Abstract
Objective
To define the clinical features and biomarkers that predict which patients with pure autonomic failure will develop Parkinson disease, dementia with Lewy bodies, or multiple system atrophy.
Methods
One hundred patients who presented with pure autonomic failure were recruited at 5 medical centers in the U.S. Seventy-four patients agreed to be followed prospectively. Patients underwent clinical evaluations including neurological rating scales, sleep questionnaires, smell test, and sympathetic and parasympathetic cardiovascular autonomic function tests.
Results
At enrollment, patients were 68(12) years old [(median (interquartile range)] and had had autonomic failure for 5(7) years. Within 4-years of follow-up, 25 of 74 subjects (34%) developed dementia with Lewy bodies (in 13), Parkinson disease (in 6), or multiple system atrophy (in 6). The presence of probable REM sleep behavior disorder was strongly associated with the development of a manifest CNS synucleinopathy (odds ratio=7.1). Patients who phenoconverted to multiple system atrophy had younger age at onset of autonomic failure, severe bladder/bowel dysfunction, preserved olfaction, and a cardiac chronotrophic response upon tilt >10 beats per minute. Those who phenoconverted to Parkinson disease or dementia with Lewy bodies had decreased olfaction, a lesser chronotrophic response to tilt, and a longer duration of illness. The small group of patients retaining the pure autonomic failure phenotype had very low plasma norepinephrine levels, slow resting heart rate, no REM sleep behavior disorder, and preserved smell.
Interpretation
Patients presenting with pure autonomic failure are at high risk of phenoconverting to a manifest CNS synucleinopathy. Specific clinical features predict future diagnosis.
Introduction
Synucleinopathies are a group of neurodegenerative disorders caused by the accumulation of misfolded α-synuclein (α-Syn) in neurons or glia or both in the central and peripheral autonomic nervous system. Experimental evidence is now accumulating to show that α-Syn spreads from cell-to-cell in a “prion-like fashion.”1 The anatomical location of α-Syn aggregation and pattern of progressive neuronal death gives rise to distinct neurological phenotypes.2, 3
Patients with synucleinopathies that present with autonomic failure affecting the cardiovascular system and symptomatic orthostatic hypotension as the main clinical feature receive a diagnosis of pure autonomic failure (PAF).4 After a period of time, in some patients, motor or cognitive deficits emerge indicating that additional neuronal populations in the central nervous system (CNS) are affected, and patients are diagnosed with Parkinson disease (PD), dementia with Lewy bodies (DLB), or multiple system atrophy (MSA).5, 6 Whether PAF is a specific synucleinopathy mostly restricted to autonomic neurons or is always an early premotor or precognitive phase of a widespread CNS disorder is unknown.
PAF was first described by Bradbury and Eggleston in 1925. Their original report featured 3 middle-aged men with orthostatic hypotension, a slow unchanging pulse rate, constipation, decreased sweating, and erectile dysfunction.7 The authors suspected that their subjects' symptoms were the result of chronic failure of the autonomic nervous system. Since then a number of clinical and neuropathological studies of similar patients have been reported, all showing degeneration of peripheral autonomic neurons and the aggregation of α-Syn in Lewy bodies within sympathetic ganglia and Lewy neurites along autonomic axons in the heart, periadrenal tissue, bladder, skin, and colon.8-13 Sparse Lewy bodies were also found in the substantia nigra and locus coeruleus but without neuronal loss, which explained the lack of motor abnormalities.8, 10, 14-17. This pathological hallmark tied PAF to PD and DLB leading to its classification as a synucleinopathy without manifest CNS involvement.
It is well known that some patients with PAF will develop PD, DLB or MSA, but phenoconversion rates from PAF to a manifest CNS synucleinopathy are lacking. In the general population, hyposmia, and rapid eye movement (REM) sleep behavior disorder (RBD) have been linked to α-Syn-driven neurodegeneration,18 and carry an increased risk for a future diagnosis of PD or DLB.19, 20 The prognostic implications of autonomic failure combined with these established risk factors are unknown and predictors of phenoconversion to MSA have yet to be determined.
We conducted a multicenter prospective study of patients presenting with PAF to define the clinical characteristics of a national cohort, determine phenoconversion rates to PD, DLB and MSA, and identify the clinical features and biomarkers that may predict phenoconversion.
Methods
Subjects
Participants were recruited through the Autonomic Disorders Consortium, a multicenter collaborative study supported by the National Institutes for Health (NIH) Rare Disease Clinical Research Network (RDCRN). Subjects were enrolled in a prospective longitudinal observational natural history study at 5 U.S. medical Centers: New York University Medical Center (New York, NY), Vanderbilt University (Nashville, TN), Mayo Clinic (Rochester, MN), NIH Intramural Research Program (Bethesda, MD), and Beth Israel Deaconess Medical Center (Boston, MA). Sites were selected for their expertise in autonomic research in neurological diseases. The protocol was developed with NIH program directors within the RDCRN. Approval for the study procedures was obtained from each local Institutional Review Board and informed consent was obtained in all cases. To standardize autonomic testing and data acquisition across sites, a manual of operations describing the study procedures was developed. Training videos developed by the Consortium were made available to ensure standardization of the clinical assessments and neurological rating scales. Source data were collected in custom-designed case report forms and captured electronically using a secure Research Electronic Data Capture (RedCap) platform.
Subjects were recruited from September 2011 to September 2015. As inclusion criteria, patients with autonomic failure were required to have neurogenic orthostatic hypotension defined as (i) a fall in blood pressure (BP) of 20 mmHg systolic or 10 mmHg diastolic within the first 3 minutes of upright tilt,21 and (ii) absence of phase IV BP overshoot after release of the Valsalva strain, consistent with sympathetic (autonomic) failure and a neurogenic cause.22 The main exclusion criteria were (i) fulfillment of diagnostic clinical criteria for PD,23 MSA,24 DLB,25 or other neurodegenerative disorders; (ii) presence of a peripheral neuropathy, amyloidosis, diabetes or autoimmune disease, including seropositivity for antibodies against the ganglionic acetylcholine receptor; (iii) other secondary causes of orthostatic hypotension including medications, dehydration, or severe anemia. Evaluations took place at 12-month intervals.
Study assessments
Autonomic function tests
At each study visit, subjects underwent a standard battery of autonomic function tests, in a quiet, temperature controlled room. Participants were free of caffeine, alcohol and nicotine from the previous evening. An intravenous forearm catheter was inserted into the antecubital vein and subjects were transferred to the tilt table for instrumentation. Continuous electrocardiographic RR intervals were recorded from 3 precordial electrodes. Beat-to beat BP was measured with finger plethysmography with the hand supported at heart level. BP was also measured at 1-minute intervals with a validated automated cuff sphygmomanometer over the brachial artery. All signals were acquired and digitized and sampled at a minimum rate of 500 Hz.
As a measure of parasympathetic function, subjects were verbally coached to breathe at 6 cycles per minute, and respiratory sinus arrhythmia during deep paced breathing was calculated from the average of the 3 longest RR intervals during expiration divided by the average the 3 shortest RR intervals during inspiration (i.e., expiratory: inspiratory ratio).22 A 300-second segment of spontaneous breathing in the supine position was selected and processed to detect RR intervals. Heart rate variability in the time- and frequency-domain were measured following standards.26
Subjects performed a standardized Valsalva maneuver, maintaining an expiratory pressure of >30-mmHg for at least 10 seconds. If BP was not higher than baseline within 10 seconds after release of the Valsalva strain (i.e., phase IV), the overshoot in BP was considered absent, indicative of sympathetic (autonomic) failure. Pressure recovery time was measured as the time (in seconds) taken for the systolic BP to return to baseline values after release of the strain.27
After the subject had fully recovered, BP and RR intervals were acquired for a further 10 minutes. While still supine, venous blood was sampled through the indwelling catheter. Subjects were then tilted upright to an angle of 60-degrees and instructed to remain immobile. After 10 minutes upright, a second set of blood samples were acquired to assay plasma norepinephrine levels using high-performance liquid chromatography with electrochemical detection. Patients also underwent a complete blood count, comprehensive metabolic panel, and urinalysis.
Neurological assessments
All patients had a full neurological evaluation captured in case report forms. In addition, to monitor the emergence of motor/cognitive deficits all patients were evaluated with disease-specific rating scales for PD and MSA, including Hoehn and Yahr,28 and Unified Multiple System Atrophy Rating Scale (UMSARS).29 Particular attention was paid to the presence of subtle motor abnormalities including mild generalized slowness/bradykinesia, decreased blinking frequency or reduced facial expressions (minimal hypomimia), reduced unilateral arm-swing when walking, and mild slowing/reduction in amplitude in rapid alternating movements.
Cognitive impairment was assessed during a clinical interview and rated with the mini-mental state examination (MMSE). If the participant was suspected to have worsening cognitive function on follow-up, formal neuropsychological testing was performed.
Sleep disturbances
Sleep-disordered breathing and probable REM sleep behavior disorder (RBD) was assessed during a clinical interview with the bed partner and/or via questionnaires.30, 31 Subjects were considered to have probable RBD if they answered yes to acting out dreams, shouting/yelling or swearing during sleep, having violent behaviors or hurt themselves or a bed partner while sleeping. Some patients also underwent polysomnography. The polysomnography diagnosis of sleep apnea and REM sleep behavior disorder was done according to the AASM International Classification of Sleep Disorders (2nd Edition).
Olfactory function
Participants were first asked if they had noticed any changes in their sense of smell. The English-language version of the University of Pennsylvania Smell Identification Test (UPSIT)32 was administered in a controlled well-ventilated setting.
Autonomic symptom assessment and disability
The severity of symptoms of orthostatic hypotension and impact on activities of daily living was assessed using the OH-questionnaire, a 10-item patient reported outcome.33 Symptoms of generalized autonomic dysfunction were assessed using the Composite Autonomic Symptom Scale (COMPASS), with 46-questions addressing orthostatic, vasomotor, sudomotor, pupillomotor, urinary, and sleep function.34 The degree of disability was rated using the UMSARS disability domain (part IV).29
Diagnostic outcomes
At each 12-month interval follow-up visit, the diagnosis of the subject was re-evaluated to determine whether he/she retained an pure autonomic failure phenotype or had developed clinical evidence of PD, DLB or MSA according to current consensus criteria.23-25 The diagnosis of PAF remained if there was persistence of autonomic failure, in the absence of significant motor or cognitive deficits.4
Data analysis and definitions
All data was reviewed, verified and audited by the RDCRN's designated Data Monitoring and Coordinating Center (DMCC). Outlying data points were flagged and discussed with investigators through email and monthly conference calls. Categorical variables were compared using the Chi-squared test. For comparison of quantitative dependent variables appropriate parametric tests were used (see table legends). Symptom onset was defined as the date the patient first noticed symptoms of orthostatic hypotension (e.g., dizziness, light-headedness, feeling about to faint, or syncope when upright). Hypertension was defined as BP >140/90 mmHg35 and bradycardia defined as a heart rate <60 bpm. Anemia was defined as hemoglobin less than 13 g/dl in men, and less than 12 g/dl in women according to the World Heath Organization (WHO) criteria.36 Cardiac baroreflex gain was assessed during the Valsalva maneuver and determined by calculating the slope of the regression line relating changes in systolic BP against RR intervals and expressed as ms/mmHg, as described.37 The risk of developing a manifest CNS synucleinopathy in the 74 subjects from the cohort was estimated with the Kaplan–Meier method. Disease-free survival rate was assessed from the date of initial orthostatic symptoms to the date of the diagnosis of manifest CNS synucleinopathy or to the last follow-up visit for censored observations (subjects who died or were lost to follow-up). Several markers were examined to determine the odds ratio of developing a manifest CNS synucleinopathy. Missing data were managed using multiple imputation. Analysis using this method has been shown to provide less biased estimates of associations than the use of complete data only or other methods such as mean imputation.38 Data were analyzed with SPSS 19.0 (SPSS, Chicago, IL, USA) and Prism (Graph-Pad Software, Inc., La Jolla, CA, USA). Data are expressed in median (interquartile range, IQR) unless otherwise specified.
Role of the funding source
Funds from the National Institutes of Health (NIH) supported the U.S. Autonomic Disorders Consortium. Members of the Consortium were involved in the study design, conduct, database development, and data collection. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Patient characteristics at entry
One hundred consecutive patients met inclusion criteria and completed the clinical evaluations at entry into the natural history study (Supplementary Table 1). A significant majority of subjects were males (70% vs. 30%, Chi-square p=0.0038). Symptoms of neurogenic orthostatic hypotension began at age 63 (14) years. At the time of enrollment, median duration of illness was 5 (7) years. Symptoms of orthostatic hypotension were the main complaint at the time of enrollment: 97% of patients reported having had symptoms of orthostatic hypotension within the last week on the OH-questionnaire.33 Additional symptoms of autonomic impairment included: constipation (58%), bladder disturbances (50%), sweating abnormalities (44%), and erectile dysfunction in men (65%). The most commonly prescribed medications were: midodrine (64%), fludrocortisone (45%), and pyridostigmine (11%).
Autonomic function testing confirmed that all patients met criteria for autonomic failure.21 Resting supine BP was 152/84 mmHg (IQR: 15/10) and the prevalence of supine hypertension was 47%. Supine heart rate was 65(5) bpm, with 13% of patients having resting bradycardia (<60 bpm). After 3-minutes of upright tilt, BP fell by -52(27) mmHg systolic and -23(23) mmHg diastolic; and standing BP was 93/58 mmHg (IQR: 34/23). BP continued to fall with prolonged tilt.
Markers of sympathetic function
At the end of the straining phase of the Valsalva maneuver, BP had fallen by -62(26) mmHg systolic and -17(12) mmHg diastolic. Late phase II rebound was absent in all patients. Despite the pronounced fall in BP, heart rate increased by only 8(8) bpm. Cardiac baroreflex gain (estimated by the slope of the relationship between changes in BP and RR intervals) was 1.8(0.2) ms/mmHg, a value significantly lower than in normal controls.39 The phase IV overshoot in BP was absent in all cases. Ten seconds after release of the strain, systolic BP remained 29(24) mmHg lower than at baseline. The latency of the Valsalva pressure recovery time was 32(25) seconds, a value much longer than in normal subjects.40 These markers are consistent with impaired baroreflex-mediated sympathetic activation.
On average, plasma norepinephrine levels in the supine position were lower than expected for age at 101(97) pg/ml and the increase in plasma norepinephrine levels after head up tilt was blunted [54%, 191(218) pg/ml], findings consistent with reduced sympathetic activity.22 A subset of patients had norepinephrine levels that remained below 100 pg/ml both supine and upright (n=18).
Markers of parasympathetic function
Seven patients had a cardiac pacemaker and 80% had normal sinus rhythm allowing parasympathetic function to be determined. During deep paced breathing, shortest RR intervals during inspiration were 855(136) ms and longest RR intervals during expiration were 937(152) ms, resulting in an E:I ratio of 1.07(1.12); 71% of patients had an E:I ratio that was below the value expected for age. Spectral measures of heart rate variability in the high frequency (HF) range during a 300-second segment of spontaneous breathing were 58(81) ms2. Measures of heart rate variability in the time domain were also reduced: rMSSD was 16(12) ms and the percentage of neighbouring RR intervals that differed by 50 ms or more (pNN50) was only 13(21)%. Taken together, these multiple markers of parasympathetic (vagal) influences on the sinoatrial node induced by respiration were universally reduced in all patients.
Clinical laboratory findings
Clinical laboratory tests were consistent with mild anemia in 47% of patients. Median hemoglobin was 13.1(1.7) mg/dl and hematocrit was 39(5) %. Serum creatinine concentration was 1.15(0.38) mg/dl with a BUN of 21(9) mg/dl. Proteinuria was present in 25% (19 out of 75) of subjects. Proteinuria was graded as mild in 20% of patients (10-20 mg/dl), moderate in 3% (1-30 mg/dl) and severe in 1% (30-100 mg/dl).
Neurological features
On entry, none of the patients had signs of cognitive impairment. Mini-mental status examination score was normal [29(1) points]. Hoehn and Yahr motor assessments were rated at 0 with no signs of disease and investigators rated 85% of patients as completely independent on UMSARS part IV. While none of the patients met clinical diagnostic criteria for PD, MSA or DLB, close review of the disease-specific rating scales revealed the presence of subtle non-specific neurological deficits. These included mild generalized slowness/bradykinesia (12%), minimal hypomimia or reduced blinking frequency (26%), reduced unilateral arm-swing when walking (12%) and mild slowing/reduction in amplitude in rapid alternating movements (22%). None of the patients had resting tremor or rigidity.
Sleep disturbances
Overall, 24% of patients reported difficulty falling asleep. Ninety-seven percent of patients had difficultly remaining asleep because of nocturia, which awoke them 3(1) times during the night. The prevalence of probable RBD at study entry was 72%. During sleep, 56% of patients shouted, yelled or swore, 33% confessed to have either hurt themselves or their bed partner while sleeping and 32% reported having fallen out of bed at night. Sixteen percent of patients had a diagnosis of sleep apnea.
Olfactory function
Only 4 patients subjectively complained of lack of smell. However, only 19% of patients had normal olfactory testing (UPSIT >34 points); whereas 8% had mild microsmia (UPSIT: 30-33 points), 6% had moderate microsmia (UPSIT: 26-29 points), 23% had severe microsmia (UPSIT: 19-25), and 44% had total anosmia (UPSIT <18).
Outcomes at follow-up
Seventy-four patients agreed to be followed longitudinally (Figure 1). Age, gender distribution and clinical features of these 74 patients were similar to the 26 that declined to be followed up, suggesting a random selection.
Figure 1. Outcomes of the natural history of pure autonomic failure.
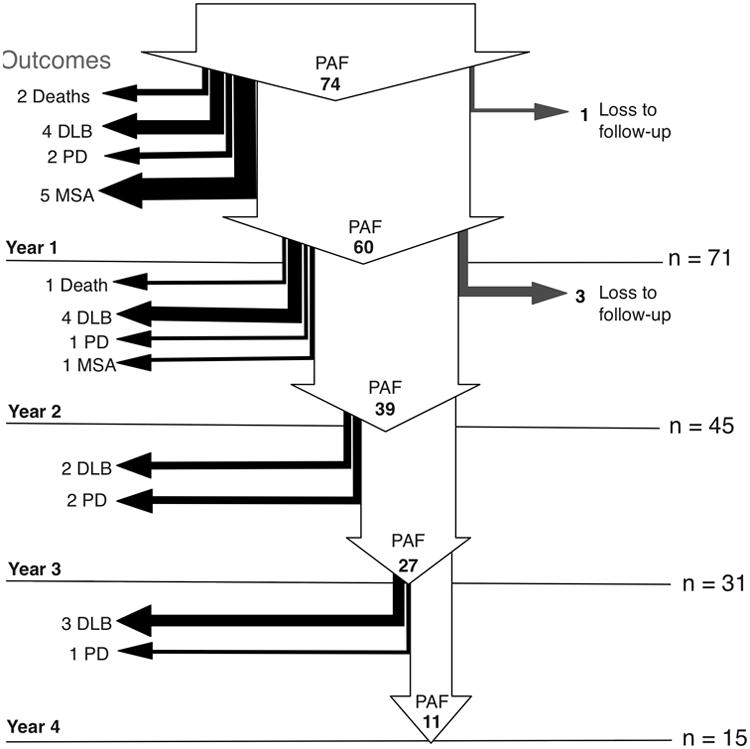
Arrows describe subject flow through the natural history study. Endpoint outcomes are listed on the left. 74 patients met eligibility criteria and agreed to follow-up longitudinal evaluations. At the time of writing, follow-up data of 1-year or more was available in 96% of patients (n=71). The first year saw 39 of patients continue with autonomic failure as the only clinical feature, and 12-patients convert to full synucleinopathy. By year two (n=45), only 39 remained with pure autonomic failure and 6 patients converted to a full synucleinopathy. By year 3 (n=31), Only 27 remained with pure autonomic failure and another 4 converted to a Lewy body disorder. In year 4 (n=15), 11 remain as pure autonomic failure Lost to follow-up rates in participants that agreed to longitudinal visits range from 1 to 3 patients/year. This 4-year prospective study suggests a >10% cumulative risk of conversion to full synucleinopathy per year. DLB, dementia with Lewy bodies; PD, Parkinson disease; PAF, pure autonomic failure.
Of the 74 patients who agreed to the yearly assessments, 2 died, and 1 declined to continue in the study. Therefore, 1-year follow-up data was available in 71 cases (96%). Of these 71, 11 patients were diagnosed with a manifest CNS synucleinopathy: 2 with PD, 4 with DLB, and 5 with MSA. This represents a 15% rate of phenoconversion.
Of the 60 remaining patients, 3 declined to continue in the study, and 12 were not yet due for the their 2-year visit. Therefore, 2-year data was available in 45 cases (60%). At that time, 6 more cases had been diagnosed with a manifest CNS synucleinopathy: 1 with PD, 4 with DLB and 1 with MSA. This represents a 13% rate of phenoconversion.
Of the 40 remaining patients, 1 died, and 8 were not yet due for the 3-year visit. Therefore, 3-year follow-up data was available in 31 cases (42%). Of these, 4 patients were diagnosed with a manifest CNS synucleinopathy: 2 with PD and 2 with DLB, representing a 13% phenoconversion rate.
Finally, of the 27 remaining patients, 12 were not due for their 4-year follow-up visit. Therefore, 4-year follow-up data was available in 15 cases (20%). Of these, 4 additional cases had developed a manifest CNS synucleinopathy: 1 with PD and 3 with DLB, representing a 27% phenoconversion rate.
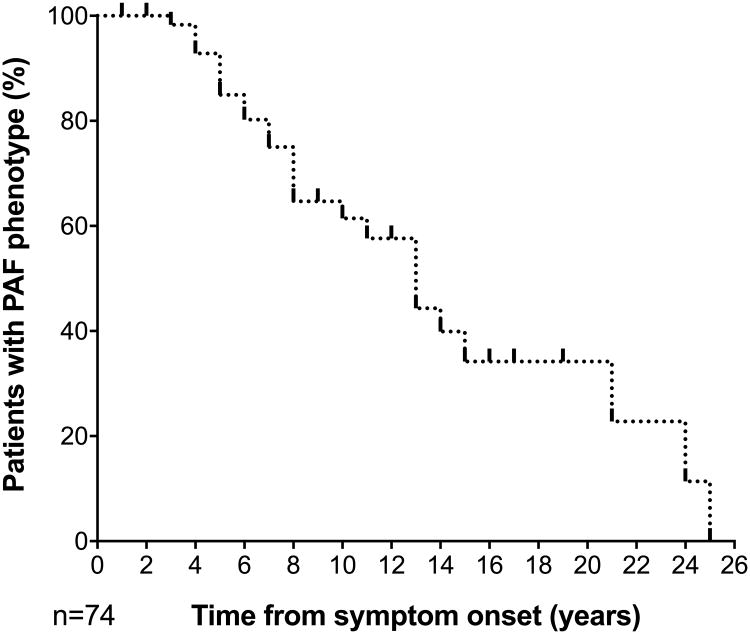
The cumulative incidence of phenoconversion during the 4-year period was 34%. Figure 2 shows a Kaplan Meier survival curve from the time of first symptom of neurogenic orthostatic hypotension to the time of diagnosis of a manifest synucleinopathy. The clinical and autonomic features of the 25 patients who phenoconverted to a manifest CNS synucleinopathy are detailed in Table 1.
Figure 2. CNS manifest synucleinopathy risk in patients with PAF.
Rates of conversion to manifest CNS synucleinopathy according to the time of onset of neurogenic orthostatic hypotension symptoms in the 74 patients from the cohort.
Table 1.
Age at onset, duration of PAF phase, and entry visit smell, REM behaviour disorder, urinary/GI symptoms, plasma norepinephrine, and heart rate in patients later diagnosed with a manifest synucleinopathy.
| # Age at onset1 /sex | PAF phase2 yrs | UPSIT score | RBD | Urinary Sx3 | GI Sx4 | NE pg/ml | HR bpm | ΔHR bpm |
|---|---|---|---|---|---|---|---|---|
| 1. 66/F | 5 | 14 | + | + | + | 134 | 65 | +5 |
|
| ||||||||
| 2. 73/M | 7 | 9 | + | + | + | 226 | 60 | +3 |
| 3. 58/M | 15 | NA | - | - | - | NA | 47 | +2 |
| 4. 49/M | 25 | NA | + | + | + | NA | 60 | 0 |
| 5. 52/M | 25 | NA | + | + + | - | NA | 64 | +10 |
| 6. 64/M | 11 | 12 | + | - | - | 162 | 66 | -1 |
|
| ||||||||
| PD: 60 (9) | 14 (9) | 11 (3) | 83% | + | + | 174 (47) | 60 (7) | 3 (4) |
|
| ||||||||
| 7. 75/M | 5 | 23 | + | + | + | 97 | 59 | +6 |
| 8. 65/F | 14 | 11 | + | - | + | 32 | 64 | 0 |
| 9. 60/F | 21 | NA | + | + | - | NA | 72 | -2 |
| 10. 72/F | 5 | NA | + | + | + | 99 | 69 | +9 |
| 11. 77/M | 6 | 11 | + | + | + | 218 | 62 | +8 |
| 12. 72/F | 8 | 23 | + | - | - | 118 | 54 | +9 |
| 13. 63/M | 8 | 11 | + | + | - | 85 | 67 | +6 |
| 14. 75/F | 5 | 23 | + | + | + + | 166 | 75 | +10 |
| 15. 54/M | 13 | 23 | + | - | - | NA | 47 | +3 |
| 16. 64/M | 13 | 29 | + | + + | - | NA | 62 | +5 |
| 17. 63/M | 13 | 23 | + | + | + + | 114 | 78 | 0 |
| 18. 71/M | 7 | 26 | + | + | + | NA | 60 | +11 |
| 19. 54/M | 10 | NA | + | + | + | NA | 83 | +10 |
|
| ||||||||
| DLB: 66 (7) | 10 (5) | 20 (7) | 100% | + | + | 116 (56) | 66 (10) | 6 (4) |
|
| ||||||||
| 20. 45/M | 4 | 37 | + | + + | + + + | 133 | 71 | +15 |
| 21. 64/M | 4 | 37 | + | + + | + + + | 379 | 71 | +11 |
| 22. 46/F | 3 | 33 | + | + + | + + + | 464 | 76 | +10 |
| 23. 57/M | 6 | NA | + | + + | + + | NA | 73 | +11 |
| 24. 47/M | 8 | NA | + | + + | + + + | NA | 65 | +15 |
| 25. 58/M | 8 | 30 | + | + + | + + | 271 | 69 | +13 |
|
| ||||||||
| MSA: 53 (8) | 6 (2) | 34 (3) | 100% | + + | + + + | 312 (143) | 71 (4) | 13 (2) |
Onset was defined as age at first symptom of orthostatic hypotension.
Duration of PAF phase was defined as time from onset of orthostatic symptoms to diagnosis of manifest CNS synucleinopathy.
Urinary symptoms severity was based on the UMSARS-I score, where ‘-’ = no symptoms; ‘+’ = mild/no medication required; and ‘+ +’ = moderate/requiring medications.
GI symptom severity was rated as constipation score in UMSARS-I, where ‘-’ = no symptoms, + = mild/occasional, no medication required, ‘++’ = moderate/frequent medication required, and ‘+++’ = severe/chronic use of laxatives, enemas, inability to have a spontaneous bowel movement).
UPSIT =University of Pennsylvania Smell Identification Test; RBD=rapid eye movement sleep behavior disorder; NE=norepinephrine; HR=heart rate, NA = not available. All men had erectile dysfunction. Cases 3, 8, and 12 did not have subtle motor signs on entry.
Patients retaining the PAF phenotype
Of the 42 patients who still had a diagnosis of PAF at their last evaluation (12 at the 1-year evaluation, 7 at the 2-year evaluation, 12 at the 3-year evaluation, and 11 at the 4-year evaluation), 30 had additional non-specific features suggesting CNS involvement (i.e., probable RBD or impaired olfaction or subtle motor signs or all). Only 12 subjects with PAF remained completely free of signs suggesting CNS involvement (Table 2). These patients were 57 (12) years old when their symptoms of neurogenic orthostatic hypotension first began, had a disease duration of 6(5) years, and their plasma norepinephrine levels were very low [median 63(37) pg/ml; in all cases <110 pg/ml]. Skin biopsy in one of these cases showed α-Syn deposition in autonomic nerves, as described.41
Table 2. Age at onset, duration of illness, and sense of smell, REM behavior disorder, urinary/GI symptoms, plasma norepinephrine, and heart rate at entry visit in patients who retained a pure autonomic failure phenotype at their last follow-up visit.
| # Age at onset /sex | Duration of illness2 yrs | UPSIT score | RBD | Urinary Sx3 | GI Sx4 | NE pg/ml | HR bpm | ΔHR bpm |
|---|---|---|---|---|---|---|---|---|
| 1. 52/M | 5 | NA | - | + + | + + | NA | 75 | +15 |
| 2. 63/M | 4 | NA | - | + | + | NA | 63 | +18 |
| 3. 53/M | 8 | NA | - | + | + | NA | 73 | 0 |
| 4. 64/F | 3 | 37 | - | + | + | 110 | 69 | +3 |
| 5. 54/M | 2 | 38 | - | + | + | 61 | 67 | +5 |
| 6. 43/M | 14 | 36 | - | - | + | 32 | 69 | +2 |
| 7. 70/F | 5 | 30 | - | - | + | 78 | 76 | +8 |
| 8. 77/F | 1 | 30 | - | - | + | 66 | 68 | +3 |
| 9. 60/F | 6 | 30 | - | + | - | 101 | 79 | +1 |
| 10. 52/M | 10 | 36 | - | + | - | NA | 62 | +10 |
| 11. 64/M | 12 | 33 | - | + | + + | 38 | 68 | +1 |
| 12. 54/M | 8 | 33 | - | + + | + + | 23 | 64 | 0 |
|
| ||||||||
| 57 (12) | 6 (5) | 33 (6) | 0% | + | + | 63 (37) | 69 (6) | +3 (6) |
Age at onset was defined as age at first symptom of orthostatic hypotension.
Duration of illness was defined as onset to final follow-up visit.
Urinary symptoms severity was based on the UMSARS-I score, where ‘-’ = no symptoms; ‘+’ = mild/no medication required; and ‘+ +’ = moderate/requiring medications.
GI symptom severity wasrated as constipation score in UMSARS-I, where ‘-’ = no symptoms, + = mild/occasional, no medication required, ‘++’ = moderate/frequent medication required, and ‘+++’ = severe/chronic use of laxatives, enemas, inability to have a spontaneous bowel movement).
UPSIT = University of Pennsylvania Smell Identification Test; RBD=rapid eye movement sleep behavior disorder; GI = gastrointestinal; NE=norepinephrine; HR=heart rate, NA = not available. All men had erectile dysfunction. No patient with PAF had subtle motor signs at the final visit.
Clinical predictors of phenoconversion
Phenoconversion to PD or DLB
All patients with PAF that went on to develop manifest PD or DLB had impaired olfactory function. All except for one had probable RBD at study entry. Age at onset of symptomatic orthostatic hypotension was 65 (11) years, and they received a diagnosis of PD/DLB 9.5 (7.5) years later. Constipation and urinary symptoms were mild and occasional, and not requiring treatment (Table 1). Careful review of their neurological examination showed subtle motor signs of CNS involvement emerging (including mild bradykinesia, minimal hypomimia/reduced blinking, mildly reduced unilateral arm swing, and mild slowing/reduction in amplitude in rapid alternating movements) at the entry visit. Supine resting heart rate was 63(7) bpm (in all cases <78 bpm), and the increase in heart rate after 3-min of upright tilt was +5(8) (in all cases <11 bpm). Supine resting plasma norepinephrine levels were 118 (66) pg/ml (in all cases <230 pg/ml).
Phenoconversion to MSA
All patients diagnosed with MSA on follow-up had probable RBD at the time of entry. In contrast to those that developed PD or DLB, they all had preserved olfactory function (UPSIT score >30 in all cases). Compared to patients who phenoconverted to PD/DLB, onset of symptomatic neurogenic orthostatic hypotension in MSA patients occurred at a younger age [52(11) years, p=0.009], and the median time to diagnosis was shorter [5(3.5) years, p=0.017]. Constipation and urinary symptoms were moderate or severe and required treatment. Supine resting heart rate was higher at 71(3) bpm (phenoconverters to MSA vs. PD/DLB; p=0.041), and the increase in heart rate after 3-min of head-up tilt was higher 12(3) bpm (in all cases >10 bpm, phenoconverters to MSA vs. PD/DLB; p=0.0001). Supine resting plasma norepinephrine was 325 (163) pg/ml (in all phenoconverters to MSA >133 pg/ml; vs. PD/DLB phenoconverters p=0.026).
Figure 2 depicts the relationship between olfaction, plasma norepinephrine levels in the supine position, and heart rate response after 3 minutes of head up tilt in each diagnostic group.
Risk prediction for phenoconversion
In patients with PAF, the presence of probable RBD was strongly associated with phenoconversion to a manifest CNS synucleinopathy (odds ratio (OR)=7.1 [95% CI: 1.5-33.5]). The combination of probable RBD and deficits in olfaction (UPSIT <30) predicted phenoconversion to PD/DLB (OR=6.3 [95% CI: 1.3-29]). The combination of probable RBD and preserved olfaction (UPSIT >30) strongly predicted the future phenoconversion to MSA (OR=22.5 [95% CI: 3.8-51]).
Autonomic risk factors were also examined. A supine heart rate >70 bpm combined with a supine plasma norepinephrine level >110 pg/ml was associated with future risk of MSA (OR=18 [95% CI: 1.9-66]). A supine heart rate <70 bpm and heart rate response to tilt <10 bpm was associated with phenoconversion to PD/DLB (OR=4.8 [95% CI: 1.4-16]). The likelihood of retaining the pure autonomic failure phenotype was higher in those with a supine heart rate <70 bpm and supine plasma norepinephrine levels <110 pg/ml (OR=5 [95% CI: 1.6-18]).
Discussion
We found that, within 4 years of enrollment in this prospective natural history study, 34% of patients with PAF phenoconverted to a manifest CNS synucleinopathy, either to DLB (18%), PD (8%), or MSA (8%). Phenoconversion best describes this change in clinical features because it is due to anatomical spread of a pathology that was already present at the time of enrollment. The risk of phenoconversion from PAF to a manifest CNS synucleinopathy was around 14% per year.
Almost all patients who phenoconverted had RBD at the time of enrollment, 88% had subtle motor deficits, and 53% had olfactory loss, all symptoms previously reported to predict phenoconversion in premotor PD cohorts.18, 19, 42-45 Therefore, the presence of RBD, olfactory loss, or subtle motor deficits should be considered as non-supportive features of PAF, because their presence indicate that CNS neurons are already affected, and thus the disease is not exclusively autonomic and is likely to progress. The diagnostic criteria of PAF should be therefore modified to reflect these findings.
A stepwise clinical progression observed in our cohorts and others,46 appears to be a characteristic feature of synucleinopathies, conceivably due to prion-like spreading.1, 2 After the onset of symptomatic neurogenic orthostatic hypotension, it took a median of 5 years to develop the motor deficits leading to the diagnosis of MSA, and nearly twice as long (9.5 years) to diagnose PD or DLB. The post-mortem pathological findings in patients with PAF are very similar to those described in cases of incidental Lewy body disease.47 Whether cases of incidental Lewy body disease had symptoms of autonomic failure during life is unknown.
Of the 42 patients who remained as PAF at their last clinical evaluation, 30 had RBD, impaired olfaction, or subtle motor signs suggesting that synuclein-driven neurodegeneration was already present in their CNS and will likely spread further resulting in phenoconversion. In contrast, 12 of these patients had neither RBD, olfactory loss, nor subtle motor deficits, implying that they have a restricted synucleinopathy affecting only autonomic neurons. In both groups there were more men than women but patients without CNS findings were younger (mid 57 vs. 69 years old). A distinctive feature of this small group was a very low plasma norepinephrine concentration with levels <110 pg/ml in all patients indicating a degree of peripheral sympathetic neuronal degeneration significantly more pronounced than in those that phenoconverted.
RBD and preserved olfaction in a patient with PAF increased the likelihood of a future diagnosis of MSA, whereas olfactory loss increased the odds of developing PD/DLB. Autonomic features were also useful pre-motor diagnostic clues. Plasma norepinephrine levels tended to be lower in patients who phenoconverted to PD/DLB and were higher in those that phenoconverted to probable MSA. Previous studies of heart rate in patients with synucleinopathies have been contradictory.48, 49 In our prospective cohort, we found heart rate to be useful for predicting phenocoversion: a lower resting heart rate and a very reduced heart rate increase of <10 bpm, despite the fall in BP, increased the risk of phenoconverting to PD or DLB; a finding consistent with selective cardiac sympathetic denervation in patients with Lewy body diseases.50, 51 Conversely, a resting heart rate above 70 bpm and a better preserved chronotrophic response to tilt was associated with future risk of phenoconversion to MSA, which is consistent with spared sympathetic innervation to the heart in these patients. Parasympathetic denervation of the heart was a feature of all patients with synucleinopathies, resulting in reduced heart rate variability at respiratory frequencies.
The age at onset of autonomic failure was also helpful to predict future diagnosis. Onset of autonomic failure in the early 50s made the diagnosis of MSA more likely, whereas onset of autonomic failure in the mid 60s made phenoconversion to PD/DLB more likely.
Similar to our findings in patients with autonomic failure, in patients with idiopathic RBD, the presence of orthostatic hypotension or hyposmia increased the risk of phenoconversion to PD or DLB.18, 19, 43-45 Our prospective data supports the recently defined research criteria for prodromal Parkinson disease.52 Until now, studies of predictive biomarkers for the development of MSA were limited to retrospective series.6, 53
Our study has limitations: pathological confirmation is lacking, probable RBD was identified only through questionnaires in most cases, and other cognitive screening instruments may be more sensitive to detect earlier cognitive impairment. The patient cohort was captured 5 years after the onset of autonomic failure, which raises the possibility that the earlier MSA phenoconverters may be under-represented. Neither metaiodobenzylguanidine (MIBG) or dopamine transporter-SPECT were performed.
It is tempting to speculate that patients who remain as PAF may have a neuroprotective advantage that prevents CNS involvement in the pathological process. Alternatively, it may just be a matter of time before they phenoconvert to a manifest CNS synucleinopathy.
Supplementary Material
Figure 3. Relationship between olfaction, plasma norepinephrine levels in the supine position (top) and heart rate response after 3 minutes of head up tilt (bottom).
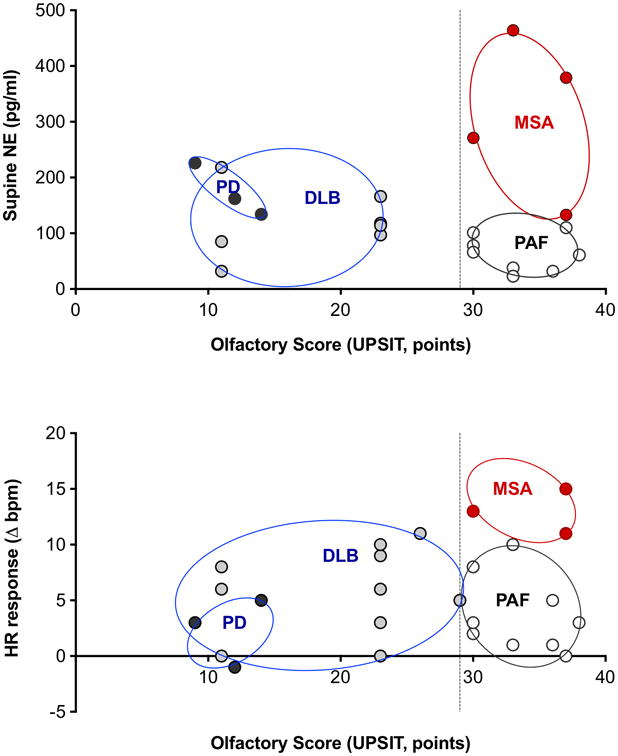
Patients with autonomic failure and were later diagnosed MSA (red) had normal olfactory function, tended to have plasma norepinephrine levels that were not low, and greater chronotrophic response to head up tilt. Those that converted to PD or DLB (grey) had impaired olfaction, tended to have lower norepinephrine levels, and a lesser heart rate response to tilt. Patients who remained as pure autonomic failure without signs of CNS involvement are shown in white. Circles represent clusters of patient groups. UPSIT: University of Pennsylvania Smell Identification Test.
Acknowledgments
Supported by NIH U54-NS065736 (All authors).
Funding source: National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN)
Footnotes
Author Contributions: Conceptualized and designed the study: HK, LNK, IB, PL, DSG
Acquisition and data analysis: HK, LNK, JAP, IB, PL, WS, DSG, AP, CS, CG, RF
Drafted the initial version of the manuscript and figures: HK, LNK, JAP
Potential Conflicts of Interest: All authors report no conflict of interests relevant to this manuscript.
References
- 1.Prusiner SB, Woerman AL, Mordes DA, et al. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A. 2015 Sep 22;112(38):E5308–17. doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCann H, Cartwright H, Halliday GM. Neuropathology of alpha-synuclein propagation and braak hypothesis. Mov Disord. 2016 Feb;31(2):152–60. doi: 10.1002/mds.26421. [DOI] [PubMed] [Google Scholar]
- 3.Peelaerts W, Bousset L, Van der Perren A, et al. alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015 Jun 18;522(7556):340–4. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- 4.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996;46(5):1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann H, Nahm K, Purohit D, Wolfe D. Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology. 2004 Sep 28;63(6):1093–5. doi: 10.1212/01.wnl.0000138500.73671.dc. [DOI] [PubMed] [Google Scholar]
- 6.Roncevic D, Palma JA, Martinez J, Goulding N, Norcliffe-Kaufmann L, Kaufmann H. Cerebellar and parkinsonian phenotypes in multiple system atrophy: similarities, differences and survival. J Neural Transm. 2014 May;121(5):507–12. doi: 10.1007/s00702-013-1133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradbury S, Eggleston C. Postural hypotension - A report of three cases. Am Heart J. 1925 Oct-Dec;1:73–86. [Google Scholar]
- 8.Roessmann U, Van den Noort S, McFarland DE. Idiopathic orthostatic hypotension. Archives of neurology. 1971;24(6):503–10. doi: 10.1001/archneur.1971.00480360037004. [DOI] [PubMed] [Google Scholar]
- 9.van Ingelghem E, van Zandijcke M, Lammens M. Pure autonomic failure: a new case with clinical, biochemical, and necropsy data. J Neurol Neurosurg Psychiatry. 1994;57(6):745–7. doi: 10.1136/jnnp.57.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hague K, Lento P, Morgello S, Caro S, Kaufmann H. The distribution of Lewy bodies in pure autonomic failure: autopsy findings and review of the literature. Acta neuropathologica. 1997 Aug;94(2):192–6. doi: 10.1007/s004010050693. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann H, Hague K, Perl D. Accumulation of alpha-synuclein in autonomic nerves in pure autonomic failure. Neurology. 2001;56(7):980–1. doi: 10.1212/wnl.56.7.980. [DOI] [PubMed] [Google Scholar]
- 12.Masuda H, Asahina M, Oide T, et al. Antemortem detection of colonic alpha-synuclein pathology in a patient with pure autonomic failure. J Neurol. 2014 Dec;261(12):2451–2. doi: 10.1007/s00415-014-7529-y. [DOI] [PubMed] [Google Scholar]
- 13.Donadio V, Incensi A, Piccinini C, et al. Skin nerve misfolded alpha-synuclein in pure autonomic failure and Parkinson disease. Ann Neurol. 2016 Feb;79(2):306–16. doi: 10.1002/ana.24567. [DOI] [PubMed] [Google Scholar]
- 14.Arai K, Kato N, Kashiwado K, Hattori T. Pure autonomic failure in association with human alpha-synucleinopathy. Neurosci Lett. 2000 Dec 22;296(2-3):171–3. doi: 10.1016/s0304-3940(00)01623-2. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DS, Holmes C, Sato T, et al. Central dopamine deficiency in pure autonomic failure. Clin Auton Res. 2008 Apr;18(2):58–65. doi: 10.1007/s10286-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 16.Terao Y, Takeda K, Sakuta M, Nemoto T, Takemura T, Kawai M. Pure progressive autonomic failure: a clinicopathological study. Eur Neurol. 1993;33(6):409–15. doi: 10.1159/000116985. [DOI] [PubMed] [Google Scholar]
- 17.Miura H, Tsuchiya K, Kubodera T, Shimamura H, Matsuoka T. An autopsy case of pure autonomic failure with pathological features of Parkinson's disease. Rinsho Shinkeigaku. 2001 Jan;41(1):40–4. [PubMed] [Google Scholar]
- 18.Postuma RB, Adler CH, Dugger BN, et al. REM sleep behavior disorder and neuropathology in Parkinson's disease. Mov Disord. 2015 Sep;30(10):1413–7. doi: 10.1002/mds.26347. [DOI] [PubMed] [Google Scholar]
- 19.Postuma RB, Iranzo A, Hogl B, et al. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann Neurol. 2015 May;77(5):830–9. doi: 10.1002/ana.24385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahlknecht P, Kiechl S, Stockner H, et al. Predictors for mild parkinsonian signs: a prospective population-based study. Parkinsonism Relat Disord. 2015 Mar;21(3):321–4. doi: 10.1016/j.parkreldis.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011 Apr;21(2):69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein DS, Low PA. Clinical evaluation of the autonomic nervous system. Continuum (Minneap Minn), Autonomic Disorders. 2007;13(6):33–49. [Google Scholar]
- 23.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases [see comments] J Neurol Neurosurg Psychiatry. 1992;55(3):181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008 Aug 26;71(9):670–6. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005 Dec 27;65(12):1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 26.Electrophysiology. TFotESoCtNASoP. Heart Rate Variabilty: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 27.Levin AB. A simple test of cardiac function based upon the heart rate changes induced by the Valsalva maneuver. Am J Cardiol. 1966;18(1):90–9. doi: 10.1016/0002-9149(66)90200-1. [DOI] [PubMed] [Google Scholar]
- 28.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008 Nov 15;23(15):2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 29.Wenning GK, Tison F, Seppi K, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS) Mov Disord. 2004 Dec;19(12):1391–402. doi: 10.1002/mds.20255. [DOI] [PubMed] [Google Scholar]
- 30.Frauscher B, Ehrmann L, Zamarian L, et al. Validation of the Innsbruck REM sleep behavior disorder inventory. Mov Disord. 2012 Nov;27(13):1673–8. doi: 10.1002/mds.25223. [DOI] [PubMed] [Google Scholar]
- 31.Postuma RB, Arnulf I, Hogl B, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012 Jun;27(7):913–6. doi: 10.1002/mds.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984 Feb;94(2 Pt 1):176–8. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012 Apr;22(2):79–90. doi: 10.1007/s10286-011-0146-2. [DOI] [PubMed] [Google Scholar]
- 34.Suarez GA, Opfer-Gehrking TL, Offord KP, Atkinson EJ, O'Brien PC, Low PA. The Autonomic Symptom Profile: a new instrument to assess autonomic symptoms. Neurology. 1999;52(3):523–8. doi: 10.1212/wnl.52.3.523. [DOI] [PubMed] [Google Scholar]
- 35.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA : the journal of the American Medical Association. 2014 Feb 5;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 36.Schatz IJ. The meaning of sitting hypotension: still unclear. Clin Auton Res. 2003 Dec;13(6):402. doi: 10.1007/s10286-003-0145-z. [DOI] [PubMed] [Google Scholar]
- 37.Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology. 2010 Nov 23;75(21):1904–11. doi: 10.1212/WNL.0b013e3181feb283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Bmj. 2009 Jun 29;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norcliffe-Kaufmann L, Kaufmann H, Martinez J, Katz SD, Tully L, Reynolds HR. Autonomic Findings in Takotsubo Cardiomyopathy. Am J Cardiol. 2016 Jan 15;117(2):206–13. doi: 10.1016/j.amjcard.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 40.Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology. 2005 Nov 22;65(10):1533–7. doi: 10.1212/01.wnl.0000184504.13173.ef. [DOI] [PubMed] [Google Scholar]
- 41.Gibbons CH, Garcia J, Wang N, Shih LC, Freeman R. The diagnostic discrimination of cutaneous alpha-synuclein deposition in Parkinson disease. Neurology. 2016 Aug 02;87(5):505–12. doi: 10.1212/WNL.0000000000002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology. 2015 Mar 17;84(11):1104–13. doi: 10.1212/WNL.0000000000001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahlknecht P, Iranzo A, Hogl B, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology. 2015 Feb 17;84(7):654–8. doi: 10.1212/WNL.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 44.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006 Jul;5(7):572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 45.Iranzo A, Santamaria J, Tolosa E. Idiopathic rapid eye movement sleep behaviour disorder: diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol. 2016 Apr;15(4):405–19. doi: 10.1016/S1474-4422(16)00057-0. [DOI] [PubMed] [Google Scholar]
- 46.Sauerbier A, Jenner P, Todorova A, Chaudhuri KR. Non motor subtypes and Parkinson's disease. Parkinsonism Relat Disord. 2016 Jan;22(1):S41–6. doi: 10.1016/j.parkreldis.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 47.DelleDonne A, Klos KJ, Fujishiro H, et al. Incidental Lewy body disease and preclinical Parkinson disease. Archives of neurology. 2008 Aug;65(8):1074–80. doi: 10.1001/archneur.65.8.1074. [DOI] [PubMed] [Google Scholar]
- 48.Pilleri M, Levedianos G, Weis L, et al. Heart rate circadian profile in the differential diagnosis between Parkinson disease and multiple system atrophy. Parkinsonism Relat Disord. 2014 Feb;20(2):217–21. doi: 10.1016/j.parkreldis.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Mollenhauer B, Trautmann E, Sixel-Doring F, et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology. 2013 Oct 1;81(14):1226–34. doi: 10.1212/WNL.0b013e3182a6cbd5. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein DS, Holmes C, Cannon RO, 3rd, Eisenhofer G, Kopin IJ. Sympathetic cardioneuropathy in dysautonomias. N Engl J Med. 1997;336(10):696–702. doi: 10.1056/NEJM199703063361004. [DOI] [PubMed] [Google Scholar]
- 51.Palma JA, Carmona-Abellan MM, Barriobero N, et al. Is cardiac function impaired in premotor Parkinson's disease? A retrospective cohort study. Mov Disord. 2013 May;28(5):591–6. doi: 10.1002/mds.25431. [DOI] [PubMed] [Google Scholar]
- 52.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2015 Oct;30(12):1600–11. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 53.Palma JA, Fernandez-Cordon C, Coon EA, et al. Prevalence of REM sleep behavior disorder in multiple system atrophy: a multicenter study and meta-analysis. Clin Auton Res. 2015 Feb;25(1):69–75. doi: 10.1007/s10286-015-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.