Abstract
Objective
To compare the survival outcomes of patients with cervical squamous cell carcinoma (SCC) and adenocarcinoma/adenosquamous carcinoma (AC/ASC) among patients with locally advanced cervical cancer that were treated with definitive radiotherapy.
Methods
The baseline characteristics and outcome data of patients with locally advanced cervical cancer who were treated with definitive radiotherapy between November 1993 and February 2014 were collected and retrospectively reviewed. A Cox proportional hazards regression model was used to investigate the prognostic significance of AC/ASC histology.
Results
The patients with AC/ASC of the cervix exhibited significantly shorter overall survival (OS) (p=0.004) and progression-free survival (PFS) (p=0.002) than the patients with SCC of the cervix. Multivariate analysis showed that AC/ASC histology was an independent negative prognostic factor for PFS. Among the patients who displayed AC/ASC histology, larger tumor size, older age, and incomplete response to radiotherapy were found to be independent prognostic factors. PFS was inversely associated with the number of poor prognostic factors the patients exhibited (the estimated 1-year PFS rates; 100.0%, 77.8%, 42.8%, 0.0% for 0, 1, 2, 3 factors, respectively).
Conclusion
Locally advanced cervical cancer patients with AC/ASC histology experience significantly worse survival outcomes than those with SCC. Further clinical studies are warranted to develop a concurrent chemoradiotherapy (CCRT) protocol that is specifically tailored to locally advanced cervical AC/ASC.
Keywords: Uterine Cervical Neoplasms; Radiotherapy; Adenocarcinoma; Carcinoma, Adenosquamous
INTRODUCTION
Cervical cancer is the most common form of cancer affecting women in developing countries and the fourth most common type of malignancy affecting women worldwide, with almost half a million cases diagnosed each year. The incidence of cervical cancer has decreased by more than 40% during the past 40 years owing to the wider implementation of cytological screening. In contrast to the marked reduction in the incidence of squamous cell carcinoma (SCC), the absolute incidence of adenocarcinoma/adenosquamous carcinoma (AC/ASC) and its relative frequency compared with SCC have increased [1]. As a result, AC/ASC of the cervix currently accounts for approximately 20% of all cervical cancers, which is significantly higher than the 5% to 10% incidence observed in the 1970s [1,2].
On the basis of the results of recent prospective randomized clinical trials investigating the role of concurrent chemoradiotherapy (CCRT) as a treatment for locally advanced cervical cancer, concurrent chemotherapy combined with pelvic radiotherapy (RT) has become the standard adjuvant treatment for cervical cancer, regardless of the histological subtype of the disease [3,4,5,6]. However, the prognosis of cervical cancer patients with AC/ASC is yet to be determined, mainly because of the lack of prospective studies focusing on the prognostic differences between AC/ASC and SCC. Although some previous retrospective studies of early stage cervical cancer patients that were treated with radical surgery did not detect any survival differences between AC/ASC and SCC [7,8], the majority of the reports about this topic suggested that patients with AC/ASC have a worse prognosis than patients with SCC [9,10,11,12,13]. However, there is little information regarding the prognostic significance of AC/ASC among patients with locally advanced cervical cancer that are treated with definitive RT [13,14,15,16], and the reported results are conflicting. Rose et al. [15] showed that AC/ASC histology is associated with worse outcomes than SCC when treated with radiation alone, but similar outcomes to SCC when treated with CCRT. Lee et al. [16] recently reported that patients with AC exhibited worse overall survival (OS) than those with SCC regardless of the treatment modality (CCRT or RT alone). In contrast, Katanyoo et al. [14] suggested that AC histology does not affect survival outcomes. Thus, the prognostic significance of AC/ASC histology in patients who are treated with definitive CCRT merits further investigation.
In the current study, we retrospectively examined the prognostic significance of AC/ASC compared with SCC in cases of locally advanced cervical cancer that were treated with definitive RT.
MATERIALS AND METHODS
1. Patients
Permission to proceed with the data acquisition and analysis was obtained from Osaka University Hospital's Institutional Review Board. Informed consent was obtained from all patients. A list of patients who were treated with definitive RT for the International Federation of Gynecology and Obstetrics (FIGO) stage IIB–IVA cervical cancer at Osaka University Hospital from November 1993 to February 2014 was generated from our institutional tumor registry. Then, through a chart review, patients with AC/ASC or SCC histology were identified. At our institution, the histological classification of cancer is performed by two independent gynecological pathologists based on the World Health Organization (WHO) staging system for tumors of the uterine cervix [17]. The patients were clinically staged according to the FIGO staging criteria.
2. Definitive RT
The patients were treated with definitive RT consisting of external beam radiotherapy (EBRT) followed by high-dose rate intracavitary brachytherapy (HDR-ICBT) with or without platinum-based concurrent chemotherapy, as described previously [18,19]. Patients who developed cervical cancer before 1999 and patients above the age of 75 were treated with definitive RT without concurrent platinum-based chemotherapy. The EBRT was delivered based on computed tomography-based treatment planning, at a dose of 2 Gy per fraction, 5 times per week. The initial 30–40 Gy were delivered to the whole pelvis with a 4-field box, and then pelvic irradiation was conducted with a 4-cm-wide central shield to reduce the radiation exposure of the organs at risk. The total pelvic sidewall dose was 50 Gy in 25 fractions. After adequate tumor regression had been achieved with EBRT, HDR-ICBT was performed. The HDR-ICBT was performed once a week during the course of the EBRT with a midline block field. The planned total dose for the HDR-ICBT was 27.2 Gy in 4 fractions. Patients in whom it was found that it was unlikely that it would be possible to irradiate the whole tumor volume with ICBT were treated with interstitial brachytherapy (ISBT), as described previously [20]. EBRT was skipped on the days on which HDR-ICBT or HDR-ISBT was performed.
3. Concurrent chemotherapy
At our institution, nedaplatin is employed as a radiosensitizing agent for patients with cervical cancer [21,22,23]. Nedaplatin (40 mg/m2) was administered intravenously each week during the course of the pelvic EBRT, as reported previously.
4. Post-treatment follow-up
The patients were followed-up regularly by both gynecological oncologists and radiation oncologists, as reported previously [24,25].
5. Assessment of treatment outcomes
1) Response evaluation
We evaluated the tumor response at 3 months after the completion of the definitive RT. The response to treatment was assessed according to the Response Evaluation Criteria in Solid Tumors after every 3 cycles of each regimen. A complete response (CR) was defined as the disappearance of all target and non-target lesions and the absence of new lesions on two consecutive assessments performed at least 4 weeks apart. A partial response (PR) was defined as at least a 30% reduction in the sum of the longest dimensions of the target lesions. Progressive disease (PD) was defined as a 20% increase in the sum of the longest dimensions of the target lesions or the development of new lesions. Stable disease (SD) implies that none of the above apply.
2) Definitions of recurrence, progression-free survival (PFS), and OS
Pelvic recurrence was defined as the presence of tumor recurrence in the cervix, vagina, or pelvic area after successful planned treatment. Distant recurrence was defined as the emergence of disease outside the pelvic region. PFS was defined as the duration of the period from the first day of treatment to the detection of tumor progression (pelvic recurrence or distant recurrence) or death from any cause. OS was defined as the time from the first day of treatment to the date of death from any cause.
6. Statistical analysis
Continuous data were compared between the groups using the Student's t test, Wilcoxon rank-sum test, or median test, as appropriate. Frequency counts and proportions were compared between the groups using the χ2 test or the two-tailed Fisher's exact test, as appropriate. We performed univariate analyses by comparing the Kaplan-Meier curves for each subgroup with the log-rank test. OS was defined as the time from the date of the initial surgical procedure to the date of death or the last follow-up. Cox proportional hazards regression analysis was carried out to identify independent predictors of survival. p-values of <0.05 were considered to be statistically significant. All analyses were performed using the software JMP® pro, version 11.2.1 (SAS Institute, Cary, NC, USA).
RESULTS
1. Patients' characteristics
Two hundred and forty-nine patients were included in this retrospective study. Of these, 225 patients (90.4%) had SCC, and 24 (9.6%) had AC/ASC. Of a total of 24 AC/ASC, 11 were mucinous, 6 were endometrioid, 1 was serous, 1 was adenosquamous, in the remaining 5 patients, information regarding the AC subclassification was not available.
The characteristics of the patients are summarized in Table 1. The clinical stages of the patients with AC/ASC were significantly less severe than those of the patients with SCC (p=0.039). Moreover, adjuvant hysterectomy was performed more frequently in AC/ASC patients than in SCC patients (p=0.006).
Table 1. Clinicopathological characteristics of the patients with SCC or AC/ASC.
| Characteristics | No. of Patients (%) | p | |||
|---|---|---|---|---|---|
| All patients (n=249) | SCC (n=225) | AC/ASC (n=24) | |||
| Age (yr) | Mean (SD) | 61.5 (12.8) | 61.4 (12.9) | 62.6 (12.4) | NS* |
| ≤50 | 51.0 (20.5) | 46.0 (20.4) | 5.0 (20.8) | NS† | |
| >50 | 198.0 (79.5) | 179.0 (79.6) | 19.0 (79.2) | ||
| Treatments | RT | 139.0 (55.8) | 129.0 (57.3) | 10.0 (41.7) | NS† |
| CCRT | 110.0 (44.2) | 96.0 (42.7) | 14.0 (58.3) | ||
| FIGO stage | IIB | 96.0 (38.6) | 81.0 (36.0) | 15.0 (62.5) | 0.039† |
| IIIA | 12.0 (4.8) | 11.0 (4.9) | 1.0 (4.2) | ||
| IIIB | 124.0 (49.8) | 118.0 (52.4) | 6.0 (25.0) | ||
| IVA | 17.0 (8.8) | 15.0 (6.7) | 2.0 (8.3) | ||
| PLN meta | Negative | 176.0 (70.7) | 157.0 (69.8) | 19.0 (79.2) | NS† |
| Positive | 73.0 (29.3) | 68.0 (30.2) | 5.0 (20.8) | ||
| Tumor size (mm) | Mean (SD) | 52.2 (16.2) | 52.6 (16.2) | 48.5 (15.5) | NS* |
| ≤40 | 65.0 (26.1) | 58.0 (25.8) | 7.0 (29.2) | NS† | |
| >40 | 184.0 (73.9) | 167.0 (84.2) | 17.0 (70.8) | ||
| Duration of RT (day) | Median (min–max) | 45.0 (7–84) | 45.0 (7–66) | 47.5 (35–84) | NS‡ |
| ≤55 | 228.0 (91.6) | 208.0 (92.4) | 20.0 (83.3) | NS† | |
| >55 | 21.0 (8.4) | 17.0 (7.6) | 4.0 (16.7) | ||
| Pretreatment Hb (g/dL) | Mean (SD) | 11.7 (1.7) | 11.7 (1.7) | 11.8 (1.9) | NS* |
| <11.0 | 74.0 (29.7) | 68.0 (30.2) | 6.0 (25.0) | NS† | |
| ≥11.0 | 175.0 (70.3) | 157.0 (69.8) | 18.0 (75.0) | ||
| Adjuvant hysterectomy | Yes | 14.0 (5.6) | 9.0 (4.0) | 5.0 (20.8) | 0.006† |
| No | 235.0 (94.4) | 216.0 (96.0) | 19.0 (79.2) | ||
All statistical tests were 2-sided.
AC, adenocarcinoma; ASC, adenosquamous carcinoma; CCRT, concurrent chemoradiotherapy; FIGO, International Federation of Gynecology and Obstetrics; NS, not significant; PLN, popliteal lymph node; RT, radiotherapy; SCC, squamous cell carcinoma; SD, standard deviation.
*The Wilcoxon rank sum test was used to analyze continuous variables (age, tumor size, and the pretreatment hemoglobin level); †Fisher's exact test and the χ2 test were used to analyze categorical variables; ‡The median test was used to analyze continuous variables (the duration of RT).
2. Response to treatment
As shown in Table 2, in the SCC group 200 patients (88.9%) achieved a CR, 20 patients (8.9%) achieved a PR, and 5 patients (2.1%) achieved SD or PD. In contrast, in the AC/ASC group 16 patients (66.7%) achieved a CR, 5 patients (20.8%) achieved a PR, and 3 patients (12.5%) achieved SD or PD. The CR ratio of the patients with AC/ASC histology was significantly lower than that of the patients with SCC histology (p=0.002).
Table 2. Response to treatment.
| CR (%) | PR (%) | SD (%) | PD (%) | Total (%) | |
|---|---|---|---|---|---|
| SCC | 200 (88.9) | 20 (8.9) | 3 (1.3) | 2 (0.9) | 225 (100.0) |
| AC/ASC | 16 (66.7) | 5 (20.8) | 3 (12.5) | 0 (0.0) | 24 (100.0) |
p<0.001.
AC, adenocarcinoma; ASC, adenosquamous carcinoma; CR, complete response; PD, progressive disease; PR, partial response; SCC, squamous cell carcinoma; SD, stable disease.
3. Pattern of recurrence
Recurrence was observed in 105 (46.6%) patients in the SCC group and 16 (66.7%) patients in the AC/ASC group. The recurrence rate of the AC/ASC group was higher than that of the SCC group; however, the difference was not statistically significant (p=0.062). In the SCC group, 44 (41.9%) patients developed pelvic recurrence, 37 (35.2%) suffered distant recurrence, and 24 (22.9%) developed both pelvic and distant recurrence. In the AC/ASC group, 11 (68.8%) patients developed pelvic recurrence, and 5 (31.2%) patients suffered distant recurrence. When the two groups were compared, it was found that the patients in the AC/ASC group were more likely to develop pelvic recurrence than those in the SCC group (p=0.045).
4. Survival outcomes
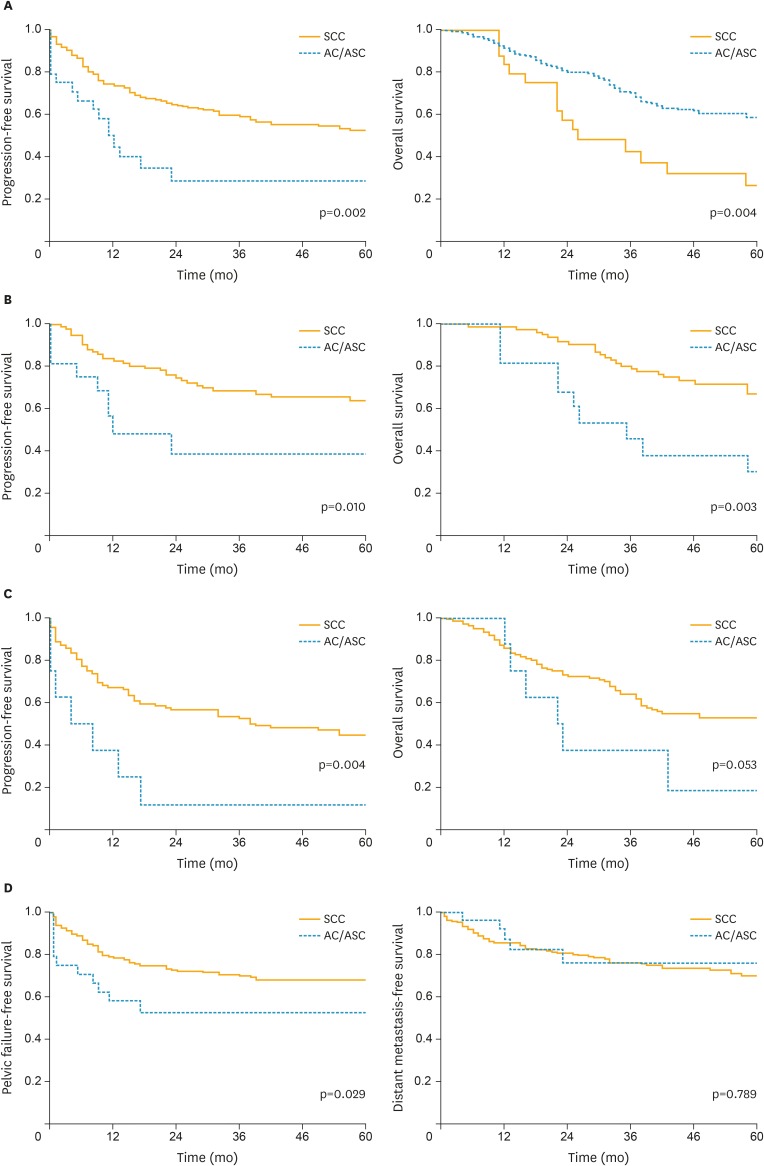
As shown in Table 3 and Fig. 1A-C, the univariate analyses demonstrated that AC/ASC histology was associated with significantly shorter PFS and OS. The estimated 5-year OS rates of the patients with AC/ASC histology and SCC histology were 26.7% and 58.6%, respectively. In the multivariate analysis (Table 3), it was found that in addition to type of treatment and incomplete response to RT, AC/ASC histology was also an independent predictor of PFS (hazard ratio [HR], 1.94; 95% confidence interval [CI], 1.07 to 3.35; p=0.031). Similar survival differences were observed in the separate analyses including patients with SCC and AC histology (Supplementary Fig. 1A).
Table 3. Univariate/multivariate analysis of prognostic factors for progression free survival (all patients).
| Characteristics | Univariate analysis of PFS | Multivariate analysis of PFS | |||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Age (yr) | ≤50 | 1 | 1 | ||
| >50 | 0.55 (0.37–0.83) | 0.006* | 0.76 (0.48–1.20) | NS | |
| Treatment | RT | 1 | 1 | ||
| CCRT | 0.97 (0.67–1.40) | NS | 0.53 (0.34–0.82) | 0.004* | |
| FIGO stage | IIB–IIIA | 1 | 1 | ||
| IIIB–IVA | 1.73 (1.19–2.55) | 0.004* | 1.49 (1.00–2.26) | 0.052 | |
| PLN meta | Negative | 1 | 1 | ||
| Positive | 1.46 (0.99–2.12) | 0.054 | 1.21 (0.79–1.83) | NS | |
| Tumor size (mm) | ≤40 | 1 | 1 | ||
| >40 | 2.24 (1.42–3.73) | <0.001* | 1.43 (0.87–2.46) | NS | |
| Histology | SCC | 1 | 1 | ||
| AC/ASC | 2.20 (1.25–3.63) | 0.008* | 1.94 (1.07–3.35) | 0.031* | |
| Duration of RT (day) | ≤55 | 1 | 1 | ||
| >55 | 1.17 (0.59–2.08) | NS | 1.00 (0.50–1.81) | NS | |
| Pretreat Hb (g/dL) | <11.0 | 1 | 1 | ||
| ≥11.0 | 0.50 (0.35–0.73) | <0.001* | 0.78 (0.52–1.21) | NS | |
| Response to treatment | CR | 1 | 1 | ||
| Non-CR | 8.47 (5.29–13.20) | <0.001* | 7.57 (4.29–13.30) | <0.001* | |
AC, adenocarcinoma; ASC, adenosquamous carcinoma; CCRT, concurrent chemoradiotherapy; CI, confidence interval; CR, complete response; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NS, not significant; PFS, progression-free survival; PLN, popliteal lymph node; RT, radiotherapy; SCC, squamous cell carcinoma.
*p-value is less than 0.05.
Fig. 1.
Clinical implications of AC/ASC histology in locally advanced cervical cancer patients.
Kaplan-Meier estimates of survival according to the histological subtype. (A) PFS and OS (all patients). (B) PFS and OS (stage IIB–IIIA patients). (C) PFS and OS (stage IIIB–IVA patients). (D) PFFS and DMFS (all patients).
AC, adenocarcinoma; ASC, adenosquamous carcinoma; DMFS, distant metastasis-free survival; OS, overall survival; PFFS, pelvic failure-free survival; PFS, progression-free survival; SCC, squamous cell carcinoma.
Given the high probability of local treatment failure in AC/ASC patients (Table 2), in Fig. 1D and Supplementary Fig. 2, the pelvic failure-free survival (PFFS) and distant metastasis-free survival (DMFS) were analyzed according to the histological subtypes. As shown, AC/ASC histology was associated with significantly shorter PFFS (all patients, p=0.029; stage IIB+IIIA, p=0.047; stage IIIB+IVA, p=0.047). However, DMFS in patients with AC/ASC histology was similar to that observed in SCC histology (all patients, p=0.789; stage IIB+IIIA, p=0.727; stage IIIB+IVA, p=0.891). In the separate analyses in which only patients with SCC and AC histology were included, the similar survival differences were observed (Supplementary Fig. 1B).
We next investigated the survival differences between AC/ASC and SCC according to the type of definitive RT administered. Among the 249 patients included in the current study, 139 (55.8%) received definitive RT alone (the RT group), and 110 (44.2%) were treated with definitive CCRT (the CCRT group). In the CCRT group, AC/ASC histology was associated with significantly shorter PFS (p<0.001) and OS (p=0.001) (Supplementary Fig. 3B). In contrast, in the RT group, the patients with AC/ASC histology demonstrated similar PFS (p=0.554) and OS (p=0.391) to the patients with SCC histology (Supplementary Fig. 3A). The multivariate analyses also produced similar results; i.e., AC/ASC histology was only found to be an independent predictor of PFS in the CCRT group (Supplementary Tables 1 and 2).
5. Prognostic factors in AC/ASC patients
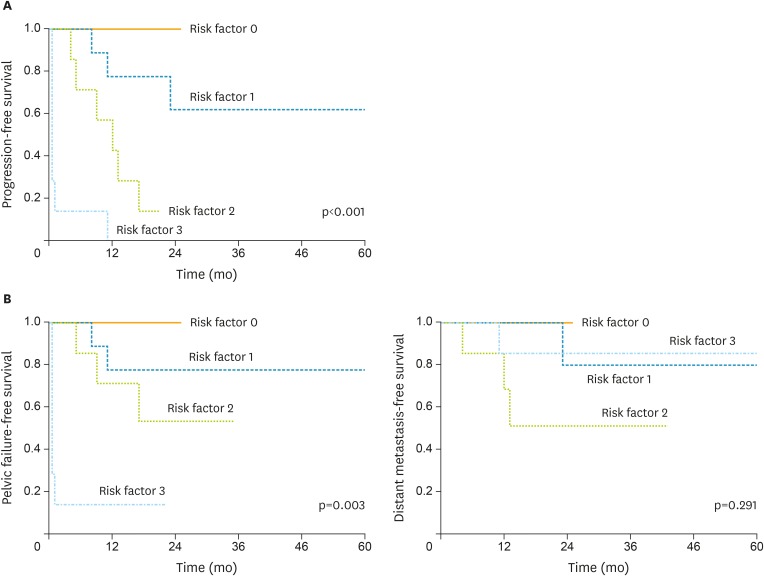
To elucidate which AC patients are at high risk of developing resistance to definitive RT, we conducted a multivariate analysis that only involved the AC/ASC patients. As shown in Table 4, larger tumor size (HR, 15.7; 95% CI, 1.53 to 458; p=0.018), older age (HR, 5.14; 95% CI, 1.12 to 32.6; p=0.035) and incomplete response to RT (HR, 11.4; 95% CI, 1.78 to 101; p=0.010) were found to be independent predictors of shorter PFS. Moreover, it was demonstrated that PFS was affected by the number of poor prognostic factors the patients exhibited (p<0.001, Fig. 2). The estimated 1-year PFS rates for those with 0, 1, 2, 3 factors were 100.0%, 77.8%, 42.8%, 0.0%, respectively.
Table 4. Prognostic factors in patients with AC/ASC histology.
| Characteristics | Univariate analysis of PFS | Multivariate analysis of PFS | |||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Age (yr) | ≤50 | 1 | 1 | ||
| >50 | 1.87 (0.58–8.25) | NS | 5.14 (1.12–32.60) | 0.035* | |
| Treatments | RT | 1 | 1 | ||
| CCRT | 1.86 (0.68–5.92) | NS | 2.95 (0.67–16.10) | NS | |
| FIGO stage | IIB–IIIA | 1 | 1 | ||
| IIIB–IVA | 1.06 (0.73–5.59) | NS | 1.60 (0.36–7.19) | NS | |
| PLN meta | Negative | 1 | 1 | ||
| Positive | 1.40 (0.39–4.05) | NS | 0.13 (0.01–1.08) | NS | |
| Tumor size (mm) | ≤40 | 1 | 1 | ||
| >40 | 11.20 (2.22–203.00) | 0.001* | 15.70 (1.53–458.00) | 0.018* | |
| Duration of RT (day) | ≤55 | 1 | 1 | ||
| >55 | 0.77 (0.12–2.81) | NS | 0.35 (0.04–1.92) | NS | |
| Pretreatment Hb (g/dL) | <11.0 | 1 | 1 | ||
| ≥11.0 | 1.36 (0.43–5.98) | NS | 0.86 (0.08–8.79) | NS | |
| Response to treatment | CR | 1 | 1 | ||
| Non-CR | 6.15 (2.04–19.40) | 0.002* | 11.40 (1.78–101.00) | 0.010* | |
AC, adenocarcinoma; ASC, adenosquamous carcinoma; CCRT, concurrent chemoradiotherapy; CI, confidence interval; CR, complete response; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NS, not significant; PFS, progression-free survival; PLN, popliteal lymph node; RT, radiotherapy.
*p-value is less than 0.05.
Fig. 2.
Survival difference according to the poor prognostic factors possessed in AC/ASC patients.
(A) Kaplan-Meier estimates of PFS. The PFS of the patients was inversely associated with the number of poor prognostic factors they possessed (p<0.001). The estimated 1-year PFS rates for those with 0, 1, 2, 3 factors were 100.0%, 77.8%, 42.8%, 0.0%, respectively. (B) Kaplan-Meier estimates of PFFS and DMFS.
AC, adenocarcinoma; ASC, adenosquamous carcinoma; DMFS, distant metastasis-free survival; PFFS, pelvic failure-free survival; PFS, progression-free survival.
DISCUSSION
In the current study, the AC/ASC patients displayed a worse prognosis than the SCC patients. The estimated 5-year OS rates of the patients that exhibited AC/ASC histology and SCC histology were 26.7% and 58.6%, respectively.
Although a previous report demonstrated that the addition of concurrent chemotherapy to pelvic RT abolished the adverse prognostic impact of AC histology [13], in the current study the patients with AC/ASC histology displayed worse survival than the patients with SCC in the CCRT group (Supplementary Fig. 2). The reason for this remains unknown, but it might be partially explained by the smaller number of patients in the present study.
In an analysis aimed at identifying prognostic factors for patients with AC/ASC, larger tumor size (>40 mm) was found to be an independent prognostic factor of reduced PFS. This is consistent with the findings of an earlier report involving 302 stage I–IV AC patients, which suggested that tumor bulk was of prognostic significance [26]. We also found that older age (>50) is an independent prognostic factor for reduced PFS in patients with AC/ASC histology. The prognostic significance of older age in locally advanced cervical AC has also been reported previously; i.e., women younger than 35 years achieved significantly better survival than those over 65 years [27]. Importantly, as shown in Fig. 2, it was found that patient survival was affected by the types of poor prognostic factors possessed by the patients. Thus, identifying such risk factors might allow the precise estimation of PFS or OS in this patient population.
The results of the current study indicate that there is a need for more effective RT protocols for treating patients with locally advanced cervical cancer who exhibit AC/ASC histology, especially older patients and those with larger tumors. One possible treatment strategy is the use of adjuvant chemotherapy after definitive RT. The aim of adjuvant chemotherapy after the completion of chemoradiation is to eradicate micrometastases that might have escaped the radiation field or were not detected and to consolidate the local effects of the CCRT. The efficacy of adjuvant chemotherapy following definitive CCRT for locally advanced cervical cancer is currently being evaluated in the OUTBACK trial, in which patients were randomly assigned to receive 4 cycles of adjuvant chemotherapy involving carboplatin and paclitaxel or to undergo observation, after cisplatin-based CCRT [28]. Next possibility is the use of neoadjuvant chemotherapy (NAC) before CCRT. The use of both NAC plus adjuvant chemotherapy in combination with CCRT in patients with AC histology has recently been reported [29]. In a recent trial, 880 patients with AC of the uterine cervix were randomized to receive CCRT or CCRT preceded by one cycle of NAC and adjuvant chemotherapy involving cisplatin and paclitaxel. The patients who received CCRT combined with NAC and adjuvant chemotherapy exhibited significantly longer disease-free survival (p<0.05), cumulative OS (p<0.05), and long-term local tumor control (p<0.05). The patients who received CCRT combined with NAC and adjuvant chemotherapy also demonstrated lower rates of local and distant failure (p<0.05). These results indicate that the addition of NAC and adjuvant chemotherapy involving paclitaxel and cisplatin to CCRT is effective in cervical cancer patients with AC histology. Another possibility is the use of targeted agents in combination with CCRT. Recently, Wright et al. [30] characterized the molecular profiles of 40 AC and 40 SCC of the cervix and found significant differences in their genetic alterations: AC and SCC: KRAS mutations, 17.5% vs. 0.0% (p=0.010); EGFR mutations, 0.0% vs. 7.5% (p=0.240); and PIK3CA mutations, 25.0% vs. 37.5% (p=0.330), respectively. Moreover, they found that PIK3CA mutations were associated with shorter OS. Thus, these genetic alterations and the resultant changes in protein expression might represent targets for future therapies for cervical AC/ASC. A recent phase II study of EGFR inhibitor erlotinib plus standard cisplatin-based CCRT has shown an encouraging result against locally advanced cervical cancer: 94.0% of patients achieved a CR with the 2-year and 3-year cumulative OS and PFS rates were 91.7% and 80.6% and 80.0% and 73.8%, respectively. mTOR-inhibitor temsirolimus has also shown clinical activity in recurrent or metastatic cervical cancer patients: with about two-thirds of patients exhibiting stable disease [31]. In contrast, in a phase II trial evaluating the efficacy of the anti-EGFR antibody cetuximab, cetuximab has shown limited activity in patients with persistent or recurrent cervical cancer [32]. However, as none of the previous and ongoing clinical trials are designed to evaluate the efficacy of a novel agent according to histologic subtypes, the activity of novel agents in AC/ASC histology remains unknown. Future clinical studies specifically targeting AC/ASC histology are warranted. Lastly, as shown in Table 3 and Fig. 1D the main problem in the treatment of locally-advanced cervical AC/ASC using RT is the lower CR rate and the resulting shorter PFFS rather than distant metastasis. Thus, adjuvant hysterectomy after RT might be more important than adjuvant chemotherapy or NAC in AC/ASC patients. We hope the efficacy of post-radiation adjuvant hysterectomy be investigated in patients with locally-advanced AC/ASC patients in the future.
The limitations of our study need to be addressed. The first is that our study was conducted at a single institution and included a relatively small number of patients. Second, due to its retrospective nature, we cannot exclude potential sources of biases, e.g., selection bias might have been introduced by the physicians when determining and allocating the treatment modalities. Third, as this study covers a long period, changes in the choice of treatments for recurrent disease, the pretreatment work-up and diagnostic procedures, and improvements in RT procedures might have affected the patients' survival. Lastly, a recent meta-analysis showed that ASC histology may be associated with poorer outcomes compared with AC histology [33]. Patient's survival may also be influenced by the subtypes of AC such as endometrioid, mucinous, clear cell, serous, or mesonephric. Moreover, due to its aggressive clinical behaviors, “gastric type mucinous AC” has gotten a lot of attention recently. However, in the current study, due to the limited number of AC/ASC patients included, we could not draw any conclusions for these issues. The prognostic significance of ASC or AC subtypes in comparison with SCC should be evaluated in the large-scale, multi-center study in the future.
In conclusion, the present study demonstrated that cervical cancer patients with AC/ASC histology experience significantly worse survival outcomes than those with SCC. Although the current guidelines for cervical cancer recommend the same CCRT protocol regardless of the histological subtype of the patient's disease, future clinical studies are warranted to evaluate and develop a CCRT protocol that is specifically tailored to locally advanced cervical AC/ASC.
ACKNOWLEDGMENTS
The authors thank their colleagues Drs. Kiyoshi Yoshino, Kenjiro Sawada, Yutaka Ueda, and Eiji Kobayashi for participating in this study.
Footnotes
Conflict of Interest: No potential conflicts of interest relevant to this article was reported.
Supplementary Materials
Clinical implications of AC histology in locally advanced cervical cancer patients. Kaplan-Meier estimates of survival according to the histological subtype. (A) PFS and OS (all patients; SCC, n=225; AC, n=23). (B) PFFS and DMFS (all patients; SCC, n=225; AC, n=23).
AC, adenocarcinoma; DMFS, distant metastasis-free survival; OS, overall survival; PFFS, pelvic failure-free survival; PFS, progression-free survival; SCC, squamous cell carcinoma.
Kaplan-Meier estimates of PFFS and DMFS between the patients with AC/ASC and SCC histology. AC/ASC histology was associated with significantly shorter PFFS. However, DMFS was not affected by histology. (A) Stage IIB–IIIA patients. (B) Stage IIIB–IVA patients.
AC, adenocarcinoma; ASC, adenosquamous carcinoma; DMFS, distant metastasis-free survival; PFFS, pelvic failure-free survival; SCC, squamous cell carcinoma.
Survival difference between the patients with AC/ASC and SCC histology according to the type of radiotherapy administered. Kaplan-Meier estimates of survival according to the histological subtype. (A) Patients treated with definitive RT alone: AC/ASC histology exhibited similar PFS (p=0.554) and OS (p=0.391) to those with SCC histology. (B) Patients treated with CCRT: AC/ASC histology demonstrated significantly shorter PFS (p<0.001) and OS (p=0.001) than those with SCC histology.
AC, adenocarcinoma; ASC, adenosquamous carcinoma; CCRT, concurrent chemoradiotherapy; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; SCC, squamous cell carcinoma.
Univariate/multivariate analysis of prognostic factors for PFS (patients treated with definitive RT)
AC, adenocarcinoma; ASC, adenosquamous carcinoma; CI, confidence interval; CR, complete response; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NS, not significant; PFS, progression-free survival; PLN, popliteal lymph node; RT, radiotherapy; SCC, squamous cell carcinoma.
Univariate/multivariate analysis of prognostic factors for PFS (patients treated with definitive CCRT)
AC, adenocarcinoma; ASC, adenosquamous carcinoma; CCRT, concurrent chemoradiotherapy; CI, confidence interval; CR, complete response; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NS, not significant; PFS, progression-free survival; PLN, popliteal lymph node; RT, radiotherapy; SCC, squamous cell carcinoma.
References
- 1.Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer. 2004;100:1035–1044. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Carstensen B, Møller H, Zappa M, Zakelj MP, Lawrence G, et al. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomarkers Prev. 2005;14:2191–2199. doi: 10.1158/1055-9965.EPI-05-0231. [DOI] [PubMed] [Google Scholar]
- 3.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 4.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 5.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 6.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 7.Shingleton HM, Bell MC, Fremgen A, Chmiel JS, Russell AH, Jones WB, et al. Is there really a difference in survival of women with squamous cell carcinoma, adenocarcinoma, and adenosquamous cell carcinoma of the cervix? Cancer. 1995;76:1948–1955. doi: 10.1002/1097-0142(19951115)76:10+<1948::aid-cncr2820761311>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Kasamatsu T, Onda T, Sawada M, Kato T, Ikeda S, Sasajima Y, et al. Radical hysterectomy for FIGO stage I–IIB adenocarcinoma of the uterine cervix. Br J Cancer. 2009;100:1400–1405. doi: 10.1038/sj.bjc.6605048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabuchi S, Okazawa M, Kinose Y, Matsuo K, Fujiwara M, Suzuki O, et al. Comparison of the prognoses of FIGO stage I to stage II adenosquamous carcinoma and adenocarcinoma of the uterine cervix treated with radical hysterectomy. Int J Gynecol Cancer. 2012;22:1389–1397. doi: 10.1097/IGC.0b013e31826b5d9b. [DOI] [PubMed] [Google Scholar]
- 10.Mabuchi S, Okazawa M, Matsuo K, Kawano M, Suzuki O, Miyatake T, et al. Impact of histological subtype on survival of patients with surgically-treated stage IA2–IIB cervical cancer: adenocarcinoma versus squamous cell carcinoma. Gynecol Oncol. 2012;127:114–120. doi: 10.1016/j.ygyno.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi T, Ishikawa H, Suzuki Y, Inoue T, Nakamura S, Kuzuya K. A comparison of prognoses of pathologic stage Ib adenocarcinoma and squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 2000;79:289–293. doi: 10.1006/gyno.2000.5935. [DOI] [PubMed] [Google Scholar]
- 12.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes after radical hysterectomy in patients with early-stage adenocarcinoma of uterine cervix. Br J Cancer. 2010;102:1692–1698. doi: 10.1038/sj.bjc.6605705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galic V, Herzog TJ, Lewin SN, Neugut AI, Burke WM, Lu YS, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012;125:287–291. doi: 10.1016/j.ygyno.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Katanyoo K, Sanguanrungsirikul S, Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol. 2012;125:292–296. doi: 10.1016/j.ygyno.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Rose PG, Java JJ, Whitney CW, Stehman FB, Lanciano R, Thomas GM. Locally advanced adenocarcinoma and adenosquamous carcinomas of the cervix compared to squamous cell carcinomas of the cervix in gynecologic oncology group trials of cisplatin-based chemoradiation. Gynecol Oncol. 2014;135:208–212. doi: 10.1016/j.ygyno.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JY, Kim YT, Kim S, Lee B, Lim MC, Kim JW, et al. Prognosis of cervical cancer in the era of concurrent chemoradiation from national database in Korea: a comparison between squamous cell carcinoma and adenocarcinoma. PLoS One. 2015;10:e0144887. doi: 10.1371/journal.pone.0144887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavassoli FA, Devilee P, editors. Pathology and genetics of tumours of the breast and female genital organs: WHO classification of tumours. Lyon: IARC Press; 2003. Tumours of the uterine cervix; pp. 259–289. [Google Scholar]
- 18.Fujiwara M, Isohashi F, Mabuchi S, Yoshioka Y, Seo Y, Suzuki O, et al. Efficacy and safety of nedaplatin-based concurrent chemoradiotherapy for FIGO Stage IB2–IVA cervical cancer and its clinical prognostic factors. J Radiat Res (Tokyo) 2015;56:305–314. doi: 10.1093/jrr/rru101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mabuchi S, Ugaki H, Isohashi F, Yoshioka Y, Temma K, Yada-Hashimoto N, et al. Concurrent weekly nedaplatin, external beam radiotherapy and high-dose-rate brachytherapy in patients with FIGO stage IIIb cervical cancer: a comparison with a cohort treated by radiotherapy alone. Gynecol Obstet Invest. 2010;69:224–232. doi: 10.1159/000273207. [DOI] [PubMed] [Google Scholar]
- 20.Isohashi F, Yoshioka Y, Koizumi M, Konishi K, Sumida I, Takahashi Y, et al. High-dose-rate interstitial brachytherapy for previously untreated cervical carcinoma. Brachytherapy. 2009;8:234–239. doi: 10.1016/j.brachy.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Mabuchi S, Kimura T. Nedaplatin: a radiosensitizing agent for patients with cervical cancer. Chemother Res Pract. 2011;2011:963159. doi: 10.1155/2011/963159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mabuchi S, Morishige K, Isohashi F, Yoshioka Y, Takeda T, Yamamoto T, et al. Postoperative concurrent nedaplatin-based chemoradiotherapy improves survival in early-stage cervical cancer patients with adverse risk factors. Gynecol Oncol. 2009;115:482–487. doi: 10.1016/j.ygyno.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Okazawa M, Mabuchi S, Isohashi F, Suzuki O, Yoshioka Y, Sasano T, et al. Impact of the addition of concurrent chemotherapy to pelvic radiotherapy in surgically treated stage IB1–IIB cervical cancer patients with intermediate-risk or high-risk factors: a 13-year experience. Int J Gynecol Cancer. 2013;23:567–575. doi: 10.1097/IGC.0b013e31828703fd. [DOI] [PubMed] [Google Scholar]
- 24.Mabuchi S, Isohashi F, Maruoka S, Hisamatsu T, Takiuchi T, Yoshioka Y, et al. Post-treatment follow-up procedures in cervical cancer patients previously treated with radiotherapy. Arch Gynecol Obstet. 2012;286:179–185. doi: 10.1007/s00404-012-2235-4. [DOI] [PubMed] [Google Scholar]
- 25.Mabuchi S, Isohashi F, Yoshioka Y, Temma K, Takeda T, Yamamoto T, et al. Prognostic factors for survival in patients with recurrent cervical cancer previously treated with radiotherapy. Int J Gynecol Cancer. 2010;20:834–840. doi: 10.1111/IGC.0b013e3181dcadd1. [DOI] [PubMed] [Google Scholar]
- 26.Chen RJ, Chang DY, Yen ML, Lee EF, Huang SC, Chow SN, et al. Prognostic factors of primary adenocarcinoma of the uterine cervix. Gynecol Oncol. 1998;69:157–164. doi: 10.1006/gyno.1998.4971. [DOI] [PubMed] [Google Scholar]
- 27.Baalbergen A, Ewing-Graham PC, Hop WC, Struijk P, Helmerhorst TJ. Prognostic factors in adenocarcinoma of the uterine cervix. Gynecol Oncol. 2004;92:262–267. doi: 10.1016/j.ygyno.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Clinical Trials.gov. Cisplatin and radiation therapy with or without carboplatin and paclitaxel in patients with locally advanced cervical cancer ( NCT01414608) [Internet] Bethesda, MD: Clinical Trials.gov; [cited 2016 Jun 27]. Available from: http://www.clinicaltrials.gov. [Google Scholar]
- 29.Tang J, Tang Y, Yang J, Huang S. Chemoradiation and adjuvant chemotherapy in advanced cervical adenocarcinoma. Gynecol Oncol. 2012;125:297–302. doi: 10.1016/j.ygyno.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 30.Wright AA, Howitt BE, Myers AP, Dahlberg SE, Palescandolo E, Van Hummelen P, et al. Oncogenic mutations in cervical cancer: genomic differences between adenocarcinomas and squamous cell carcinomas of the cervix. Cancer. 2013;119:3776–3783. doi: 10.1002/cncr.28288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tinker AV, Ellard S, Welch S, Moens F, Allo G, Tsao MS, et al. Phase II study of temsirolimus (CCI-779) in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix. A trial of the NCIC Clinical Trials Group (NCIC CTG IND 199) Gynecol Oncol. 2013;130:269–274. doi: 10.1016/j.ygyno.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Santin AD, Sill MW, McMeekin DS, Leitao MM, Jr, Brown J, Sutton GP, et al. Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;122:495–500. doi: 10.1016/j.ygyno.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JY, Lee C, Hahn S, Kim MA, Kim HS, Chung HH, et al. Prognosis of adenosquamous carcinoma compared with adenocarcinoma in uterine cervical cancer: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2014;24:289–294. doi: 10.1097/IGC.0000000000000063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical implications of AC histology in locally advanced cervical cancer patients. Kaplan-Meier estimates of survival according to the histological subtype. (A) PFS and OS (all patients; SCC, n=225; AC, n=23). (B) PFFS and DMFS (all patients; SCC, n=225; AC, n=23).
AC, adenocarcinoma; DMFS, distant metastasis-free survival; OS, overall survival; PFFS, pelvic failure-free survival; PFS, progression-free survival; SCC, squamous cell carcinoma.
Kaplan-Meier estimates of PFFS and DMFS between the patients with AC/ASC and SCC histology. AC/ASC histology was associated with significantly shorter PFFS. However, DMFS was not affected by histology. (A) Stage IIB–IIIA patients. (B) Stage IIIB–IVA patients.
AC, adenocarcinoma; ASC, adenosquamous carcinoma; DMFS, distant metastasis-free survival; PFFS, pelvic failure-free survival; SCC, squamous cell carcinoma.
Survival difference between the patients with AC/ASC and SCC histology according to the type of radiotherapy administered. Kaplan-Meier estimates of survival according to the histological subtype. (A) Patients treated with definitive RT alone: AC/ASC histology exhibited similar PFS (p=0.554) and OS (p=0.391) to those with SCC histology. (B) Patients treated with CCRT: AC/ASC histology demonstrated significantly shorter PFS (p<0.001) and OS (p=0.001) than those with SCC histology.
AC, adenocarcinoma; ASC, adenosquamous carcinoma; CCRT, concurrent chemoradiotherapy; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; SCC, squamous cell carcinoma.
Univariate/multivariate analysis of prognostic factors for PFS (patients treated with definitive RT)
AC, adenocarcinoma; ASC, adenosquamous carcinoma; CI, confidence interval; CR, complete response; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NS, not significant; PFS, progression-free survival; PLN, popliteal lymph node; RT, radiotherapy; SCC, squamous cell carcinoma.
Univariate/multivariate analysis of prognostic factors for PFS (patients treated with definitive CCRT)
AC, adenocarcinoma; ASC, adenosquamous carcinoma; CCRT, concurrent chemoradiotherapy; CI, confidence interval; CR, complete response; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NS, not significant; PFS, progression-free survival; PLN, popliteal lymph node; RT, radiotherapy; SCC, squamous cell carcinoma.