Abstract
Purpose of review
Urine- and serum-based biomarkers are useful for assessing individuals’ exposure to environmental factors. However, variations in urinary creatinine (a measure of dilution) or serum lipid levels, if not adequately corrected for, can directly impact biomarker concentrations and bias exposure-disease association measures.
Recent findings
Recent methodological literature has considered the complex relationships between creatinine or serum lipid levels, exposure biomarkers, outcomes, and other potentially relevant factors using directed acyclic graphs and simulation studies. The optimal measures of urinary dilution and serum lipids have also been investigated.
Summary
Existing evidence supports the use of covariate-adjusted standardization plus creatinine adjustment for urinary biomarkers and standardization plus serum lipid adjustment for lipophilic, serum-based biomarkers. It is unclear which urinary dilution measure is best, but all serum lipid measures performed similarly. Future research should assess methods for pooled biomarkers and for studying diseases and exposures that affect creatinine or serum lipids directly.
Keywords: creatinine adjustment, lipid adjustment, biomarkers, environmental exposures
Introduction
Biomarkers measured in urine or serum are commonly used to assess exposure to environmental factors in epidemiologic studies. While investigators typically recognize a need to express biomarker concentrations in a way that accounts for inter and intra-individual differences in urine dilution or serum lipid levels to minimize measurement error, there is substantial debate regarding the optimal approach to accomplish this.
When biomarkers are measured in urine, 24-hour urine samples are considered the “gold standard” of exposure measurement but investigators often rely on spot urine samples due to convenience and cost (1). Biomarker concentrations in spot urine samples vary based on the water content of the urine sample and are thus affected by inter- and intra-individual differences in hydration. Creatinine, a metabolite of creatine, is used to correct for hydration status because its production is constant with no diurnal patterns and it is eliminated mostly via glomerular filtration at a relatively stable rate within an individual. In general, dividing chemical concentrations by creatinine can therefore account for variability due to urinary dilution.
Similarly, concentrations of lipophilic serum-based biomarkers vary depending on serum lipid levels. Philips et al. showed that differences in chlorinated hydrocarbon concentrations between fasting and non-fasting samples were proportional to differences in fasting and non-fasting total serum lipid levels (2). Correcting chemical concentrations for serum lipid levels eliminated differences by fasting status, demonstrating that the lipid content of serum should be accounted for in studies of lipophilic chemicals.
Investigators have employed a variety of statistical approaches to account for individual differences in urinary dilution and serum lipid levels. The most common approaches are standardization (also called correction) and covariate adjustment. The standardization approach accounts for urinary dilution (or serum lipid levels) by dividing the biomarker concentration by the creatinine (or serum lipid) concentration measured in the same sample. This ratio is thought to represent the residual biomarker concentration after accounting for dilution (or serum lipid levels) and is used to model exposure and internal dose. In the covariate adjustment approach, uncorrected exposure biomarker concentration is modeled and creatinine (or serum lipid) concentration is included as an adjustment variable in regression analyses.
Although commonly employed, the standardization and covariate adjustment approaches may be problematic in many scenarios. Creatinine and serum lipid levels can be affected by individual factors, and inclusion of these factors in statistical models can induce biased associations between standardized biomarkers and health (3). These relationships are further complicated by the fact that urinary or serum biomarker concentrations are usually proxies for concentrations in the more relevant target tissue. Given these considerations, it becomes clear that the choice of correction method should be based on the specific causal relationships under study.
In this review, we discuss influential papers and recent work evaluating approaches to account for variation in exposure biomarkers due to urinary dilution and serum lipid levels. We discuss a novel method, covariate-adjusted standardization, and demonstrate its utility in urinary biomarker studies. We also review recent work examining alternative measures of urinary dilution or serum lipid levels and suggest areas for future research on creatinine and lipid adjustment in studies relying on biomarkers to estimate environmental exposure.
Creatinine adjustment
In their seminal 2005 paper, Barr et al. (3) discuss the importance of spot urines as a matrix for bio-monitoring when 24-hour urine samples are not available. The authors give special consideration to the physiology of creatinine formation and clearance, how various biological factors affect this physiology, and the use of urinary creatinine concentrations as a proxy for urinary dilution levels that vary within individuals over time. Using data from 22,245 participants from the National Health and Nutrition Examination Survey (NHANES), they demonstrate how creatinine levels vary according to a number of biologic factors, including age, sex, race/ethnicity, body mass index (BMI), fat free mass, and kidney function. When these factors are associated with disease, they can induce an association between creatinine and disease and bias the estimated association between creatinine-standardized biomarker levels and disease. To resolve this issue, Barr et al. recommend controlling for creatinine by including it as a covariate in the regression model when assessing the relationship between a urine-based biomarker and a health outcome, instead of using the classical standardization approach.
A study by Christensen et al (4) used simulations to evaluate the classical standardization and covariate adjustment approaches for urinary biomarker measures, as well as measures based on excretion rate and estimated daily intakes. The authors showed that, as expected, when individuals were randomly assigned an intake dose of di-2-ethylhexyl phthalate, the randomly generated intakes, excretion rates, and urine concentrations of di-2-ethylhexyl phthalate were not associated with BMI or waist circumference. However, after correcting the random urinary phthalate concentrations for creatinine (either through standardization or covariate adjustment), the exposure measures were associated with BMI and waist circumference despite the random exposure assignment. As the observed associations could be the result of an association between creatinine and waist circumference or creatinine and BMI, additional simulations using outcomes unrelated to creatinine could provide further insights on the relative performance of method in other common scenarios.
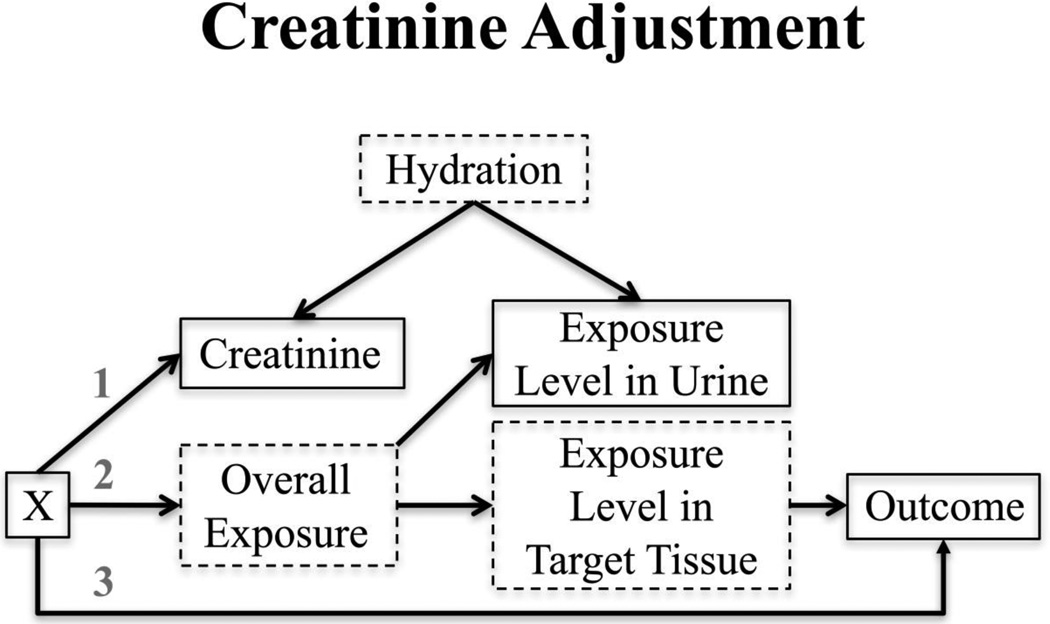
O’Brien et al. (2016) (5) tested the validity of classical standardization, adjusting for creatinine as a covariate, and other methodological approaches to creatinine adjustment using a directed acyclic graph (DAG) framework and simulation studies. Based on the observations of Barr et al. (3) that factors such as age, sex, race/ethnicity, and BMI are associated with creatinine levels, the authors constructed a DAG (Figure 1). Here, the exposure levels measured in urine are a proxy for those in a more outcome-relevant target tissue and hydration levels affect both urinary exposure levels and creatinine levels. Target tissue exposure levels and hydration are both unmeasured, as are overall exposure levels. One or more factor Xs (e.g. age, sex, race/ethnicity, and BMI) can influence creatinine (arrow 1), and may also affect overall exposure (arrow 2) or the outcome (arrow 3).
Figure 1.
Variables with solid outlines are observed; those with dashed outlines are unobserved. X represents one or more covariates that could potentially affect creatinine (arrow 1), overall exposure (arrow 2), and/or the outcome (arrow 3). Examples of X include age, sex, BMI, and race. (Reproduced from: O’Brien et al., 2016;124(2):220-7, with permission from Environmental Health Perspectives) [5••].
In their simulations, O’Brien et al. compared seven different methodological approaches to creatinine adjustment, including classical standardization (urinary exposure concentration divided by creatinine concentration), adjusting for creatinine as a covariate (as suggested by Barr et al. (3)), and a novel approach called covariate-adjusted standardization. Covariate-adjusted standardization is a two-step process. First, creatinine is modeled as a function of the measured covariates that affect creatinine (i.e. any factor Xs). Using this regression model, individuals are assigned predicted creatinine values based on their X values. The observed creatinine values are then divided by the predicted creatinine values to calculate the Cratio, which represents a measure of the residual effect of hydration on creatinine. Under the assumption that hydration is responsible for the difference between exposure levels in urine versus the target tissue, the ratio that results from dividing the urinary biomarker levels by the Cratio should represent the target tissue exposure levels (measured in the same units as the urinary biomarker). Therefore, the estimated effect of the derived ratio on the outcome should be an unbiased estimate of the true effect of the exposure on the outcome if the assumptions are met. This interpretation was confirmed in simulation studies, with the new covariate-adjusted standardization approach outperforming other methods, demonstrating low bias and confidence interval coverage consistent with 95% in all scenarios. The novel method performed particularly well when creatinine was also included as a covariate in the regression model (hereto referred to as covariate-adjusted standardization plus creatinine adjustment).
In addition to the applied example in the original paper by O’Brien et al., (5) the covariate-adjusted standardization plus creatinine adjustment approach has been utilized in several other epidemiologic studies (6–9). For example, Buckley et al. (6) applied this approach to estimate associations between environmental phenol biomarker concentrations and childhood fat mass in a New York City birth cohort. In sensitivity analyses, the authors compared covariate-adjusted standardization plus creatinine adjustment to traditional approaches and found that while the point estimates did not materially differ, the covariate-adjusted standardization plus creatinine adjustment method resulted in more precise interval estimates than the other creatinine adjustment approaches. This study also demonstrates how the covariate-adjusted standardization plus creatinine adjustment method can be adapted to Bayesian and prospective settings.
Creatinine excretion is not a perfect measure of glomerular filtration and elimination can also occur through active secretion and passive diffusion. Therefore, correcting for creatinine may not be appropriate if the environmental exposure of interest is not eliminated by a similar renal clearance mechanism (e.g. mercury or lead) (1, 10). Other recent methodological studies of urinary biomarkers have considered this issue. Traditionally, 24-hour urine collections are treated as the gold standard of exposure measurement, though there is likely substantial inter- and intra-individual variation in this metric as well. Given this caveat, Hoet et al. (11) found that standardization by specific gravity produced trace element concentrations that were often more similar to those observed in the 24-hour urine samples than those standardized to creatinine. However, there was no single best method across all trace elements. Furthermore, the authors did not apply covariate-adjusted standardization to either creatinine or specific gravity, which is also known to be associated with factors such as age, sex, and body size (12). Yeh et al. (13) propose the use of osmolality to measure urinary dilution rather than creatinine. Like creatinine and specific gravity, this measure demonstrated high variability by factors such as age, sex, race/ethnicity, and BMI. Therefore, the relative utility of creatinine, specific gravity, and osmolality as measures of dilution remains unclear, and may depend on the renal elimination mechanism(s) of the biomarker(s) of interest (10, 14). For example, it has been proposed that specific gravity may be preferable to creatinine in studies of phthalates, at least some of which are eliminated by active tubular secretion (15). Additional research may help to determine the predominant filtration or secretion mechanism for each chemical of interest and to better justify the corresponding optimal hydration measure(s).
Urine flow rate can also be used to adjust biomarker concentrations for hydration. Several studies using simulated or NHANES data have compared traditional approaches to those using urine flow rate to calculate biomarker excretion rates (4, 16, 17). In these studies, urine flow rate is estimated by dividing void volume by time since last void and biomarker excretion rate is then computed by multiplying urine flow rate by urinary analyte concentration. Excretion rates can also be adjusted for body weight. While further work is needed to determine how best to utilize urine flow rate, future studies may benefit from collecting the information necessary to calculate these measures.
Serum lipid adjustment
The groundwork for modern approaches to serum lipid adjustment methods was laid by Phillips et al. (1989) (2) and Schisterman et al. (2005) (18). Phillips et al. clearly demonstrated how chlorinated hydrocarbon levels varied markedly within individuals over the course of a single 24-hour period, depending on recent fat intake. The differences were equalized after concurrent serum lipid levels were accounted for using classical standardization (chlorinated hydrocarbon level ÷ serum lipid level). In their manuscript, Phillips et al. defined a formula for quantifying total serum lipid levels as a combination of total cholesterol, free cholesterol, triglycerides, and phospholipids.
To test whether classical standardization outperformed alternative methods for different applications, Schisterman et al. (18) simulated data based on eight potential causal scenarios, illustrated using DAGs. They examined a variety of assumptions, including whether the exposure affected the outcome or serum lipid levels directly and whether there were other covariates causally related to the exposure, the outcome, serum lipids, or the ratio between the exposure and serum lipids. They compared the results of classical standardization, serum lipid adjustment and a two-stage model. The two-stage model was originally proposed by Hunter et al. (19), and is implemented by first calculating the residual term that remains after modeling the exposure biomarker as a predictor of serum lipid levels, and then including the residual term from the first model in a regression analysis of the effect of the exposure biomarker on the outcome. The results of Schisterman et al.’s simulation studies indicated that the classical standardization approach was often not the most appropriate method, and that adjusting for serum lipid levels as a covariate or using a two-stage model resulted in lower bias and mean squared error.
Li et al. took a different approach to the problem, considering lipids as a concomitant variable that is not a confounder but can nevertheless improve the precision of the estimate if included in the data analysis (20). In their simulation study, they assume that the standardization approach is generally correct, but that (1/serum lipid levels) may not be the most appropriate correction factor. They describe how a Box-Cox transformation approach can be used to optimize fit. However, the authors found that in the specific simulations and examples they considered, the optimal correction factor was equivalent to classical standardization for most of the chemicals they examined.
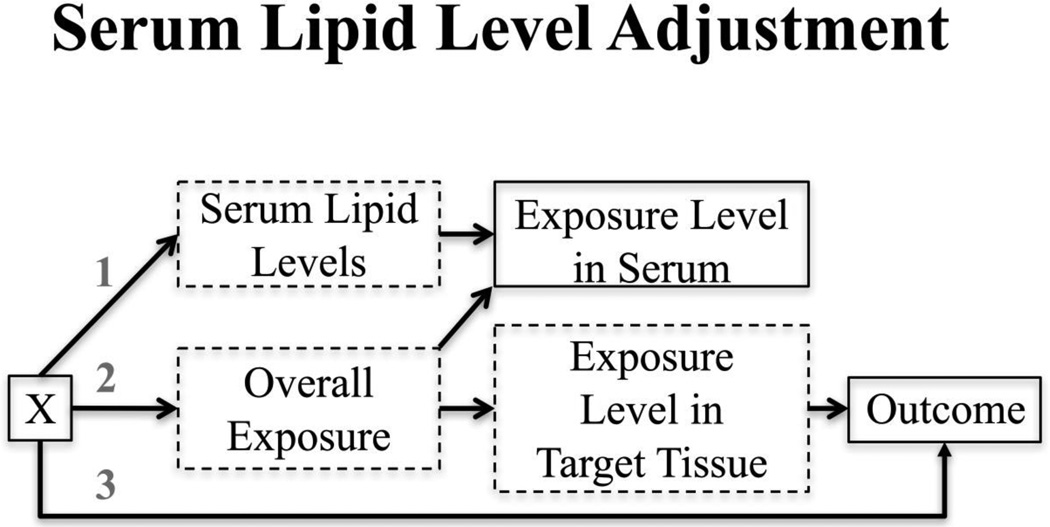
In their 2016 paper, O’Brien et al. (5) expanded on the scenarios originally proposed by Schisterman et al. (18) with a few crucial changes. First of all, O’Brien et al. constructed DAGs (Figure 2) in which serum exposure levels were not causally related to the outcome of interest, but rather a proxy for levels in tissue that is more relevant to the outcome of interest. Both the serum exposure levels and target tissue exposure levels are directly related to overall (unmeasured) exposure levels. Similar to the DAG for creatinine adjustment, O’Brien et al. allowed for other factors (denoted X) to influence serum lipid levels (arrow 1), overall exposure (arrow 2) or the outcome (arrow 3). Here, X could be sex, age, or BMI (21). Serum lipid levels were assumed to affect serum exposure levels directly, in contrast to the creatinine example in which creatinine served as a proxy measure for hydration. The other major departure from the Schisterman et al. simulation study was that O’Brien et al. modeled exposure levels as z-scores rather than continuous variables to ensure that ratio measures and linear (or log-linear) measures were compared on the same measurement scale. Under these revised assumptions, the classical standardization methods out-performed the other approaches. The authors recommended combining standardization with covariate adjustment for serum lipid levels to further control for residual confounding.
Figure 2.
Variables with solid outlines are observed; those with dashed outlines are unobserved. X represents one or more covariates that could potentially affect serum lipid levels (arrow 1), overall exposure (arrow 2), and/or the outcome (arrow 3). Examples of X include age, sex, BMI, and race. (Reproduced from: O’Brien et al., 2016;124(2):220-7, with permission from Environmental Health Perspectives) [5••].
Although not as divisive as the debate over how best to quantify hydration, there is also some controversy over how best to calculate total serum lipid levels. The original formula introduced by Phillips et al. requires that total cholesterol, free cholesterol, triglycerides, and phospholipids be measured for each subject. The authors also proposed a modified version that includes measured triglycerides and total cholesterol, but estimated free cholesterol and phospholipids (2). Their original analysis found that the second “short” formula was less successful at eliminating the differences between chlorinated hydrocarbon levels in fasting versus non-fasting samples, but a re-investigation by Bernert et al. (22) found that all of the total serum lipid estimates were highly correlated. Bergonzi et al. (23) and Rylander et al. (24) reached similar conclusions, providing further justification for using the less expensive approach of measuring only triglycerides and total cholesterol, with or without the inclusion of estimated phospholipids and free cholesterol levels.
Remaining issues in need of further research
Though not included in the DAGs presented here (Figures 1 and 2), creatinine may also be directly affected by the diseases or environmental exposures under study. For example, certain conditions, including some kidney diseases or diseases associated with muscle atrophy, could affect creatinine production or excretion. This would potentially limit the utility of creatinine as a proxy for hydration levels in these settings. Similarly, individuals with proteinuria, glucosuria, diarrhea, or other conditions may have altered specific gravity or osmolality measures. Such circumstances require careful research on the mechanisms involved in eliminating the exposure biomarker and selected hydration measure. If both are excreted via the same mechanism(s), the correction may still be valid even if the excretion rates are altered in affected individuals.
Exposure to a nephrotoxicant such as cadmium could directly affect tubular secretion or other kidney functions (25). Here again, a more detailed understanding of the physiological effects of the exposure and disease of interest on renal function is required to select the most appropriate hydration correction metric. If the covariates fit the causal diagram described in Figure 1, covariate-adjusted standardization may still be the most useful mathematical approach to account for factors that influence exposure, disease, and/or the hydration measure of interest, but are independent of hydration itself.
The exposure or outcome of interest could also affect serum lipid levels directly, though the direction of any causal association is difficult to ascertain (26, 27). Here, an additional concern is whether all serum lipid level components are affected similarly, or if triglycerides, phospholipids, total cholesterol, and free cholesterol are each uniquely affected. Once again, a more detailed understanding of disease and biomarker physiology is necessary to understand how the biomarker distribution would be altered for specific scenarios. With this information, it is theoretically possible to re-calibrate serum lipid level measures to account for measurable differences in the distribution of the lipid components prior to standardizing the exposure biomarker concentrations across those levels.
Another area in need of further research is the optimal methodological approach to adjust for creatinine or serum lipid levels in pooled samples. Spot and 24-hour urine samples are “snapshot” measures of recent exposure and may not reflect long-term average exposure, especially for non-persistent chemicals, such as phthalates or bisphenol A (28, 29). Pooling multiple biomarker samples from the same individuals over a specified follow-up period is a valid, cost-saving approach to studying longer-term average environmental exposure (30). Some designs also allow for samples to be pooled across individuals (31). Because it produces a ratio measure, classical standardization is particularly problematic for studies utilizing pooled samples, as the average of ratios is not mathematically equivalent to the ratio of averages. When pooling an individual’s biomarker samples from multiple time points over a follow-up period, there is no clear justification why one approach should be a more valid measure than the other for capturing average exposure levels. Covariate-adjusted standardization has the benefit of not being a ratio measure, but the method’s utility in capturing longitudinal exposure levels has not been thoroughly explored.
Conclusions
Inter- and intra-individual differences in urinary dilution and serum lipid levels need to be accounted for when assessing the health effects of environmental exposures measured in urine or serum. Recent methodological investigations support the use of covariate-adjusted standardization plus creatinine adjustment for urinary biomarkers and standardization plus serum lipid adjustment for lipophilic, serum-based biomarkers. There is no universally accepted measure of urinary dilution, but all approaches to quantifying total serum lipid levels produce similar results. We recommend using causal diagrams, such as DAGs, to examine relationships among variables in studies involving diseases or environmental exposures that affect serum lipid levels or creatinine (or other dilution measures) directly, with careful consideration of the specific physiologic factors involved. Future research should address how best to account for creatinine and serum lipids when working with biomarker data that has been pooled within or across individuals.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Katie M. O’Brien, Kristen Upson, and Jessie P. Buckley declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of outstanding importance
- 1.Heavner DL, Morgan WT, Sears SB, Richardson JD, Byrd GD, Ogden MW. Effect of creatinine and specific gravity normalization techniques on xenobiotic biomarkers in smokers' spot and 24-h urines. J Pharm Biomed Anal. 2006;40(4):928–942. doi: 10.1016/j.jpba.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: Effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 3. Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environmental Health Perspectives. 2004;113(2):192–200. doi: 10.1289/ehp.7337. This is a seminal paper that provided evidence that creatinine levels are associated with various other factors, including age, sex, race, fat free mass, and body mass index. This paper is highly cited as justification for adjusting for creatinine as a covariate when assessing the health effects of environmental exposures measured in urine.
- 4.Christensen K, Sobus J, Phillips M, Blessinger T, Lorber M, Tan YM. Changes in epidemiologic associations with different exposure metrics: a case study of phthalate exposure associations with body mass index and waist circumference. Environ Int. 2014;73:66–76. doi: 10.1016/j.envint.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 5. O'Brien KM, Upson K, Cook NR, Weinberg CR. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ Health Perspect. 2016;124(2):220–227. doi: 10.1289/ehp.1509693. With the aid of directed acyclic graphs and simulation studies, these authors compared existing methods for creatinine and lipid adjustment with a new method, called covariate-adjusted standardization. They show that the new method is appropriate for assessing urinary biomarkers and that classical standardization works well for lipophilic, serum-based biomarkers.
- 6.Buckley JP, Herring AH, Wolff MS, Calafat AM, Engel SM. Prenatal exposure to environmental phenols and childhood fat mass in the Mount Sinai Children's Environmental Health Study. Environ Int. 2016;91:350–356. doi: 10.1016/j.envint.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo J, Su L, Zhao X, Xu Z, Chen G. Relationships between urinary antimony levels and both mortalities and prevalence of cancers and heart diseases in general US population, NHANES 1999–2010. Sci Total Environ. 2016;571:452–460. doi: 10.1016/j.scitotenv.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Tsai HJ, Wu CF, Tsai YC, Huang PC, Chen ML, Wang SL, et al. Intake of Phthalate-tainted Foods and Serum Thyroid Hormones in Taiwanese Children and Adolescents. Sci Rep. 2016;6:30589. doi: 10.1038/srep30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Schaumberg DA, Park SK. Cadmium and lead exposure and risk of cataract surgery in U.S. adults. Int J Hyg Environ Health. 2016 doi: 10.1016/j.ijheh.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. Am Ind Hyg Assoc J. 1993;54(10):615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 11.Hoet P, Deumer G, Bernard A, Lison D, Haufroid V. Urinary trace element concentrations in environmental settings: is there a value for systematic creatinine adjustment or do we introduce a bias? J Expo Sci Environ Epidemiol. 2016;26(3):296–302. doi: 10.1038/jes.2015.23. [DOI] [PubMed] [Google Scholar]
- 12.Suwazono Y, Akesson A, Alfven T, Jarup L, Vahter M. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers. 2005;10(2–3):117–126. doi: 10.1080/13547500500159001. [DOI] [PubMed] [Google Scholar]
- 13.Yeh HC, Lin YS, Kuo CC, Weidemann D, Weaver V, Fadrowski J, et al. Urine osmolality in the US population: implications for environmental biomonitoring. Environ Res. 2015;136:482–490. doi: 10.1016/j.envres.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver VM, Kotchmar DJ, Fadrowski JJ, Silbergeld EK. Challenges for environmental epidemiology research: are biomarker concentrations altered by kidney function or urine concentration adjustment? J Expo Sci Environ Epidemiol. 2016;26(1):1–8. doi: 10.1038/jes.2015.8. [DOI] [PubMed] [Google Scholar]
- 15.Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal Variability of Urinary Phthalate Metabolite Levels in Men of Reproductive Age. Environmental Health Perspectives. 2004;112(17):1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hays SM, Aylward LL, Blount BC. Variation in urinary flow rates according to demographic characteristics and body mass index in NHANES: potential confounding of associations between health outcomes and urinary biomarker concentrations. Environ Health Perspect. 2015;123(4):293–300. doi: 10.1289/ehp.1408944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middleton DR, Watts MJ, Lark RM, Milne CJ, Polya DA. Assessing urinary flow rate, creatinine, osmolality and other hydration adjustment methods for urinary biomonitoring using NHANES arsenic, iodine, lead and cadmium data. Environ Health. 2016;15(1):68. doi: 10.1186/s12940-016-0152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schisterman EF, Whitcomb BW, Buck Louis GM, Louis TA. Lipid Adjustment in the Analysis of Environmental Contaminants and Human Health Risks. Environmental Health Perspectives. 2005;113(7):853–857. doi: 10.1289/ehp.7640. This is the first paper to apply a directed acyclic graph and simulation approach to the issue of serum lipid adjustment. This paper is highly cited as justification for adjusting for serum lipids as a covariate or using a two-stage model when assessing the health effects of lipophilic, environmental exposures measured in serum.
- 19.Hunter DJ, Hankinson SE, Laden F, Colditz GA, Manson JE, Willett WC, et al. Plasma organochlorine levels and the risk of breast cancer. The New England journal of medicine. 1997;337(18):1253–1259. doi: 10.1056/NEJM199710303371801. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Longnecker MP, Dunson DB. Lipid adjustment for chemical exposures: accounting for concomitant variables. Epidemiology. 2013;24(6):921–928. doi: 10.1097/EDE.0b013e3182a671e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costanza MC, Cayanis E, Ross BM, Flaherty MS, Alvin GB, Das K, et al. Relative contributions of genes, environment, and interactions to blood lipid concentrations in a general adult population. Am J Epidemiol. 2005;161(8):714–724. doi: 10.1093/aje/kwi103. [DOI] [PubMed] [Google Scholar]
- 22.Bernert JT, Turner WE, Patterson DG, Jr, Needham LL. Calculation of serum "total lipid" concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere. 2007;68(5):824–831. doi: 10.1016/j.chemosphere.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 23.Bergonzi R, De Palma G, Tomasi C, Ricossa MC, Apostoli P. Evaluation of different methods to determine total serum lipids for normalization of circulating organochlorine compounds. Int Arch Occup Environ Health. 2009;82(10):1241–1247. doi: 10.1007/s00420-009-0426-5. [DOI] [PubMed] [Google Scholar]
- 24.Rylander L, Nilsson-Ehle P, Hagmar L. A simplified precise method for adjusting serum levels of persistent organohalogen pollutants to total serum lipids. Chemosphere. 2006;62(3):333–336. doi: 10.1016/j.chemosphere.2005.04.107. [DOI] [PubMed] [Google Scholar]
- 25.Moriguchi J, Ezaki T, Tsukahara T, Furuki K, Fukui Y, Okamoto S, et al. Comparative evaluation of four urinary tubular dysfunction markers, with special references to the effects of aging and correction for creatinine concentration. Toxicology Letters. 2003;143(3):279–290. doi: 10.1016/s0378-4274(03)00181-4. [DOI] [PubMed] [Google Scholar]
- 26.Goncharov A, Haase RF, Santiago-Rivera A, Morse G, Akwesasne Task Force on the E. McCaffrey RJ, et al. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ Res. 2008;106(2):226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel CJ, Cullen MR, Ioannidis JP, Butte AJ. Systematic evaluation of environmental factors: persistent pollutants and nutrients correlated with serum lipid levels. Int J Epidemiol. 2012;41(3):828–843. doi: 10.1093/ije/dys003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engel LS, Buckley JP, Yang G, Liao LM, Satagopan J, Calafat AM, et al. Predictors and variability of repeat measurements of urinary phenols and parabens in a cohort of Shanghai women and men. Environ Health Perspect. 2014;122(7):733–740. doi: 10.1289/ehp.1306830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meeker JD, Calafat AM, Hauser R. Urinary phthalate metabolites and their biotransformation products: predictors and temporal variability among men and women. J Expo Sci Environ Epidemiol. 2012;22(4):376–385. doi: 10.1038/jes.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrier F, Giorgis-Allemand L, Slama R, Philippat C. Within-subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-based Studies. Epidemiology. 2016;27(3):378–388. doi: 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyles RH, Mitchell EM, Weinberg CR, Umbach DM, Schisterman EF. An efficient design strategy for logistic regression using outcome- and covariate-dependent pooling of biospecimens prior to assay. Biometrics. 2016 doi: 10.1111/biom.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]