention was to increase physical activity and decrease energy intake. Details of the design, intervention, and study procedures have been published previously [26,27]. All study procedures were approved by the Institutional Review Boards of each site and participants provided written informed consent.
Study Population
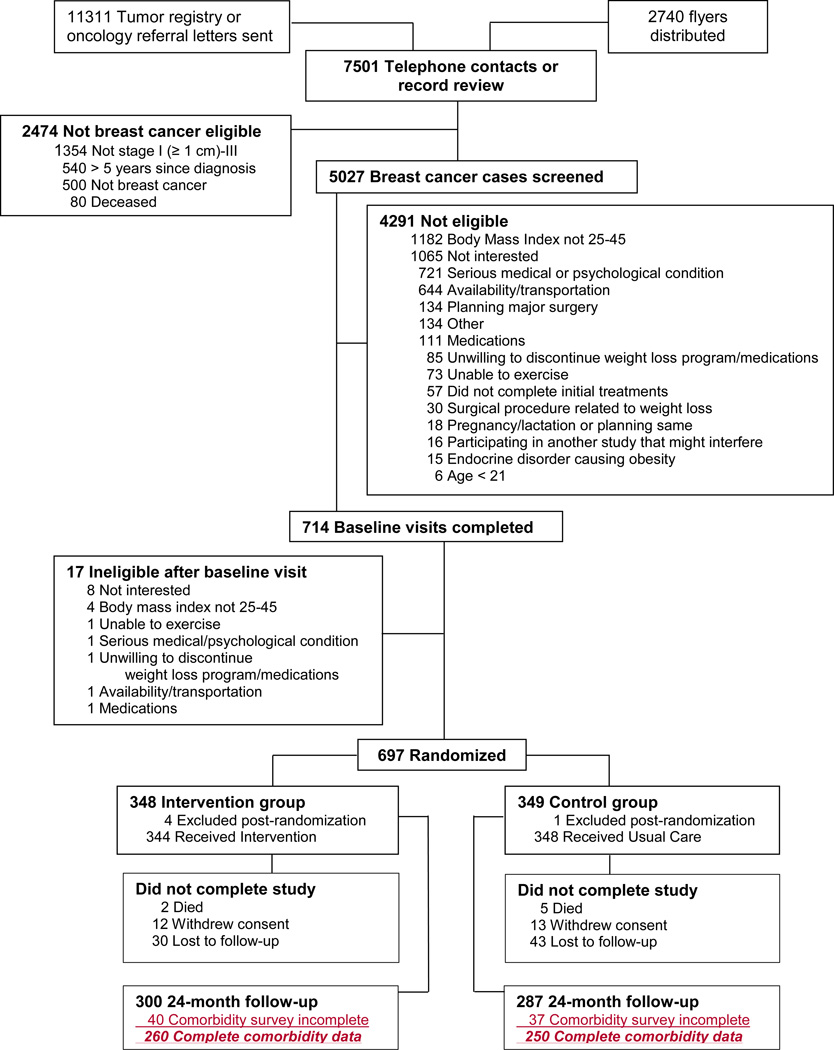
From 2010 to 2012, 692 women with a previous diagnosis of invasive breast cancer were recruited and enrolled from four clinical sites (San Diego, CA; Denver, CO; St. Louis, MO; and Birmingham, AL). Women who were ≥21 years of age with a diagnosis of breast cancer (stages I [≥1 cm], II, or III) within the previous five years who had completed all initial therapies except endocrine therapy, had a body mass index (BMI) 25–45 kg/m2, and were able to comply with study procedures were included in this trial. Women were excluded if they had a history of malignancies other than initial breast cancer diagnosis or non-melanoma skin cancer, serious psychiatric illness, and any medical condition substantially limiting moderate physical activity. Participants were randomized with equal distribution to either the intervention or control arm and were blocked on age (older/younger than 55 years), breast cancer stage (I vs lI and III), and study site. The consort diagram is presented in Figure 1.
Figure 1.
Interventions
Specific details of the weight loss intervention aimed at increasing physical activity and decreasing energy intake have been reported previously [27]. Briefly, the intervention was based on the behavioral determinants model [28] which is constructed on the Social Cognitive Theory [29–31]. Based on this theory, active goal-setting of weight, dietary intake, and physical activity was encouraged; successes of short-term goals contributed to increased self-efficacy which was instrumental in participants obtaining their weight goals. The intervention was designed as a 12-month intervention and follow-up one year later. Participants randomized to the intervention group participated in weekly one-hour closed group sessions for 4 months followed by sessions every other week for two months. Groups consisted of an average of 15 participants and were led by 1–2 leaders with training in nutrition, psychology, and/or exercise physiology. Following the intensive phase of the intervention, the groups met monthly from 6 to 12 months. Brief personalized telephone and/or email guidance was used to further reinforce and individualize the approaches discussed in the group sessions. From 6 to 24 months, additional support and reinforcement of self-management was also provided through quarterly tailored print newsletters. The newsletters were tailored based on individually collected information about dietary intake, physical activity, and weight, and included guidance to promote increase physical activity and regulation of dietary intake. Minimal weight loss support was provided in the second year of the study.
Participants randomized to the control group were provided an individualized weight loss counseling session at baseline and at 6 months. These sessions were based on standard resource materials that were available in the public domain and included weight management and current physical activity recommendations. During the first year of the trial, control group participants were invited to attend optional seminars on healthy living other than weight control every other month.
Measures
Current comorbidities were self-reported at baseline for 12 conditions (heart disease, high blood pressure, lung disease, diabetes, ulcers or stomach disease, kidney disease, liver disease, anemia or other blood disease, depression, osteo- or degenerative-arthritis, rheumatoid arthritis, and back pain), and text fields provided for other medical problems. Any new medical conditions or problems were assessed at the 6-, 12-, 18-, and 24-month follow-up clinic visits. Hospitalization that resulted in at least one overnight stay within the last year, emergency room visits within the last year, and medical conditions with concurrent prescription medications other than those for cancer treatment were assessed by self-report at baseline. New hospitalizations and emergency room visits, new medical conditions, and any changes to prescription medications were also reported at all follow-up clinic visits. Medical documentation was obtained for all hospitalizations, emergency room visits, and new cardiovascular events and cancers.
Trained staff measured height at baseline as well as weight at baseline and 6-, 12-, 18- and 24-month follow-up clinic visits. Using the height and weight measures, BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2).
Age, education, race, Hispanic ethnicity, smoking status, and menopausal status were reported on baseline questionnaires whereas information on breast cancer diagnosis and treatments (including surgery and chemotherapy, and endocrine therapy) was abstracted from medical records.
Statistical Analyses
The outcome measures for this evaluation were any new medical conditions, any hospital visits, any ER visits, and conditions in which non-cancer medications were prescribed with a specific focus on four leading categories of medications (antihypertensives, lipid-lowering agents, diabetes medications, and acid reflux medications). Of the 587 participants who provided weight data at 24 months, 516 had comorbidity measures at 12 months and 510 had comorbidity measures at 24 months.
Participant characteristics were evaluated to compare those women randomized to the intervention versus those in the control group using t-tests for continuous variables and chi-squared tests for categorical variables. Changes in comorbidity measures were analyzed using chi-squared tests for categorical outcomes (any new medical conditions, four leading conditions in which non-cancer medications were prescribed, any ER visits, or hospitalizations) at 12- and 24-month time periods. To further explore the association between any new medical conditions by study arm, a Kaplan-Meier survival curve was used to evaluate the time until the date of the first reported new medical condition. Censoring occurred at the last reported study visit if no new medical condition was reported. The log-rank test was used to assess statistically significant differences in the Kaplan-Meier survival curve. Additionally, logistic regression analysis was used to evaluate the association between any new medical conditions and changes in body weight at the 12-month time period and then 24-month time period adjusted for age and number of comorbid conditions at baseline. Statistical analyses were based on two-sided statistical tests with an alpha-level of 0.05. Analyses were conducted using SAS version 9.4 (Cary, North Carolina).
Results
The majority of the participants in this study were college-educated, non-Hispanic white, non-smokers, who were postmenopausal and had been diagnosed with estrogen receptor positive breast cancer, stage I or II. There were no statistically significant differences in study characteristics at baseline by groups, including comorbid conditions (Table 1). The predominant comorbid conditions at baseline were hypertension (33.1% in the intervention group versus 31.6% in the control group), depression (23.6% in the intervention group versus 19.0% in the control group) and osteoarthritis (7.6% in the intervention group versus 7.8% in the control group), as well as “other” comorbidities among which thyroid disease was most commonly cited. The mean number of conditions for which participants were taking a non-cancer prescription medication was 2.3 at study entry. The mean number of comorbid conditions was 1.1 (SD 1.1). Overall, 34.7% of women reported one comorbid condition, 17.2% reported two conditions, and 11.6% reported three or more conditions at baseline. The overall completion of the study was 85% [26], and women who did not complete the 24-month study visit (n=105) had the same number of mean baseline comorbid conditions, i.e., 1.1 (SD 1.1), as compared to women who completed the study.
Table 1.
Characteristics of the ENERGY study participants at baseline (study entry).
| Intervention N=344 |
Control N=348 |
P-valuea | |
|---|---|---|---|
| Age at study entry, years (mean [SD]) | 56 (9) | 56 (10) | 0.61 |
| Age at study entry, year categories % | |||
| 30–44 | 13.1 | 9.8 | |
| 44–54 | 30.5 | 33.1 | |
| 55–64 | 35.5 | 35.6 | |
| ≥65 | 20.9 | 21.6 | |
| Race/Ethnicity % | 0.77 | ||
| Non-Hispanic White | 77.0 | 81.0 | |
| African-American | 10.5 | 10.1 | |
| Hispanic | 7.6 | 5.8 | |
| Mixed/Other | 4.9 | 3.2 | |
| Education % | 0.95 | ||
| ≤High school graduate | 14.8 | 13.8 | |
| Some college | 25.6 | 27.3 | |
| College graduate | 26.7 | 28.2 | |
| Some postgraduate education | 32.9 | 30.8 | |
| Postmenopausal at study entry % | 79.9 | 82.8 | 0.42 |
| Smoking status at baseline % | 0.65 | ||
| Never | 66.3 | 63.2 | |
| Former | 30.5 | 32.8 | |
| Current | 3.2 | 3.7 | |
| Years between diagnosis and study entry, mean (SD) | 2.61 (1.38) | 2.78 (1.40) | 0.10 |
| Breast cancer stage % | 0.55 | ||
| I | 30.2 | 30.5 | |
| II | 50.3 | 53.2 | |
| III | 19.5 | 16.4 | |
| Tumor estrogen receptor status % | 0.93 | ||
| Positive | 75.9 | 73.3 | |
| Negative | 22.7 | 21.6 | |
| Chemotherapy % | 0.60 | ||
| Yes | 77.0 | 75.3 | |
| No | 23.0 | 24.7 | |
| Endocrine modulating Therapy % | 0.73 | ||
| None | 25.92 | 25.9 | |
| SERM only | 20.1 | 22.4 | |
| Any Aromatase Inhibitor | 54.1 | 51.7 | |
| Specific comorbidities at baseline % | |||
| Heart Disease | 1.7 | 3.2 | 0.23 |
| High Blood Pressure | 33.1 | 31.6 | 0.67 |
| Diabetes | 6.4 | 5.2 | 0.49 |
| Depression | 23.6 | 19.0 | 0.14 |
| Lung Disease | 1.2 | 3.5 | 0.05 |
| Ulcer or stomach disease | 4.7 | 4.0 | 0.69 |
| Kidney Disease | 0.0 | 0.3 | 0.58 |
| Liver Disease | 0.3 | 0.3 | 0.99 |
| Anemia or other blood disease | 1.7 | 1.2 | 0.51 |
| Osteoarthritis, degenerative arthritis | 7.6 | 7.8 | 0.51 |
| Rheumatoid arthritis | 0.9 | 2.3 | 0.13 |
| Back pain | 6.7 | 4.3 | 0.17 |
| Other | 22.4 | 19.5 | 0.36 |
| Conditions in which non-cancer medications were prescribed, mean(SD) |
2.3(1.9) | 2.3(2.0) | 0.69 |
| Conditions in which non-cancer medications were prescribed % |
|||
| Acid reflux medications | 21.8 | 19.5 | 0.73 |
| Allergy medications | 15.4 | 11.8 | 0.32 |
| Anxiety medications | 11.6 | 12.4 | 0.94 |
| Antihypertensives | 30.8 | 28.7 | 0.74 |
| Antihyperlipidemics | 23.3 | 22.1 | 0.87 |
| Arthritis medications | 12.9 | 10.5 | 0.38 |
| Depression Medications | 20.9 | 21.6 | 0.98 |
| Insomnia Medications | 14.0 | 13.2 | 0.78 |
| Osteoporosis Medications | 10.2 | 10.1 | 0.85 |
| Pain Medications | 11.3 | 9.5 | 0.61 |
| Thyroid Medications | 20.9 | 21.6 | 0.93 |
p-values are reported from t-tests for continuous variables and chi-squared tests for categorical variables.
At 12 months, there were significantly fewer intervention than control participants who reported new medical conditions (19.6% vs. 32.2%, p<0.001); however, by the 24-month time point there was no longer a significant difference between the groups (Table 2). A summary of the disease categories for new conditions is reported in the supplemental table. There was no difference between the intervention and control groups in conditions for which non-cancer medications were prescribed, hospital visits, or ER visits at either the 12- or 24-month follow-up time points.
Table 2.
New medical conditions, prescription medications, hospital visits, and emergency room visits at baseline, 12- and 24-month time points among the ENERGY trial cohort.
| Baseline (study entry) | 12-Month Follow-Up | 24-Month Follow-Up | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention N=344 |
Control N=348 | p-valuea | Intervention N=271 |
Control N=245 |
p-valuea | Intervention N=260 |
Control N=250 |
p-valuea | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||||
| Any new medical condition | --- | --- | --- | --- | --- | 53 | 19.6 | 79 | 32.2 | 0.001 | 68 | 26.2 | 55 | 22.0 | 0.27 |
| Leading conditions in which non-cancer medications were prescribed | |||||||||||||||
| Antihypertensives % | 106 | 30.8 | 100 | 28.7 | 0.47 | 84 | 31.1 | 79 | 32.2 | 0.81 | 84 | 32.4 | 83 | 33.3 | 0.83 |
| Lipid-lowering agents % | 80 | 23.3 | 77 | 22.1 | 0.66 | 66 | 24.4 | 63 | 25.7 | 0.72 | 69 | 26.5 | 66 | 26.4 | 0.97 |
| Diabetes Medications % | 21 | 6.1 | 19 | 5.5 | 0.69 | 19 | 7.0 | 17 | 6.9 | 0.88 | 15 | 5.8 | 19 | 7.6 | 0.48 |
| Acid Reflux Medications % | 75 | 21.8 | 68 | 19.5 | 0.43 | 65 | 24.1 | 60 | 24.5 | 0.54 | 53 | 20.5 | 55 | 22.1 | 0.52 |
| Hospital visitb % | 87 | 25.3 | 87 | 25.0 | 0.86 | 22 | 8.1 | 24 | 9.9 | 0.49 | 18 | 6.9 | 13 | 5.2 | 0.42 |
| Emergency room visitb % | 56 | 16.3 | 50 | 14.4 | 0.49 | 18 | 6.7 | 18 | 7.4 | 0.77 | 22 | 8.5 | 18 | 7.2 | 0.59 |
p-values are from chi square tests.
Hospital visits and emergency room visits at baseline captures the preceding 12-months whereas the 12-month and 24-month time points capture the time period since the last clinic visit.
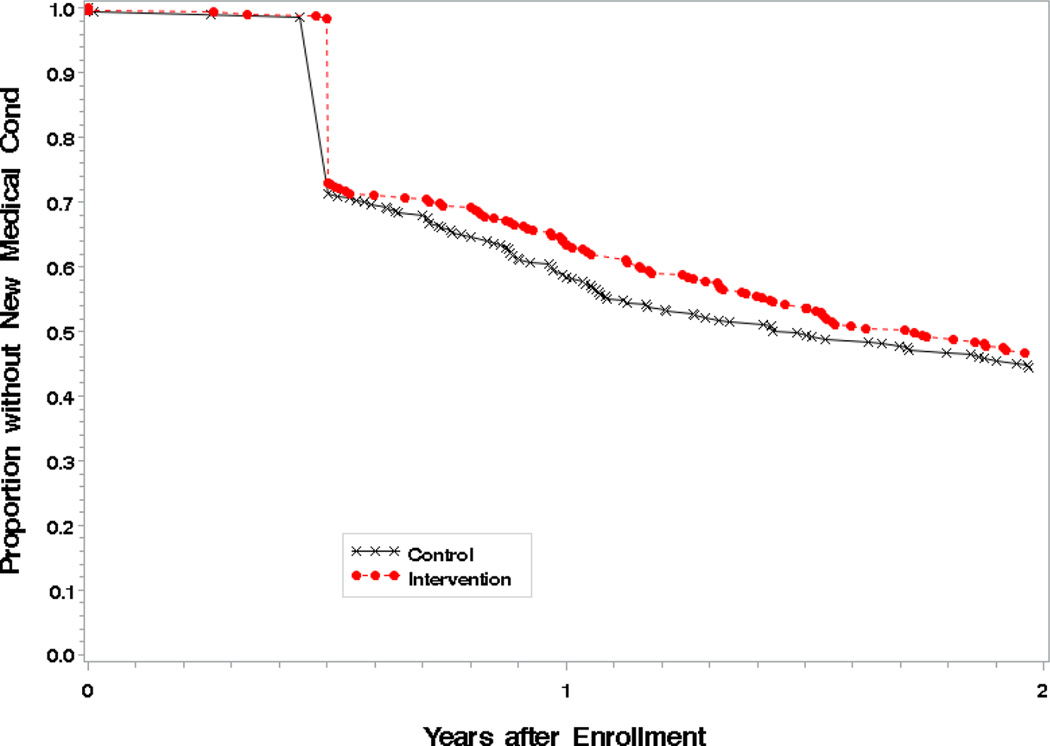
The Kaplan-Meier curve showed separation between the groups beginning at 6 months with the largest difference around 12 months (p=0.04) followed by a decrease during the second year (p=0.41, Figure 2). Additionally, when logistic regression analysis of any new comorbid conditions at 12 months by weight change was conducted, fewer new medical conditions were associated with weight loss after adjustment (p=0.04).
Figure 2.
Changes in any new medical conditions during 24 months of follow-up.
Discussion
Following breast cancer diagnosis, healthy weight management is an essential component of self-care strategies to prevent health outcomes such as recurrence, secondary primary cancers, and risk for other chronic diseases [32]. The ENERGY trial is the largest randomized controlled trial of a behavioral weight loss intervention conducted to date among overweight and obese early stage breast cancer survivors. The weight loss intervention was associated with a lower number of new medical conditions as compared to the control group (19.6% vs. 32.2%, p<0.001) immediately following completion of the intervention period. Findings from this trial demonstrate that the weight loss intervention, which promoted modest weight loss, had an impact in the short term rates of new medical conditions. In addition, there were no between-group differences in the four medical conditions for which non-cancer medications were prescribed, hospitalizations, or emergency room visits.
Intentional weight loss is an effective strategy for improving obesity-associated comorbidities and reducing the development of new medical conditions in non-cancer adult populations [33–38]. Surgical methods of weight loss result in larger weight loss, better weight maintenance, and improved comorbidities as compared to weight loss achieved through lifestyle changes; however, adverse events have been noted with surgery and not everyone will qualify for the surgical option [33,39].
Within the ENERGY trial, the largest weight loss (−6.0% in the intervention group versus -1.5% in the control group) that was observed occurred during the first year of the intervention when the active weight loss phase was being implemented, and this corresponds to the significant finding of lower comorbidities among the intervention group. Furthermore, fewer comorbid conditions were reported at the 12-month time point with weight loss. Noteworthy, there were differences in new cardiovascular conditions at the 12-month time point; 3 (1.1%) in the intervention versus 8 (3.2%) in the control group. In the second year of the trial, support was minimal and weight was regained. Although a mediation model was beyond the scope of this analysis, the independent association of weight change with both the intervention [26] and comorbid conditions at both 12- and 24-months mirrors what was observed for the intervention and suggests that the intervention influences new comorbid conditions primarily through weight changes. In the general population of overweight and obese adults, clinical guidelines recommend initial weight loss of 5–10% for health benefits [40]; weight loss of this magnitude (6%) was observed immediately following the one-year intervention.
Findings from this study reinforce the need for continued support of weight loss maintenance to prevent weight regain and subsequent comorbidities. Within the ENERGY trial, 55% of the intervention group had lost 5% or more of their initial weight however this decreased to 44% by 24-months [26]. Weight regain is common after weight loss; a systematic review suggests that on average 50% of weight loss is regained at one year [41]. Interventions that can extend treatment and support periods as well as incorporate successful strategies such as continued self-monitoring, increased physical activity, and regular meal planning along with cognitive restructuring to mitigate responses to lapses [42–44] will be more effective in maintaining weight loss and reducing the development of obesity-related diseases.
Excess adiposity contributes to the prevalence of comorbid conditions among survivors as well as to the development of new comorbid conditions [4,5]. Although comorbid conditions are common among women with a previous diagnosis of breast cancer ranging from 42% to 54% [2–4], 63.5% of ENERGY participants reported at least one comorbidity at study entry. This somewhat higher prevalence of comorbidities at baseline is likely attributable to the targeted enrollment of overweight and obese breast cancer survivors, which has been previously shown to result in higher Charlson comorbidity index scores among breast cancer survivors [4].
As with all population-based research, this trial had limitations that need to be considered when interpreting these findings. Overall completion of the study was high (85%) [26] and there was no difference in mean number of baseline comorbidities between women who did versus did not complete the 24-month study visit. Nevertheless, this study recruited primarily educated, non-Hispanic white women with early stage breast cancer who were overweight or obese; thus, these findings can only be generalized to this population. Due to a limited number of racial and ethnic minorities in the current trial, possible difference by race and ethnicity could not be fully evaluated. Medications and changes to medications were self-reported; however, the dosages of medications were not collected and thus, we were unable to evaluate any possible changes in the dosage of medications associated with the intervention. Finally, the data on new medical conditions were self-reported followed by medical record confirmation for cardiovascular disease and new cancers.
In conclusion, modest weight loss demonstrated in the ENERGY behavioral weight loss intervention reduced incident comorbidities following an intensive weight loss intervention. These results support the effort to prescribe and encourage healthy weight management efforts for overweight and obese breast cancer survivors, and highlight the need to provide continued support so that weight loss and health benefits are maintained in the long term.
Supplementary Material
Acknowledgments
This study was supported by NCI grant CA148791. Management of stored biological samples at the UCSD Coordinating Center was facilitated by the Diet and Physical Activity Shared Resource of the Moores UCSD Cancer Center (NCI Cancer Center Support Grant CA23100). The Colorado Clinical Translational Sciences Institute grant (NIH CTSI Grant TR001082) supported study activities at the University of Colorado.
This publication also was made possible by grant number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or NIH. We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri, for the use of the Tissue Procurement Core, which provided sample storage and processing (supported in part by an NCI Cancer Center Support Grant CA91842).
The authors thank the data and safety monitoring committee: Bernard Rosner, PhD, Joanne Mortimer, MD, Ken Fujioka MD, Frank Greenway, MD, and Linda Litzau. The authors thank Catherine Alfano, PhD (Program Officer) and Julia Rowland, PhD, NIH Office of Cancer Survivorship, and also Robert Croyle, PhD, NIH Division of Cancer Control and Population Sciences, for their assistance, guidance and support.
Appendix
The ENERGY Trial Group
University of California, San Diego: Cheryl L. Rock, PhD, RD, Bilge Pakiz, EdD, Barbara A. Parker, MD, Christine Zoumas, MS, RD, Shirley W. Flatt, MS, Hava Shoshana Barkai, MS, RD, Dennis D. Heath, MS, Lea Jacinto, Mila Pruitt.
University of California, Los Angeles: Patricia A. Ganz, MD.
University of Colorado Denver: Tim Byers, MD, MPH, Rebecca Sedjo, PhD, Holly Wyatt, MD, Anthony Elias, MD, James Hill, PhD, Kim Gorman, MS, RD, Carmen Faust, MPH, Jhenny Hernandez, MBA, Anna Van Pelt, MPH.
Washington University in St. Louis: Graham Colditz, MD, Kathleen Wolin, ScD, Jingxia Liu, PhD, Michael Naughton, MD, Casey Fagin, MA, Jennifer Tappenden, Sonya Izadi.
University of Alabama at Birmingham: Wendy Demark-Wahnefried, PhD, RD, Helen Krontiras, MD, Maria Azrad, PhD, RD, Cindy Blair, PhD, Lahnor Powell, DO, Laura Lee Goree, MS, RD.
Footnotes
Clinical Trial Number: NCT01112839 on clinicaltrials.gov
References
- 1.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst 20. 2011;103(14):1101–1111. doi: 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nechuta S, Lu W, Zheng Y, Cai H, Bao PP, Gu K, Zheng W, Shu XO. Comorbidities and breast cancer survival: a report from the Shanghai Breast Cancer Survival Study. Breast Cancer Res Treat. 2013;139:227–235. doi: 10.1007/s10549-013-2521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braithwaite D, Moore DH, Satariano WA, Kwan ML, Hiatt RA, Kroenke C, Caan BJ. Prognostic impact of comorbidity among long-term breast cancer survivors: results from the LACE study. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1115–1125. doi: 10.1158/1055-9965.EPI-11-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leach CR, Weaver KE, Aziz NM, Alfano CM, Bellizzi KM, Kent EE, Forsythe LP, Rowland JH. The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. J Cancer Surv. 2015;9(2):239–251. doi: 10.1007/s11764-014-0403-1. [DOI] [PubMed] [Google Scholar]
- 6.Ording AG, Boffetta P, Garne JP, Nyström PM, Cronin-Fenton D, Frøsley T, Sillliman R, Sørensen HT, Lash TL. Relative mortality rates from incident chronic diseases among breast cancer survivors - A 14year follow-up of five-year survivors diagnosed in Denmark between 1994 and 2007. Eur J Cancer. 2015;51(6):767–775. doi: 10.1016/j.ejca.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Wu AH, Kurian AW, Kwan ML, John EM, Lu Y, Keegan TH, Gomez SL, Cheng I, Shariff-Marco S, Caan BJ, Lee VS, Sullivan-Halley J, Tseng CC, Bernstein L, Sposto R, Vigen C. Diabetes and other comorbidities in breast cancer survival by race/ethnicity: the California Breast Cancer Survivorship Consortium (CBCSC) Cancer Epidemiol Biomarkers Prev. 2015;24(2):361–368. doi: 10.1158/1055-9965.EPI-14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patterson RE, Flatt SW, Saquib N, Rock CL, Caan BJ, Parker BA, Laughlin GA, Erickson K, Thomson CA, Bardwell WA, Hajek RA, Pierce JP. Medical comorbidities predict mortality in women with a history of early stage breast cancer. Breast Cancer Res Treat. 2010;122:859–865. doi: 10.1007/s10549-010-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 20. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pakiz B, Ganz PA, Sedjo RL, Flatt SW, Demark-Wahnefried W, Liu J, Wolin KY, Rock CL. Correlates of quality of life in overweight or obese breast cancer survivors at enrollment into a weight loss trial. Psychooncology. 2015 doi: 10.1002/pon.3820. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malnick SD, Knobler H. The medical complications of obesity. QJM. 2006;99(9):565–579. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- 12.Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118(Suppl. 8):2277–2287. doi: 10.1002/cncr.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 16. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 14.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 15.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12:282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 16.Su HI, Sammel MD, Springer E, Freeman EW, DeMichele A, Mao JJ. Weight gain is associated with increased risk of hot flashes in breast cancer survivors on aromatase inhibitors. Breast Cancer Res Treat. 2010;124:205–211. doi: 10.1007/s10549-010-0802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caan BJ, Emond JA, Su HI, Flatt SW, Gold EB, Newman VA, Rock CL, Thomson CA, Pierce JP. Effect of postdiagnosis weight change on hot flash status among early-stage breast cancer survivors. J Clin Oncol. 2012;30:1492–1497. doi: 10.1200/JCO.2011.36.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsythe LP, Alfano CM, George SM, McTiernan A, Baumgartner KB, Bernstein L, Ballard-Barbash R. Pain in long-term breast cancer survivors: the role of body mass index, physical activity, and sedentary behavior. Breast Cancer Res Treat. 2013;137:617–630. doi: 10.1007/s10549-012-2335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imayama I, Alfano CM, Neuhouser ML, George SM, Wilder Smith A, Baumgartner RN, Baumgartner KB, Bernstein L, Wang CY, Duggan C, Ballard-Barbash R, McTiernan A. Weight, inflammation, cancer-related symptoms and health related quality of life among breast cancer survivors. Breast Cancer Res Treat. 2013;140:159–176. doi: 10.1007/s10549-013-2594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetes Prevention Program Research Group. 10–year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esposito K, Maiorino MI, Petrizzo M, Bellastella G, Giugliano D. Remission of type 2 diabetes: is bariatric surgery ready for prime time? Endocrine. 2015;48(2):417–421. doi: 10.1007/s12020-014-0463-z. [DOI] [PubMed] [Google Scholar]
- 23.Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, Pownall HJ, Johnson KC, Safford MM, Kitabchi AE, Pi-Sunyer FX, Wing RR, Bertoni AG Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308(23):2489–2496. doi: 10.1001/jama.2012.67929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redmon JB, Bertoni AG, Connelly S, Feeney PA, Glasser SP, Glick H, Greenway F, Hesson LA, Lawlor MS, Montez M, Montgomery B Look AHEAD Research Group. Effect of the look AHEAD study intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care. 2010;33(6):1153–1158. doi: 10.2337/dc09-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espeland MA, Glick HA, Bertoni A, Brancati FL, Bray GA, Clark JM, Curtis JM, Egan C, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Hazuda HP, Hill JO, Hire D, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Killean T, Kitabchi AE, Knowler WC, Kriska A, Lewis CE, Miller M, Montez MG, Murillo A, Nathan DM, Nyenwe E, Patricio J, Peters AL, Pi-Sunyer X, Pownall H, Redmon JB, Rushing J, Ryan DH, Safford M, Tsai AG, Wadden TA, Wing RR, Yanovski SZ, Zhang P Look AHEAD Research Group. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014;37(9):2548–2556. doi: 10.2337/dc14-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rock CL, Flatt SW, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PA, Wolin KY, Elias A, Krontiras H, Liu J, Naughton M, Pakiz B, Parker BA, Sedjo RL, Wyatt H. Results of the Exercise and Nutrition to Enhance Recovery and Good health for You (ENERGY) trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol. 2015;33(28):3169–3176. doi: 10.1200/JCO.2015.61.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rock CL, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PA, Wolin KY, Elias A, Krontiras H, Liu J, Naughton M, Pakiz B, Parker BA, Sedjo RL, Wyatt H Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial Group. Reducing breast cancer recurrence with weight loss, a vanguard trial: the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial. Contemp Clin Trials. 2013;34(2):282–295. doi: 10.1016/j.cct.2012.12.003. Epub 2012 Dec 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallis JF, Calfas KJ, Alcaraz JE, Gehrman C, Johnson MF. Potential mediators of change in a physical activity promotion course for university students: Project GRAD. Ann Behav Med. 1999;212:149–158. doi: 10.1007/BF02908296. [DOI] [PubMed] [Google Scholar]
- 29.Bandura A. Social learning theory. Englewood Cliffs: Prentice-Hall; 1977. [Google Scholar]
- 30.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 31.Bandura A. Self-efficacy: the exercise of self-control. New York: W.H. Freeman; 1997. [Google Scholar]
- 32.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 33.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, Pownall HJ, Johnson KC, Safford MM, Kitabchi AE, Pi-Sunyer FX, Wing RR, Bertoni AG Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308(23):2489–2496. doi: 10.1001/jama.2012.67929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redmon JB, Bertoni AG, Connelly S, Feeney PA, Glasser SP, Glick H, Greenway F, Hesson LA, Lawlor MS, Montez M, Montgomery B Look AHEAD Research Group. Effect of the look AHEAD study intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care. 2010;33(6):1153–1158. doi: 10.2337/dc09-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espeland MA, Glick HA, Bertoni A, Brancati FL, Bray GA, Clark JM, Curtis JM, Egan C, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Hazuda HP, Hill JO, Hire D, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Killean T, Kitabchi AE, Knowler WC, Kriska A, Lewis CE, Miller M, Montez MG, Murillo A, Nathan DM, Nyenwe E, Patricio J, Peters AL, Pi-Sunyer X, Pownall H, Redmon JB, Rushing J, Ryan DH, Safford M, Tsai AG, Wadden TA, Wing RR, Yanovski SZ, Zhang P Look AHEAD Research Group. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014;37(9):2548–2556. doi: 10.2337/dc14-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietz WH, Baur LA, Hall K, Puhl RM, Taveras EM, Uauy R, Kopelman P. Management of obesity: improvement of health-care training and systems for prevention and care. Lancet. 2015;385(9986):2521–2533. doi: 10.1016/S0140-6736(14)61748-7. [DOI] [PubMed] [Google Scholar]
- 40.American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel, 2013. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity (Silver Spring) 2014;22(Suppl 2):S5–S39. doi: 10.1002/oby.20821. [DOI] [PubMed] [Google Scholar]
- 41.Barte JC, ter Bogt NC, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, Bemelmans WJ. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev. 2010;11:899–906. doi: 10.1111/j.1467-789X.2010.00740.x. [DOI] [PubMed] [Google Scholar]
- 42.Sarwer DB, von Sydow Green A, Vetter ML, Wadden TA. Behavior therapy for obesity: Where are we now? Curr Opin Endocrinol Diabetes Obes. 2009;16:347–352. doi: 10.1097/MED.0b013e32832f5a79. [DOI] [PubMed] [Google Scholar]
- 43.Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, Hill DR. Long-term maintenance of weight loss: Current status. Health Psychol. 2000;19(suppl 1):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 44.Middleton KM, Patidar SM, Perri MG. The impact of extended care on the long-term maintenance of weight loss: A systematic review and meta-analysis. Obes Rev. 2012;13:509–517. doi: 10.1111/j.1467-789X.2011.00972.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.