Abstract
Objective
S. lugdunensis is considered to be more aggressive than other coagulase negative staphylococci (CoNS). There are gaps in knowledge regarding the importance of isolating S. lugdunensis from different sources and in different patient subsets. Our objective was to describe the spectrum, clinical manifestations, and outcomes of infections caused by S. lugdunensis in patients with cancer.
Methods
A retrospective review of all cancer patients from whom S. lugdunensis was isolated in pure culture from clinically significant sites.
Results
Between 2011 – 2014, 2,263 CoNS were isolated, of them 45 S. lugdunensis were isolated in pure culture and were included in this analysis. Only 3 patients were neutropenic. Skin and skin structure infections (SSSIs) occurred most often (36 cases) followed by 5 blood stream infections, one of which had destructive endocarditis, and 4 infections at other sites. Of the 36 SSSIs, 29 were related to surgical or invasive procedures, and 6 of these involved an implanted medical device. All isolates were susceptible to vancomycin, 98% to levofloxacin and 89% to oxacillin. All patients responded to therapy.
Conclusions
Cancer patients including those with neutropenia, do not appear to have an increased frequency of infections caused by S. lugdunensis. SSSIs are predominant, and are often associated with surgical procedures and/or implanted medical devices. Blood stream infections caused by S. lugdunensis are uncommon but may have an increased rate of serious complications such as endocarditis. Nevertheless, these organisms are generally susceptible to multiple classes of antimicrobial agents and the overall response to therapy is high.
Keywords: Staphylococcus lugdunensis, cancer patients, skin/skin structure infection
Introduction
Patients with cancer develop bacterial infections frequently, especially but not exclusively during episodes of neutropenia.[1] Recent epidemiologic surveys in adult and pediatric cancer patients have documented the predominance of Gram-positive organisms over Gram-negative bacilli as causes of microbiologically documented infections in this setting. Coagulase-negative staphylococci (CoNS) are normal inhabitants of human skin. Although they are isolated frequently from various sites including the bloodstream, they are often dismissed as commensals or contaminants, especially if only one of several cultures is positive. Even in cancer patients, CoNS are considered to be of low virulence and seldom cause life-threatening infections. Staphylococcus lugdunensis was first described in 1988 and named after Lyon, the French city where it was first isolated.[2] Taxonomically they belong to the CoNS. Several recent reports however, emphasize that these organisms appear to be more virulent than other CoNS species, and like Staphylococcus aureus, cause serious infections such as native and prosthetic valve infective endocarditis, bloodstream infections (including catheter-related infections), bone and joint infections, meningitis, brain abscesses, and other device related infections. [3–6] There is evidence that S. lugdunensis binds directly to von-Willebrand factor which enables it to cause more aggressive infections including endocarditis compared to other CoNS.[7] Consequently, many investigators recommend that S. lugdunensis should not be considered a commensal or contaminant without careful review and investigation, even in patients with only one positive blood culture. [8]
Many clinical microbiology laboratories still do not perform or recommend routine identification of CoNS from sterile sites to species level.[9] Additionally, many recent reports have focused on serious infections such as endocarditis, without delineating the entire spectrum of infection caused by S. lugdunensis. As a result, there may be some reporting bias and gaps in knowledge regarding some aspects of infections caused by S. lugdunensis, including the overall frequency and the clinical significance of isolating these organisms from various sites. Furthermore, as best as we can determine, there are no published data on the frequency, clinical spectrum, severity, antimicrobial susceptibility, management and outcomes of S. lugdunensis infections in cancer patients.
Our institution, (The University of Texas, MD Anderson Cancer Center in Houston) has been designated a Comprehensive Cancer Center by the National Cancer Institutes (NCI) and provides care exclusively for cancer patients. Our center has approximately 620 in-patient beds, with approximately 25,000 admissions annually. Our institutional microbiology laboratory which processes all clinical cultures at our institution, began identifying CoNS isolated from clinically significant sites, to species level, in 2011. This has provided us with the opportunity to review and report on our clinical experience of S. lugdunensis infections in cancer patients.
Methods
Microbiology and identification
CoNS were identified to species level by our microbiology laboratory if they had been isolated from the following sites: the bloodstream (including blood obtained from peripheral and central venous catheters), and sterile body fluids such as the cerebrospinal fluid (CSF), pleural fluid, synovial fluid, and urine. Additionally, cultures obtained from closed abscesses and skin and skin structure infections including surgical site infections were processed similarly. CoNS isolated from chronic open wounds such as diabetic foot ulcers or decubitus ulcers were not identified to species level and were therefore not included in this report. In order to have complete data, patients with only one positive blood culture were included. CoNS including S. lugdunensis were identified by biochemical methods as follows. Catalase positive cocci were initially tested using the StaphAurex latex agglutination method (Remel Inc., Lenexa, Kansas USA). Agglutination-positive and coagulase-negative isolates were tested for further speciation by the Vitek II system (Biomeriux Inc., Durham, North Carolina, USA) and those confirmed as S. lugdunensis were further characterized using the S. lugdunensis AST Interpretation Criteria. Susceptibility testing was performed using the automated Vitek II system. Seven isolates were tested against newer antimicrobial agents using Clinical Laboratory Standards Institute (CLSI) approved broth dilution methodology.
Patients and data collection
All patients from whom S. lugdunensis was isolated from the sites outlined above between January 2011 and March 2014 were identified by reviewing the electronic database of our institutional microbiology laboratory. Clinical data were then collected retrospectively by reviewing the medical records of these patients. These data included patient demographics (age, gender), underlying malignancies, type of stem cell transplant (if performed), co-morbidities using the Charlson co-morbidity index, clinical spectrum and manifestations, pertinent laboratory data, management (including antibiotic therapy, surgical intervention, and catheter management, and management of implanted devices), and outcomes.
Statistical analysis
Baseline demographic data including relevant statistical data for categorical variables were summarized and the proportion of CoNS infections caused by S. lugdunensis was determined. One-way analysis of variance, chi-squared test, and Fisher’s exact test were used to assess the demographic and clinical differences between neutropenic (absolute neutrophil count <500/mm3) and non-neutropenic patients. The statistical differences between the outcomes of patients with implanted devices were also assessed using the same tests. Significance was based on two-tailed analysis with alpha = 0.05, using SPSS software version 21 (IBM Corporation, Chicago, IL, USA). This study was reviewed and approved by our Institutional Review Board (protocol number PA12-1181) and informed consent was waived.
Results
Patient Demographics and General Clinical features
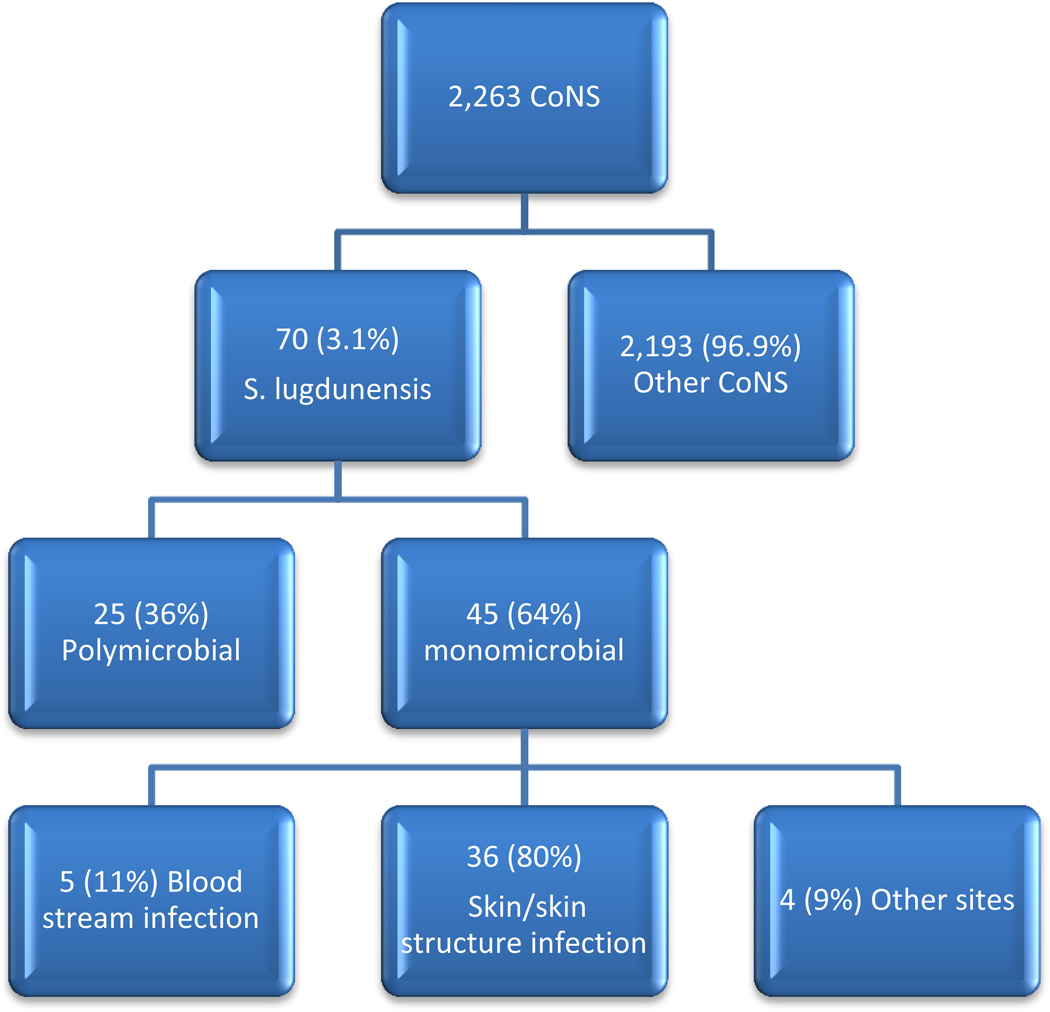
During the study period, S. lugdunensis was isolated from blood and sterile body fluids, wounds and closed abscesses in 70 cancer patients, representing 3.1% of all cultures positive for CoNS (70 of 2,263). Of these, 45 (64%) had S. lugdunensis isolated in pure culture (monomicrobial infection) whereas 25 had a polymicrobial infection (Figure 1). Although it is likely that many polymicrobial infections were clinically significant, they were excluded from this analysis because it was not possible to determine the specific role of S. lugdunensis in these infections. General demographics of patients with monomicrobial infections are shown in Table 1. The median age of patients was 51 years (range 2–80 years). Twenty six (58%) were female. Thirty eight (84%) had an underlying solid organ malignancy with 24 (53%) of them breast cancer being the most common. Only 3 patients (7 %) were neutropenic (absolute neutrophil count [ANC] ≤ 500/mm3) at time of the infection and only 4 (9%) had received corticosteroid therapy within 7 days prior to development of infection. Our statistical analysis failed to show any statistical significance or impact of steroid administration on morbidity or mortality.
Figure 1.
Frequency and nature of S. lugdunensis infections, identified in cancer patients.
Table 1.
Demographic features of 45 cancer patients with monomicrobial staphylococcus lugdunensis infections
| Feature | Result (% Frequency) | |
|---|---|---|
| Age | Median | 51 years |
| Range | 2 – 80 years | |
| Gender | Female | 26 (58) |
| Male | 19 (42) | |
| Underlying malignancy | Solid tumor* | 38 (84) |
| Hematologic** | 7 (16) | |
| Surgical procedure | 29 (64) | |
| Implanted medical device | 6 (13) | |
| Corticosteroid use | 4 (9) | |
| Neutropenia | 3 (7) | |
| Leukocytosis | 4 (9) | |
breast cancer (53%) and melanoma (24%) were the most common underlying solid tumors
four of the 7 patients with hematologic malignancy were hematopoietic cell transplant recipients
Fever (temperature > 38.3°C) was present in 22 patients (49 %) at the onset of the infection, including 4/5 patients (80%) with bloodstream infections, but in only 18/40 patients (45%) with other infections. Local findings (erythema/cellulitis, induration, skin breakdown, discharge, wound dehiscence) were common in patients with SSSIs (32 patients or 71%). Other less common clinical manifestations included arthralgias and myalgias, and non-specific central nervous system complaints such as headaches and confusion. Neutropenia occurred in only 3 patients (7 %), one of whom had a blood stream infection (BSI), and surprisingly was not a significant predisposing factor for S. lugdunensis infection. Leukocytosis was also uncommon and occurred in only 4 patients (9%). We found no correlation between the nature and severity of S. lugdunensis infection and underlying co-morbid conditions as measured with the Charlson comorbidity index. Twenty nine patients, including all patients with blood stream infection had a score of 4 or less indicating low co-morbidity, whereas most patients with a score of 5 or greater suggesting greater co-morbidity, had localized SSSIs
Infection characteristics
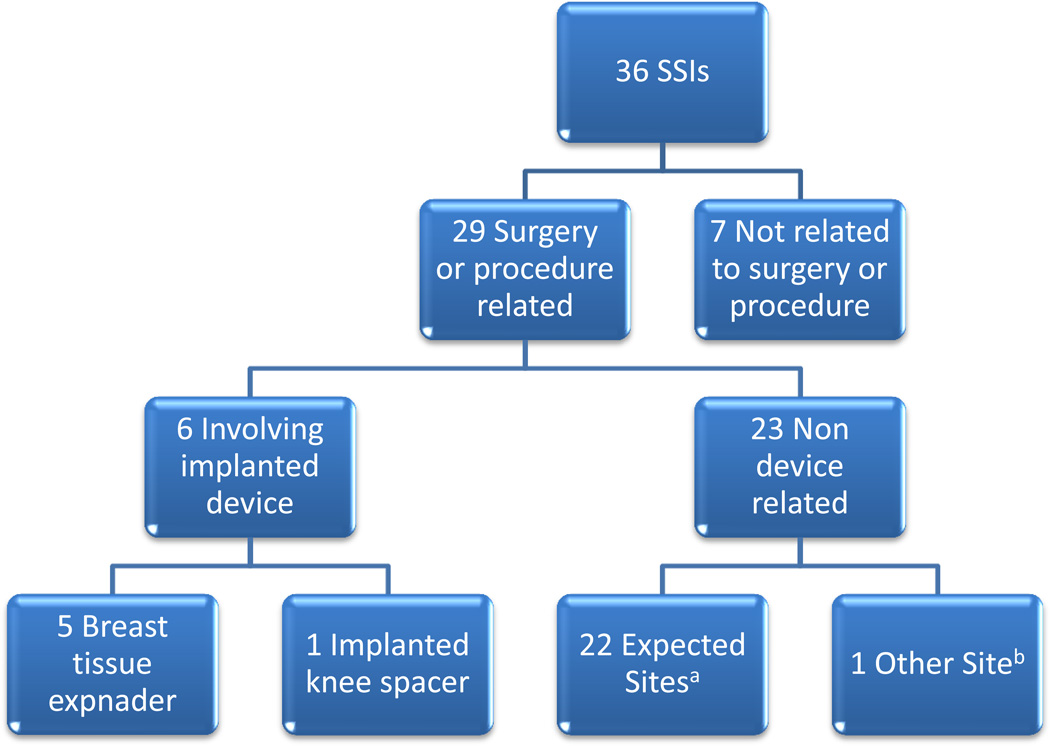
Skin and skin structure infections (SSSIs) occurred most often, accounting for 36 of the 45 (80%) S. lugdunensis infections (Figure 1). Of these, 29 (81 %) were associated with surgical or invasive procedures, whereas 7 (19 %) were unrelated to any procedure. Six of forty five (13%) SSSIs were associated with implanted medical devices, 5 of which (83 %) were breast tissue expanders and one was an implanted knee spacer. The most common site of SSSIs was the breast (19 cases, 53%). Inguinal, perineal, abdominal, axillary, scalp, and mandible accounted for the rest of the cases.
Bloodstream infections were documented in 5 patients (11%). Of these, one patient had a positive surveillance central venous catheter (CVC) culture prior to hematopoietic cell transplantation. Another patient had a high colony count (> 1000 CFU/ml) catheter-related BSI. Two other patients with BSI had uncomplicated infections. These patients underwent extensive investigations including trans-thoracic echocardiography and radiographic/nuclear medicine imaging to look for endocarditis, abscesses, or other distant foci of infection. None were documented. The fifth patient with BSI developed rapidly progressive native aortic valve endocarditis. There were 4 other sites of infection (9%) including 2 urinary tract infections (both following genito-urinary manipulation), one infected Ommaya reservoir following repeated access, and one empyema following repeated thoracentesis procedures.
Antimicrobial susceptibility
All isolates were susceptible to vancomycin, gentamicin, tetracyclines, and rifampin, 98 % were susceptible to levofloxacin, and 89 % were methicillin susceptible (Table 2). This susceptibility pattern is quite different to that of other CoNS species isolated at our institution (i.e. S. epidermidis, S. haemolyticus and S. hominis), greater than 90 % of which are resistant to the quinolones and methicillin. Seven isolates were available for testing against newer agents including ceftaroline, daptomycin, dalbavancin, linezolid, and telavancin and were uniformly susceptible to these agents (Table 2).
Table 2.
Antimicrobial susceptibility of S. lugdunesis strains isolated from monomicrobial infections
| Antimicrobial agent | No. of isolates tested | No (%) susceptibility |
|---|---|---|
| Clindamycina | 45 | 37 (82) |
| Erythromycina | 44 | 37 (84) |
| Gentamicina | 45 | 45 (100) |
| Levofloxacina | 44 | 43 (98) |
| Oxacillina | 45 | 40 (89) |
| Rifampina | 45 | 45 (100) |
| Tetracyclinea | 44 | 44 (100) |
| Vancomycina | 45 | 45 (100) |
| Ceftarolineb | 7 | 7 (100) |
| Dalbavancinb | 7 | 7 (100) |
| Daptomycinb | 7 | 7 (100) |
| Linezolideb | 7 | 7 (100) |
| Telavancinb | 7 | 7 (100) |
| Tigecyclineb | 7 | 7 (100) |
| TMP-SMX*b | 7 | 7 (100) |
Trimethoprim-sulfamethoxazole
testing performed by the institutional microbiology laboratory using the automated Vitek I
testing performed by the research laboratory using broth microdilution methodology in accordance with CLSI guidelines
Treatment and Outcomes
Overall, 42 of the 45 patients (93 %) received antimicrobial therapy with a median of 17 days (range 0 to 56), whereas 3 patients were treated with surgical intervention alone and did not receive antimicrobial agents, The infection resolved in all 45 patients. The median duration of follow up was 273 days (range 23 – 684 days). Multiple different antimicrobial agents were used for therapy. The most common were beta-lactams, quinolones, daptomycin, trimethoprim-sulfamethoxazole (TMP-SMX), vancomycin, and clindamycin, either alone or in combination. All 5 patients with BSIs received and responded to antimicrobial therapy. The patient with a positive surveillance CVC culture prior hematopoietic cell transplantation had his CVC removed and received a 16-day course of daptomycin. Catheter removal in addition to antimicrobial therapy with vancomycin was also necessary in the patient with high colony count (>1000 CFU/ml) bloodstream infection. In contrast, the patient with endocarditis of the native aortic valve, required valve replacement in addition to prolonged antimicrobial therapy (56 days) with daptomycin. He ultimately responded to therapy and is well after 3 years of follow up. The remaining 2 patients with S. lugdunensis BSI had uncomplicated clinical courses.
Of the 6 patients with an infection involving an implanted device, 3 had the device removed as primary therapy with resolution of the infection (2 received post removal antibiotics whereas one did not). In the remaining 3 cases, all involving breast tissue expanders, an attempt was made to preserve the foreign body. These patients underwent radiological guided aspiration of the fluid surrounding the expanders, combined with a prolonged courses of antibiotics. With this strategy, infection resolved in two of these patients. The third patient required removal of the expander due to unresolved infection. Only 4 patients (9%) had other infections, all associated with procedures such as repeated thoracentesis of a malignant effusion, repeated Omaya reservoir access, and genito-urinary manipulation. All four responded to antimicrobial therapy.
Discussion
Taxonomically, S. lugdunensis has been placed among the coagulase-negative staphylococci. Like other CoNS, these organisms are an integral part of the normal human skin flora but they occupy different niches compared to other CoNS species and S. aureus. Studies have shown that although S. lugdunensis can be isolated from almost all cutaneous sites, the most common sites of isolation are the inguinal folds, the perineum, the axilla, and the toes.[10] Thus, it is not surprising that the most common infections caused by S. lugdunensis are SSSIs predominantly located at these sites, including infections following cesarean sections, infections associated with vasectomy or femoral artery catheterization, and those associated with scrotal wounds.[11] Non-puerperal breast infections have also been previously reported.[12–14] Indeed, 31/36 (86 %) of SSSIs documented in our cohort of patients involved these sites. This ecologic distribution of S. lugdunensis may also explain the low frequency of BSIs including catheter-related infections caused by S. lugdunensis, since peripheral and/or central venous catheters are seldom placed at these sites.
There are some reports from the general adult and pediatric patient population documenting that S. lugdunensis account for 2% to 7.3% of all CoNS isolates.[9, 14] Our data confirm this relatively low rate of occurrence, in patients with cancer. As mentioned above, one possible reason might be the unique ecological niche that these organisms occupy as part of the normal skin flora. Additionally, many patients with hematologic malignancies receive antimicrobial prophylaxis during periods of neutropenia with agents such as TMP-SMX and/or the fluoroquinolones which have activity against these organisms, and might suppress or abort infections. This might explain why infections caused by S. lugdunensis occur infrequently in neutropenic patients, and are documented primarily in patients with solid tumors without neutropenia who do not receive antimicrobial prophylaxis. Indeed, the majority of infections caused by S. lugdunensis in our cancer patients occurred in patients with solid tumors, often related to surgical procedures or implanted medical devices, whilst bloodstream infections were much less common. This spectrum of infection is quite unlike that seen with other CoNS species which predominantly cause catheter-related BSIs.
Typically, rates of true BSI range from 10% to 25% when CoNS are isolated from blood cultures and endocarditis is a rare event.[15] Previous reports of S. lugdunensis isolated from the blood stream have emphasized the invasiveness and the destructiveness of this pathogen.[14, 16] In our cohort, five patients had a BSI involving S. lugdunensis. Only one developed destructive native aortic valve endocarditis as previously mentioned. The remaining four cases of BSI were similar to what is typically observed with CoNS species, with no evidence of invasive or destructive disease. Our rate of invasive disease appears to be higher than the reported rate for other CoNS such as S. epidermidis. However, the number of patients with BSI in our cohort is small.
As mentioned earlier, S. lugdunensis was isolated from polymicrobial infections in 25 patients, with a spectrum similar to that of monomicrobial infections (i.e. most were SSSIs 18 cases, 72%, data not shown). The role of S. lugdunensis in these polymicrobial infections was difficult to determine, therefore they were excluded from this analysis. However, it is quite likely (based on the similarity of these infections to monomicrobial S. lugdunensis infections) that these infections are also clinically significant, and should be treated accordingly.
CoNS isolated from patients with previous exposures to the health care system are usually resistant to multiple antimicrobial agents. Streit and colleagues reported that 87.5% of isolates were resistant to oxacillin, 93.5% were resistant to penicillin, 65.6% were resistant to ciprofloxacin, 73% were resistant to erythromycin, 52% were resistant to clindamycin and 48% were resistant to TMP-SMX[17]. More recent studies evaluating the in-vitro activities of various agents against large numbers of Gram-positive isolates from North America and Europe have shown a similar susceptibility/resistance pattern. [18, 19] S. lugdunensis isolates differ from other CoNS species in their antimicrobial susceptibility patterns as well. The majority of isolates (> 90%) are susceptible to methicillin or oxacillin, aminoglycosides, macrolides, quinolones, tetracyclines, rifampin, TMP-SMX, and agents like fucidic acid and mupirocin.[13, 20, 21] Tan and colleagues tested 106 strains of S. lugdunensis for susceptibility to various antimicrobial agents and found > 95 % to be susceptible to cefoxitin, clindamycin, TMP-SMX, and erythromycin.[20] Giormezis and colleagues tested 38 strains and found them all to be cefoxitin and oxacillin susceptible and none of them carried the mecA gene.[21] Moreover, all these isolates were also susceptible to daptomycin, vancomycin, teicoplanin, linezolid, gentamicin, ciprofloxacin, and rifampin. Our results are similar and demonstrate a low overall rate of resistance to most agents tested (table 2). Although a limited number of isolates (7) were available to us for testing against newer agents, they were uniformly susceptible to daptomycin, telavancin, dalbavancin, ceftaroline, and linezolid. Isolated reports of oxacillin resistance are beginning to appear, with some isolates carrying the mecA and SCCmec type V gene [22–25]. Fortunately, such strains are still quite uncommon.
Despite the emphasis by many authors on the tendency of S. lugdunensis to cause aggressive/invasive infections, this experience has not been universal. (Table 3) In a review of 20 cases of S. lugdunensis bacteremia, Ebright and colleagues reported that most of these cases were of short duration, did not produce prolonged fever, and were not associated with secondary suppurative complications or mortality.[26] In a report of 36 significant S. lugdunensis infections, Kleiner et al did not document any cases of aggressive intravascular infections.[14] In another report from, German and associates concluded that the prevalence and clinical significance of S. lugdunensis in pediatric patients was low. These authors recommended against the routine identification of CoNS to species level from non-sterile sites, and only marginally justified this approach from sterile site specimens such as blood.[9] A more recent report, also in pediatric patients, found no cases of invasive disease among seven cases of bacteremia.[27] Our experience lies somewhere in between. Whilst the overall frequency of S. lugdunensis infection in our patients was low, one of our 5 patients with BSI did develop aggressive native aortic valve endocarditis. Given this diversity in clinical significance and manifestations, and some recent changes in antimicrobial susceptibility/resistance patterns, it appears that S. lugdunensis infections are still evolving, and should continue to be closely monitored and reported.
Table 3.
Summary of reports in the literature not focusing exclusively on endocarditis or serious systemic infections caused by Staphylococcus lugdunensis*.
| Author (ref #) |
Year | Number of cases |
Bacteremia | Endocarditis | Other site |
SSSI | Device / catheter related |
Mortality | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| Ebright[26] | 1990– 2002 |
6 | 6 | 1 | 1 | 0 | 5 | 0 | Bacteremia events only |
|
Zinkernagel [28] |
1996 – 2005 |
28 | 28 | 13 | 3 | 1 | 11 | 3 | Bacteremia events only |
| Choi [29] | 1999 – 2009 |
63 | 15 | 4 | 3 | 1 | 11 | 3 | All sites |
| Lin [30] | 2002 – 2012 |
48 | 41 | 4 | 6 | 2 | 15 | 10 | All sites |
| Wu [31] | 2005 – 2009 |
32 | 3 | 0 | 0 | 44 | 3 | 1 | All sites |
| Liang [4] | 2006 – 2011 |
11 | 11 | 3 | 4 | 3 | 1 | 1 | Bacteremia events only |
| Klotchko [6] | 2006 – 2009 |
70 | 21 | 5 | 9 | 35 | 5 | 2 | All sites |
| Kleiner [14] | 2006 – 2007 |
36 | 1 | 0 | 5 | 30 | 0 | 0 | All sites |
| Sato [27] | 2009– 2014 |
7 | 7 | 0 | 1 | 1 | 5 | 0 | Pediatric bacteremia events only |
| Nesher ** | 2011– 2014 |
45 | 5 | 1 | 4 | 36 | 6 | 0 | Cancer patients – all sites |
Case reports and reports focusing exclusively on endocarditis were not included.
Current study
The primary drawback of our study is its retrospective nature. Consequently, these infections were not investigated and/or managed in a uniform and predefined manner. Additionally, we did not have access to many of the S. lugdunensis isolates since our clinical microbiology laboratory does not routinely store such specimens. This prevented us from conducting further analysis of these strains (such as time-kill studies). However, the strength of our data relates to the fact that during the study period, all clinically significant CoNS isolates, including all S. lugdunensis strains, were identified to species level, enabling us to provide accurate, unbiased, and complete information on the frequency and spectrum of S. lugdunensis infections in our cancer patient population, as well as pertinent information on the treatment and outcomes of these infections.
In conclusion, S. lugdunensis causes infection much less often than other CoNS species in patients with cancer. Patients with solid tumors appear to be infected more often, probably because many patients with hematologic malignancies receive antimicrobial prophylaxis with agents (TMP-SMX, fluoroquinolones) that are active against S. lugdunensis. Therefore, neutropenia does not appear to be a significant predisposing factor for S. lugdunensis infection. SSSIs are the predominant infections and are often associated with surgical procedures and/or implanted medical devices, whereas bloodstream infections including those that are catheter-related, are infrequent. However, when BSIs do occur, complications such as endocarditis and deep abscesses may be more common. Consequently, within the limitations of our retrospective study and the lack of prospective data on the true incidence of S. lugdunensis blood stream infections, these organisms should not be dismissed as contaminants even when isolated from a solitary blood culture. Unlike other CoNS species, S. lugdunensis isolates are susceptible to most commonly used antimicrobial agents (including methicillin, other beta-lactams, quinolones, macrolides, TMP-SMX, and several newer anti-staphylococcal agents), and the overall response to therapy is high.
Figure 2.
Details of S. lugdunensis skin and skin structure infection in cancer patients
SSIs – skin and skin structure infections
a - Expected sites included: breast – 15; inguinal, abdominal wall, perineal – 8; and axillary – 1
b - Other sites associated with mandibular surgery
Acknowledgments
Conflict of interest: This study was supported in part by the NIH/NCI under award number P30CA016672 and used the Cancer Center Support Grant resources the authors had full control of the data, analysis and writing of the manuscript and no others had any input.
References
- 1.Nesher L, Rolston KV. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection. 2014;42(1):5–13. doi: 10.1007/s15010-013-0525-9. [DOI] [PubMed] [Google Scholar]
- 2.Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont P. Staphylococcus lugdunensis sp. Nov. And staphylococcus schleiferi sp. Nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38:168–172. [Google Scholar]
- 3.Frank KL, Del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: How daring to be different works for staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111–133. doi: 10.1128/CMR.00036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang M, Mansell C, Wade C, Fisher R, Devlin G. Unusually virulent coagulase-negative staphylococcus lugdunensis is frequently associated with infective endocarditis: A waikato series of patients. N Z Med J. 2012;125(1354):51–59. [PubMed] [Google Scholar]
- 5.Shah NB, Osmon DR, Fadel H, Patel R, Kohner PC, Steckelberg JM, et al. Laboratory and clinical characteristics of staphylococcus lugdunensis prosthetic joint infections. J Clin Microbiol. 2010;48(5):1600–1603. doi: 10.1128/JCM.01769-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klotchko A, Wallace MR, Licitra C, Sieger B. Staphylococcus lugdunensis: An emerging pathogen. South Med J. 2011;104(7):509–514. doi: 10.1097/SMJ.0b013e31821e91b1. [DOI] [PubMed] [Google Scholar]
- 7.Liesenborghs L, Peetermans M, Claes J, Veloso TR, Vandenbriele C, Criel M, et al. Shear-resistant binding to von willebrand factor allows staphylococcus lugdunensis to adhere to the cardiac valves and initiate endocarditis. J Infect Dis. 2016;213(7):1148–1156. doi: 10.1093/infdis/jiv773. [DOI] [PubMed] [Google Scholar]
- 8.Fadel HJ, Patel R, Vetter EA, Baddour LM. Clinical significance of a single staphylococcus lugdunensis-positive blood culture. J Clin Microbiol. 2011;49(4):1697–1699. doi: 10.1128/JCM.02058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.German GJ, Wang B, Bernard K, Stewart N, Chan F, Pacheco AL, et al. Staphylococcus lugdunensis: Low prevalence and clinical significance in a pediatric microbiology laboratory. Pediatr Infect Dis J. 2013;32(1):87–89. doi: 10.1097/INF.0b013e3182755f58. [DOI] [PubMed] [Google Scholar]
- 10.Bieber L, Kahlmeter G. Staphylococcus lugdunensis in several niches of the normal skin flora. Clin Microbiol Infect. 2010;16(4):385–388. doi: 10.1111/j.1469-0691.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 11.Bellamy R, Barkham T. Staphylococcus lugdunensis infection sites: Predominance of abscesses in the pelvic girdle region. Clin Infect Dis. 2002;35(3):E32–E34. doi: 10.1086/341304. [DOI] [PubMed] [Google Scholar]
- 12.Lina B, Vandenesch F, Reverdy ME, Greenland T, Fleurette J, Etienne J. Non-puerperal breast infections due to staphylococcus lugdunensis. Eur J Clin Microbiol Infect Dis. 1994;13(8):686–687. doi: 10.1007/BF01974001. [DOI] [PubMed] [Google Scholar]
- 13.Hellbacher C, Törnqvist E, Söderquist B. Staphylococcus lugdunensis: Clinical spectrum, antibiotic susceptibility, and phenotypic and genotypic patterns of 39 isolates. Clin Microbiol Infect. 2006;12(1):43–49. doi: 10.1111/j.1469-0691.2005.01296.x. [DOI] [PubMed] [Google Scholar]
- 14.Kleiner E, Monk AB, Archer GL, Forbes BA. Clinical significance of staphylococcus lugdunensis isolated from routine cultures. Clin Infect Dis. 2010;51(7):801–803. doi: 10.1086/656280. [DOI] [PubMed] [Google Scholar]
- 15.Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559–566. doi: 10.1086/502584. [DOI] [PubMed] [Google Scholar]
- 16.Sabe MA, Shrestha NK, Gordon S, Menon V. Staphylococcus lugdunensis: A rare but destructive cause of coagulase-negative staphylococcus infective endocarditis. Eur Heart J Acute Cardiovasc Care. 2014 doi: 10.1177/2048872614523350. [DOI] [PubMed] [Google Scholar]
- 17.Streit JM, Jones RN, Sader HS, Fritsche TR. Assessment of pathogen occurrences and resistance profiles among infected patients in the intensive care unit: Report from the sentry antimicrobial surveillance program (north America, 2001) Int J Antimicrob Agents. 2004;24(2):111–118. doi: 10.1016/j.ijantimicag.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Sahm DF, Deane J, Bien PA, Locke JB, Zuill DE, Shaw KJ, et al. Results of the surveillance of tedizolid activity and resistance program: In vitro susceptibility of gram-positive pathogens collected in 2011 and 2012 from the united states and europe. Diagn Microbiol Infect Dis. 2015;81(2):112–118. doi: 10.1016/j.diagmicrobio.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Biedenbach DJ, Arhin FF, Moeck G, Lynch TF, Sahm DF. In vitro activity of oritavancin and comparator agents against staphylococci, streptococci and enterococci from clinical infections in europe and north America, 2011–2014. Int J Antimicrob Agents. 2015;46(6):674–681. doi: 10.1016/j.ijantimicag.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393–2395. doi: 10.1128/JCM.00740-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giormezis N, Kolonitsiou F, Makri A, Vogiatzi A, Christofidou M, Anastassiou ED, et al. Virulence factors among staphylococcus lugdunensis are associated with infection sites and clonal spread. Eur J Clin Microbiol Infect Dis. 2015;34(4):773–778. doi: 10.1007/s10096-014-2291-8. [DOI] [PubMed] [Google Scholar]
- 22.Yen TY, Sung YJ, Lin HC, Peng CT, Tien N, Hwang KP, et al. Emergence of oxacillin-resistant staphylococcus lugdunensis carrying staphylococcal cassette chromosome mec type v in central taiwan. J Microbiol Immunol Infect. 2014 doi: 10.1016/j.jmii.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Shen D, Guo J, Wang K, Wang H, Yan Z, et al. Clinical and microbiological characterization of staphylococcus lugdunensis isolates obtained from clinical specimens in a hospital in china. BMC Microbiol. 2012;12:168. doi: 10.1186/1471-2180-12-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tseng SP, Lin YT, Tsai JC, Hung WC, Chen HJ, Chen PF, et al. Genotypes and phenotypes of staphylococcus lugdunensis isolates recovered from bacteremia. J Microbiol Immunol Infect. 2013 doi: 10.1016/j.jmii.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Pereira EM, Schuenck RP, Nouér SA, Santos KR. Methicillin-resistant staphylococcus lugdunensis carrying sccmec type v misidentified as mrsa. Braz J Infect Dis. 2011;15(3):293–295. doi: 10.1016/s1413-8670(11)70192-1. [DOI] [PubMed] [Google Scholar]
- 26.Ebright JR, Penugonda N, Brown W. Clinical experience with staphylococcus lugdunensis bacteremia: A retrospective analysis. Diagn Microbiol Infect Dis. 2004;48(1):17–21. doi: 10.1016/j.diagmicrobio.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Sato M, Kubota N, Horiuchi A, Kasai M, Minami K, Matsui H. Frequency, clinical manifestations, and outcomes of staphylococcus lugdunensis bacteremia in children. J Infect Chemother. 2016;22(5):298–302. doi: 10.1016/j.jiac.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Zinkernagel AS, Zinkernagel MS, Elzi MV, Genoni M, Gubler J, Zbinden R, et al. Significance of staphylococcus lugdunensis bacteremia: Report of 28 cases and. Infection. 2008;36(4):314–321. doi: 10.1007/s15010-008-7287-9. [DOI] [PubMed] [Google Scholar]
- 29.Choi SH, Chung JW, Lee EJ, Kim TH, Lee MS, Kang JM, et al. Incidence, characteristics, and outcomes of staphylococcus lugdunensis bacteremia. J Clin Microbiol. 2010;48(9):3346–3349. doi: 10.1128/JCM.00609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin JF, Cheng CW, Kuo AJ, Liu TP, Yang CC, Huang CT, et al. Clinical experience and microbiologic characteristics of invasive staphylococcus lugdunensis infection in a tertiary center in northern taiwan. J Microbiol Immunol Infect. 2015;48(4):406–412. doi: 10.1016/j.jmii.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Wu AB, Wang MC, Tseng CC, Lin WH, Teng CH, Huang AH, et al. Clinical and microbiological characteristics of community-acquired staphylococcus lugdunensis infections in southern taiwan. J Clin Microbiol. 2011;49(8):3015–3018. doi: 10.1128/JCM.01138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]