Abstract
Objective
Obesity is associated with insulin resistance and adipose tissue inflammation. Reactive oxygen species (ROS) increase in adipose tissue during the development of obesity. We previously showed that in response to excess nutrients like glucose and palmitate, adipocytes generated ROS via NADPH oxidase (NOX) 4, the major adipocyte isoform, instead of using mitochondrial oxidation. However, the role of NOX4-derived ROS in the development of whole body insulin resistance, adipocyte inflammation, and recruitment of macrophages to adipose tissue during the development of obesity is unknown.
Approach and Results
In this study, control C57BL/6 mice and mice in which NOX4 has been deleted specifically in adipocytes were fed a high fat, high sucrose (HFHS) diet. During the development of obesity in control mice, adipocyte NOX4 and PPP activity were transiently increased. Primary adipocytes differentiated form mice with adipocytes deficient in NOX4 showed resistance against high glucose or palmitate-induced adipocyte inflammation. Mice with adipocytes deficient in NOX4 showed a delayed onset of insulin resistance during the development of obesity, with an initial reduction in adipose tissue inflammation that normalized with prolonged HFHS feeding.
Conclusions
These findings imply that NOX4-derived ROS may play a role in the onset of insulin resistance and adipose tissue inflammation. As such, therapeutics targeting NOX4-mediated ROS production could be effective in preventing obesity-associated conditions such as insulin resistance.
Keywords: Adipocyte, Reactive oxygen species, Obesity, NADPH oxidase, Insulin resistance
Introduction
Excess energy derived from glucose or fatty acids is stored as triglycerides in adipocytes and leads to obesity, which is characterized by adipocyte hypertrophy and accumulation of macrophages in adipose tissue1–3. In obesity, adipocytes secrete chemotactic factors such as monocyte chemotactic protein-1 (MCP-1)4–6 and serum amyloid A3 (SAA3)5, 6, contributing to recruitment of immune cells such as macrophages7–9. Both adipocytes and macrophages secrete pro-inflammatory molecules, which may promote insulin resistance and systemic inflammation. During the development of obesity, reactive oxygen species (ROS) have been implicated as contributors to both the onset and the progression of insulin resistance10, 11. ROS in visceral adipose tissue are significantly increased in genetically obese mice and mice made obese by consumption of a high-fat diet12. However, the temporal contributions of adipose tissue ROS, inflammation, and immune cell infiltration to obesity-associated insulin resistance remain poorly defined.
We previously showed that excess glucose and palmitate are not metabolized to a major extent via mitochondrial oxidation in adipocytes. Instead excess nutrients activate NADPH oxidase (NOX) and the pentose phosphate pathway (PPP), which is a major source of cellular NADPH and leads to NOX activation13. NOXs are membrane-bound enzyme complexes that transfer electrons from NADPH to oxygen, generating superoxide. We have also found that NOX4 is the major NOX isoform in cultured murine and human adipocytes13. Moreover, NOX4-derived ROS were stimulated by excess glucose as well as palmitate, leading to increased chemotactic factor expression in cultured adipocytes13. Other studies showed that NOX4 and PPP activity increase in adipose tissue with diet induced obesity (DIO), and treatment with the NOX inhibitor apocynin, or the PPP inhibitor dehydroepiandrosterone (DHEA), reduces ROS generation and obesity-related inflammation12,14. Despite the relationship between obesity, ROS production and NOX4 activity, the role of adipocyte NOX4-derived ROS in the onset of insulin resistance, adipocyte inflammation, and recruitment of macrophages to adipose tissue during the development of obesity has not yet been explored in vivo.
In this study, we determined whether NOX4 and/or PPP activity are altered during the development of insulin resistance in mice challenged with a high fat, high sucrose (HFHS) diet. Further, we utilized a new mouse model in which NOX4 has been deleted specifically in adipocytes to characterize the pathophysiological role of NOX4 in an obesogenic diet-induced obesity model that develop insulin resistance over time. We now show that adipocyte NOX4 and PPP activity are transiently increased during the development of obesity. Moreover, mice with adipocytes deficient in NOX4 show a delayed onset of insulin resistance with reduced adipose tissue inflammation. However, with prolonged HFHS diet feeding, mice with adipocyte-specific NOX4 deficiency show the same extent of insulin resistance and adipose tissue inflammation as control wild type mice. Collectively, these data suggest that NOX4-derived ROS from adipocytes may play a role in the onset of insulin resistance and adipose tissue inflammation. Such enhanced understanding of the interplay between ROS, adipocyte inflammation, and adipose tissue macrophage accumulation could provide novel directions for prevention of obesity-associated conditions such as insulin resistance.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
PPP and NOX4 activity are transiently increased during the development of obesity in control wild type mice
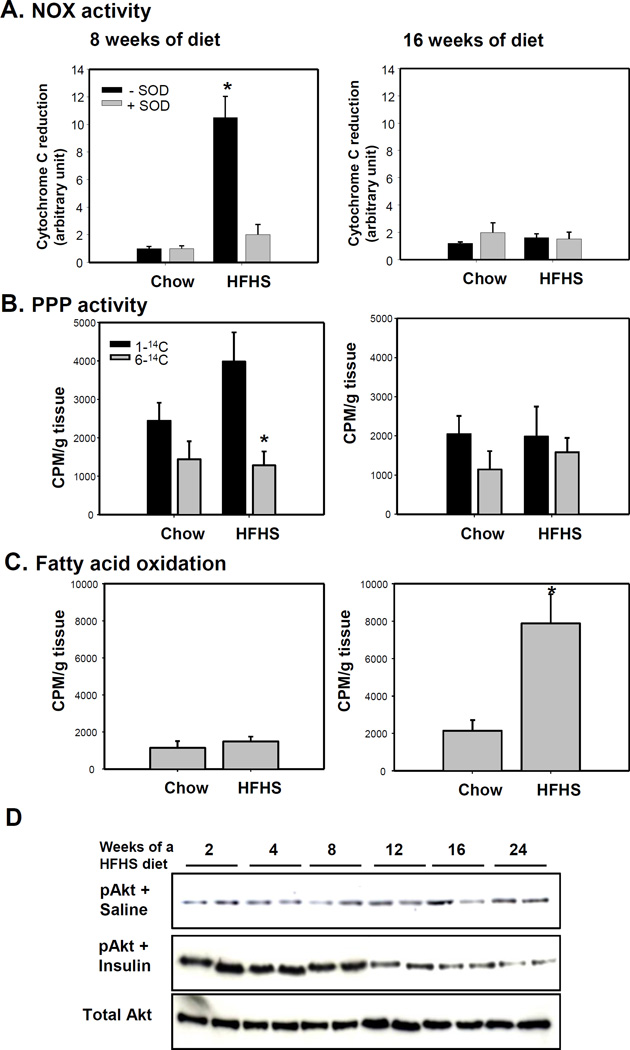
To determine whether PPP and NOX4 activities change during the progression of obesity, we fed male C57BL/6 mice fed either a HFHS or chow (control) diets for 8 and 16 weeks as we have done previously15. SOD-inhibitable NOX4 and PPP activity in epididymal adipocytes was increased after 8 weeks of HFHS diet, while these activities were diminished after 16 weeks on the HFHS diet (Figure 1A–B). Since overloading the mitochondrial oxidation system leads to leakage of electrons as a source of ROS and β-oxidation of FFAs could also be a potential source of mitochondrial-derived ROS, we measured fatty acid-oxidation. Fatty acid-oxidation was only increased after 16 weeks on the HFHS diet (Figure 1C). To determine whether increasing fatty acid-oxidation is due to increasing mitochondrial abundance, we measured mitochondrial DNA (mtDNA) abundance relative to nuclear DNA. We did not find any significant change in mtDNA/nuclear DNA ratio between 8 and 16 weeks on HFHS or chow diets (data not shown). Insulin-dependent phosphorylation of Akt in epididymal adipocytes was high for the first 8 weeks of HFHS feeding, and gradually waned after 12 weeks (Figure 1D). These data suggest that NOX4-derived ROS were generated by HFHS feeding, but were diminished coincidently with the onset of insulin resistance. Conversely, ROS derived from fatty acid oxidation were generated after adipocytes became insulin resistant.
Figure 1. NOX and PPP activity are increased before adipocytes become insulin resistant while β-oxidation is increased after adipocytes become insulin resistant during the progression of obesity.
C57BL/6 mice were fed HFHS or chow diets for the indicated time periods (n=5). A–C; At sacrifice, the AE fraction from epididymal fat was harvested and analyzed for NOX activity (A), PPP activity (B), or fatty acid-oxidation (C) using 0.5 µCi of [1–14C] or [6–14C] glucose, and 0.5 µCi of [1–14C] oleate, respectively. *P<0.001 vs chow diet. SOD = superoxide dismutase. ANOVA and Bonferroni post-hoc test for figure 1A and B, and Student-t test for figure 1C. D; 15min prior to sacrifice, mice received insulin or saline injections, and the AE fraction from epididymal fat was harvested and analyzed by immunoblotting using anti-phospho-Akt or total Akt antibodies. Data are representative of at least 3 independent experiments (n=2).
Adipocyte-specific NOX4-deficient mice show delayed onset of insulin resistance
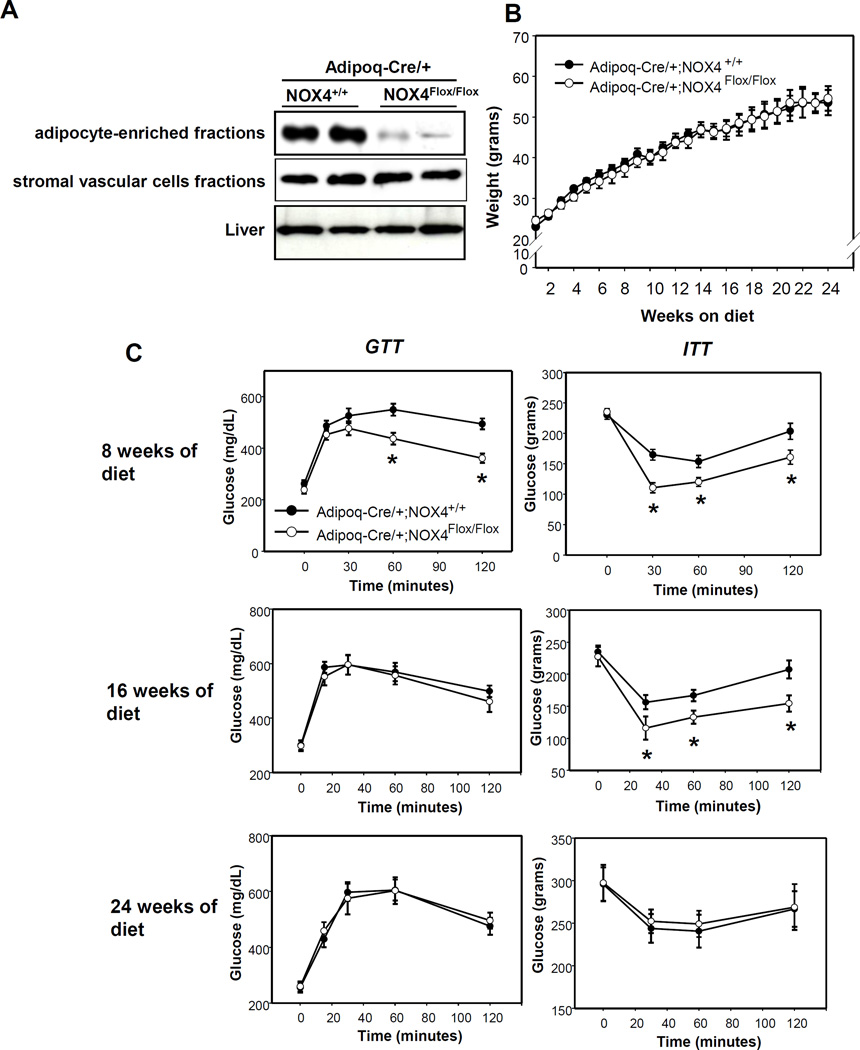
To investigate the pathophysiological role of adipocyte-specific NOX4 in a mouse model of obesogenic diet-induced obesity, we have generated Adipoq-Cre/+;NOX4Flox/Flox mice as described in Methods. To verify adipocyte-specific deletion of NOX4, we performed Western blot analysis after obtaining lysates of the AE and stromal vascular cell (SVC) fraction from epididymal white adipose tissue (EWAT) and whole liver. As shown in Figure 2A, the AE fraction from Adipoq-Cre/+;NOX4Flox/Flox EWAT shows almost complete loss of NOX4 protein, while expression is maintained in SVC fractions and liver. To assess the effect of adipocyte-specific NOX4 deletion on metabolic homeostasis, we compared body weight gain, glucose tolerance and insulin tolerance in Adipoq-Cre/+;NOX4Flox/Flox and Adipoq-Cre/+;NOX4+/+ control mice fed a HFHS diet for up to 24 weeks. No differences in body weights between genotypes were observed during the course of the study (Figure 2B). Adipoq-Cre/+;NOX4Flox/Flox mice showed improved glucose and insulin tolerance after 8 weeks, improved insulin tolerance after 16 weeks, and no improvements after 24 weeks of HFHS feeding, suggesting a transient improvement in insulin sensitivity during the development of obesity. Consistent with this, fasting insulin levels were low in Adipoq-Cre/+;NOX4Flox/Flox mice at 8 weeks and GTT insulin levels was also low in in Adipoq-Cre/+;NOX4Flox/Flox mice at 12 and 16 weeks (Table 1). In addition, plasma adiponectin levels were higher in Adipoq-Cre/+;NOX4Flox/Flox mice at 12 and 16 weeks (Table 1), suggesting the potential involvement of this adipokine in the improvements in insulin sensitivity seen at these time points. We have also measured NOX activity in AE fractions from EWAT in Adipoq-Cre/+;NOX4Flox/Flox and Adipoq-Cre/+;NOX4+/+ control mice fed a HFHS diet for 8, 12, and 16 weeks (Supplemental Figure IIA). Nox4 was highly expressed in AE fractions while other NOX family members (Nox1, 2, 3, 5, Duox1/2) were barely detectable. To determine whether there are macrophages present in the AE fractions, we measured macrophage markers using RT-PCR and western blotting. F4/80 or Cd11b, macrophage markers, were minimally expressed while Adipoq was highly expressed in AE fractions (data not shown). Also,F4/80 protein was only detected in SVC fractions from EWAT in control mice fed a HFHS diet for 16 weeks (Supplemental Figure IIB). Consistent with NOX4 activity in C57BL/6 mice, Adipoq-Cre/+;NOX4+/+ control mice showed that NOX4 activity was increased in 8 weeks of HFHS diet and diminished after 16 weeks. Moreover, NOX4 activity was not detected in Adipoq-Cre/+;NOX4Flox/Flox mice fed a HFHS diet for 8, 12, and 16 weeks (Supplemental Figure II).
Figure 2. Adipocyte-specific deficiency of NOX4 improves the insulin sensitivity before 16 weeks, but not after 24 weeks on a HFHS diet during the development of obesity.
Adipoq-Cre/+;NOX4+/+ and Adipoq-Cre/+;NOX4Flox/Flox mice were fed a HFHS diet for the indicated time periods (n=6). A; Deletion of NOX4 in the adipocyte-enriched fraction from epididymal adipose tissue by Adipoq-Cre. The AE and SVC fractions from epididymal adipose tissues of Adipoq-Cre/+;NOX4+/+ and Adipoq-Cre/+;NOX4Flox/Flox mice were isolated and analyzed by Western blotting using a NOX4 antibody. Data are representative of at least 3 independent experiments (n=2). B and C; Body weight (B), GTT and ITT (C) were measured at the indicated time points. *P < 0.01 vs. Adipoq-Cre/+;NOX4+/+ in HFHS. ANOVA and Bonferroni post-hoc test.
Table 1.
Plasma variables during indicated weeks on HFHS diet
| Measurements | Genotype | Weeks on a diet | ||||
|---|---|---|---|---|---|---|
| 4 | 8 | 12 | 16 | 24 | ||
| Insulin (ng/mL) |
Control |
1.47 ± 0.16 |
7.33 ± 3.23 |
12.48 ± 4.07 |
11.01 ± 2.76 |
12.30 ± 2.99 |
| NOX4−/− |
1.06 ± 0.21 |
2.76 ± 2.76 |
9.67 ± 4.45 |
9.46 ± 3.54 |
11.68 ± 3.78 |
|
| GTT insuin (ng/mL) |
Control | 2.81 ± 0.70 |
6.41 ± 3.53 |
8.81 ± 2.42 |
11.01 ± 2.23 |
11.19 ± 1.92 |
| NOX4−/− | 1.97 ± 0.53 |
4.16 ± 3.13 |
4.22 ± 1.41 |
6.42 ± 1.99 |
9.43 ± 3.37 |
|
| Adiponectin (µg/mL) |
Control | 24.59 ± 3.22 |
24.99 ± 1.54 |
15.06 ± 1.00 |
15.57 ± 2.45 |
16.79 ± 4.15 |
| NOX4−/− | 24.18 ± 2.74 |
25.52 ± 6.06 |
21.53 ± 2.65 |
20.65 ± 2.74 |
16.84 ± 2.62 |
|
| SAA (µg/mL) |
Control | 3.78 ± 1.62 |
5.86 ± 1.95 |
39.98 ± 14.18 |
59.58 ± 41.14 |
35.23 ± 7.53 |
| NOX4−/− | 4.50 ± 2.14 |
12.18 ± 6.55 |
36.23 ± 51.87 |
44.86 ± 33.86 |
43.21 ± 13.94 |
|
| Triglycerides (mg/dL) |
Control |
67.98 ± 8.25 |
94.16 ± 21.98 |
57.14 ± 7.61 |
63.60 ± 14.39 |
63.17 ± 12.54 |
| NOX4−/− |
36.43 ± 11.77 |
39.17 ± 11.58 |
40.69 ± 14.18 |
43.22 ± 4.40 |
45.17 ± 10.67 |
|
| Cholesterol (mg/dL) |
Control | 155.26 ± 11.44 |
173.54 ± 13.21 |
272.28 ± 36.19 |
266.68 ± 12.36 |
287.20 ± 37.72 |
| NOX4−/− | 111.52 ± 43.27 |
144.96 ± 44.26 |
190.62 ± 47.31 |
206.84 ± 54.93 |
206.09 ± 46.67 |
|
Vales represent mean ± SD (n=4–6 per group). Bold font P< 0.05 vs control mice on indicated weeks on a diet.
To investigate whether adipocyte-specific NOX4 ablation affects oxidative stress in adipose tissue, we have measured a particular membrane lipid peroxidation porduct, 4-hydroxynonenal (4-HNE) which is well known as an indicator of ROS, in EWAT from Adipoq-Cre/+;NOX4Flox/Flox and Adipoq-Cre/+;NOX4+/+ control mice fed a HFHS diet for 8, 12, and 16 weeks (Supplemental Figure IA). 4-HNE immunostaining was reduced in 8 and 12 weeks in EWAT from Adipoq-Cre/+;NOX4Flox/Flox mice compared with control mice, while 4-HNE showed equal staining after 16 weeks in EWAT from both mice (Supplemental Figure IA). These data indicate that the absence of NOX4 in adipocytes contributes to reduced oxidative stress in EWAT during the initial development of HFHS diet-induced obesity that is eventually lost with prolonged HFHS feeding.
Deletion of NOX4 from adipocytes does not alter differentiation capacity, but protects adipocytes from nutrient-induced inflammation
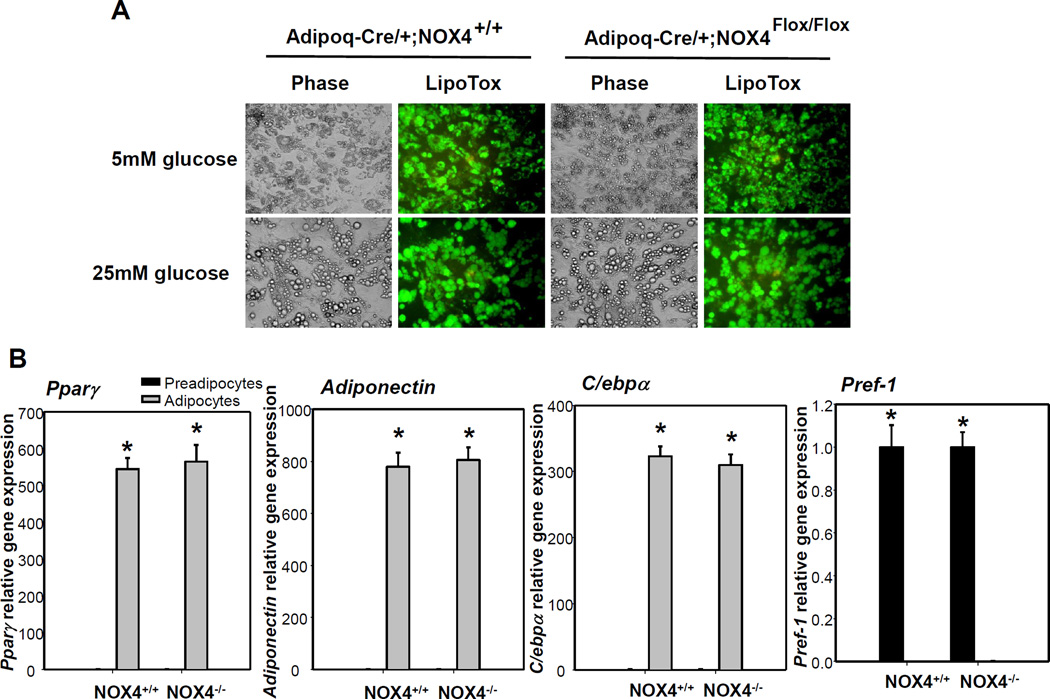
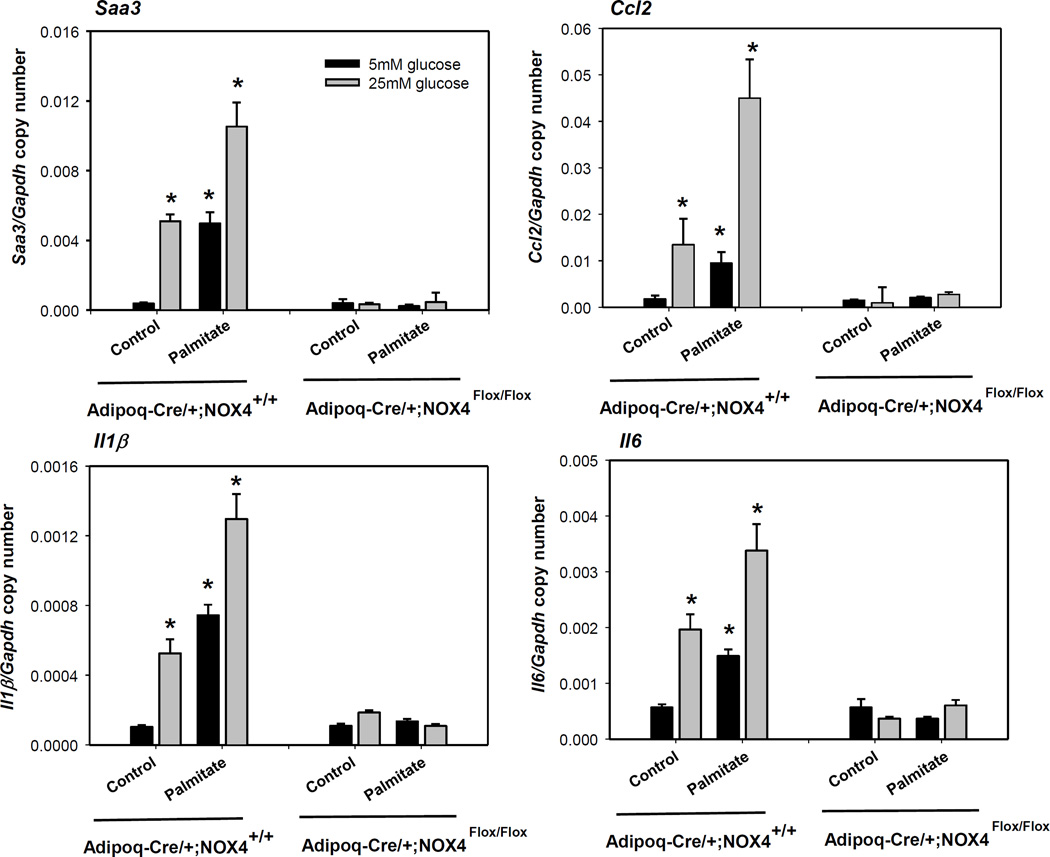
Since NOX4-derived ROS are key modulators of adipocyte differentiation16, we next determined whether blunted adipogenesis occurs in Adipoq-Cre/+;NOX4Flox/Flox mice. Differentiated adipocytes cultured from SVC isolated from EWAT of Adipoq-Cre/+;NOX4Flox/Flox and Adipoq-Cre/+;NOX4+/+ mice both showed appropriate levels of lipid droplets (Figure 3A). In addition, expression of adipogenesis genes (Pparγ, Adiponectin and C/ebpα) was not altered in Adipoq-Cre/+;NOX4Flox/Flox mice, compared with Adipoq-Cre/+;NOX4+/+ mice (Figure 3B). Similarly, gene expression of Pref-1, a marker for pre-adipocytes, was equally diminished in fully differentiated adipocytes from both Adipoq-Cre/+;NOX4Flox/Flox and Adipoq-Cre/+;NOX4+/+ mice (Figure 3B). These data suggest that the extent of adipocyte differentiation between wild type control and adipocyte-specific NOX4 deleted mice did not differ. Interestingly, primary adipocytes differentiated form Adipoq-Cre/+;NOX4Flox/Flox mice showed resistance against high glucose or palmitate-induced adipocyte inflammation (Figure 4). This data indicate that deficiency of NOX4 in adipocytes has a protective effect on exposure of excess nutrients in adipocytes, without altering pre-adipocyte differentiation capacity.
Figure 3. Adipocyte-specific deficiency of NOX4 does not affect adipogenesis from pre-adipocytes.
Pre-adipocytes from EWAT from Adipoq-Cre/+;NOX4+/+ and Adipoq-Cre/+;NOX4Flox/Flox mice were differentiated into adipocytes. A; The extent of differentiation was determined by lipid droplet staining using HCS LipidTOX. Primary adipocytes were also photographed (original magnification ×400). Representative fluorescence images of 3 independent experiments are shown. B; Pparg, Adiponectin, C/ebpa, and Pref-1 gene expression was measured in pre-adipocytes and differentiated adipocytes from Adipoq-Cre/+;NOX4+/+ and Adipoq-Cre/+;NOX4Flox/Flox mice by RT-PCR. Data are representative of at least 3 independent experiments (n=3). *P < 0.01 vs. pre-adipocytes from Adipoq-Cre/+;NOX4+/+. Student-t test.
Figure 4. Adipocyte-specific deficiency of NOX4 inhibits both high glucose- and palmitate-induced adipocyte inflammation on differentiated primary adipocytes.
Primary adipocytes differentiated from Adipoq-Cre/+;NOX4+/+ and Adipoq-Cre/+;NOX4Flox/Flox mice were cultured in 5 or 25 mmol/L glucose with or without palmitate (250 µmol/L) for 7 days. Saa3, Mcp-1, Il1β and Il6 gene expression was analyzed by RT-PCR. Data are representative of at least 3 independent experiments (n=3). *P < 0.05 vs. 5 mM glucose. ANOVA and Bonferroni post-hoc test.
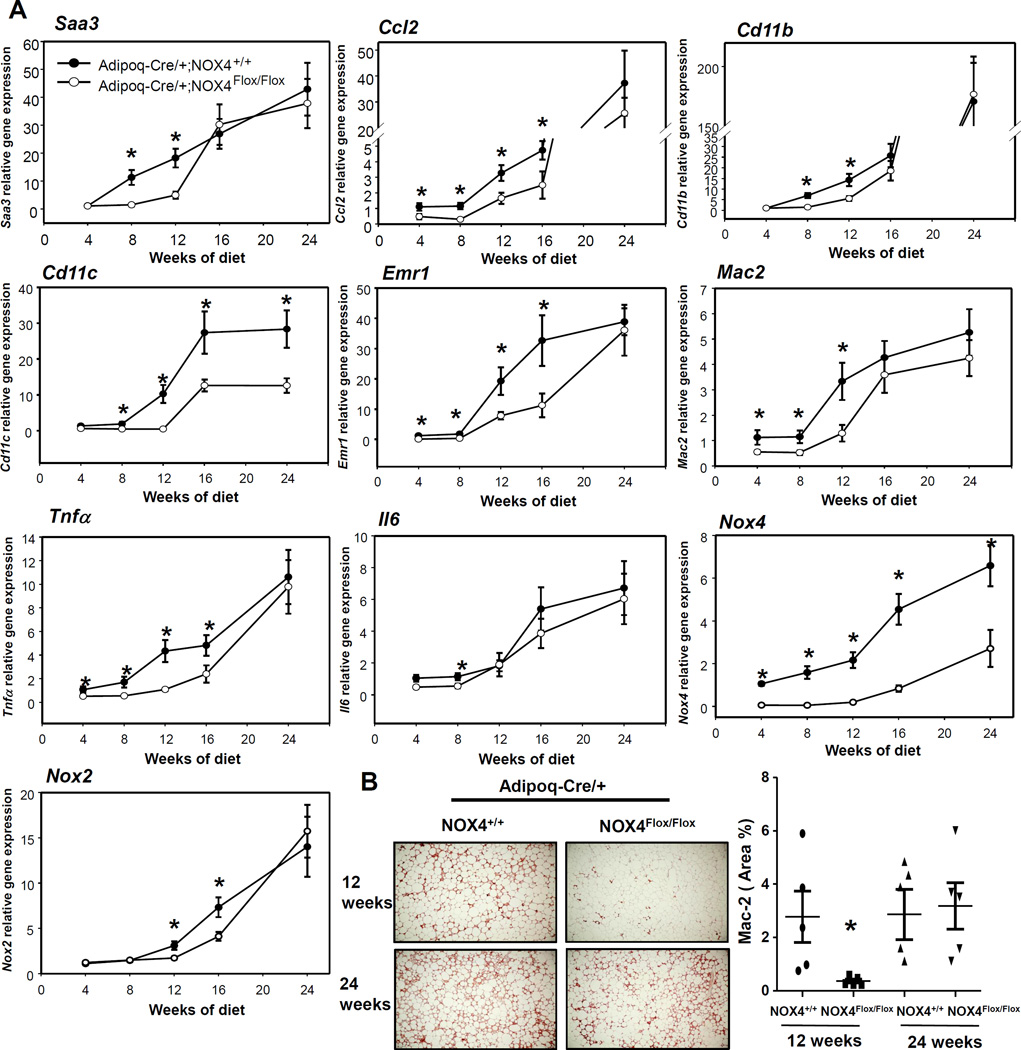
Adipocyte-specific deficiency of NOX4 initially reduces adipose tissue inflammation during the development of obesity
To determine the effect of ablation of adipocyte-derived NOX4 on adipose tissue inflammation at different stages during the development of obesity, we performed a time course experiment with Adipoq-Cre/+;NOX4Flox/Flox and Adipoq-Cre/+;NOX4+/+ mice fed a HFHS diet for up to 24 weeks. Expression of macrophage chemotactic factor genes Saa3 and Ccl2 was decreased in EWAT of Adipoq-Cre/+;NOX4Flox/Flox versus littermate controls (Adipoq-Cre/+;NOX4+/+ mice) until 16 and 24 weeks on HFHS diet, respectively (Figure 5A). Similarly, mRNA expression of macrophage markers (Mac2, Cd11b, Cd11c and Emr1) was decreased in EWAT of Adipoq-Cre/+;NOX4Flox/Flox versus littermate controls (Adipoq-Cre/+;NOX4+/+ mice) before 12 weeks, with no changes after 16 weeks on HFHS diet (Figure 5A). Consistently, immunohistochemical staining also showed a decrease in Mac2 protein in EWAT of Adipoq-Cre/+;NOX4Flox/Flox mice compared to Adipoq-Cre/+;NOX4+/+ mice after 12 weeks, but not 24 weeks on the HFHS diet (Figure 5B). Similarly, gene expression of the pro-inflammatory cytokines Tnfα and Il6 was decreased in EWAT of Adipoq-Cre/+;NOX4Flox/Flox mice compared to Adipoq-Cre/+;NOX4+/+ mice fed the HFHS diet before 12 weeks during the development of obesity, but not after 16 weeks (Figure 5A). Interestingly, Mac2 staining mirrored 4-HNE staining in adjacent sections (Supplemental Figure IB). These findings suggest that the absence of NOX4 in adipocytes exerts an anti-inflammatory effect on EWAT during HFHS diet-induced obesity that is eventually lost with prolonged HFHS feeding.
Figure 5. Adipocyte-specific deficiency of NOX4 initially improves the adipose tissue inflammation during the development of obesity.
Adipoq-Cre/+;NOX4+/+ and Adipoq-Cre/+;NOX4Flox/Flox mice were fed a HFHS diet for the indicated times (n=6). A; Saa3, Ccl2, Tnfa, Il6, Cd11b, Cd11c, Emr1, Mac2, Nox2 and Nox4 gene expression was measured in epididymal fat by RT-PCR. B; Epididymal fat isolated from Adipoq-Cre/+;NOX4+/+ and Adipoq-Cre/+;NOX4Flox/Flox mice fed a HFHS diet for 12 or 24 weeks was analyzed by immunohistochemistry using a Mac2 antibody which detects macrophages (n=5). Tissues were photographed using microscopy (original magnification ×60) and quantified using Image Pro Plus software. *P < 0.05 vs. Adipoq-Cre/+;NOX4+/+ in HFHS. ANOVA and Bonferroni post-hoc test.
Since NOX expression levels could be changed during the development of obesity in adipose tissue, we measured mRNA levels of Nox4 and Nox2 in Adipoq-Cre/+;NOX4+/+ and Adipoq-Cre/+;NOX4Flox/Flox mice. Nox2 and Nox4 gene expression was gradually increased during HFHS feeding in wild-type control mice, while only NOX2 gene expression was increased in Adipoq-Cre/+;NOX4Flox/Flox mice (Figure 5A).
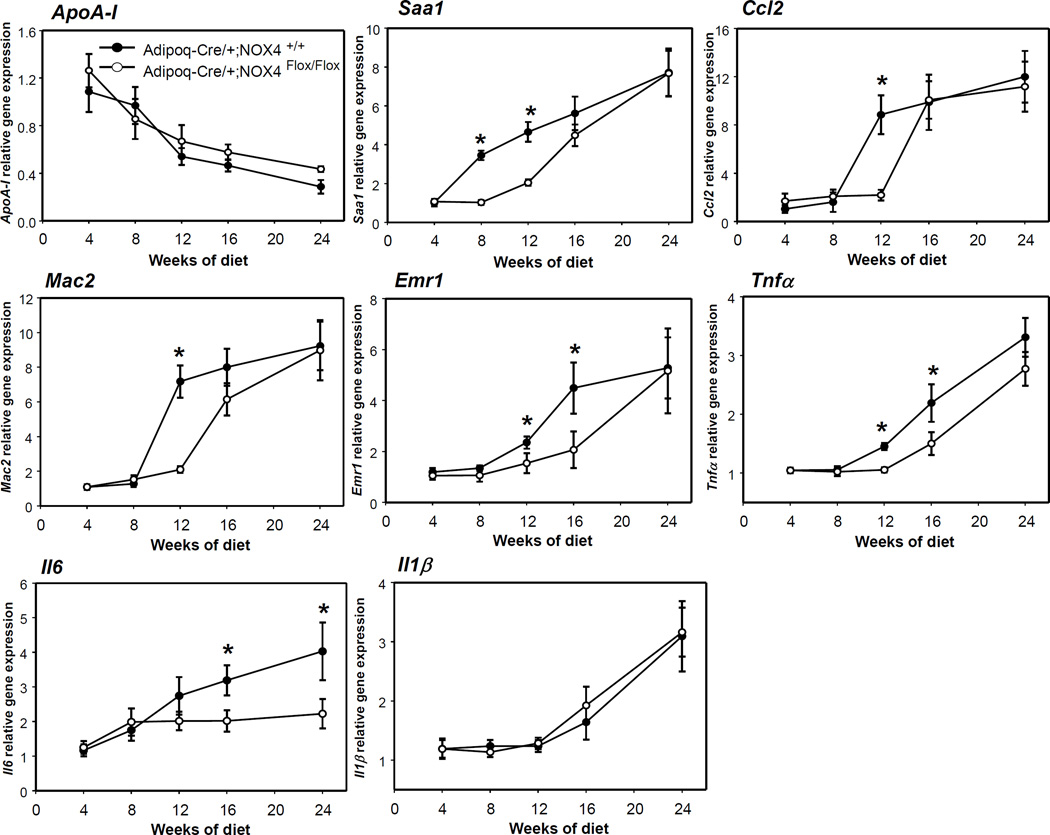
Liver inflammation is temporally improved by adipocyte-specific deficiency of NOX4 during the development of obesity
Since adipocyte-specific ablation of NOX4 could impact hepatic physiology via altered adipokine secretion, we measured expression of various genes from the liver in our mouse model of adipocyte NOX4 deficiency. Pro-inflammatory gene expression of Saa1, Ccl2, Tnfα and Il6 was decreased in the livers of Adipoq-Cre/+;NOX4Flox/Flox mice versus littermate controls (Adipoq-Cre/+;NOX4+/+ mice) after 12 and 16 weeks of HFHS feeding (Figure 6). However, there were no differences in expression of apolipoprotein A-I (apoA-I) and Il1β gene expression between Adipoq-Cre/+;NOX4+/+ and Adipoq-Cre/+;NOX4Flox/Flox mice during the development of obesity (Figure 6). Similarly, gene expression of macrophage markers Mac2 and Emr1 was decreased in the livers of Adipoq-Cre/+;NOX4Flox/Flox mice versus littermate controls at the 12 week time point (Figure 6). Even though plasma SAA levels were not changed in Adipoq-Cre/+;NOX4Flox/Flox and Adipoq-Cre/+;NOX4+/+ mice at any time points, Adipoq-Cre/+;NOX4Flox/Flox mice showed lower triglyceride and cholesterol levels compared with Adipoq-Cre/+;NOX4+/+ mice (Table 1).
Figure 6. Adipocyte-specific deficiency of NOX4 initially improves the liver tissue inflammation during the progression of obesity.
Adipoq-Cre/+;NOX4+/+ and Adipoq-Cre/+;NOX4Flox/Flox mice were fed a HFHS diet for the indicated times (n=6). ApoA-I, Saa1, Ccl2, Tnfα, Il1β, il6, Emr1 and Mac2 gene expression was measured in liver by RT-PCR. *P < 0.05 vs. Adipoq-Cre/+;NOX4+/+ in HFHS. ANOVA and Bonferroni post-hoc test.
Collectively, these data suggest that adipocyte specific deletion of NOX4 could have beneficial effects on other organs such as the liver, which could contribute to the observed whole-body insulin sensitization.
Discussion
Previously, we have shown that excess glucose and saturated fatty acids generate ROS in cultured adipocytes, and have confirmed that the source of these ROS is NOX413. In this study we now show that NOX4 activity is initially increased in adipose tissue during the development of obesity when adipocytes are still insulin-sensitive, and eventually decreases prolonged HFHS feeding diet when adipocytes become insulin-resistant. Furthermore, in response to an obesogenic diet, adipocyte-specific ablation of NOX4 shows the delayed onset of insulin resistance and improves adipose tissue inflammation as well as liver inflammation during the development of obesity.
Global NOX4 deficiency has been reported to worsen adipose tissue inflammation in a mouse model of diet-induced obesity17. Since NOX4 activity is essential for the differentiation of pre-adipocytes into mature adipocytes16, blunted adipogenesis in the absence of NOX4 would reduce the number of adipocytes, allowing the remaining adipocytes to become more hypertrophic, thereby leading to adipose tissue inflammation. Consistent with this, expression of the adipogenesis genes Pparγ and C/ebpα was reduced in global NOX4 knockout mice17. Therefore, it is imperative to investigate NOX4 activity in mice during the pathophysiological progression of obesity where adipogenesis is intact, and to study the effect of adipocyte-specific deficiency of NOX4. Indeed, different from the situation with global NOX4 deficiency in which adipocyte differentiation is blunted17, our study showed that adipogenesis from the adipocyte-specific NOX4 deficient mice is intact, and the adipocyte-specific NOX4 deficient mice showed the same degree of adiposity as control mice. We speculate that our adipocyte-specific NOX4 deficient mice show intact adipogenesis because pre-adipocytes still express NOX4, while in the global NOX4 knockout mice they do not.
Others reported that high insulin treatment of human adult adipose-derived stem cells (ACS) caused NOX4-derived ROS generation, leading to prevention of proliferation and adipogenesis, and induction of apoptosis in these cells18. This report raises the question of whether the progression in adipogenesis could be affected by NOX4 ablation in adipocyte-specific NOX4 knockout mice. However, in our study, pre-adipocytes isolated from wild type control and adipocyte-specific NOX4 deleted mice showed no inhibition of proliferation and adipogenesis, and no induction of apoptosis by high insulin treatment during the progression of adipogenesis.
How does the adipocyte-specific deficiency of NOX4 show beneficial effects on insulin sensitivity and tissue inflammation only transiently during the development of obesity? During the development of obesity, when insulin responsiveness is intact and insulin activates downstream protein kinases, including insulin receptor substrate (IRS) proteins and Akt/PKB19, 20, energy flux from nutrient excess flows into lipogenesis, in which excess glucose and free fatty acids (FFA) are used for triglyceride synthesis by adipocytes. In these stages, PPP generates pentose from the 6 carbon glucose and is a major source of cellular NADPH. During the initial stage of energy excess, adipocytes will continue to actively store triglycerides derived from excess nutrients, and will demonstrate increased PPP activity and NADPH content, which results in NOX4-derived ROS generation21. We speculate that these NOX4-derived ROS initiate the onset of adipocyte insulin resistance and adipose tissue inflammation. We expect that adipocyte-specific deficiency of NOX4 will retard the initiation of adipocyte inflammation and insulin resistance during this stage. Supporting this concept, our study showed increased NOX4 and PPP activity before adipocytes become insulin resistant during the development of obesity. Consequently, our study showed that adipocyte-specific deletion of NOX4, which is an important source of ROS generation, initially led to reduced adipose tissue inflammation and improved insulin resistance during the progression of obesity.
Why then does the adipocyte-specific deficiency of NOX4 fail to show beneficial effects on insulin sensitivity and tissue inflammation after prolonged HFHS feeding? In the obese state, insulin responsiveness is blunted and phosphorylation of IRS and Akt is reduced19, 20, 22, and uptake of glucose and FFA by adipocytes is reduced. Hormone-sensitive lipase is activated leading to the release of FFA from triglyceride stores23, and energy flux in this state flows to lipolysis rather than lipogenesis. We propose that when adipocyte glucose uptake is reduced by insulin resistance, adipocytes start to use FFA from triglyceride stores for energy. This alteration of energy flux into fatty acid-oxidation could overwhelm the capacity of mitochondria, leading to leakage of electrons. Indeed, our study showed that after adipocytes became insulin resistant in vivo, oxidation of FFA is increased, with the potential that ROS could be generated in mitochondria. Since others also showed that several proteins in the mitochondrial respiratory chain or matrix were decreased by prolonged high fat diet feeding, despite unchanged mitochondrial abundance24, it would also be possible that mitochondrial remodeling during the development of obesity could evoke extensive leakage of protons, leading to the generation of mitochondria-derived ROS. Thus, mitochondria-derived ROS could now take the place of NOX4 and play a pivotal role in ROS generation and adipose tissue inflammation. Another possibility is that recruited and activated immune cells that infiltrate obese adipose tissue can promote the generation of ROS by NOX2, which is predominantly expressed in activated T cells and macrophages8, 25–27. Whole body deficiency of NOX2 shows attenuation of adipose tissue inflammation and insulin resistance in mice fed a high fat diet28. Also, NOX2-derived ROS itself could take the place of NOX4 and has effects on adipose tissue inflammation, promoting the onset of insulin resistance in adipocytes during the progression of obesity. This potential possibility must be investigated in future studies.
We interestingly found that 4-HNE staining was co-localized with Mac2 staining. While it is possible that infiltrated macrophages in adipose tissue generate ROS through NOX2 activation, resulting in lipid peroxidation. However, the origin of ROS-mediated lipid peroxidation and how different sources of ROS affect insulin resistance and adipose tissue inflammation will need to be examined closely in further studies.
We have observed that liver inflammation was ameliorated by adipocyte-specific ablation of NOX4. Adipocytes routinely secrete adipokines that enable complex homeostatic cross talk with other tissues such as the liver. It is well established that both adipose tissue and hepatic inflammation are associated with obesity29. Our results showed that plasma adiponectin, an anti-inflammatory adipokine derived from adipocytes that has been shown to promote insulin sensitivity, was sustained in mice with adipocyte specific-NOX4 ablation. This elevated level of circulating adiponectin could have a beneficial effect on the liver, potentially resulting in the reduced liver inflammation as well as reduced plasma triglycerides and cholesterol we have shown. Consistent with this notion, several other reports showed that increased adiponectin was associated with increased activity of AMPK and reduced NOX4-derived ROS, and showed improvements in heart and podocyte function in obesity and diabetes30, 31. In contrast to previous speculation that adiponectin reduces NOX4 activity, leading to improvement of tissue function30, 31, our study demonstrates that ablation of NOX4 activity increases plasma adiponectin, leading to improved liver function. We also speculate that the reason why adiponectin levels are elevated after 12 and 16 weeks of HFHS feeding in mice with adipocyte specific-NOX4 ablation is due to the fact that reduced inflammation in adipose tissue leads to increased adiponectin production from adipocytes.
It is unknown whether ROS directly promote insulin resistance and inflammation, or are a casual bystander associated with insulin resistance and inflammation in adipocytes. In this study, we demonstrated that NOX4-derived ROS could play a role in the initiation of adipocyte inflammation and the onset of insulin resistance during the development of obesity. The findings from this study offer translational implications related to possibilities in which modulation of NOX4 activity could be a therapeutic target to prevent obesity-associated insulin resistance.
Supplementary Material
Highlights.
NADPH oxidase and pentose phosphate pathway are transiently activated during the development of obesity in mice.
Adipocyte-specific deletion of NADPH oxidase 4, the major isoform in adipocytes, delays the onset of insulin resistance in mice fed a high fat high sucrose diet.
Adipocyte-specific deletion of NADPH oxidase 4 protects mice from adipose tissue and hepatic inflammation, and improves plasma lipids.
Acknowledgments
We would like to Dr. Alan Chait for his excellent advice and editing this manuscript.
Sources of Funding
This work was supported in part by National Institutes of Health grants HL092969, HL094352, DK035816, K01 AT007177, a University of Washington Nutrition Obesity Research Center Pilot and Feasibility Award (P30 DK035816), a University of Washington Diabetes Research Center Pilot and Feasibility Award (P30 DK017047), and a Beginning Grant-in-Aid from the American Heart Association.
Abbreviations
- NOX
NADPH oxidase
- MCP-1
monocyte chemotactic protein-1
- CCL2
chemokine (C-C motif) ligand 2
- PPP
pentose phosphate pathway
- SAA
serum amyloid A
- FFA
free fatty acid
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- ROS
reactive oxygen species
- HFHS
a high fat, high sucrose diet
- ApoA-I
apolipoprotein A-I
- SVC
stromal vascular cells
- AE
adipocyte-enriched
- EWAT
epididymal white adipose tissue
- IRS
insulin receptor substrate
Footnotes
Disclosures
No potential conflicts of interest relevant to this article were reported.
Author contributions
LJD, MO, LG, SW, TW, KDO and CYH conducted experiments. All authors interpreted the data. LJD, KDO and CYH supervised the work, interpreted the data, and wrote the manuscript.
REFERENCES
- 1.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr Ccr2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han CY, Subramanian S, Chan CK, Omer M, Chiba T, Wight TN, Chait A. Adipocyte-derived serum amyloid a3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 2007;56:2260–2273. doi: 10.2337/db07-0218. [DOI] [PubMed] [Google Scholar]
- 6.Han CY, Kargi AY, Omer M, Chan CK, Wabitsch M, O'Brien KD, Wight TN, Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: Dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59:386–396. doi: 10.2337/db09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and th1 polarization in adipose tissue during diet-induced obesity in c57bl/6 mice. Obesity (Silver Spring) 2010;18:1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. Cd8+ effector t cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 9.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Blüher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: A primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 10.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 11.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han CY, Umemoto T, Omer M, Den Hartigh LJ, Chiba T, LeBoeuf R, Buller CL, Sweet IR, Pennathur S, Abel ED, Chait A. Nadph oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes. J Biol Chem. 2012;287:10379–10393. doi: 10.1074/jbc.M111.304998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleary MP, Zisk JF. Anti-obesity effect of two different levels of dehydroepiandrosterone in lean and obese middle-aged female zucker rats. Int J Obes. 1986;10:193–204. [PubMed] [Google Scholar]
- 15.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A, Kirk EA, O'Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese ldl receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schröder K, Wandzioch K, Helmcke I, Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol. 2009;29:239–245. doi: 10.1161/ATVBAHA.108.174219. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Mouche S, Sajic T, Veyrat-Durebex C, Supale R, Pierroz D, Ferrari S, Negro F, Hasler U, Feraille E, Moll S, Meda P, Deffert C, Montet X, Krause KH, Szanto I. Deficiency in the nadph oxidase 4 predisposes towards diet-induced obesity. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2011.279. [DOI] [PubMed] [Google Scholar]
- 18.Scioli MG, Cervelli V, Arcuri G, Gentile P, Doldo E, Bielli A, Bonanno E, Orlandi A. High insulin-induced down-regulation of erk-1/igf-1r/fgfr-1 signaling is required for oxidative stress-mediated apoptosis of adipose-derived stem cells. J Cell Physiol. 2014;229:2077–2087. doi: 10.1002/jcp.24667. [DOI] [PubMed] [Google Scholar]
- 19.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summers SA, Whiteman EL, Birnbaum MJ. Insulin signaling in the adipocyte. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):S67–S70. doi: 10.1038/sj.ijo.0801509. [DOI] [PubMed] [Google Scholar]
- 21.Han CY. Roles of reactive oxygen species on insulin resistance in adipose tissue. Diabetes Metab J. 2016 doi: 10.4093/dmj.2016.40.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 23.Sethi JK, Hotamisligil GS. The role of tnf alpha in adipocyte metabolism. Semin Cell Dev Biol. 1999;10:19–29. doi: 10.1006/scdb.1998.0273. [DOI] [PubMed] [Google Scholar]
- 24.Cummins TD, Holden CR, Sansbury BE, Gibb AA, Shah J, Zafar N, Tang Y, Hellmann J, Rai SN, Spite M, Bhatnagar A, Hill BG. Metabolic remodeling of white adipose tissue in obesity. Am J Physiol Endocrinol Metab. 2014;307:E262–E277. doi: 10.1152/ajpendo.00271.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 27.Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox -serine phosphorylated by convergent mapks mediates neutrophil nadph oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–2043. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepping JK, Freeman LR, Gupta S, Keller JN, Bruce-Keller AJ. Nox2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. Am J Physiol Endocrinol Metab. 2013;304:E392–E404. doi: 10.1152/ajpendo.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res. 2013;43:51–64. doi: 10.1111/j.1872-034X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Z, Qi W, Yu Y, Du S, Wu J, Liu J. Effect of exenatide on the cardiac expression of adiponectin receptor 1 and nadph oxidase subunits and heart function in streptozotocin-induced diabetic rats. Diabetol Metab Syndr. 2014;6:29. doi: 10.1186/1758-5996-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.