Abstract
Purpose
Cancer-related fatigue (CRF) is a prevalent and distressing side effect of cancer and its treatment that remains inadequately understood and poorly managed. A better understanding of the factors contributing to CRF could result in more effective strategies for the prevention and treatment of CRF. The objectives of this study were to examine the prevalence, severity and potential predictors for the early onset of CRF after chemotherapy cycle 1 in breast cancer patients.
Methods
We report on a secondary data analysis of 548 female breast cancer patients from a phase III multi-center randomized controlled trial examining antiemetic efficacy. CRF was assessed by the Brief Fatigue Inventory at pre- and post-chemotherapy cycle 1 as well as by the four-day diary.
Results
The prevalence of clinically relevant post-CRF was 75%. Linear regression showed that pre-treatment CRF, greater nausea, disturbed sleep, and younger age were significant risk factors for post-CRF (adjusted R2=0.39; P<0.0001). Path modeling showed that nausea severity influenced post-CRF both directly and indirectly by influencing disturbed sleep. Similarly, pre-treatment CRF influenced post-CRF directly as well as indirectly through both nausea severity and disturbed sleep. Pearson correlations showed that changes in CRF over time were significantly correlated with concurrent changes in nausea severity (r=0.41; P<0.0001) and in disturbed sleep (r=0.20; P<0.0001).
Conclusion
This study showed a high prevalence (75%) of clinically relevant CRF in breast cancer patients following their initial chemotherapy, and that nausea severity, disturbed sleep, pre-treatment CRF, and age were significant predictors of symptom.
Keywords: Breast Cancer, Cancer-related Fatigue, Nausea, Disturbed Sleep
Introduction
Breast cancer is the most frequently diagnosed cancer among females, with more than 3 million new cases of invasive and in situ breast cancer in the United States estimated for 2016 [1]. Cancer-related fatigue (CRF) is one of the most common and debilitating side effects experienced by breast cancer patients, even more so than pain, nausea, or vomiting [2]. The National Comprehensive Cancer Network (NCCN) practice guidelines attribute CRF to both cancer and its treatment [3]. It is defined as a “distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion that is related to cancer and its treatment and interferes with functioning,” and is not proportional to recent activity or the amount of rest or sleep [3,4]. The prevalence and severity of CRF is much higher for breast cancer patients receiving chemotherapy than other therapies [5]. Fifty-eight to 94 percent of breast cancer patients report some level of CRF during chemotherapy [6,7], with moderate-severe fatigue levels occurring in 26 to 60% of patients [8,9]. Despite the high prevalence and severity of CRF, it often goes unassessed, undiagnosed and unmanaged [10].
CRF during cancer treatment can impact the patient’s ability to tolerate treatment and may result in treatment discontinuation [11]. CRF also interferes with activities of daily life and has a considerable impact on physical, mental and social well-being; thereby, disrupting vocational and social activities as well as interpersonal relationships [5]. This results in diminished quality of life (QOL) [12] and possibly reduced survival [13]. CRF also has a profound effect on employment and financial status, with decreased working hours, more sick leaves, increased disability and even inability to return to work [14].
These issues affect not just cancer patients but also their family members and caregivers [14]. The etiology of CRF involves a variety of physiological, biochemical, behavioral and psychological factors [15,16]. However, the overall knowledge about the predictors of and risk factors for CRF is still limited and inconclusive, and currently, there is no well-established and effective treatment for CRF [16].
A better understanding of the predictors of CRF is important for developing preventive strategies, identifying patients at risk, and providing tailored and individualized interventions to reduce the burden of CRF and improve QOL in breast cancer patients. The main objectives of the present study were to examine the prevalence, severity, and potential predictors for the early onset of CRF in breast cancer patients undergoing initial chemotherapy. We also correlated changes over time in nausea severity and disturbed sleep with concurrent changes in CRF in our study population.
Methods
Study design and patients
This study is a secondary analysis of a subset of participants from a previously completed trial [17]. The original study was a multicenter, randomized, double-blind, placebo-controlled phase III clinical trial examining antiemetic efficacy for the prevention of delayed nausea in cancer patients during the first cycle of chemotherapy [17]. The University of Rochester Cancer Center (URCC) NCI’s Community Oncology Research Program (NCORP) Research Base (previously called URCC CCOP) recruited patients from 15 geographically unique private-practice oncology groups in the U.S. from May 2007 to September 2010. Eligible participants were chemotherapy-naive outpatients at least 18 years of age with any cancer diagnosis who were scheduled to receive their first treatment with a chemotherapy regimen containing any of the five following highly emetogenic chemotherapy agents: doxorubicin, epirubicin, cisplatin, carboplatin, or oxaliplatin. Participants were randomized to one of the four antiemetic arms.
The institutional review boards of the University of Rochester and of each participating site approved the protocol for the original study, and participants provided written informed consent. This trial is registered with ClinicalTrials.gov, number NCT00475085.
Assessments
Participants completed on-study questionnaires on enrollment, providing demographic and clinical information.
Brief Fatigue Inventory (BFI)
CRF was assessed by the revised BFI, which is a unidimensional, 9-item, patient-report instrument with first three items measuring current, usual and worst fatigue severity levels while the remaining six items measuring interference caused by fatigue on different aspects of the patient’s life, namely activity, mood, walking, work, relationships, and enjoyment of life. Each item ranges from 0 (= no fatigue/does not interfere) to 10 (= fatigue as bad as you can imagine/completely interferes) [18]. The global BFI score is the average of all nine items. The single-item “fatigue at its worst” is used to represent fatigue severity levels [18]. Scores of 1–3, 4–6, and 7–10 are categorized as mild, moderate, and severe fatigue, respectively, [18] while clinically relevant CRF is considered to be ≥ 4 on the single-item “fatigue at its worst” [3]. Its reliability and validity with internal consistency coefficient (α=0.96) have been demonstrated in cancer patients [18]. The BFI also correlates well with other well-established instruments such as the Functional Assessment of Cancer Therapy-Fatigue and the Profile of Mood States Fatigue subscale [18]. The participants completed the BFI questionnaire prior to the start of chemotherapy (i.e., pre-treatment CRF) and on the third day after the completion of chemotherapy cycle 1 (i.e., post-CRF).
CRF Diary Measure
CRF was also assessed by a secondary measure, a self-report four-day diary [19,20] that was completed after chemotherapy cycle 1 on the day of chemotherapy and on the three following days. Participants reported “fatigue at its worst” once a day on an 11-point scale from 0 (= no fatigue) to 10 (= as bad as you can imagine). Change scores of CRF from day 1 to day 4 were calculated.
Nausea
Nausea was assessed by a self-report four-day diary [19,20] that was completed by the participants over a four-day period. Each day was divided into four segments (morning, afternoon, evening, night), and participants reported the severity of nausea for each period on the day of chemotherapy cycle 1 and on the three following days. Nausea was rated on a 7-point scale from 1 (= no nausea) to 7 (= extremely nauseated). Post-chemotherapy cycle 1 mean nausea scores were obtained from the latter three reporting periods (afternoon, evening, night) on Day 1 (since the chemotherapy was given during the day) and from the 12 reporting periods from Days 2, 3 and 4. In addition, change scores of mean nausea from day 1 to day 4 were calculated.
Any Occurrence of Vomiting
Whether or not the participants vomited after the completion of the chemotherapy cycle 1 was assessed by the same self-report four-day diary with the variable “any occurrence of vomiting (yes/no)” being created from the latter three reporting periods (afternoon, evening, night) on Day 1 and from the 12 reporting periods from Days 2, 3 and 4.
Disturbed Sleep
Disturbed sleep was assessed daily using the self-report four-day diary. Participants reported any “disturbed sleep the night before” on an 11-point scale from 0 (= none) to 10 (= as bad as you can imagine). Post-chemotherapy cycle1 mean disturbed sleep was calculated from the latter three days. In addition, change scores of disturbed sleep from day 2 to day 4 were calculated.
Statistical Analyses
The original trial had 944 cancer patients. For the present analysis, we included the 548 cancer patients who had a diagnosis of breast cancer, were female, and had no missing data for the variables of interest. Descriptive statistics were used to describe baseline characteristics and to examine the prevalence and severity of post-CRF after the completion of chemotherapy cycle 1. For identifying potential predictors of post-CRF as well as mediation effects of predictors (i.e., direct and indirect effects), we performed (i) Pearson and Spearman rank correlations for continuous and categorical variables, respectively; (ii) linear regression models; and (iii) path modeling with Maximum likelihood estimation. The assumptions of normality, linearity, and homoscedasticity were investigated by the residual scatterplots. Independent variables such as pre-treatment CRF, disturbed sleep, nausea severity, any occurrence of vomiting, age, ethnicity, and previous radiation therapy were included in the linear regression analysis only if their correlations with post-CRF were significant, and the correlations between the independent variables were ≤ 0.7. For the path model, we included direct paths from age, pre-treatment CRF, disturbed sleep, nausea severity, any occurrence of vomiting on post-CRF. We also included the following direct paths from: any occurrence of vomiting on disturbed sleep; nausea severity on both any occurrence of vomiting and disturbed sleep; and age and pre-treatment CRF on disturbed sleep, nausea severity, and any occurrence of vomiting, respectively. We also used Pearson correlations to examine the associations of changes in CRF over four days with concurrent changes in nausea severity and disturbed sleep. Assumptions underlying all analyses were checked and no outliers were found; therefore, analyses included all evaluable patients. All statistical analyses were performed at a two-tailed 5% level of significance. We used SPSS version 22 and STATA IC version 14 for analyses as appropriate.
Results
Demographic and Clinical Characteristics
Table 1 shows the baseline characteristics of the 548 female breast cancer participants included in the analyses. Mean (SD) age was 54 (10) years, 91% were white, 97% were non-Hispanic or Latino, 66% were married, 63% had at least a partial or complete college degree or higher, and 84% had received previous surgery. Ninety three percent of women received anthracycline treatment (i.e., doxorubicin or epirubicin) while the remaining 7% received a non-anthracycline regimen (i.e., carboplatin or oxaliplatin). Clinically relevant CRF (i.e., moderate-severe levels) was reported by 290 patients (53%) as assessed by “fatigue at its worst” question on the BFI. Prior to the start of chemotherapy, mean fatigue severity level (i.e., score ≥ 1) and pre-treatment CRF (i.e., global BFI score) were 4.09 (SD = 2.90) and 2.33 (SD = 2.06), respectively.
Table 1.
Baseline characteristics of participants
| Characteristics | Total Sample (N = 548) |
|
|---|---|---|
| Age | Mean (SD) | 54.3 (10.5) |
| Range | 22–80 | |
| Gender | Female | 548 |
| Race | White | 91.2% |
| Black/African American | 7.8% | |
| Asian | 0.5% | |
| American Indian/Native Alaskan | 0.4% | |
| Ethnicity | Non-Hispanic/Latino | 97.4% |
| Hispanic/Latino | 2% | |
| Unknown | 0.5% | |
| Marital Status | Married | 66.1% |
| Divorced/widowed/separated | 21.8% | |
| Single | 12.2% | |
| Education | High School or less | 36.8% |
| Some college | 33.4% | |
| Bachelor’s degree | 21.2% | |
| Graduate degree | 8.8% | |
| Previous Treatments | Previous Surgery | 84.5% |
| Previous Chemotherapy | 0.9% | |
| Previous Radiation Therapy | 1.3% | |
| Other | 0.7% | |
| Anthracycline | Yes - Doxorubicin | 91.2% |
| Yes - Epirubicin | 1.8% | |
| No - Carboplatin | 6.8% | |
| No - Oxaliplatin | 0.2% | |
| Clinically relevant CRFa | Moderate-severe level (score ≥4) | 52.9% |
| Fatigue severity level (score ≥1)a | Mean (SD) | 4.09 (2.90) |
| Pre-treatment CRFb | Mean (SD) | 2.33 (2.06) |
NOTE: Data might not add to 100% because of rounding.
Abbreviations: SD, standard deviation; CRF, cancer-related fatigue.
By single-item “fatigue at its worst” from the Brief Fatigue Inventory.
By global Brief Fatigue Inventory score.
Prevalence and Severity of post-CRF
Post-CRF i.e., the global BFI score was 3.76 (SD = 2.40). Mean fatigue severity level (score ≥ 1) at post-chemotherapy cycle 1, as evaluated using the single-item “fatigue at its worst,” was 5.79 (SD = 2.90) with 95% of patients experiencing some level of fatigue. 75% of participants reported clinically relevant CRF (i.e., score ≥ 4) and the number of patients experiencing mild, moderate, and severe CRF were 113 (21%), 156 (28%), and 254 (46%), respectively.
Correlates of post-CRF
Post-CRF i.e., the global BFI score was moderately correlated with physical factors namely, pre-treatment CRF (r=0.44; P<0.0001), disturbed sleep (r=0.47; P<0.0001) and nausea severity (r=0.43; P<0.0001). A weak correlation with any occurrence of vomiting (r=0.23; P<0.0001) was also observed. Post-CRF also showed weak negative correlations with age (r=−0.21; P<0.0001) and ethnicity (r=−0.10; P=0.020) while weak positive correlation with previous radiation therapy (r=0.10; P=0.014). There were no significant correlations of post-CRF with anthracycline treatment or with other variables (Table 2).
Table 2.
Correlates of cancer-related fatigue at post-chemotherapy cycle 1
| Characteristic | post-CRFa | P-value |
|---|---|---|
| Agec | −0.21 | <0.0001 |
| Pre-treatment CRFac | 0.44 | <0.0001 |
| Disturbed sleepbc | 0.47 | <0.0001 |
| Nausea severitybc | 0.43 | <0.0001 |
| Any occurrence of vomiting (Yes/No)bd | 0.23 | <0.0001 |
| Previous radiation therapy (Yes/No)d | 0.10 | 0.014 |
| Ethnicityd | −0.10 | 0.020 |
| Raced | 0.06 | 0.140 |
| Anthracycline (Yes/No)d | −0.003 | 0.939 |
| Antiemetic intervention armd | −0.06 | 0.141 |
Abbreviations: CRF, cancer-related fatigue.
By global Brief Fatigue Inventory score.
By self-report four-day diary.
Using Pearson correlations.
Using Spearman rank correlations.
Predictors of post-CRF
Regression analyses showed that younger age, greater pre-treatment CRF, more disturbed sleep, and greater nausea severity were significant risk factors for post-CRF, as measured by the global BFI score, while any occurrence of vomiting was not a predictor (Table 3). Taken together, age, pre-treatment CRF, disturbed sleep and nausea severity accounted for 39% of the variance in post-CRF (adjusted R2=0.39; F=51.42; P<0.0001).
Table 3.
Predictors of cancer-related fatigue at post-chemotherapy cycle 1 using linear regression model
| Characteristic | β | Standardized β |
SE | t | P-value |
|---|---|---|---|---|---|
| Age | −0.02 | −0.09 | 0.01 | −2.58 | 0.010 |
| Pre-treatment CRFa | 0.37 | 0.32 | 0.04 | 9.17 | <0.0001 |
| Disturbed sleepb | 0.26 | 0.28 | 0.04 | 7.38 | <0.0001 |
| Nausea severityb | 0.43 | 0.20 | 0.09 | 4.97 | <0.0001 |
| Any occurrence of vomiting (Yes/No)b | 0.37 | 0.07 | 0.20 | 1.80 | 0.073 |
| Ethnicity | −0.08 | −0.02 | 0.15 | −0.53 | 0.597 |
| Previous radiation therapy | 1.31 | 0.06 | 0.72 | 1.82 | 0.069 |
Abbreviations: CRF, cancer-related fatigue; β, coefficient; SE, standard error.
By global Brief Fatigue Inventory score.
By self-report four-day diary.
The path model for post-CRF fit the data very well with root mean square error of approximation (RMSEA) <0.0001 and comparative fit index (CFI) =1.000. We note that a RMSEA <0.1 is considered ideal and that CFI is interpreted similar to R2 in a regression with CFI >0.95 is ideal. For post-CRF, the only statistically significant direct positive effects (path coefficient, coef) were from: pre-treatment CRF (coef=0.38; P<0.0001), disturbed sleep (coef=0.26; P<0.0001), and nausea severity (coef=0.45; P<0.0001) and direct negative effects were from age (coef=−0.02; P=0.007) (Fig. 1). The statistically significant indirect effects on post-CRF were from: nausea severity via disturbed sleep (coef=0.20; P<0.0001); pre-treatment CRF via both disturbed sleep (coef=0.07; P<0.0001) and nausea severity (coef=0.04; P=0.003); and age via nausea severity (coef=−0.01; P<0.0001). Disturbed sleep did not have a significant indirect effect on post-CRF. In addition, there were no significant direct or indirect effects of any occurrence of vomiting on post-CRF.
Figure 1.
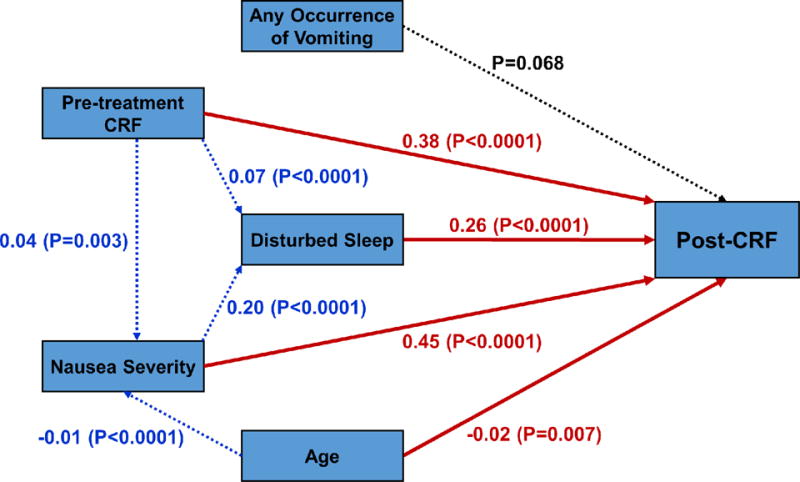
Diagram for the path model. Statistically significant direct paths to post-CRF from nausea severity, disturbed sleep, pre-treatment CRF and age are colored in red while dotted blue lines show the statistically significant indirect paths (i.e., via other variables) to post-CRF, with selected path coefficients marked (P≤0.05). Direct paths to post-CRF that are not statistically significant are in black.
The CRF change score over four days after chemotherapy cycle 1 as measured by the four-day diary was significantly correlated with both concurrent changes in nausea severity (r=0.41; P<0.0001) and disturbed sleep (r=0.20; P<0.0001). This implies that an improvement in nausea severity is moderately associated with an improvement in CRF while improvement in disturbed sleep is weakly associated with improvement in CRF.
Discussion
To the best of our knowledge, the present secondary analysis is the first report using a large sample size to describe the prevalence and the direct and indirect effects of predictors for the early onset of CRF after the first cycle of chemotherapy in a uniform cohort of female breast cancer patients undergoing treatment.
Our findings show that a high proportion (75%) of female breast cancer patients experience clinically relevant CRF (i.e., moderate-severe levels) following the completion of cycle 1 of mostly anthracycline-containing chemotherapy regimens, with 46% experiencing severe CRF. This observation is consistent with previous studies that have shown that 26–60% of breast cancer patients experience moderate-severe fatigue during adjuvant chemotherapy [8,9]. A study by Jacobsen et al. examined fatigue prior to each of the first four chemotherapy cycles and showed that the prevalence and severity of any level of fatigue significantly increased after starting chemotherapy but showed a stable pattern later [6]. The majority of our patients (93%) were receiving anthracycline-based regimens, which are known to be associated with multiple adverse effects [21]. Anthracyclines are commonly used for neo/adjuvant chemotherapy for breast cancer [22] and can adversely affect normal tissues, such as muscles, which can result in fatigue [21]. The presence of nontrivial CRF in 75% of our patients undergoing initial treatment indicates that fatigue management is needed for a majority of patients, especially those undergoing anthracycline-based treatments. Otherwise unmanaged fatigue might affect their ability to tolerate chemotherapy as well as result in reduced QOL.
We identified four predictors of CRF following the first cycle of chemotherapy. Among treatment-related behavioral/physical symptoms, we found greater nausea severity, disturbed sleep, and pre-treatment CRF to be significant predictors of post-CRF. While nausea is not typically reported as a predictor of CRF [23,24], some studies have found a relationship between these two symptoms [25–27]. Our finding on nausea could be because of the fatiguing effects of nausea itself. The NCCN guidelines suggest that the amount of food consumed or nutritional intake may be affected by nausea and may lead to increased fatigue [3]. Consistent with some of the previous studies [23,24,28], our results did not show vomiting to be a potential predictor of CRF, although there was a trend towards significance. However, some studies have shown an association of vomiting with CRF possibly due to malnutrition or anemia [27,25].
Similar to our results, other studies have also shown CRF to be significantly associated with various sleep parameters such as decreased sleep quality and insomnia [29–31]. One study in 78 breast cancer patients showed that fatigue, measured at the second and fourth chemotherapy cycles, was greater in patients with less stable sleep-wake patterns who often napped [32]. This is not surprising given that CRF and sleep disorders are usually reported as part of a symptom cluster, suggesting that CRF and disturbed sleep may have a common underlying etiology [33]. Further, pre-treatment CRF is known to be one of the strongest and persistent predictors of post-treatment fatigue, indicating that some of the risk factors of CRF may be present before the initiation of cancer treatments [30]. Cancer itself, medical comorbidities, physical inactivity, muscle weakness, physiological factors such as pain, psychological factors such as depression and stress, and other physical symptoms such as impaired sleep are some of the probable contributing factors for pre-treatment CRF [6,34,35].
Our data also showed that higher levels of nausea severity not only directly influenced post-CRF but also indirectly through its effect on disturbed sleep. This indirect effect of nausea severity suggests that an improvement in nausea may improve sleep which in turn may improve CRF. Similarly, pre-treatment CRF also indirectly influenced post-CRF via its effects on both disturbed sleep and nausea severity besides having a direct effect on post-CRF. Further, our study showed that improvement in CRF over time was significantly correlated with concurrent improvements in nausea severity and disturbed sleep.
Among the demographic variables, only age was a significant predictor of CRF. Younger patients experienced greater CRF than older patients, although the contribution from age was small for our study. This finding is consistent with prior studies which showed that younger age was associated with higher levels of CRF in breast cancer patients [36] and survivors [31], most likely due to more aggressive treatments given to younger patients [37,38]. However, several studies have also shown mixed results regarding the relationship between age and CRF with some even reporting higher levels of CRF in older patients [39,40].
The symptom of CRF undoubtedly reflects multiple etiologies that contribute to its development and are likely to vary over the course of treatment and with type of treatment as well as with time. CRF has been compared to chronic pain, for which behavioral factors play a prominent role in its development and maintenance [8].
Similarly, behavioral factors may play an analogous role in the development of CRF suggesting that it can be considered as a symptom that arises in response to other symptoms. Since our results indicate that nausea severity and disturbed sleep may contribute to the early-onset of CRF especially for breast cancer patients undergoing anthracycline-based regimens and given the prevalence of nausea and disturbed sleep to be as high as 70% and 60%, respectively, in breast cancer patients [41–43], interventions targeting nausea and disturbed sleep could be promising treatments for CRF particularly in this patient population. In fact, a recent study by Heckler et al. showed that cognitive behavioral therapy for insomnia (CBT-I) resulted in a significant reduction of fatigue in cancer survivors with chronic insomnia [44]. It would be equally interesting to study changes in CRF in a trial in cancer patients designed for treating nausea or both nausea and sleep disorder.
The findings of this study are useful for hypothesis generation and designing future studies. However, the interpretation of these results is limited as the present study is a secondary data analysis of a large multi-center clinical trial that examined antiemetic efficacy in cancer patients but was not designed to identify mediators of CRF. Second, the four-day diary measures have limitations, as they are unidimensional in nature assessing only the occurrence and severity of the symptoms and may be less sensitive than multi-question assessments to detect changes in symptoms with disease progression. However, the four-day diary has been used in other large multi-center trials [45]. Third, since post-CRF, nausea severity and disturbed sleep were measured at the same time point (i.e., after chemotherapy cycle 1), the path analyses are only suggestive of possible relationships. Lastly, our analyses were done only with female breast cancer patients, as a method of controlling for cancer type, and mostly for anthracycline-based chemotherapy regimens; therefore, our findings may not be generalizable to men or to individuals with other cancers or undergoing other cancer treatments. Although these limitations affect generalizability, the current study addresses a critical gap in CRF literature by providing initial insight into the potential predictors and mediators of CRF during the first cycle of mostly anthracycline-containing chemotherapy for breast cancer patients.
Conclusion
This study showed a high prevalence (75%) of early onset clinically relevant CRF in breast cancer patients undergoing mostly anthracycline-based chemotherapy treatment, indicating the need for effective management of initial fatigue especially in this group. Nausea severity, disturbed sleep, pre-treatment CRF, and younger age were significant predictors of CRF after the completion of chemotherapy cycle 1. Nausea severity had direct as well as indirect influence via disturbed sleep on CRF. Additionally, improvement in CRF was significantly associated with improvements in nausea severity and disturbed sleep. Further research is needed to determine whether early management of CRF, nausea severity and disturbed sleep during adjuvant therapy might reduce risk for CRF later in the disease trajectory.
Acknowledgments
Supported by NCI grants U10 CA37420, R25 CA102618 and UG1 CA189961.
Funding
This study was funded by the NCI grants U10 CA37420, R25 CA102618 and UG1 CA189961. Study medication was provided by MGI Pharma, Bloomington, MN.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest. Dr. Roscoe has full control of the primary data and agrees to allow the journal to review the data if requested.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118(8 Suppl):2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Cancer-Related Fatigue Panel: National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Cancer-Related Fatigue, version 1. 2016:1–56. [Google Scholar]
- 4.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Support Care Cancer. 1996;4(2):82–96. doi: 10.1007/BF01845757. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt ME, Chang-Claude J, Vrieling A, Heinz J, Flesch-Janys D, Steindorf K. Fatigue and quality of life in breast cancer survivors: temporal courses and long-term pattern. Journal of cancer survivorship: research and practice. 2012;6(1):11–19. doi: 10.1007/s11764-011-0197-3. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manage. 1999;18(4):233–242. doi: 10.1016/s0885-3924(99)00082-2. doi:S0885-3924(99)00082-2 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Greene D, Nail LM, Fieler VK, Dudgeon D, Jones LS. A comparison of patient-reported side effects among three chemotherapy regimens for breast cancer. Cancer Pract. 1994;2(1):57–62. [PubMed] [Google Scholar]
- 8.Andrykowski MA, Schmidt JE, Salsman JM, Beacham AO, Jacobsen PB. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J Clin Oncol. 2005;23(27):6613–6622. doi: 10.1200/JCO.2005.07.024. doi:23/27/6613 [pii]10.1200/JCO.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. Journal of the National Cancer Institute Monographs. 2004;(32):40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 10.Borneman T, Koczywas M, Sun VC, Piper BF, Uman G, Ferrell B. Reducing patient barriers to pain and fatigue management. J Pain Symptom Manage. 2010;39(3):486–501. doi: 10.1016/j.jpainsymman.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 12.Scott JA, Lasch KE, Barsevick AM, Piault-Louis E. Patients’ experiences with cancer-related fatigue: a review and synthesis of qualitative research. Oncol Nurs Forum. 2011;38(3):E191–203. doi: 10.1188/11.ONF.E191-E203. [DOI] [PubMed] [Google Scholar]
- 13.Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, Mouridsen HT. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast cancer research and treatment. 2007;105(2):209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 14.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK, Vogelzang NJ. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 15.Wang XS. Pathophysiology of cancer-related fatigue. Clin J Oncol Nurs. 2008;12(5 Suppl):11–20. doi: 10.1188/08.CJON.S2.11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XS, Woodruff JF. Cancer-related and treatment-related fatigue. Gynecol Oncol. 2015;136(3):446–452. doi: 10.1016/j.ygyno.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roscoe JA, Heckler CE, Morrow GR, Mohile SG, Dakhil SR, Wade JL, Kuebler JP. Prevention of delayed nausea: a University of Rochester Cancer Center Community Clinical Oncology Program study of patients receiving chemotherapy. J Clin Oncol. 2012;30(27):3389–3395. doi: 10.1200/JCO.2011.39.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Burish TG, Carey MP, Krozely MG, Greco FA. Conditioned side effects induced by cancer chemotherapy: prevention through behavioral treatment. Journal of consulting and clinical psychology. 1987;55(1):42–48. doi: 10.1037//0022-006x.55.1.42. [DOI] [PubMed] [Google Scholar]
- 20.Carey MP, Burish TG. Etiology and treatment of the psychological side effects associated with cancer chemotherapy: a critical review and discussion. Psychol Bull. 1988;104(3):307–325. doi: 10.1037/0033-2909.104.3.307. [DOI] [PubMed] [Google Scholar]
- 21.Gilliam LA, St Clair DK. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal. 2011;15(9):2543–2563. doi: 10.1089/ars.2011.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NCCN Breast Cancer: National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Breast Cancer, version 2. 2016:1–202. [Google Scholar]
- 23.Minton O, Alexander S, Stone PC. Identification of factors associated with cancer related fatigue syndrome in disease-free breast cancer patients after completing primary treatment. Breast cancer research and treatment. 2012;136(2):513–520. doi: 10.1007/s10549-012-2284-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Son BH, Hwang SY, Han W, Yang JH, Lee S, Yun YH. Fatigue and depression in disease-free breast cancer survivors: prevalence, correlates, and association with quality of life. J Pain Symptom Manage. 2008;35(6):644–655. doi: 10.1016/j.jpainsymman.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Garabeli Cavalli Kluthcovsky AC, Urbanetz AA, de Carvalho DS, Pereira Maluf EM, Schlickmann Sylvestre GC, Bonatto Hatschbach SB. Fatigue after treatment in breast cancer survivors: prevalence, determinants and impact on health-related quality of life. Support Care Cancer. 2012;20(8):1901–1909. doi: 10.1007/s00520-011-1293-7. [DOI] [PubMed] [Google Scholar]
- 26.Paiva CE, Paiva BS. Prevalence, predictors, and prognostic impact of fatigue among Brazilian outpatients with advanced cancers. Support Care Cancer. 2013;21(4):1053–1060. doi: 10.1007/s00520-012-1625-2. [DOI] [PubMed] [Google Scholar]
- 27.Stobaus N, Muller MJ, Kupferling S, Schulzke JD, Norman K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutrition and cancer. 2015;67(5):818–824. doi: 10.1080/01635581.2015.1040520. [DOI] [PubMed] [Google Scholar]
- 28.Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. European journal of cancer. 2009;45(3):384–392. doi: 10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Rissling M, Natarajan L, Fiorentino L, Mills PJ, Dimsdale JE, Sadler GR, Parker BA, Ancoli-Israel S. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep. 2012;35(2):237–245. doi: 10.5665/sleep.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Development of fatigue in cancer survivors: a prospective follow-up study from diagnosis into the year after treatment. J Pain Symptom Manage. 2013;45(2):213–222. doi: 10.1016/j.jpainsymman.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Banthia R, Malcarne VL, Ko CM, Varni JW, Sadler GR. Fatigued breast cancer survivors: the role of sleep quality, depressed mood, stage and age. Psychol Health. 2009;24(8):965–980. doi: 10.1080/08870440802110831. doi:10.1080/08870440802110831 905088851 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, Andrews PL. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 2002;10(4):329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 33.Roscoe JA, Kaufman ME, Matteson-Rusby SE, Palesh OG, Ryan JL, Kohli S, Perlis ML, Morrow GR. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(Suppl 1):35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 34.Pertl MM, Hevey D, Collier S, Lambe K, O’Dwyer AM. Predictors of fatigue in cancer patients before and after chemotherapy. J Health Psychol. 2014;19(6):699–710. doi: 10.1177/1359105313477675. [DOI] [PubMed] [Google Scholar]
- 35.Goedendorp MM, Gielissen MF, Verhagen CA, Peters ME, Bleijenberg G. Severe fatigue and related factors in cancer patients before the initiation of treatment. Br J Cancer. 2008;99(9):1408–1414. doi: 10.1038/sj.bjc.6604739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhruva A, Dodd M, Paul SM, Cooper BA, Lee K, West C, Aouizerat BE, Swift PS, Wara W, Miaskowski C. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs. 2010;33(3):201–212. doi: 10.1097/NCC.0b013e3181c75f2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer S, Kuhnt S, Zwerenz R, Eckert K, Hofmeister D, Dietz A, Giesinger J, Hauss J, Papsdorf K, Briest S, Brown A. Age- and sex-standardised prevalence rates of fatigue in a large hospital-based sample of cancer patients. Br J Cancer. 2011;105(3):445–451. doi: 10.1038/bjc.2011.251. doi:10.1038/bjc.2011.251bjc2011251 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soltow D, Given BA, Given CW. Relationship between age and symptoms of pain and fatigue in adults undergoing treatment for cancer. Cancer Nurs. 2010;33(4):296–303. doi: 10.1097/NCC.0b013e3181ce5a1a. [DOI] [PubMed] [Google Scholar]
- 39.Hamre H, Zeller B, Kanellopoulos A, Ruud E, Fossa SD, Loge JH, Aukrust P, Halvorsen B, Mollnes TE, Kiserud CE. Serum cytokines and chronic fatigue in adults surviving after childhood leukemia and lymphoma. Brain Behav Immun. 2013;30:80–87. doi: 10.1016/j.bbi.2013.01.006. doi:10.1016/j.bbi.2013.01.006S0889-1591(13)00009-3 [pii] [DOI] [PubMed] [Google Scholar]
- 40.Butt Z, Rao AV, Lai JS, Abernethy AP, Rosenbloom SK, Cella D. Age-associated differences in fatigue among patients with cancer. J Pain Symptom Manage. 2010;40(2):217–223. doi: 10.1016/j.jpainsymman.2009.12.016. doi:10.1016/j.jpainsymman.2009.12.016S0885-3924(10)00312-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Booth CM, Clemons M, Dranitsaris G, Joy A, Young S, Callaghan W, Trudeau M, Petrella T. Chemotherapy-induced nausea and vomiting in breast cancer patients: a prospective observational study. J Support Oncol. 2007;5(8):374–380. [PubMed] [Google Scholar]
- 42.Fiorentino L, Ancoli-Israel S. Insomnia and its treatment in women with breast cancer. Sleep Med Rev. 2006;10(6):419–429. doi: 10.1016/j.smrv.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol. 2009;27(31):5233–5239. doi: 10.1200/JCO.2008.21.6333. [DOI] [PubMed] [Google Scholar]
- 44.Heckler CE, Garland SN, Peoples AR, Perlis ML, Shayne M, Morrow GR, Kamen C, Hoefler J, Roscoe JA. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer. 2016;24(5):2059–2066. doi: 10.1007/s00520-015-2996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan JL, Heckler CE, Roscoe JA, Dakhil SR, Kirshner J, Flynn PJ, Hickok JT, Morrow GR. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer. 2012;20(7):1479–1489. doi: 10.1007/s00520-011-1236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


